Abstract
Interstitial cystitis/bladder pain syndrome is a debilitating condition of unknown etiology characterized by persistent pelvic pain with lower urinary tract symptoms and comprises a wide variety of potentially clinically useful phenotypes with different possible etiologies. Current clinicopathological and genomic evidence suggests that interstitial cystitis/bladder pain syndrome should be categorized by the presence or absence of Hunner lesions, rather than by clinical phenotyping based on symptomatology. The Hunner lesion subtype is a distinct inflammatory disease with proven bladder etiology characterized by epithelial denudation and enhanced immune responses frequently accompanied by clonal expansion of infiltrating B cells, with potential engagement of infection. Meanwhile, the non-Hunner lesion subtype is a non-inflammatory disorder with little evidence of bladder etiology. It is potentially associated with urothelial malfunction and neurophysiological dysfunction, and frequently presents with somatic and/or psychological symptoms, that commonly result in central nervous sensitization. Animal models of autoimmune cystitis and neurogenic sensitization might serve as disease models for the Hunner lesion and non-Hunner lesion subtypes, respectively. Here, we revisit the taxonomy of interstitial cystitis/bladder pain syndrome according to current research, and discuss its potential pathophysiology and representative animal models. Categorization of interstitial cystitis/bladder pain syndrome based on cystoscopy is mandatory to design optimized treatment and research strategies for each subtype. A tailored approach that specifically targets the characteristic inflammation and epithelial denudation for the Hunner lesion subtype, or the urothelial malfunction, sensitized/altered nervous system and psychosocial problems for the non-Hunner lesion subtype, is essential for better clinical management and research progress in this complex condition.
Keywords: animal model, autoimmune cystitis, bladder pain syndrome, interstitial cystitis, pathophysiology
Introduction
IC/BPS refers to a symptom syndrome complex characterized by persistent pain perceived to be related to the urinary bladder in conjunction with urinary frequency and/or urgency, and presents with a wide variety of clinical phenotypes.1–4 Although the whole picture of IC/BPS remains unclear, IC/BPS with Hunner lesions is currently a well-recognized clear clinical entity, characterized by the cystoscopic finding known as Hunner lesions, reddish mucosal lesions accompanied by abnormal capillary structures (Fig. 1). Past studies have shown that this entity presents with distinct clinicopathological and genomic characteristics compared with other forms of IC/BPS.5–11 This evidence suggests that IC/BPS with Hunner lesions comprises a potentially distinct disease entity with different etiology in the IC/BPS umbrella. In the past, many clinical studies were carried out to develop promising therapies for IC/BPS. However, most failed to achieve positive results. Likewise, a wide range of basic studies on the pathogenesis of IC/BPS have been carried out, but the results were variable and inconsistent. These unexpected and confusing results are likely due to multiple clinical conditions (i.e. pathogenetically different entities) having been combined in a single (and small) cohort.12 In addition, the unknown etiology, diverse clinical symptoms and varying histological manifestations also hinder clearly showing the landscape of IC/BPS. In this article, we review recent evidence on IC/BPS categorization to describe the evolving taxonomy of IC/BPS, discuss the potential pathophysiologies and animal models, and suggest future strategies to achieve better clinical management and research progress of IC/BPS based on these emerging concepts.
Fig. 1.

A Hunner lesion. (a) A Hunner lesion is a reddish mucosal lesion lacking the normal capillary structure, frequently covered by fibrin clots. (b) Narrow-band imaging cystoscopy of the Hunner lesion emphasizes the abnormal capillary structure converging toward the lesion.
Categorization of IC/BPS
The workshop on IC/BPS phenotyping at the 4th International Consultation on Interstitial Cystitis Japan meeting discussed potential categorization of IC/BPS in terms of their clinical characteristics, epidemiology and bladder histology.4,8 It was unanimously agreed that IC/BPS with Hunner lesions is a proven, clinically relevant subtype that should be regarded as a distinct disease entity. However, other forms of IC/BPS could not be clearly defined because of their obscure pathophysiology and histology, and their varied clinical manifestations. Thus, IC/BPS can be categorized into two subtypes at present: (i) IC/BPS with Hunner lesions, which is also known as the ESSIC BPS type 3; and (ii) IC/BPS without Hunner lesions, which includes the ESSIC BPS type 1 and 2. These two subtypes, which are distinguished simply by the cystoscopic presence or absence of Hunner lesions, present with different, but overlapping, clinical characteristics and cannot be clinically differentiated in the absence of cystoscopic findings (Table 1). IC/BPS with Hunner lesions is characterized by an older age at diagnosis, more severe bladder-centric symptoms, reduced bladder capacity, fewer comorbid non-bladder conditions and more favorable outcomes on endoscopic treatment compared with IC/BPS without Hunner lesions.7,9 IC/BPS without Hunner lesions is frequently accompanied by non-bladder symptoms, including other common systemic pain problems (“bladder-beyond” pain), psychosocial health problems and affect dysregulation.13 Recent studies have shown that these clinical characteristics of IC/BPS without Hunner lesions strongly overlap with those of widely known somatoform disorders or FSSs, such as irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome and migraines.14–16 This evidence suggests that there might be a subgroup of patients with IC/BPS without Hunner lesions representing a FSS that manifests somatic symptoms related to the pelvis. Bladder histology is clearly distinct between the two forms of IC/BPS (Fig. 2). IC/BPS with Hunner lesions has a proven bladder histology that manifests epithelial denudation and chronic inflammatory changes, such as lymphoplasmacytic and MC infiltration, stromal fibrosis, and edema.17,18 Of note, these histological changes are not confined to Hunner lesions, but can be observed in the entire bladder (i.e. pancystitis).19 Meanwhile, IC/BPS without Hunner lesions shows few histological changes.17–19 This histological evidence showed that IC/BPS with Hunner lesions is truly a chronic inflammatory disease of the bladder, whereas IC/BPS without Hunner lesions is a non-inflammatory disorder with no obvious bladder etiology. Our recent whole transcriptome analysis of IC/BPS emphasized this concept by showing the distinct gene expression profile of IC/BPS with Hunner lesions, in which the genes involved in immune responses and infection were upregulated (Fig. 3; Table 2). In contrast, the gene expression profile of IC/BPS without Hunner lesions was similar to that of non-IC controls.11 In addition, there are few documented clinical examples of a patient converting between the two forms of IC/BPS. Based on this evidence, the workshop concluded that it would be logical if IC/BPS with Hunner lesions be excluded from the current IC/BPS umbrella and defined as IC, and the remaining forms (IC/BPS without Hunner lesions) be designated as BPS.4
Table 1.
Differences in clinical characteristics between IC/BPS with and without Hunner lesions (comparison with the counterpart)
| IC/BPS with Hunner lesions | IC/BPS without Hunner lesions | Reference | |
|---|---|---|---|
| Age at diagnosis | Older | Younger | 5–8, 143, 144 |
| Extent of symptoms | Bladder-centric | Bladder-beyond | 7 |
| Bladder-centric symptom severity | Severer | Equally or less severe | 7, 11, 140 |
| Bladder capacity | Smaller | Larger | 5–8, 11 |
| Comorbid conditions† | Fewer | More | 7 |
| Endoscopic surgery‡ | Effective | Less effective | 9, 10, 28 |
| Bladder pathology | Proven | Unproven | 6, 8, 11, 17–19, 22, 65, 66, 103 |
Non-bladder conditions including somatoform disorders, psychosocial health problems or affect dysregulation.
Elimination/steroidal injection for Hunner lesions.
Fig. 2.

Histological features of IC/BPS. (a) IC/BPS with Hunner lesions (lesion area). Dense subepithelial inflammatory infiltrates, epithelial denudation and increased neovascularization are observed, often accompanied by lymph follicles (magnification: ×100). (b) IC/BPS with Hunner lesions (non-lesion area). Note that similar inflammatory changes are observed in a non-lesion area (magnification: ×200). (c) IC/BPS without Hunner lesions. Few inflammatory changes with retained urothelium (magnification: ×200).
Fig. 3.
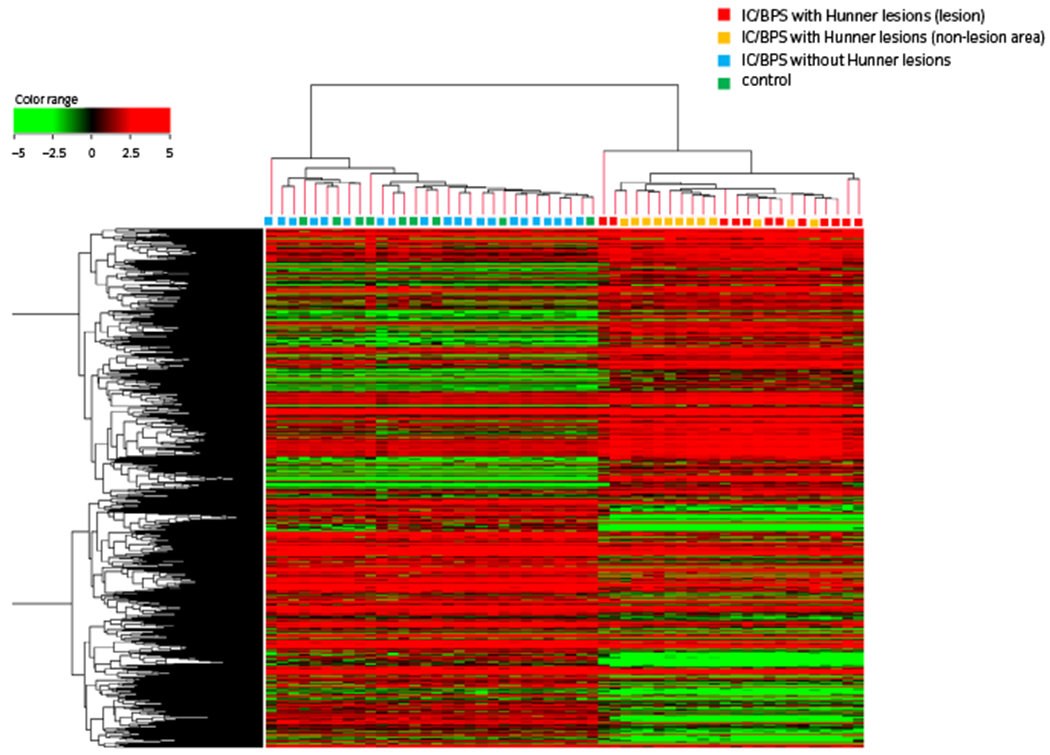
Genomic landscape of IC/BPS, modified from the results of Akiyama et al.11 IC/BPS with Hunner lesions (red: lesion area, yellow: non-lesion area) shows a distinct gene expression pattern from IC/BPS without Hunner lesions (blue) and controls (green). The gene expression pattern of IC/BPS without Hunner lesions is similar to that of controls.
