Abstract
Background
Safety of rechallenge of immune checkpoint inhibitor (ICI) after grade ≥2 immune-related adverse events (irAEs) leading to ICI discontinuation remains unclear.
Methods
All adverse drug reactions involving at least one ICI reported up to December 31, 2019 were extracted from the French pharmacovigilance database. Patients were included if they experienced at least one grade ≥2 irAE resulting in ICI discontinuation, with subsequent ICI rechallenge. The primary outcome was the recurrence of at least one grade ≥2 irAE in these patients after ICI rechallenge.
Results
We included 180 patients: 61.1% were men (median age of 66 years), 43.9% had melanoma and 78.9% were receiving anti-programmed cell death 1. First ICI discontinuation was related to 191 irAEs. After ICI rechallenge, 38.9% of the patients experienced at least one grade ≥2 irAE. Among them, 70.0% experienced the same irAE, 25.7% a distinct irAE, and 4.3% both the same and a distinct irAE. Lower recurrence rates of irAEs were associated with rechallenge with the same ICI treatment (p=0.02) or first endocrine irAEs (p=0.003). Gastrointestinal irAEs were more likely to recur (p=0.007). The median duration from ICI discontinuation to rechallenge and the severity of the initial irAE did not predict recurrent irAEs after ICI rechallenge (p=0.53 and p=0.40, respectively).
Conclusions
In this study, 61.1% of the patients who discontinued ICI treatment for grade ≥2 irAEs experienced no recurrent grade ≥2 irAEs after ICI rechallenge. Although ICI rechallenge appears to be safe under close monitoring, it should always be discussed balancing usefulness of rechallenge, patient comorbidities and risk of recurrence of first irAE(s). Due to inherent bias associated with pharmacovigilance studies, further prospective studies are needed to assess risk factors that may influence patient outcomes after ICI rechallenge.
Keywords: immunotherapy, autoimmunity
Introduction
Immune checkpoint inhibitors (ICIs) are the new standard of care in the treatment of many different types of cancer.1 ICIs promote antitumor immune response by inhibiting cytotoxic T-lymphocyte antigen-4 (CTLA-4) or programmed cell death 1 or ligand 1 (PD-1/PDL-1) or both, leading to durable responses when compared with standard chemotherapy.2–9 Because of the rapid and broad expansion of their indications, ICIs have been prescribed to a growing number of patients, resulting in increased understanding of their safety profile.
ICI use is associated with immune-related adverse events (irAEs), related to their T-cell mechanism of action. IrAEs can affect nearly any organ system, with varying degrees of severity.10 ICI therapy can generally be continued with close monitoring in the presence of grade 1 irAEs. Grade ≥2 irAEs require corticosteroids and temporary or permanent ICI discontinuation.11–13 Severe irAEs leading to ICI discontinuation affect approximately 15%, 10% and 5% of patients receiving anti-CTLA4, anti-PD-1 or anti-PD-L1 agents, respectively, and 50% of patients receiving both nivolumab and ipilimumab.10 11 13 14 According to the current guidelines, permanent discontinuation of ICI treatment is generally recommended for grade 4 irAEs. The decision to resume ICI treatment after grade ≥2 irAEs often remains challenging. Indeed, ICI rechallenge can be associated not only with significant antitumor response,15 16 but also with a theoretically increased significant risk of irAE(s) in comparison with naïve patients. Indeed, few studies have explored the issue of ICI rechallenge after an irAE. The largest series of ICI rechallenges after an irAE focused only on the recurrence rate of the same irAE after the rechallenge of the same ICI, thereby covering only part of the possible clinical scenario.17 Many other studies have also been based on small samples of patients.15 16 18 19 So far, the recurrence rate of irAEs varies from 39% to 55%.20 In this study, we aimed to characterize the safety of ICI rechallenge after initial grade ≥2 irAEs, which led to subsequent ICI discontinuation.