Table 2.
Significantly enriched biological pathways in IC/BPS with Hunner lesions (selected results from Akiyama et al.11)
| Pathway ID | Kyoto Encyclopedia of Genes and Genomes pathway name (no. genes) | No. enriched genes | P-value |
|---|---|---|---|
| Upregulated pathways | |||
| hsa01521 | EGFR tyrosine kinase inhibitor resistance (79) | 37 | <0.0001 |
| hsa05163 | Human cytomegalovirus infection (225) | 71 | |
| hsa05167 | Kaposi sarcoma-associated herpesvirus infection (186) | 61 | |
| hsa05165 | Human papillomavirus infection (339) | 85 | |
| map09020 | Pathways in cancer (526) | 120 | |
| hsa04062 | Chemokine signaling pathway (185) | 54 | |
| hsa05220 | Chronic myeloid leukemia (76) | 30 | |
| hsa05169 | Epstein–Barr virus infection (201) | 56 | |
| hsa04722 | Neurotrophin signaling pathway (119) | 38 | |
| hsa04150 | mTOR signaling pathway (151) | 41 | |
| hsa04662 | B-cell receptor signaling pathway (71) | 27 | |
| hsa04664 | Fc epsilon RI signaling pathway (68) | 23 | |
| hsa04650 | Natural killer cell-mediated cytotoxicity (131) | 38 | |
| hsa04064 | NF-kappa B signaling pathway (95) | 29 | |
| hsa04621 | NOD-like receptor signaling pathway (168) | 44 | |
| hsa04210 | Apoptosis (138) | 71 | |
| hsa05221 | Acute myeloid leukemia (66) | 22 | <0.0005 |
| hsa04666 | Fc gamma R-mediated phagocytosis (91) | 27 | |
| hsa04660 | T-cell receptor signaling pathway (101) | 29 | |
| hsa04151 | PI3K-Akt signaling pathway (354) | 75 | |
| hsa04659 | Th17 cell differentiation (107) | 28 | <0.001 |
| hsa04612 | Antigen processing and presentation (77) | 22 | |
| hsa04658 | Th1 and Th2 cell differentiation (92) | 25 | |
| hsa04672 | Intestinal immune network for IgA production (49) | 16 | |
| hsa04668 | TNF signaling pathway (110) | 26 | <0.005 |
| hsa04620 | TLR signaling pathway (104) | 25 | |
| hsa04066 | HIF-1 signaling pathway (100) | 24 | |
| hsa05100 | Bacterial invasion of epithelial cells (74) | 19 | <0.01 |
| hsa04370 | VEGF signaling pathway (59) | 16 | |
| hsa04915 | Estrogen signaling pathway (137) | 37 | |
| hsa04670 | Leukocyte transendothelial migration (112) | 25 | <0.05 |
| hsa04010 | MAPK signaling pathway (295) | 54 | |
| hsa00532 | GAG biosynthesis – chondroitin sulfate/dermatan sulfate (20) | 7 | |
| hsa04540 | Gap junction (88) | 19 | |
| hsa04350 | TGF-beta signaling pathway (84) | 18 | |
| hsa00534 | GAG biosynthesis – heparan sulfate/heparin (20) | 7 | |
| hsa04640 | Hematopoietic cell lineage (97) | 20 | |
| hsa05120 | Epithelial cell signaling in Helicobacter pylori infection (68) | 15 | |
| hsa04750 | Inflammatory mediator regulation of TRP channels (97) | 20 | |
| Down-regulated pathways | |||
| hsa01521 | AMPK signaling pathway (120) | 28 | <0.0005 |
| hsa05163 | Aldosterone-regulated sodium reabsorption (37) | 12 | <0.001 |
| hsa04072 | Phospholipase D signaling pathway (146) | 29 | <0.005 |
| hsa00270 | Cysteine and methionine metabolism (45) | 11 | <0.05 |
| hsa00564 | Glycerophospholipid metabolism (97) | 19 | |
| hsa03320 | Peroxisome proliferator-activated receptor signaling pathway (72) | 15 | |
| hsa04330 | Notch signaling pathway (48) | 11 | |
| hsa01230 | Biosynthesis of amino acids (74) | 15 | |
| hsa00563 | Glycosylphosphatidylinositol-anchor biosynthesis (25) | 7 | |
Glomerulations (mucosal bleeding after bladder overdistension) have been considered another characteristic disease marker of IC/BPS. However, recent studies have cast doubt on its significance in the diagnosis and etiology of IC/BPS.11,20 To obtain biological evidence that could shed light on this controversial matter, we carried out genomic and histological analyses of the microvasculature in bladder biopsy samples from patients with IC/BPS without Hunner lesions. Consequently, the presence of glomerulations did not affect gene expression, or the degree of subepithelial inflammation and neovascularization.11 These findings suggest that glomerulations might not be a phenotypic feature of IC/BPS. However, we cannot conclusively discount the significance of glomerulations in IC/BPS at present. Therefore, we propose that, for the time being, the appearance of glomerulations during bladder hydrodistension should be recorded, although not used for diagnostic or therapeutic decision-making.
Pathophysiology of IC/BPS
IC/BPS with Hunner lesions
The underlying mechanisms that perpetuate inflammation in IC/BPS with Hunner lesions remain elusive. However, we found that accumulation of plasma cells and frequent expansion of light chain-restricted B cells in the bladder are the characteristic histological features of IC/BPS with Hunner lesions (Fig. 4).19 A recent RNA-seq analysis showed that genes involved in biological pathways related to cell proliferation, immune system and infectious disease were significantly upregulated in IC/BPS with Hunner lesions (Table 2).11 In the past, deposits of immunoglobulin and complement, and increased expression of chemokine receptor CXCR3 and its ligands (CXCL9, 10 and 11), in the bladder tissue of patients with IC/BPS with Hunner lesions has been reported.21–23 This evidence suggests that an immunological inflammatory process frequently accompanied by B-cell clonal expansion might underlie the pathophysiology in a subgroup of patients with IC/BPS with Hunner lesions.
Fig. 4.

Light chain-restricted B cells infiltrating in IC/BPS with Hunner lesions. (a–c) Light chain restriction in the infiltrating plasma cells in IC/BPS with Hunner lesions. (a) Hematoxylin–eosin staining shows dense plasma cell infiltration in the subepithelial layer. (b,c) In situ hybridization for the (b) kappa chain or (c) lambda chain. Most plasma cells are kappa chain-restricted (magnification: ×200).
Urothelial denudation is another characteristic histological feature of IC/BPS with Hunner lesions. Specifically, full layers of the urothelium are frequently sloughed off at Hunner lesion sites, whereas partial layers remain attached at non-lesion sites.18 This entire loss of the urothelial barrier at lesion sites could permit urinary stimuli to directly come into contact with afferent peripheral nerves in the bladder. This could be one possible explanation for the hypersensitive bladder symptoms; that is, occasionally susceptible to the change in urine composition in patients with IC/BPS, as consumption of specific diets exacerbate the symptoms.24–27 Clinical evidence that treatments targeted to Hunner lesions have favorable effects, despite the pancystitic nature of this form, might support this notion.9–10,28 However, whether there is a causal relationship between the urothelial denudation and the subepithelial inflammation in patients with IC/BPS with Hunner lesions remains to be determined. We next to discuss the potential pathophysiologies of IC/BPS with Hunner lesions, focusing on the urothelial deficiency and characteristic subepithelial inflammatory process featured by frequent B-cell clonal expansion.
Primary autoimmunity to the bladder tissue
Simply, we could associate the urothelial denudation and enhanced immune responses in IC/BPS with Hunner lesions with autoimmunity to the bladder tissue. In the past, anti-urothelial autoantibodies have been found in patients with IC.21,29,30 High incidence and titers of autoantibodies in the serum and bladder of patients with IC have also been reported.31,32 In this context, it is of interest that bladder disorders are commonly noted in patients with systemic autoimmune diseases, such as Sjogren’s syndrome, systemic lupus erythematosus and autoimmune thyroiditis, with histological evidence of deposits of immunoglobulin and complement in the bladder.33 Furthermore, the lower urinary tract symptoms in these patients resemble those in patients with IC/BPS with Hunner lesions.34,35 In addition, female preponderance and an increased prevalence of comorbid systemic autoimmune disorders are well-known epidemiological features of IC/BPS.36–38 Collectively, this evidence strongly suggests the possible autoimmune nature of IC/BPS with Hunner lesions. In animal models, forced autoimmunity against the bladder tissue can induce chronic cystitis that resembles human IC/BPS in histology and symptomatology.39–41 However, specific autoantibodies against bladder tissue have not been reported in patients with IC/BPS with Hunner lesions, thus this hypothesis remains controversial. Recently, we found that the immune responses in IC/BPS with Hunner lesions might be related to bladder infection.11,19 In this context, it is of great interest that female IC patients have a higher prevalence of positive urine cultures42,43 or altered urine bacterial flora than controls.44,45 Furthermore, an increased frequency of Epstein–Barr virus infection was reported in patients with IC/BPS with Hunner lesions.46 Infection is known to drive autoimmune pathogenesis in individuals with genetic susceptibility.47–49 Urothelial barrier dysfunction might help microbial invasion into the bladder mucosa during this process.50 Taken together, this evidence suggests that autoimmune responses against the urinary bladder might develop in conjunction with infection in IC/BPS with Hunner lesions. Further studies on the connection between autoimmunity and epithelial denudation and infection in IC/BPS with Hunner lesions are warranted.