Methods
Data source
Data were extracted from the French pharmacovigilance database (FPVD). Briefly, the FPVD includes all adverse drug reactions (ADRs) spontaneously reported to the 31 French Regional Pharmacovigilance Centers since 1985. French regulations require healthcare professionals to report all ADRs to the Regional Pharmacovigilance Centre on which they depend. All ADRs are diagnosed by healthcare specialists on the basis of clinical data, laboratory findings and/or imaging if necessary. All ADRs reports are then reviewed by pharmacologists with expertize in this field. Causality is assessed according to the French method for causality assessment of ADRs.21 Cases of ADRs are then recorded in the database, using the Medical Dictionary for Regulatory Activities.22
Eligibility criteria
All ADRs involving at least one ICI (including nivolumab, pembrolizumab, cemiplimab, ipilimumab, atezolizumab, durvalumab and avelumab) coded as suspected or interacting drugs according to the WHO criteria and reported up to December 31, 2019 were extracted from the FPVD. Patients were included if they experienced at least one grade ≥2 irAE resulting in substantial ICI discontinuation, with subsequent ICI rechallenge. Patients must have discontinued ICI treatment for a period at least equal to twice the duration of a cycle (ie, for at least 4 weeks for a 2 weekly dosing regimen, 6 weeks for a 3 weekly dosing regimen, 8 weeks for a monthly dosing regimen and 12 weeks for a 6 weekly dosing regimen).
For each included case, the following data were collected: patient data (age, sex, body mass index (BMI)), characteristics of the ICIs (type, indication (including number of prior courses), duration between discontinuation and rechallenge, reason for ICI rechallenge), characteristics of the irAEs (type, time to occurrence, severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) V.5.0, management (including corticosteroids and other immunosuppressive agents) and outcomes).
Clinical outcomes
The primary outcome was the recurrence of at least one grade ≥2 irAE after ICI rechallenge in patients who discontinued ICI treatment for at least one grade ≥2 irAE. We specifically evaluated the recurrence rate of the same grade ≥2 irAE after ICI rechallenge, the recurrence rate of a distinct irAE after ICI rechallenge, and the characteristics of the patients and the irAEs. The recurrence of endocrine irAEs was defined as the need to increase significantly the dose of hormone replacement therapy after ICI rechallenge. The factors associated with irAE recurrence after ICI rechallenge were considered as secondary outcomes.
Statistical analysis
In the descriptive statistical analyzes, continuous variables were described by mean and SD, or median and IQR and categorical variables by the number and proportion of subjects in each class. All variables with less than 20% of missing data were compared between groups using Student’s t-test or Wilcoxon’s test for continuous variables and Pearson’s χ2 test or Fisher’s exact test for categorical variables. The threshold for statistical significance was set at p<0.05. All statistical analyzes were performed using SAS software (V.9.4).
Results
Description of the study population
Patient and first ICI treatment characteristics
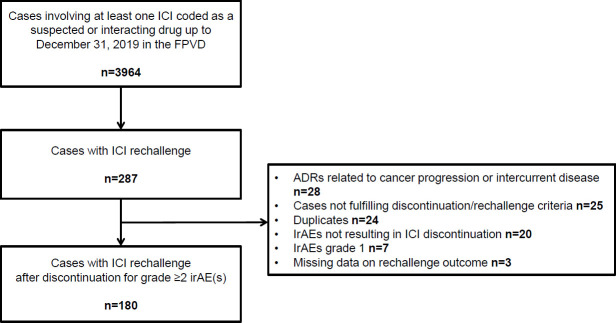
A total of 180 patients were included in the study (figure 1). Patient characteristics are summarized in table 1. Sixty-one per cent were men, the median (IQR) age was 66 (55–72) years. Melanoma was the most common tumor type (43.9%), followed by lung cancer (41.1%) and renal cell carcinoma (6.1%). Seventy-nine per cent of the patients were receiving an anti-PD-1 agent, 10.0% an anti-CTLA-4 and anti-PD-1 combination, 6.0% an anti-CTLA-4 agent and 5.0% an anti-PD-L1 agent. ICI treatment was initiated as a first-line treatment in 22.8% of the patients.
Figure 1.
Flow chart. ADR, adverse drug reaction; FPVD, French pharmacovigilance database; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event.
Table 1.
Characteristics of the study population
| All patients (n=180) |
IrAE recurrence (n=70) |
No irAE recurrence (n=110) |
P value | ||||
| Median age, years (IQR) | 66 | (55–72) | 66 | (59–70) | 66 | (54–72) | 0.98 |
| Male gender, n (%) | 110 | (61.1) | 47 | (67.1) | 63 | (57.3) | 0.19 |
| Median BMI (IQR) | 24.4 | (21.8–26.9) | 26.0 | (21.9–27.7) | 23.8 | (21.6–26.6) | –* |
| Missing data, n (%) | 62 | (34.4) | 19 | (27.1) | 43 | (39.1) | |
| Cancer type, n (%) | 0.12 | ||||||
| Melanoma | 79 | (43.9) | 33 | (47.2) | 46 | (41.8) | |
| Lung cancer | 74 | (41.1) | 25 | (35.7) | 49 | (44.6) | |
| Renal cell carcinoma | 11 | (6.1) | 8 | (11.4) | 3 | (2.7) | |
| Lymphoma | 5 | (2.8) | 1 | (1.4) | 4 | (3.6) | |
| Others† | 10 | (5.6) | 3 | (4.3) | 7 | (6.4) | |
| Missing data | 1 | (0.5) | 0 | (0.0) | 1 | (0.9) | |
| Initial ICI type, n (%) | 0.44 | ||||||
| Anti-PD-1 | 142 | (78.9) | 53 | (75.7) | 89 | (80.9) | |
| Anti-PDL-1 | 9 | (5.0) | 5 | (7.1) | 4 | (3.6) | |
| Anti-CTLA-4 | 11 | (6.1) | 3 | (4.3) | 8 | (7.3) | |
| Anti-PD-1+anti-CTLA-4 | 18 | (10.0) | 9 | (12.9) | 9 | (8.2) | |
| Treatment line, n (%) | –* | ||||||
| First-line | 41 | (22.8) | 15 | (21.4) | 26 | (23.6) | |
| Second-line | 67 | (37.2) | 29 | (41.4) | 38 | (34.5) | |
| Third-line | 19 | (10.5) | 9 | (12.9) | 10 | (9.1) | |
| Fourth line and over | 16 | (8.9) | 4 | (5.7) | 12 | (11.0) | |
| Missing data | 37 | (20.6) | 13 | (18.6) | 24 | (21.8) | |
| Median time to first irAE, days (IQR) | 84 | (39–155) | 112 | (50–219) | 63 | (34–135) | 0.01 |
| Missing data, n (%) | 3 | (1.7) | 2 | (2.9) | 1 | (0.9) | |
| Median time to first irAE, treatment cycle (IQR) | 4 | (2.0–9.0) | 5 | (2.0–10.0) | 4 | (2.0–7.5) | 0.27 |
| Missing data, n (%) | 13 | (7.2) | 7 | (10.0) | 6 | (5.5) | |
| Grade of first irAE, n (%) | 0.40 | ||||||
| 2 | 92 | (51.1) | 33 | (47.1) | 59 | (53.6) | |
| 3/4 | 88 | (48.9) | 37 | (52.9) | 51 | (46.4) | |
| Treatment of first irAE, n (%) | |||||||
| Systemic corticosteroids | 98 | (54.4) | 42 | (60.0) | 56 | (50.9) | 0.10 |
| Other immunosuppressant add-on | 9 | (5.0) | 6 | (8.6) | 3 | (2.7) | 0.08 |
| Anti-TNF agent/vedolizumab | 5 | (2.8) | 4 | (5.7) | 1 | (0.9) | |
| Mycophenolate mofetil | 2 | (1.1) | 0 | (0.0) | 2 | (1.8) | |
| Hydroxychloroquine | 1 | (0.5) | 1 | (1.4) | 0 | (0.0) | |
| Methotrexate | 1 | (0.5) | 1 | (1.4) | 0 | (0.0) | |
| Missing data | 4 | (2.2) | 4 | (5.7) | 0 | (0.0) | |
| Reason for ICI rechallenge, n (%) | 0.76 | ||||||
| Continued/maintenance therapy | 156 | (86.7) | 60 | (85.7) | 96 | (87.3) | |
| Progression | 24 | (13.3) | 10 | (14.3) | 14 | (12.7) | |
| Rechallenge of the same ICI or ICI combination, n (%) | 153 | (85.0) | 54 | (77.1) | 99 | (90.0) | 0.02 |
| Median duration from ICI discontinuation to rechallenge, days (IQR) | 56 | (42–84) | 56 | (42–84) | 56 | (42–85) | 0.53 |
*More>20.0% missing data.