Inflammatory reaction secondary to bladder tissue injury
The immunological inflammatory reaction of IC/BPS with Hunner lesions might be elicited secondarily against endogenous pathogens derived from cellular components (damage-associated molecular pattern) exposed to the immune system after bladder tissue cell injury caused by mechanical/chemical insult, aberrant metabolism or infection. Ketamine-induced cystitis, which is known to mimic IC/BPS with Hunner lesions clinically, is thought to be caused by direct damage to the urothelium by urine metabolites of ketamine.51 Parsons et al. suggested that cationic urinary components could potentially be cytotoxic to the urothelial cells, resulting in bladder mucosal injury.52,53 Some dietary metabolites, medications and nutritional supplements also exert cytotoxic effects on the urothelium.26 Altered levels of antiproliferative or growth factors, and increased apoptotic activity have been also reported in IC/BPS.54,55 Chronic urinary tract infection could lead to increased urothelial cell apoptosis.56 As a result, secondary to these bladder tissue cell disruptions, an inflammatory reaction might be induced against released endogenous pathogens, including proteins and peptides, polysaccharides and proteoglycan, nucleic acids, and phospholipids through pattern recognition receptors.57 Furthermore, persistent inflammatory stress could evoke clonal expansion of infiltrating lymphocytes.58,59 This hypothesis might explain the fact that autoantibodies reported in the past were not specific to IC/BPS.
Neurogenic inflammation
MC infiltration in IC/BPS:
MC infiltration has been considered a characteristic disease marker for IC/BPS.60–66 Before the year 2000, Giemsa staining, toluidine blue staining, periodic acid-Schiff staining and c-kit immunohistochemistry were used to identify MCs in routine pathology. However, these staining methods are not specific for human MCs.61,65,66 Since the 2000s, when a human MC-specific antibody (an anti-tryptase antibody) was developed, emerging evidence, including our recent study, has cast doubt on the significance of MC infiltration in IC/BPS.67–70 We evaluated MC densities in IC/BPS with and without Hunner lesions, and equally inflamed non-IC controls using an anti-tryptase antibody; each subtype of IC/BPS and its corresponding control (i.e. IC/BPS with Hunner lesions and non-IC chronic cystitis, or IC/BPS without Hunner lesions and normal bladder, respectively) had a comparable degree of background lymphoplasmacytic infiltration. The results showed that MC densities did not differ between IC/BPS and controls with equal background inflammation.70 Other recent studies have also suggested that increased MC density might not be a specific histological feature of IC/BPS.67–69
Nerve–MC interaction axis:
Enhanced release of neuropeptides from the sensory and/or sympathetic nerves leads to persistent afferent nerve sensitization and local inflammatory changes.71 These processes, referred to as neurogenic inflammation, are mediated by MCs. Neurotransmitters released by peripheral neurons, including vasoactive peptides, calcitonin gene-related peptide, tachykinins or substance P, induce MC degranulation and the release of pro-inflammatory mediators, such as histamine, serotonin, tryptase, TNF-α and NGF, resulting in local bladder inflammation. These inflammatory mediators act back on the afferent neurons in a positive feedback loop, resulting in increased release of neuropeptides that further exacerbate the MC degranulation and inflammatory response (known as the nerve–MC interaction axis).72–79 Persistent afferent nerve stimulation results in altered neural plasticity and central nervous sensitization in the dorsal root ganglia and the upper spinal cord, contributing to symptom persistence in IC/BPS.80 Chitinase-like protein (YKL-40), another protein contained within MC granules, also promotes stromal edema and fibrosis.81 Thus, MCs play a role in diverse pathophysiological processes including the synthesis of pro-inflammatory cytokines, leukocyte recruitment, afferent nerve sensitization and vascular remodeling.82 However, as aforementioned, a skeptical view on the role of MCs in IC/BPS is emerging based on recent studies. Gamper et al. reported that not only MC counts, but also the degree of MC degranulation did not differ significantly between patients with IC/BPS and overactive bladder and non-IC controls without bladder pain, suggesting that the functional contribution of MCs to the pathophysiology of IC/BPS might be questionable as well.69 Given the disproportionately low numbers of infiltrating MCs compared with other infiltrating inflammatory cells in IC/BPS with Hunner lesions, it might well be unlikely that MCs play a pivotal role in its pathogenesis. Meanwhile, based on this current evidence, the role of MCs in IC/BPS without Hunner lesions should not be completely discounted, as MCs have been implicated in other disorders characterized by afferent hypersensitivity and neurogenic inflammation, that are known to frequently overlap with IC/BPS without Hunner lesions.83–86 The role of neurogenic inflammation and MC infiltration in IC/BPS should be revisited in light of categorization of IC/BPS and proper controls.
Increased angiogenesis in the bladder mucosa
Increased angiogenesis is considered another pathogenic feature of IC/BPS, as it could be associated with the formation of glomerulations in conjunction with urothelial deficiency.50,87,88 However, recent studies have cast doubt on the specificity of glomerulations in IC/BPS.11,20 Our quantitative analysis of the subepithelial microvasculature showed increased microvessel density in IC/BPS with Hunner lesions, and showed that microvessel density did not differ according to the presence or absence of glomerulations in IC/BPS without Hunner lesions.11 Increased levels of VEGF, a pro-inflammatory growth factor that induces neovascularization, have also been reported in IC/BPS with Hunner lesions.89 Given the robust chronic inflammatory changes in IC/BPS with Hunner lesions, increased angiogenesis might result from the subepithelial inflammation, rather than being the cause of the inflammation. Comparison with non-IC chronic cystitis, as we did in the MC assessment, might help validate the specificity of VEGF in IC/BPS with Hunner lesions.
Thus, the etiology of IC/BPS with Hunner lesions might be multifactorial, as the variable cystoscopic appearance of Hunner lesions implies. Given the evidence from clinical, epidemiological and basic research studies described in this review, genetic, environmental and infectious factors might all play a role in the pathogenesis of IC/BPS with Hunner lesions.
IC/BPS without Hunner lesions
The key to understanding the pathophysiology of IC/BPS without Hunner lesions is exploring the mechanisms underlying nociceptive amplification in the absence of overt bladder pathology.
BPS as a somatoform disorder
Growing evidence suggests a potential connection between IC/BPS without Hunner lesions and somatoform disorders. Chen et al. showed that somatic symptoms could be linked to biological pathways that increase the risk of IC/BPS without Hunner lesions.16 A study that carried out magnetic resonance imaging of the brain of patients with UCPPS and fibromyalgia, and pain-free controls showed similar abnormal brain activity in patients with UCPPS and fibromyalgia.90 The relationship between specific dietary intake and symptom changes is commonly seen in IC/BPS and FSSs,24–27,91–95 in which some dietary metabolites might act as excitatory neurotransmitters, resulting in the central nervous sensitization.96,97 These findings suggest that IC/BPS without Hunner lesions might share its pathogenetic neurophysiological process affecting the CNS with somatoform disorders or FSSs.98 The underlying pathophysiology of FSSs remains unclear, but aberrant neuroimmune or endocrine processes with certain stressors might be responsible for central nervous sensitization and systemic hypersensitivity.99
Urothelial alterations
Parsons et al. postulated that abnormalities of the GAG layer might be associated with the urothelial dysfunction of IC/BPS.100 The GAG layer, which consists of glycoproteins and proteoglycans, plays a pivotal role in protective barrier function.101 Disruption of the GAG layer leads to increased urothelial permeability. The use of conventional intravesical therapies with GAG family components, such as heparan sulfate, chondroitin sulfate or hyaluronic acid, in IC/BPS is based on the hypothesis that replenishment of the GAG layer might promote urothelial layer recovery.102 In contrast, Liu et al. reported that tight junction proteins, such as E-cadherin and zonula occludens-1, are downregulated in patients with IC/BPS without Hunner lesions compared with patients with stress urinary incontinence or overactive bladder syndrome.103 Recurrent urinary tract infection could lead to downregulation of E-cadherin expression of the urothelium,56 which might explain the symptom exacerbations exposed after bacterial infection in patients with IC/BPS.104 The umbrella cells of patients with IC/BPS, including those without Hunner lesions, showed denudation, defects in integrity and microplicae, and severe pleomorphism by electron microscopy, and the degree of the umbrella cell defects correlated with symptom severity.50 These urothelial alterations impair its barrier function, resulting in increased permeability, which enables urinary stimuli to gain access to the subepithelial tissue and stimulate the afferent neurons persistently, leading to central nervous amplification with subtle histological bladder disruption undetectable by optical microscopy.105
Altered nociceptive sensory pathways
Neurogenic inflammation contributes to bladder afferent hypersensitivity and stromal fibrotic/edematous changes through nerve–MC interactions, as aforementioned. Kim et al. reported that fibrosis and MC counts are increased in IC/BPS without Hunner lesions, suggesting the potential involvement of neurogenic inflammation in its pathophysiology.106 Elevated gene expression of NGF and transient receptor potential vanilloid type 2 in IC/BPS without Hunner lesions was also reported.23 These findings might indicate the sensory pathways that are altered in patients with IC/BPS without Hunner lesions.