†Including urothelial cancer (n=3 (1.7%)), head and neck cancer (n=2 (1.1%)), cutaneous squamous cell carcinoma (n=2 (1.1%)), glioblastoma (n=1 (0.5%)), Merkel cell carcinoma (n=1 (0.5%)) and colorectal cancer (n=1 (0.5%)).
BMI, body mass index; CTLA-4, cytotoxic T-lymphocyte antigen-4; ICI, immune checkpoint inhibitor; irAE, immune related adverse event; PD-1, programmed cell death 1; PDL-1, programmed cell death 1 ligand 1; TNF, tumor necrosis factor.
Clinical features of grade ≥2 irAEs leading to ICI discontinuation
One hundred and ninety-one irAEs led to ICI discontinuation in 180 patients (table 2). Ten patients (5.6%) had ≥2 irAEs leading to ICI discontinuation. The most frequent irAEs resulting in ICI discontinuation included gastrointestinal disorders (24.6%, including colitis (19.4%) and pancreatic disorders (4.7%)), endocrine disorders (17.8%), hepatitis (16.2%), respiratory disorders (11.0%) and skin disorders (9.4%). Fifty-two per cent of irAEs were grade 2, 46.5% grade 3 and 1.5% grade 4. The median (IQR) time to occurrence of first irAE was 84 (39–155) days.
Table 2.
Characteristics of the immune-related adverse events
| irAEs (n*) | Initial irAE(s)† (n=191) |
Second irAEs after ICI rechallenge‡ (n=77) |
||||||
| Initial irAEs (%) | Grade | Systemic corticosteroids |
Second irAEs (%) | Grade | Systemic corticosteroids |
|||
| 2 | 3/4 | 2 | 3/4 | |||||
| Gastrointestinal disorders (71) | 24.6 | 17 | 30 | 35§ | 31.2 | 15 | 9 | 20¶ |
| Colitis (57) | 19.4 | 13 | 24 | 30 | 26.0 | 12 | 8 | 17 |
| Pancreatic disorders (13) | 4.7 | 3 | 6 | 5 | 5.2 | 3 | 1 | 3 |
| Gastritis (1) | 0.5 | 1 | – | – | – | – | – | – |
| Endocrine disorders (41) | 17.8 | 23 | 11 | 4 | 9.1 | 4 | 3 | 1 |
| Hyperthyroidism (13) | 6.3 | 10 | 2 | 4 | 1.3 | 1 | – | 1 |
| Hypophysitis (10) | 4.7 | 5 | 4 | – | 1.3 | 1 | – | – |
| Diabetes (8) | 2.1 | 2 | 2 | – | 5.2 | 1 | 3 | – |
| Hypothyroidism (5) | 2.6 | 4 | 1 | – | 1.3 | – | – | – |
| Adrenal insufficiency (5) | 2.1 | 2 | 2 | – | 1.3 | 1 | – | – |
| Hepatitis (39) | 16.2 | 12 | 19 | 17§ | 10.4 | 2 | 6 | 7 |
| Respiratory disorders (28) | 11.0 | 15 | 6 | 15 | 9.1 | 5 | 2 | 4 |
| Pneumonitis (24) | 9.4 | 12 | 6 | 15 | 7.8 | 4 | 2 | 4 |
| Pulmonary sarcoidosis (2) | 1.0 | 2 | – | – | – | – | – | – |
| Pulmonary embolism (2) | 0.5 | 1 | – | – | 1.3 | 1 | – | – |
| Skin disorders (28) | 9.4 | 9 | 9 | 8§ | 13.0 | 6 | 4 | 6 |
| Musculoskeletal disorders (17) | 5.8 | 8 | 3 | 11§ | 7.8 | 5 | 1 | 3¶ |
| Arthritis/arthralgia (14) | 4.7 | 6 | 3 | 9 | 6.5 | 5 | – | 2 |
| Myositis (3) | 1.0 | 2 | – | 2 | 1.3 | – | 1 | 1 |
| Renal and urinary disorders (16) | 5.2 | 6 | 4 | 5 | 7.8 | 6 | – | 6 |
| Neurological disorders (8) | 3.1 | 4 | 2 | 5 | 2.6 | 1 | 1 | 1 |
| Hematological disorders (8) | 3.1 | 2 | 4 | 4 | 2.6 | 2 | – | – |
| Ocular disorders (7) | 2.6 | 3 | 2 | 1 | 2.6 | 2 | – | 2 |
| Cardiac disorders (5) | 1.0 | – | 2 | – | 3.9 | 2 | 1 | 2 |
| Total (268) | 100.0 | 99 | 92 | 105** | 100.0 | 50 | 27 | 52†† |
Detailed data on recurrent irAEs are shown in online supplemental table.
The bold values refer to organ involvement. Non bold values refer to more precise diseases.
*Including initial and second irAEs.
†191 grade ≥2 irAEs led to discontinuation in 180 patients.
‡77 recurrent and/or new onset irAEs occurred in 70 patients.
§Immunosuppressive drugs were required in 11 initial irAEs (nine patients): colitis (n=5), hepatitis (n=3), arthritis (n=2) and lichenoid dermatitis (n=1).
¶Immunosuppressive drugs were required in 6 second irAEs (six patients): colitis (n=5) and arthritis (n=1).
**Missing data in four initial irAEs.
††Missing data in 13 second irAEs.
ICI, immune checkpoint inhibitor; IrAE, immune-related adverse event.
jitc-2020-001622supp001.pdf (91.9KB, pdf)
Among the 180 patients, 98 (54.4%) received corticosteroid treatment. Among them, 9 (5.0%) required immunosuppressive drugs, including anti-tumor necrosis factor (anti-TNF) agent or vedolizumab for colitis (n=5, 2.8%), mycophenolate mofetil for hepatitis (n=2, 1.1%), hydroxychloroquine for lichenoid dermatitis (n=1, 0.5%) and methotrexate for rheumatoid arthritis (n=1, 0.5%).
Characteristics of ICI rechallenge after discontinuation for grade ≥2 irAE(s)
On resuming ICI after grade ≥2 irAEs, 159 patients (88.3%) received an anti-PD-1 agent and 153 patients (85.0%) the same ICI drug or the same ICIs combination. Ninety-one per cent of irAEs resolved to grade 1 or lower before ICI rechallenge. Fifty-two patients (28.9%, missing data n=23) were on systemic corticosteroids when they resumed ICI treatment. One hundred and fifty-six patients (86.7%) resumed ICI treatment as a maintenance therapy, and 24 (13.3%) for disease progression. Median (IQR) duration from ICI discontinuation to rechallenge was 56 (42–84) days (table 1).
Outcomes
Safety of ICI rechallenge after discontinuation for grade ≥2 irAE(s)
After ICI rechallenge, 70 patients (38.9%) experienced at least one grade ≥2 irAE (n=77 irAEs) (table 2). Six patients (8.6%) experienced ≥2 recurrent (same or distinct) irAEs. Sixty-five per cent of irAEs were grade 2, 33.8% grade 3 and 1.2% grade 4. The median (IQR) time to occurrence of the second irAE was 46 (16–103) days.
Among the 70 patients, 49 (70.0%) experienced a recurrence of the first irAE, 18 (25.7%) a new irAE, and 3 (4.3%) both a recurrence of the first irAE and a new irAE after ICI rechallenge.