Animal models
Potential animal models of IC/BPS with Hunner lesions
For many years, animal models induced by local or systemic administration of noxious stimuli, such as hydrochloric acid and cyclophosphamide, have been used for IC/BPS research, in which induction of “chronic” cystitis requires repetitive administration and the inflamed bladder manifests few dense lymphocytic infiltrates (e.g. lymphoid follicles) seen in IC/BPS with Hunner lesions.107,108 However, current evidence has emphasized that an immunological inflammatory process accompanied by frequent B-cell clonal expansion might underlie the pathophysiology of IC/BPS with Hunner lesions. In this context, EAC models can most closely mimic IC/BPS with Hunner lesions. EAC models were first reported in the early 1990s, and have continually emerged since then (Table 3). At present, EAC models can be categorized into four groups: (i) EAC models induced by bladder tissue homogenate; (ii) EAC models induced by bladder urothelial antigens; (iii) spontaneous EAC model; and (iv) transgenic EAC models. These models are capable of recapitulating one or more key clinical correlates seen in patients with IC/BPS, offering a unique property for investigating certain specific aspects of human IC/BPS, such as bladder inflammation, pelvic/bladder pain and voiding dysfunction.
Table 3.
Animal models of autoimmune cystitis
| Species | Strain | Induction of autoimmune cystitis | Antigen | Bladder inflammation [Ref] | Pelvic/bladder pain | Voiding dysfunction | MC increment |
|---|---|---|---|---|---|---|---|
| Mouse | Balb/cAN | Immunize with syngeneic bladder homogenate | Unknown | 109, 110 | 109 | 109 | |
| Mouse | SWXJ (H-2q.s) | Immunize with syngeneic bladder homogenate | Unknown | 111, 112 | 111 | 111, 112 | |
| Rat | Lewis | Immunize with syngeneic bladder homogenate | A 12-kDa protein | 115, 116 | 115, 116 | ||
| Mouse | SWXJ (H-2q.s) | Immunize with recombinant UPK II | UPK II | 40 | 40 | ||
| Mouse | Balb/c-Fcgr2b−/−Pdcd1−/− | Spontaneous | UPK IIIa | 120 | 120 | 120 | |
| Mouse | Balb/c | Immunize with UPK3A65-84 peptide | UPK 3A | 39, 119 | 39, 119 | 39, 119 | |
| Mouse | Balb/cJ | Immunize with UPK3A65-84 peptide | UPK 3A | 118 | 118 | 118 | |
| Mouse | C57BL/6 | Immunize with syngeneic bladder homogenate | Unknown | 113, 114 | 113, 114 | 113, 114 | 113, 114 |
| Mouse | C57BL/6 | Immunize with TRPM8 T2 peptide | TRPM8 | 117 | 117 | 117 | 117 |
| Mouse | URO-OVA | Adoptive transfer of OVA-specific CD8+ T cells | Transgenic OVA | 41, 122–124 | 124 | 124 | 41 |
| Mouse | URO-OVA | Adoptive transfer of OVA-specific CD4+ T cells | Transgenic OVA | 128 | |||
| Mouse | URO-OVA | Adoptive transfer of OVA-specific T and B cells | Transgenic OVA | Figure 6 | (Upo) | (Upo) | |
| Mouse | URO-OVA/OT-I | Spontaneous | Transgenic OVA | 41, 123, 127 | 127 | 127 |
EAC models induced by bladder tissue homogenate
During the early stage of EAC development, investigators used homogenized bladder tissues for immunization to induce EAC in rodents. This EAC type has been developed in Balb/cAN,109,110 SWXJ(H-2q.s)111,112 and C57BL/6 mice,113,114 as well as in Lewis rats.115,116 In these models, bladders histologically showed thickened lamina propria, perivascular lymphocytic infiltration, increased urothelial permeability, increased vascularity and glomerulations, and detrusor MC accumulation. They also showed functional changes, such as increased urinary frequency and suprapubic pain behavior, and decreased bladder capacity.113,114 These findings remained for several months, and were transferable to naïve syngeneic animals through adoptive splenocyte transfer.109,115 The successful induction of cystitis by adoptive transfer showed the capacity of primed immune cells to recognize and respond to normal bladder tissues. These EAC models have been applied in therapeutic studies, including the use of angiotensin II type 1 receptor antagonist,110 anti-CXCL10 antibody112 and NK1R antagonist.114 All treatments showed favorable effects on reducing cystitis and associated symptoms in the EAC models.
EAC models induced by bladder urothelial antigens
Since the past decade, investigators have used known bladder urothelial antigens for immunization to induce EAC in mice. Altuntas et al. used recombinant mouse UPK II to induce EAC in mice.40 This model showed T-cell infiltration in the urothelium, upregulated gene expression of IFN-γ, TNF-α, IL-17A and IL-1β, and voiding dysfunction. Zhang et al. used T2 peptide, an antigenic epitope of TRPM8 protein to induce EAC in mice.117 The EAC mice showed extensive T-cell infiltration, edema and MC accumulation in the bladder, and showed increased suprapubic pain and urinary frequency. Izgi et al. used an UPK3A-derived immunogenic peptide (UPK3A 65–84) to induce EAC in mice.39 The mice showed antigen-specific lymph node cell proliferation, serum antibody responses, upregulated gene expression of IFN-γ, TNF-α, and IL-1β, and increased suprapubic pain and voiding dysfunction. The induced immune responses were characterized by selective activation of CD4+ T cells with a Th1-like phenotype, whereas no CD8+ T-cell activation was observed. Bicer et al. reported that the pelvic pain in the EAC mice was mediated by chemokine CCL2-driven MCs in the bladder, as treatment with MC stabilizer cromolyn or histamine receptors 1/2 antagonists inhibited pelvic pain.118 Also, mice defective in CCL2 or chemokine C-C motif receptor 2 gene showed markedly reduced pelvic pain along with reduced MC accumulation after EAC induction.118 Recently, Li et al. reported that local treatment with low-energy shock wave improved bladder inflammation and pain, and reduced levels of TNF-α and NGF in bladder and serum in the EAC mice.119
Spontaneous EAC model
Sugino et al. reported that double knockout mice in low affinity type IIb Fc receptor for immunoglobulin G and programmed cell death 1 presented anti-urothelial autoantibodies, such as anti-UPKIIIa antibody, and therefore could spontaneously develop EAC during the normal aging process.120 This model showed degenerated urothelium, such as poorer plaque formation and ablated umbrella cells, increased number of c-kit-, CD4-, CD11c- and B220-positive cells, and upregulated gene expression of TNF-α and IL-1β, as well as voiding dysfunction.
Transgenic EAC models
We have strived for developing transgenic EAC models since 2004, and reported our first model URO-OVA in 2007.41 The URO-OVA mice express the well-defined model antigen, OVA, as a “self” antigen on the urothelium, and develop bladder inflammation after adoptive transfer of OVA-specific CD8+ T cells from OT-I mice that express the transgenic CD8+ TCR specific for the OVA257-264 epitope peptide (H2-Kb).121 The bladder shows profound cellular infiltration, including MCs, edema, mucosal hyperemia and epithelial hyperplasia, with a peak at 7–14 days, and upregulated gene expression for TNF-α, NGF, substance P, IL-6, IFN-γ and MCP-1 after cystitis induction.41,122,123 In parallel with bladder inflammation, the URO-OVA mice show increased pelvic/bladder nociceptive responses and irritative voiding symptoms.124 Thus, this model recapitulates the key pathological features of IC/BPS with Hunner lesions, providing a novel EAC model for IC/BPS research.
Our early studies showed the responsiveness of the URO-OVA model to intravesical treatment with DMSO,123 one of the mainstays in the pharmacological treatment of human IC/BPS. The DMSO administration significantly reduced bladder inflammation and gene expression for IL-6, TNF-α, NGF, MCP-1 and IFN-γ. The studies also showed that DMSO impaired effector CD8+ T-cell infiltration in vivo and viability in vitro.
The URO-OVA model presents altered TLR4 activation,124 which is consistent with our recent findings suggesting a significant role of TLR4 in IC/BPS pain.125 To determine the role of TLR4, we generated URO-OVATLR4−/− mice that retained the urothelial OVA expression, but lacked TLR4 expression.124 Interestingly, we observed that URO-OVATLR4−/− mice showed reduced pelvic/bladder nociceptive responses, although similar bladder inflammation and voiding dysfunction to URO-OVA mice were observed. We further treated the URO-OVA EAC mice with TAK-242, a well-defined TLR4 selective inhibitor,126 and observed significant pain relief in the treated mice.124 Our study showed the role of TLR4 in autoimmune-associated pelvic/bladder pain.
To develop a transgenic EAC model that can more closely mimic human IC/BPS, we generated URO-OVA/OT-I mice through crossbreeding between URO-OVA and OT-I mice. The F1 generation acquires both urothelial OVA expression and endogenous OVA-specific CD8+ T cells and, therefore, can spontaneously develop bladder inflammation over time during the normal aging process.41,123,127 The EAC initiates at 4 weeks, and appears mild at 4 weeks, moderate at 6 weeks and severe at 8 weeks that sustains up to 20 weeks tested (Fig. 5). Like the URO-OVA mice, the URO-OVA/OT-I mice show pelvic/bladder pain and voiding dysfunction after EAC development, providing a valuable model for identifying the mechanisms underlying the initiation, progression, and maintenance of chronic cystitis and associated symptoms.127
Fig. 5.

(a) Spontaneous autoimmune cystitis in a URO-OVA/OT-1 mouse at 20 weeks-of-age. Dense mononuclear cell infiltration, vasodilation, mucosal hyperemia and epithelial hyperplasia are observed in the bladder (magnification: ×200). (b) An enlargement of the area outlined by the rectangular dot box (magnification: ×400).
In addition to CD8+ T cells, CD4+ T cells are capable of inducing EAC in the URO-OVA mice. We observed that adoptive transfer of OT-II CD4+ T cells that express the transgenic TCR specific for the I-Ab/OVA323–339 epitope peptide induced EAC in the URO-OVA mice.128 Our studies showed that antigen-specific CD4+ T cells functioned as direct effector cells and induced EAC independent of CD8+ T cells.
In addition to T cells, B cells are also implicated in the pathophysiology of IC/BPS with Hunner lesions, as described in this review. To evaluate the role of B cells, we generated antigen-specific B cells, along with antigen-specific T cells, through immunization of C57BL/6 mice with adjuvant-emulsified OVA protein. We observed that URO-OVA mice developed EAC after adoptive transfer of splenocytes (a mixture of T and B cells) from the OVA-immunized mice. The bladder showed remarkable cellular infiltration, vascularity and mucosal hyperemia with a peak at 14–28 days after cystitis induction (Fig. 6). In parallel with bladder inflammation, the EAC mice showed increased urinary frequency and pain behavior, and decreased micturition volume (our Upo). The determination of the role of B cells in the EAC is currently underway.
Fig. 6.

(a) EAC in a URO-OVA mouse at 21 days after adoptive transfer of splenocytes from OVA-immunized C57BL/6 mice. Remarkable cellular infiltration, vascularity, mucosal hyperemia, stromal edema and epithelial hyperplasia are observed in the bladder (magnification: ×200). (b) An enlargement of the area outlined by the rectangular dot box (magnification: ×400).
The EAC models have been used in IC/BPS research for nearly three decades. However, the current EAC models have certain limitations as follow. First, all models are induced by known or unknown bladder tissue antigens. Unfortunately, direct evidence for the involvement of bladder tissue antigens in human IC/BPS is still lacking. Second, in IC/BPS with Hunner lesions, the bladder urothelium manifests denudation, erosions or thinning; whereas, the majority of EAC models show urothelial hyperplasia, which is not a characteristic histological feature of IC/BPS with Hunner lesions. Third, all studies used female mice and the sex impact on the EAC is not clear. However, despite these limitations, these EAC models most closely mimic the pathophysiology of IC/BPS with Hunner lesions, providing a unique translational property for research in the field.
Potential animal models of IC/BPS without Hunner lesions
Animals with neurogenic cystitis, which show neural hyperexcitability without overt bladder etiology, can be used as models of IC/BPS without Hunner lesions. Pelvic organ cross-sensitization can result in neurogenic cystitis with little evidence of bladder etiology, and might explain the frequent overlap between UCPPS, including IC/BPS, and irritable bowel syndrome or vulvodynia.129 Ustinova et al. showed in a rat model that colonic irritation could induce bladder afferent sensitization and increase MC infiltration.129 Montalbetti et al. showed that, in rats, increased urothelial permeability caused by disrupted urothelial tight junctions resulted in bladder afferent nerve hyperexcitability and upstream central nerve sensitization with few inflammatory changes, and without altering epithelial quantity or umbrella cell polarity/differentiation.130,131 In contrast, emerging evidence has suggested a potential connection between bladder/pelvic hypersensitivity and psychological/physiological stress, involving functional alteration of the CNS, in humans. Jasmin et al. showed that CNS dysfunction induced by retrograde infection of the bladder with pseudorabies virus led to neurogenic inflammation in the bladder, resulting in MC degranulation and elevated urinary histamine content.132,133 Chronic WAS is another well-known model highly relevant to human IC/BPS without Hunner lesions. Rats exposed to WAS showed bladder hypersensitivity symptoms including a decreased threshold of micturition, increased urinary frequency and hyperalgesia.134–136 The rats also showed increased subepithelial MC infiltration and urinary NGF levels,137,138 with increased engagement and connectivity of brain micturition neuronal circuits and motor cortex regions.135 In addition, this WAS-induced bladder hypersensitivity was exacerbated by early life stress,139 which could mimic the potential relationship between childhood relational affect dysregulation and the development of IC/BPS without Hunner lesions in humans.140 Interestingly, a recent study reported the potential link between WAS-induced chronic stress and urothelial dysfunction, which was mediated by autonomic mechanisms and mitochondrial malfunction.137,141 Taken together, these models might facilitate the development of novel therapies and future research on the pathogenesis of IC/BPS without Hunner lesions.
Summary and future perspectives
In this article, we emphasized the critical differences between IC/BPS with and without Hunner lesions. IC/BPS with Hunner lesions is an inflammatory disease of the urinary bladder potentially associated with enhanced immune responses and infection, whereas IC/BPS without Hunner lesions is a non-inflammatory disorder with little evidence of bladder etiology. Thus, the IC/BPS umbrella does not represent a series of one disorder, but comprises a variety of “distinct” disorders.
On the basis of symptomatology, IC/BPS can be categorized into “bladder-centric” or “bladder-beyond” phenotypes. The former can be represented by IC/BPS with Hunner lesions that presents with more severe bladder/urethral pain, smaller bladder capacity, specific clinical prognosis and few comorbid systemic conditions. The latter category is represented by IC/BPS without Hunner lesions that frequently presents with somatic symptoms, and affects dysregulation, larger anatomical bladder capacity and more comorbid systemic conditions. However, clinical phenotyping based on patient demographics, the presence of other comorbid diseases and symptom severity, as well as characteristics measured by validated scoring systems, such as UPOINT and the Interstitial Cystitis Symptom Indices, cannot distinguish the presence or absence of Hunner lesions.142–144 Meanwhile, bladder histology could differentiate these two forms. IC/BPS with Hunner lesions has a proven bladder histology that manifests epithelial denudation and chronic inflammatory changes characterized by pancystitis and frequent expansion of infiltrating B cells, whereas IC/BPS without Hunner lesions shows little histological changes in the bladder. Therefore, we propose that categorization of IC/BPS should be carried out based on cystoscopic (and histological) examination at initial diagnosis to ensure specific clinical and investigational methodologies optimized for each subtype. For example, local fulguration and steroid injections, intravesical instillation of DMSO, and cyclosporine A administration should be indicated to patients with Hunner lesions. In contrast, a neuromodulation therapy and/or a multidisciplinary treatment strategy modeled on the management of related somatoform disorders and forms of affect dysregulation might be rationale for patients with IC/BPS without Hunner lesions. Taken together, a tailored approach that targets the chronic inflammation and epithelial denudation for IC/BPS with Hunner lesions, or the sensitized/altered nervous system, urothelial malfunction and psychosocial problems for IC/BPS without Hunner lesions, might be reasonable, and could lead to better outcomes in clinical management and future research ofIC/BPS.
Acknowledgments
The authors are financially supported by the Japan Intractable Diseases Research Foundation (to YA), KAKENHI Grants-in-Aid from the Japanese Society for the Promotion of Science (JSPS) (grant numbers 19K18552 [to YA], 19K07433 [to DM]), a Health Labor Sciences Research Grant from the Ministry of Health, Labor and Welfare (grant number 18060798 [to YH]), and National Institute of Diabetes and Digestive and Kidney Diseases (grant number R01-DK-111396 [to YL]).
Abbreviations & Acronyms
- BPS
bladder pain syndrome
- CCL2
C-C motif ligand 2
- CNS
central nervous system
- DMSO
dimethyl sulfoxide
- EAC
experimental autoimmune cystitis
- ESSIC
International Society for the Study of BPS
- FSS
functional somatic syndrome
- GAG
glycosaminoglycan
- IC
interstitial cystitis
- IFN-γ
interferon-gamma
- IL
interleukin
- MC
mast cell
- NGF
nerve growth factor
- OVA
ovalbumin
- TCR
T-cell receptor
- TNF
tumor necrosis factor
- TLR
Toll-like receptor
- UCPPS
urological chronic pelvic pain syndrome
- UPK
uroplakin
- Upo
unpublished observation
- VEGF
vascular endothelial growth factor
- WAS
water avoidance stress
Footnotes
Conflict of interest
None declared.