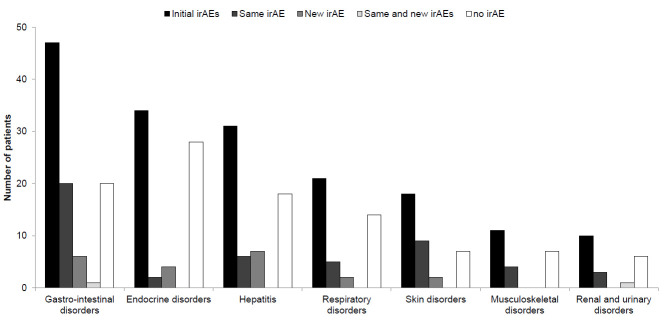
The recurrence rate of irAEs varied according to the organs involved in the first irAEs (online supplemental table). Twenty-one out of 47 patients (44.7%) experienced recurrent gastrointestinal irAEs, 2 of 34 (5.9%) recurrent endocrine irAEs, 9 of 18 (50.0%) recurrent skin irAEs, 1 of 6 (16.7%) recurrent encephalitis and 1 of 2 (50.0%) recurrent myocarditis (figure 2).
Figure 2.
Patient outcomes after immune checkpoint inhibitor rechallenge according to the type of irAEs. irAE, immune-related adverse event.
Most patients (68.6%) required systemic corticosteroids, and 6 (8.6%) an immunosuppressive drug (anti-TNF agent or vedolizumab (n=5) and methotrexate (n=1)). No death related to recurrent irAE was reported, and 76.6% of second irAEs resolved to grade 1 or lower. Forty-seven patients (67.1%) discontinued ICI treatment due to recurrent irAEs.
Factors associated with irAE(s) recurrence
In patients with recurrent grade ≥2 irAEs, the median time to first irAE was longer compared with patients without irAE after ICI rechallenge (p=0.01) (table 1). The patients rechallenged with the same ICI drug or ICIs combination had lower rates of recurrence than those rechallenged with another ICI drug or ICIs combination (p=0.02). The patients with initial gastrointestinal irAEs were more likely to have recurrent grade ≥2 irAEs after ICI rechallenge in comparison with other patients (p=0.007). By contrast, initial endocrine irAEs were less likely to recur (p=0.003). The median duration from ICI discontinuation to rechallenge and the severity of the initial irAE did not predict recurrent irAEs after ICI rechallenge (p=0.53 and p=0.40, respectively).
Discussion
This study aimed to provide complementary data on safety of ICI rechallenge after grade ≥2 irAE(s), which have led to substantial ICI discontinuation in 180 patients. Herein, we found that 61.1% of the patients who discontinued ICI treatment for grade ≥2 irAE(s) experienced no recurrent grade ≥2 irAE(s) after ICI rechallenge.
Data on safety of ICI rechallenge after grade ≥2 irAE(s) leading to ICI discontinuation are scarce. Recent studies with small sample sizes were mainly focused on the rechallenge of anti-PD-1 or anti-PD-L1 after discontinuation for irAEs. Anti-PD-1 and anti-PDL-1 rechallenge was associated with occurrence of a second grade ≥2 irAE in 55.0% of patients treated for various types of cancers.18 In 68 patients with anti-PD-L1 for advanced non-small cell lung cancer who stopped treatment due to irAEs, 55.0% experienced the same or a distinct irAE after anti-PD-L1 rechallenge.15 In the largest published series of ICI rechallenges after irAEs (n=452), Dolladille et al explored recurrence of the same irAE after rechallenge with the same ICI and found a 28.8% recurrence rate of the same irAE, which is quite similar to the rate observed in our study.17
In the present study, no patient died after ICI rechallenge. Almost half of the patients experienced initial grade ≥3 irAEs, while a third of the patients with recurrent irAEs experienced grade ≥3 irAEs, suggesting that ICI rechallenge was not associated with more severe irAEs. Furthermore, we did not observe any statistically significant difference between the group with irAE recurrence and the group with no irAE recurrence with regard to first irAE(s) severity. ICI rechallenge should always be considered with caution after a grade 4 irAE.11 ICI rechallenge was associated with recurrence of a distinct irAE in 1 of 3 patients with initial grade 4 irAEs. In addition, due to life-threatening risk, ICI rechallenge after neurological or cardiac irAEs is not recommended. In our study, 1 out of 6 patients and 1 out of 2 patients experienced recurrent encephalitis and myocarditis respectively, raising the question of the benefit/risk ratio of ICI rechallenge in these patients.
In this study, the time to first irAE was longer in the recurrence group compared the free-recurrence group. By contrast, Simonaggio et al observed that the first irAEs occurred earlier in the patients who experienced new or recurrent irAEs after ICI rechallenge.18 Such discrepancy could be related to the wide profile of irAEs, and to the longer duration between ICI discontinuation and rechallenge in our cohort (median of 8 weeks vs 3.8 weeks). This latter aspect could lead to exclude early worsening of pre-existing irAEs after ICI rechallenge, wrongly considered as irAEs recurrence.
Patients with initial gastrointestinal irAEs were more likely to have recurrent grade ≥2 irAEs after ICI rechallenge compared with those with no gastrointestinal irAEs. By contrast, initial endocrine disorders were less likely to recur. These findings are consistent with those previously reported by Dolladille et al.17 These authors observed a higher recurrence rate for colitis compared with others irAEs, while the recurrence rate of adrenal irAEs was lower than that for other irAEs.
The question of which ICI should be restarted after ICI discontinuation for severe irAEs is essential. In our study, the rechallenge of the same ICI drug or the same ICI combination was associated with a lower rate of irAE recurrence. This finding is particularly interesting for patients who progress after ICI discontinuation for irAE. Most studies have only reported the safety of PD-1 rechallenge as maintenance therapy after anti-PD-1/anti-CTLA-4 discontinuation for severe irAEs. For example, Pollack et al concluded that patients who experienced colitis or hypophysitis after anti-PD-1/anti-CTLA-4 combination could safely resume anti-PD-1.19 In a retrospective multicenter study in 167 patients, Abu-Sbeih et al also found that anti-PD-1/anti-PDL-1 rechallenge was associated with a lower risk of recurrent colitis (OR 0.30, 95% CI 0.11 to 0.81).23
This study has several strengths. First, all irAEs reports in the FPVD had been diagnosed by healthcare specialists and validated by a pharmacologist with expertize in the field. Second, this study provides additional data on safety of ICI rechallenge in a large cohort of patients treated in real-life practice with a broad spectrum of ICI regimens for varying types of cancers. It is also the first study to propose a standard definition of ICI rechallenge after discontinuation for irAEs. ICI rechallenge was only considered if the patients discontinued ICI treatment for a period at least equal to twice the duration of a cycle. Delyon et al suggested that the median duration between ICI discontinuation and rechallenge should be taken into account when considering ICI rechallenge.24 Indeed, as discussed earlier, a short temporary discontinuation could be associated with worsening of pre-existing irAEs, rather than recurrence of irAEs. However, in this study, the duration from ICI discontinuation to rechallenge was not associated with a higher risk of recurrence of irAEs.
Our study presents some limitations. The pharmacovigilance system is based on spontaneous reporting of ADRs, which can result in under reporting of less severe, for example, grade 2 irAEs, and/or expected ADRs.25 26 Given retrospective data collection, several data, such as number of prior courses or BMI were missing. Although the recurrence rate of irAEs in our study is quite similar to that previously reported, it could have been underestimated in the cases with insufficient retrospective update or when under-reporting concerned recurrent irAEs after ICI rechallenge. Despite these limitations, our results highlight the value of spontaneously reporting databases to characterize the safety profile of ICIs.
Conclusions
In conclusion, 61.1% of the patients who discontinued ICI treatment for grade ≥2 irAEs experienced no recurrent grade ≥2 irAEs after ICI rechallenge. ICI rechallenge after discontinuation for irAEs was not associated with more severe recurrent irAEs. Gastrointestinal disorders were more likely to recur after ICI rechallenge, whereas endocrine disorders had a lower recurrence rate. Due to inherent bias associated with pharmacovigilance studies, further prospective studies are needed to assess risk factors that influence patient outcomes after ICI rechallenge. Hence, even though ICI rechallenge appears to be safe under close monitoring, it should always be discussed in a multidisciplinary team meeting in light of usefulness of rechallenge, patient comorbidities and risk of recurrence of first irAE(s).