References
- 1.Hanno PM, Erickson D, Moldwin R, Faraday MM. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J. Urol 2015; 193: 1545–53. [DOI] [PubMed] [Google Scholar]
- 2.Homma Y, Ueda T, Tomoe H et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder updated in 2015. Int. J. Urol 2016; 23: 542–9. [DOI] [PubMed] [Google Scholar]
- 3.van de Merwe JP, Nordling J, Bouchelouche P et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur. Urol 2008; 53: 60–7. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama Y, Hanno P. Phenotyping of interstitial cystitis/bladder pain syndrome. Int. J. Urol 2019; 26(Suppl 1): 17–9. [DOI] [PubMed] [Google Scholar]
- 5.Peeker R, Fall M. Toward a precise definition of interstitial cystitis: further evidence of differences in classic and nonulcerdisease. J. Urol 2002; 167:2470–2. [PubMed] [Google Scholar]
- 6.Logadottir Y, Fall M, Kabjorn-Gustafsson C, Peeker R. Clinical characteristics differ considerably between phenotypes of bladder pain syndrome/interstitial cystitis. Scand. J. Urol. Nephrol 2012; 46: 365–70. [DOI] [PubMed] [Google Scholar]
- 7.Peters KM, Killinger KA, Mounayer MH, Boura JA. Are ulcerative and nonulcerative interstitial cystitis/painful bladder syndrome 2 distinct diseases? A study of coexisting conditions. Urology 2011; 78: 301–8. [DOI] [PubMed] [Google Scholar]
- 8.Whitmore KE, Fall M, Sengiku A, Tomoe H, Logadottir Y, Kim YH. Hunner lesion versus non-Hunner lesion interstitial cystitis/bladder pain syndrome. Int. J. Urol 2019; 26(Suppl 1): 26–34. [DOI] [PubMed] [Google Scholar]
- 9.Chennamsetty A, Khourdaji I, Goike J, Killinger KA, Girdler B, Peters KM. Electrosurgical management of Hunner ulcers in a referral center’s interstitial cystitis population. Urology 2015; 85: 74–8. [DOI] [PubMed] [Google Scholar]
- 10.Hillelsohn JH, Rais-Bahrami S, Friedlander JI et al. Fulguration for Hunner ulcers: long-term clinical outcomes. J. Urol 2012; 188: 2238–41. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama Y, Maeda D, Katoh H et al. Molecular taxonomy of interstitial cystitis/bladder pain syndrome based on whole transcriptome profiling by next-generation RNA sequencing of bladder mucosal biopsies. J. Urol 2019; 202: 290–300. [DOI] [PubMed] [Google Scholar]
- 12.Nickel JC, Moldwin R, Hanno P et al. Targeting the SHIP1 pathway fails to show treatment benefit in interstitial cystitis/bladder pain syndrome: lessons learned from evaluating potentially effective therapies in this enigmatic syndrome. J. Urol 2019; 202: 301–8. [DOI] [PubMed] [Google Scholar]
- 13.Warren JW, Howard FM, Cross RK et al. Antecedent nonbladder syndromes in case-control study of interstitial cystitis/painful bladder syndrome. Urology 2009; 73: 52–7. [DOI] [PubMed] [Google Scholar]
- 14.Warren JW. Bladder pain syndrome/interstitial cystitis as a functional somatic syndrome. J. Psychosom. Res 2014; 77: 510–5. [DOI] [PubMed] [Google Scholar]
- 15.Chen IC, Lee M, Wu SL, Lin HH, Chang KM, Lin H. Somatic symptoms are sensitive in predicting interstitial cystitis/bladder pain syndrome. Int. J. Psychiatry Med 2017; 52: 48–61. [DOI] [PubMed] [Google Scholar]
- 16.Chen IC, Lee MH, Lin HH, Wu SL, Chang KM, Lin HY. Somatoform disorder as a predictor of interstitial cystitis/bladder pain syndrome: Evidence from a nested case-control study and a retrospective cohort study. Medicine 2017; 96: e6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logadottir Y, Delbro D, Lindholm C, Fall M, Peeker R. Inflammation characteristics in bladder pain syndrome ESSIC type 3C/classic interstitial cystitis. Int. J. Urol 2014; 21(Suppl 1): 75–8. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama Y, Homma Y, Maeda D. Pathology and terminology of interstitial cystitis/bladder pain syndrome: a review. Histol. Histopathol 2019; 34: 25–32. [DOI] [PubMed] [Google Scholar]
- 19.Maeda D, Akiyama Y, Morikawa T et al. Hunner-type (classic) interstitial cystitis: a distinct inflammatory disorder characterized by pancystitis, with frequent expansion of clonal B-cells and epithelial denudation. PLoS One 2015; 10: e0143316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wennevik GE, Meijlink JM, Hanno P, Nordling J. The role of glomerulations in bladder pain syndrome: a review. J. Urol 2016; 195: 19–25. [DOI] [PubMed] [Google Scholar]
- 21.Mattila J, Linder E. Immunoglobulin deposits in bladder epithelium and vessels in interstitial cystitis: possible relationship to circulating anti-intermediate filament autoantibodies. Clin. Immunol. Immunopathol 1984; 32:81–9. [DOI] [PubMed] [Google Scholar]
- 22.Akiyama Y, Morikawa T, Maeda D et al. Increased CXCR3 expression of infiltrating plasma cells in Hunner type interstitial cystitis. Sci. Rep 2016; 6:28652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Homma Y, Nomiya A, Tagaya M et al. Increased mRNA expression of genes involved in pronociceptive inflammatory reactions in bladder tissue of interstitial cystitis. J. Urol 2013; 190: 1925–31. [DOI] [PubMed] [Google Scholar]
- 24.Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J. Urol 1993; 149: 465–9. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie L Metabolic appraisal of the effects of dietary modification on hypersensitive bladder symptoms. Br. J. Urol 1993; 72: 293–7. [DOI] [PubMed] [Google Scholar]
- 26.Friedlander JI, Shorter B, Moldwin RM. Diet and its role in interstitial cystitis/bladder pain syndrome (IC/BPS) and comorbid conditions. BJU Int. 2012; 109: 1584–91. [DOI] [PubMed] [Google Scholar]
- 27.Bassaly R, Downes K, Hart S. Dietary consumption triggers in interstitial cystitis/bladder pain syndrome patients. Female Pelvic Med. Reconstr. Surg 2011; 17: 36–9. [DOI] [PubMed] [Google Scholar]
- 28.Funaro MG, King AN, Stern JNH, Moldwin RM, Bahlani S. Endoscopic injection of low dose triamcinolone: a simple, minimally invasive, and effective therapy for interstitial cystitis with Hunner lesions. Urology 2018; 118: 25–9. [DOI] [PubMed] [Google Scholar]
- 29.Keay S, Zhang CO, Trifillis AL, Hebel JR, Jacobs SC, Warren JW. Urine autoantibodies in interstitial cystitis. J. Urol 1997; 157: 1083–7. [PubMed] [Google Scholar]
- 30.Neal DE Jr, Dilworth JP, Kaack MB. Tamm-Horsfall autoantibodies in interstitial cystitis. J. Urol 1991; 145: 37–9. [DOI] [PubMed] [Google Scholar]
- 31.Jokinen EJ, Alfthan OS, Oravisto KJ. Antitissue antibodies in interstitial cystitis. Clin. Exp. Immunol 1972; 11: 333–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Ochs RL, Stein TW Jr, Peebles CL, Gittes RF, Tan EM. Autoantibodies in interstitial cystitis. J. Urol 1994; 151: 587–92. [DOI] [PubMed] [Google Scholar]
- 33.Boye E, Morse M, Huttner I, Erlanger BF, MacKinnon KJ, Klassen J. Immune complex-mediated interstitial cystitis as a major manifestation of systemic lupus erythematosus. Clin. Immunol. Immunopathol 1979; 13: 67–76. [DOI] [PubMed] [Google Scholar]
- 34.Haarala M, Alanen A, Hietarinta M, Kiilholma P. Lower urinary tract symptoms in patients with Sjogren’s syndrome and systemic lupus erythematosus. Int. Urogynecol. J. Pelvic Floor Dysfunct 2000; 11: 84–6. [DOI] [PubMed] [Google Scholar]
- 35.Leppilahti M, Tammela TL, Huhtala H, Kiilholma P, Leppilahti K, Auvinen A. Interstitial cystitis-like urinary symptoms among patients with Sjogren’s syndrome: a population-based study in Finland. Am. J. Med 2003; 115: 62–5. [DOI] [PubMed] [Google Scholar]
- 36.Peeker R, Atanasiu L, Logadottir Y. Intercurrent autoimmune conditions in classic and non-ulcer interstitial cystitis. Scand. J. Urol. Nephrol 2003; 37:60–3. [DOI] [PubMed] [Google Scholar]
- 37.van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nat. Clin. Pract. Urol 2007; 4: 484–91. [DOI] [PubMed] [Google Scholar]
- 38.Wen JY, Lo TS, Chuang YC et al. Risks of interstitial cystitis among patients with systemic lupus erythematosus: A population-based cohort study. Int. J. Urol 2019; 26: 897–902. [DOI] [PubMed] [Google Scholar]
- 39.Izgi K, Altuntas CZ, Bicer F et al. Uroplakin peptide-specific autoimmunity initiates interstitial cystitis/painful bladder syndrome in mice. PLoS One 2013; 8: e72067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altuntas CZ, Daneshgari F, Sakalar C et al. Autoimmunity to uroplakin II causes cystitis in mice: a novel model of interstitial cystitis. Eur. Urol 2012; 61: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, Evanoff DP, Chen X, Luo Y. Urinary bladder epithelium antigen induces CD8+ T cell tolerance, activation, and autoimmune response. J. Immunol 2007; 178: 539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keay S, Schwalbe RS, Trifillis AL, Lovchik JC, Jacobs S, Warren JW. A prospective study of microorganisms in urine and bladder biopsies from interstitial cystitis patients and controls. Urology 1995; 45: 223–9. [DOI] [PubMed] [Google Scholar]
- 43.Haarala M, Kiilholma P, Lehtonen OP. Urinary bacterial flora of women with urethral syndrome and interstitial cystitis. Gynecol. Obstet. Invest 1999; 47: 42–4. [DOI] [PubMed] [Google Scholar]
- 44.Siddiqui H, Lagesen K, Nederbragt AJ, Jeansson SL, Jakobsen KS. Alterations of microbiota in urine from women with interstitial cystitis. BMC Microbiol. 2012; 12: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abernethy MG, Rosenfeld A, White JR, Mueller MG, Lewicky-Gaupp C, Kenton K. Urinary microbiome and cytokine levels in women with interstitial cystitis. Obstet. Gynecol 2017; 129: 500–6. [DOI] [PubMed] [Google Scholar]
- 46.Jhang JF, Hsu YH, Peng CW, Jiang YH, Ho HC, Kuo HC. Epstein–Barr virus as a potential etiology of persistent bladder inflammation in human interstitial cystitis/bladder pain syndrome. J. Urol 2018; 200: 590–6. [DOI] [PubMed] [Google Scholar]
- 47.Sener AG, Afsar I. Infection and autoimmune disease. Rheumatol. Int 2012; 32: 3331–8. [DOI] [PubMed] [Google Scholar]
- 48.Manfredo Vieira S, Hiltensperger M, Kumar V et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018; 359: 1156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minamitani T, Yasui T, Ma Y et al. Evasion of affinity-based selection in germinal centers by Epstein–Barr virus LMP2A. Proc. Natl. Acad. Sci. USA 2015; 112: 11612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jhang JF, Ho HC, Jiang YH, Lee CL, Hsu YH, Kuo HC. Electron microscopic characteristics of interstitial cystitis/bladder pain syndrome and their association with clinical condition. PLoS One 2018; 13: e0198816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jhang JF, Hsu YH, Kuo HC. Possible pathophysiology of ketamine-related cystitis and associated treatment strategies. Int. J. Urol 2015; 22: 816–25. [DOI] [PubMed] [Google Scholar]
- 52.Parsons CL, Bautista SL, Stein PC, Zupkas P. Cyto-injury factors in urine: a possible mechanism for the development of interstitial cystitis. J. Urol 2000; 164: 1381–4. [PubMed] [Google Scholar]
- 53.Parsons CL, Shaw T, Berecz Z, Su Y, Zupkas P, Argade S. Role of urinary cations in the aetiology of bladder symptoms and interstitial cystitis. BJU Int. 2014; 114: 286–93. [DOI] [PubMed] [Google Scholar]
- 54.Keay S, Kleinberg M, Zhang CO, Hise MK, Warren JW. Bladder epithelial cells from patients with interstitial cystitis produce an inhibitor of heparin-binding epidermal growth factor-like growth factor production. J. Urol 2000; 164: 2112–8. [PubMed] [Google Scholar]
- 55.Keay S, Seillier-Moiseiwitsch F, Zhang CO, Chai TC, Zhang J. Changes in human bladder epithelial cell gene expression associated with interstitial cystitis or antiproliferative factor treatment. Physiol. Genomics 2003; 14: 107–15. [DOI] [PubMed] [Google Scholar]
- 56.Chuang FC, Kuo HC. Increased urothelial cell apoptosis and chronic inflammation are associated with recurrent urinary tract infection in women. PLoS One 2013; 8: e63760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marshak-Rothstein A Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol 2006; 6: 823–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Uccelli A, Laxer KD et al. Local-clonal expansion of infiltrating T lymphocytes in chronic encephalitis of Rasmussen. J. Immunol 1997; 158: 1428–37. [PubMed] [Google Scholar]
- 59.Pereira MI, Medeiros JA. Role of Helicobacter pylori in gastric mucosa-associated lymphoid tissue lymphomas. World J. Gastroenterol 2014; 20: 684–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kastrup J, Hald T, Larsen S, Nielsen VG. Histamine content and mast cell count of detrusor muscle in patients with interstitial cystitis and other types of chronic cystitis. Br. J. Urol 1983; 55: 495–500. [DOI] [PubMed] [Google Scholar]
- 61.Aldenborg F, Fall M, Enerback L. Proliferation and transepithelial migration of mucosal mast cells in interstitial cystitis. Immunology 1986; 58: 411–6. [PMC free article] [PubMed] [Google Scholar]
- 62.Lynes WL, Flynn SD, Shortliffe LD et al. Mast cell involvement in interstitial cystitis. J. Urol 1987; 138: 746–52. [DOI] [PubMed] [Google Scholar]
- 63.Christmas TJ, Rode J. Characteristics of mast cells in normal bladder, bacterial cystitis and interstitial cystitis. Br. J. Urol 1991; 68: 473–8. [DOI] [PubMed] [Google Scholar]
- 64.Theoharides TC, Sant GR, El-Mansoury M, Letourneau R, Ucci AA, Meares EM Jr. Activation of bladder mast cells in interstitial cystitis: a light and electron microscopic study. J. Urol 1995; 153: 629–36. [DOI] [PubMed] [Google Scholar]
- 65.Peeker R, Enerback L, Fall M, Aldenborg F. Recruitment, distribution and phenotypes of mast cells in interstitial cystitis. J. Urol 2000; 163: 1009–15. [PubMed] [Google Scholar]
- 66.Yamada T, Murayama T, Mita H, Akiyama K. Subtypes of bladder mast cells in interstitial cystitis. Int. J. Urol 2000; 7: 292–7. [DOI] [PubMed] [Google Scholar]
- 67.Larsen MS, Mortensen S, Nordling J, Horn T. Quantifying mast cells in bladder pain syndrome by immunohistochemical analysis. BJU Int. 2008; 102: 204–7. [DOI] [PubMed] [Google Scholar]
- 68.Liu H-T, Jiang Y-H, Kuo H-C. Alteration of urothelial inflammation, apoptosis, and junction protein in patients with various bladder conditions and storage bladder symptoms suggest common pathway involved in underlying pathophysiology. Low. Urin. Tract Symptoms 2015; 7: 102–7. [DOI] [PubMed] [Google Scholar]
- 69.Gamper M, Regauer S, Welter J, Eberhard J, Viereck V. Are mast cells still good biomarkers for bladder pain syndrome/interstitial cystitis? J. Urol 2015; 193: 1994–2000. [DOI] [PubMed] [Google Scholar]
- 70.Akiyama Y, Maeda D, Morikawa T et al. Digital quantitative analysis of mast cell infiltration in interstitial cystitis. Neurourol. Urodyn 2018; 37: 650–7. [DOI] [PubMed] [Google Scholar]
- 71.Geppetti P, Nassini R, Materazzi S, Benemei S. The concept of neurogenic inflammation. BJU Int. 2008; 101(Suppl 3): 2–6. [DOI] [PubMed] [Google Scholar]
- 72.Krumins SA, Broomfield CA. C-terminal substance P fragments elicit histamine release from a murine mast cell line. Neuropeptides 1993; 24: 5–10. [DOI] [PubMed] [Google Scholar]
- 73.Frieling T, Cooke HJ, Wood JD. Serotonin receptors on submucous neurons in guinea pig colon. Am. J. Physiol 1991; 261: G1017–23. [DOI] [PubMed] [Google Scholar]
- 74.Frieling T, Cooke HJ, Wood JD. Histamine receptors on submucous neurons in guinea pig colon. Am. J. Physiol 1993; 264: G74–80. [DOI] [PubMed] [Google Scholar]
- 75.Leon A, Buriani A, Dal Toso R et al. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 1994; 91: 3739–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Houwelingen AH, Kool M, de Jager SC et al. Mast cell-derived TNF-alpha primes sensory nerve endings in a pulmonary hypersensitivity reaction. J. Immunol 2002; 168: 5297–302. [DOI] [PubMed] [Google Scholar]
- 77.van der Kleij HP, Ma D, Redegeld FA, Kraneveld AD, Nijkamp FP, Bienenstock J. Functional expression of neurokinin 1 receptors on mast cells induced by IL-4 and stem cell factor. J. Immunol 2003; 171: 2074–9. [DOI] [PubMed] [Google Scholar]
- 78.De Jonge F, De Laet A, Van Nassauw L et al. In vitro activation of murine DRG neurons by CGRP-mediated mucosal mast cell degranulation. Am. J. Physiol. Gastrointest. Liver Physiol 2004; 287: G178–91. [DOI] [PubMed] [Google Scholar]
- 79.Ito A, Hagiyama M, Oonuma J. Nerve-mast cell and smooth muscle-mast cell interaction mediated by cell adhesion molecule-1, CADM1. J. Smooth Muscle Res 2008; 44: 83–93. [DOI] [PubMed] [Google Scholar]
- 80.Steers WD, Tuttle JB. Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat. Clin. Pract. Urol 2006; 3: 101–10. [DOI] [PubMed] [Google Scholar]
- 81.Richter B, Roslind A, Hesse U et al. YKL-40 and mast cells are associated with detrusor fibrosis in patients diagnosed with bladder pain syndrome/interstitial cystitis according to the 2008 criteria of the European Society for the Study of Interstitial Cystitis. Histopathology 2010; 57: 371–83. [DOI] [PubMed] [Google Scholar]
- 82.Anand P, Singh B, Jaggi AS, Singh N. Mast cells: an expanding pathophysiological role from allergy to other disorders. Naunyn Schmiedebergs Arch. Pharmacol 2012; 385: 657–70. [DOI] [PubMed] [Google Scholar]
- 83.Weston AP, Biddle WL, Bhatia PS, Miner PB Jr. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig. Dis. Sci 1993; 38: 1590–5. [DOI] [PubMed] [Google Scholar]
- 84.O’Sullivan M, Clayton N, Breslin NP et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol. Motil 2000; 12: 449–57. [DOI] [PubMed] [Google Scholar]
- 85.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J. Neuroimmunol 2004; 146: 1–12. [DOI] [PubMed] [Google Scholar]
- 86.Santos J, Guilarte M, Alonso C, Malagelada JR. Pathogenesis of irritable bowel syndrome: the mast cell connection. Scand. J. Gastroenterol 2005; 40: 129–40. [DOI] [PubMed] [Google Scholar]
- 87.Tamaki M, Saito R, Ogawa O, Yoshimura N, Ueda T. Possible mechanisms inducing glomerulations in interstitial cystitis: relationship between endoscopic findings and expression of angiogenic growth factors. J. Urol 2004; 172: 945–8. [DOI] [PubMed] [Google Scholar]
- 88.Kiuchi H, Tsujimura A, Takao T et al. Increased vascular endothelial growth factor expression in patients with bladder pain syndrome/interstitial cystitis: its association with pain severity and glomerulations. BJU Int. 2009; 104: 826–31. [DOI] [PubMed] [Google Scholar]
- 89.Lee JD, Lee MH. Increased expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor associated with glomerulation formation in patients with interstitial cystitis. Urology 2011; 78: 971.e11–5. [DOI] [PubMed] [Google Scholar]