Acknowledgments
The authors would like to thank the 31 centers of the French pharmacovigilance network and Jeffrey Arsham for his help in the editing.
Footnotes
Contributors: Conception and design: MA, TL, MP. Data collection: MA, TL. Analysis and interpretation of data: MA, TL, MP, M-CP-P. Writing, review and/or revision of the manuscript: all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: In accordance with French regulations, formal approval by an investigational review board is not required for this type of study (performed here by the French pharmacovigilance Network). As all data recorded in the FPVD are anonymous, informed consent is waived. The FPVD is registered with the French data protection agency (Commission Nationale de l’Informatique et des Libertés, CNIL) (deliberation no. 2014-032, July 10, 2014).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request to the corresponding author.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. Hirsch L, Zitvogel L, Eggermont A, et al. PD-Loma: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br J Cancer 2019;120:3–5. 10.1038/s41416-018-0294-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 3. Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020;126:4156–67. 10.1002/cncr.33033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924–33. 10.1200/JCO.2017.74.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22. 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 6. Beköz H, Karadurmuş N, Paydaş S, et al. Nivolumab for relapsed or refractory Hodgkin lymphoma: real-life experience. Ann Oncol 2017;28:2496–502. 10.1093/annonc/mdx341 [DOI] [PubMed] [Google Scholar]
- 7. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810–21. 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 9. Mansfield AS, Każarnowicz A, Karaseva N, et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol 2020;31:310–7. 10.1016/j.annonc.2019.10.021 [DOI] [PubMed] [Google Scholar]
- 10. Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 11. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016;27:559–74. 10.1093/annonc/mdv623 [DOI] [PubMed] [Google Scholar]
- 13. Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv264–6. 10.1093/annonc/mdy162 [DOI] [PubMed] [Google Scholar]
- 14. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139–48. 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 15. Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and efficacy of Re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 2018;6:1093–9. 10.1158/2326-6066.CIR-17-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abou Alaiwi S, Xie W, Nassar AH, et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J Immunother Cancer 2020;8:e000144. 10.1136/jitc-2019-000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol 2020;6:865–7. 10.1001/jamaoncol.2020.0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of Readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 2019;5:1310. 10.1001/jamaoncol.2019.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 2018;29:250–5. 10.1093/annonc/mdx642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakajima EC, Lipson EJ, Brahmer JR. Challenge of rechallenge: when to resume immunotherapy following an immune-related adverse event. J Clin Oncol 2019;37:2714–8. 10.1200/JCO.19.01623 [DOI] [PubMed] [Google Scholar]
- 21. Bégaud B, Evreux JC, Jouglard J, et al. [Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France]. Therapie 1985;40:111–8. [PubMed] [Google Scholar]
- 22. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999;20:109–17. 10.2165/00002018-199920020-00002 [DOI] [PubMed] [Google Scholar]
- 23. Abu-Sbeih H, Ali FS, Naqash AR, et al. Resumption of immune checkpoint inhibitor therapy after immune-mediated colitis. J Clin Oncol 2019;37:2738–45. 10.1200/JCO.19.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Delyon J, Lourenço N, Vu L-T, et al. Recurrence of immune-mediated colitis upon immune checkpoint inhibitor resumption: does time matter? J Clin Oncol 2019;37:3563–4. 10.1200/JCO.19.01891 [DOI] [PubMed] [Google Scholar]
- 25. Hazell L, Shakir SAW. Under-reporting of adverse drug reactions : a systematic review. Drug Saf 2006;29:385–96. 10.2165/00002018-200629050-00003 [DOI] [PubMed] [Google Scholar]
- 26. Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf 2009;32:19–31. 10.2165/00002018-200932010-00002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001622supp001.pdf (91.9KB, pdf)