- 90.Kutch JJ, Ichesco E, Hampson JP et al. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain 2017; 158: 1979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J. Am. Diet. Assoc 2009; 109: 1204–14. [DOI] [PubMed] [Google Scholar]
- 92.Haugen M, Kjeldsen-Kragh J, Nordvag BY, Forre O. Diet and disease symptoms in rheumatic diseases – results of a questionnaire based survey. Clin. Rheumatol 1991; 10: 401–7. [DOI] [PubMed] [Google Scholar]
- 93.Arranz LI, Canela MA, Rafecas M. Fibromyalgia and nutrition, what do we know? Rheumatol. Int 2010; 30: 1417–27. [DOI] [PubMed] [Google Scholar]
- 94.Alpay K, Ertas M, Orhan EK, Ustay DK, Lieners C, Baykan B. Diet restriction in migraine, based on IgG against foods: a clinical double-blind, randomised, cross-over trial. Cephalalgia 2010; 30: 829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Logan AC, Wong C. Chronic fatigue syndrome: oxidative stress and dietary modifications. Altern. Med. Rev 2001; 6: 450–9. [PubMed] [Google Scholar]
- 96.Holton KF, Kindler LL, Jones KD. Potential dietary links to central sensitization in fibromyalgia: past reports and future directions. Rheum. Dis. Clin. North Am 2009; 35: 409–20. [DOI] [PubMed] [Google Scholar]
- 97.Olney JW. Excitotoxins in foods. Neurotoxicology 1994; 15: 535–44. [PubMed] [Google Scholar]
- 98.Bourke JH, Langford RM, White PD. The common link between functional somatic syndromes may be central sensitisation. J. Psychosom. Res 2015; 78: 228–36. [DOI] [PubMed] [Google Scholar]
- 99.Anderson G, Berk M, Maes M. Biological phenotypes underpin the physio-somatic symptoms of somatization, depression, and chronic fatigue syndrome. Acta Psychiatr. Scand 2014; 129: 83–97. [DOI] [PubMed] [Google Scholar]
- 100.Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J. Urol 1991; 145: 732–5. [DOI] [PubMed] [Google Scholar]
- 101.Klingler CH. Glycosaminoglycans: how much do we know about their role in the bladder? Urologia 2016; 83(Suppl 1): 11–4. [DOI] [PubMed] [Google Scholar]
- 102.Janssen DA, van Wijk XM, Jansen KC, van Kuppevelt TH, Heesakkers JP, Schalken JA. The distribution and function of chondroitin sulfate and other sulfated glycosaminoglycans in the human bladder and their contribution to the protective bladder barrier. J. Urol 2013; 189: 336–42. [DOI] [PubMed] [Google Scholar]
- 103.Liu HT, Shie JH, Chen SH, Wang YS, Kuo HC. Differences in mast cell infiltration, E-cadherin, and zonula occludens-1 expression between patients with overactive bladder and interstitial cystitis/bladder pain syndrome. Urology 2012; 80: 225e13–18. [DOI] [PubMed] [Google Scholar]
- 104.Stanford E, McMurphy C. There is a low incidence of recurrent bacteriuria in painful bladder syndrome/interstitial cystitis patients followed longitudinally. Int. Urogynecol. J. Pelvic Floor Dysfunct 2007; 18: 551–4. [DOI] [PubMed] [Google Scholar]
- 105.Zeng Y, Wu XX, Homma Y et al. Uroplakin III-delta4 messenger RNA as a promising marker to identify nonulcerative interstitial cystitis. J. Urol 2007; 178: 1322–7. [DOI] [PubMed] [Google Scholar]
- 106.Kim A, Han JY, Ryu CM et al. Histopathological characteristics of interstitial cystitis/bladder pain syndrome without Hunner lesion. Histopathology 2017; 71: 415–24. [DOI] [PubMed] [Google Scholar]
- 107.Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol. Urodyn 2011; 30: 673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Birder L, Andersson KE. Animal modelling of interstitial cystitis/bladder pain syndrome. Int. Neurourol. J 2018; 22: S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bullock AD, Becich MJ, Klutke CG, Ratliff TL. Experimental autoimmune cystitis: a potential murine model for ulcerative interstitial cystitis. J. Urol 1992; 148: 1951–6. [DOI] [PubMed] [Google Scholar]
- 110.Phull H, Salkini M, Purves T, Funk J, Copeland D, Comiter CV. Angiotensin II plays a role in acute murine experimental autoimmune cystitis. BJU Int. 2007; 100: 664–7. [DOI] [PubMed] [Google Scholar]
- 111.Lin YH, Liu G, Kavran M et al. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model for interstitial cystitis. BJU Int. 2008; 102: 1724–30. [DOI] [PubMed] [Google Scholar]
- 112.Singh UP, Singh NP, Guan H et al. The severity of experimental autoimmune cystitis can be ameliorated by anti-CXCL10 Ab treatment. PLoS One 2013; 8: e79751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jin XW, Liu BK, Zhang X, Zhao ZH, Shao Y. Establishment of a novel autoimmune experimental model of bladder pain syndrome/interstitial cystitis in C57BL/6 mice. Inflammation 2017; 40: 861–70. [DOI] [PubMed] [Google Scholar]
- 114.Liu BK, Jin XW, Lu HZ, Zhang X, Zhao ZH, Shao Y. The effects of neurokinin-1 receptor antagonist in an experimental autoimmune cystitis model resembling bladder pain syndrome/interstitial cystitis. Inflammation 2019; 42: 246–54. [DOI] [PubMed] [Google Scholar]
- 115.Luber-Narod J, Austin-Ritchie T, Banner B et al. Experimental autoimmune cystitis in the Lewis rat: a potential animal model for interstitial cystitis. Urol. Res 1996; 24: 367–73. [DOI] [PubMed] [Google Scholar]
- 116.Mitra S, Dagher A, Kage R, Dagher RK, Luber-Narod J. Experimental autoimmune cystitis: further characterization and serum autoantibodies. Urol. Res 1999; 27: 351–6. [DOI] [PubMed] [Google Scholar]
- 117.Zhang L, Ihsan AU, Cao Y et al. An immunogenic peptide, T2 induces interstitial cystitis/painful bladder syndrome: an autoimmune mouse model for interstitial cystitis/painful bladder syndrome. Inflammation 2017; 40: 2033–41. [DOI] [PubMed] [Google Scholar]
- 118.Bicer F, Altuntas CZ, Izgi K et al. Chronic pelvic allodynia is mediated by CCL2 through mast cells in an experimental autoimmune cystitis model. Am. J. Physiol. Renal Physiol 2015; 308: F103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H, Zhang Z, Peng J et al. Treatment with low-energy shock wave alleviates pain in an animal model of uroplakin 3A-induced autoimmune interstitial cystitis/painful bladder syndrome. Investig. Clin. Urol 2019; 60: 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sugino Y, Nishikawa N, Yoshimura K et al. BALB/c-Fcgr2bPdcd1 mouse expressing anti-urothelial antibody is a novel model of autoimmune cystitis. Sci. Rep 2012; 2: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clarke SR, Barnden M, Kurts C, Carbone FR, Miller JF, Heath WR. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol 2000; 78: 110–7. [DOI] [PubMed] [Google Scholar]
- 122.Liu W, Deyoung BR, Chen X, Evanoff DP, Luo Y. RDP58 inhibits T cell-mediated bladder inflammation in an autoimmune cystitis model. J. Autoimmun 2008; 30: 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim R, Liu W, Chen X, Kreder KJ, Luo Y. Intravesical dimethyl sulfoxide inhibits acute and chronic bladder inflammation in transgenic experimental autoimmune cystitis models. J. Biomed. Biotechnol 2011; 2011: 937061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui X, Jing X, Lutgendorf SK et al. Cystitis-induced bladder pain is Toll-like receptor 4 dependent in a transgenic autoimmune cystitis murine model: a MAPP Research Network animal study. Am. J. Physiol. Renal Physiol 2019; 317: F90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schrepf A, Bradley CS, O’Donnell M et al. Toll-like receptor 4 and comorbid pain in interstitial cystitis/bladder pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav. Immun 2015; 49: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ii M, Matsunaga N, Hazeki K et al. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol. Pharmacol 2006; 69: 1288–95. [DOI] [PubMed] [Google Scholar]
- 127.Kogan P, Xu S, Wang Y et al. Sub-noxious intravesical lipopolysaccharide triggers bladder inflammation and symptom onset in a transgenic autoimmune cystitis model: a MAPP network animal study. Sci. Rep 2018; 8: 6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu W, Chen X, Evanoff DP, Luo Y. Urothelial antigen-specific CD4+ T cells function as direct effector cells and induce bladder autoimmune inflammation independent of CD8+ T cells. Mucosal Immunol. 2011; 4:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol. Urodyn 2010; 29: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Montalbetti N, Rued AC, Taiclet SN, Birder LA, Kullmann FA, Carattino MD. Urothelial tight junction barrier dysfunction sensitizes bladder afferents. eNeuro 2017; 4: ENEURO.0381-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Montalbetti N, Rued AC, Clayton DR et al. Increased urothelial paracellular transport promotes cystitis. Am. J. Physiol. Renal Physiol 2015; 309: F1070–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jasmin L, Janni G, Manz HJ, Rabkin SD. Activation of CNS circuits producing a neurogenic cystitis: evidence for centrally induced peripheral inflammation. J. Neurosci 1998; 18: 10016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jasmin L, Janni G, Ohara PT, Rabkin SD. CNS induced neurogenic cystitis is associated with bladder mast cell degranulation in the rat. J. Urol 2000; 164: 852–5. [DOI] [PubMed] [Google Scholar]
- 134.Lee UJ, Ackerman AL, Wu A et al. Chronic psychological stress in high-anxiety rats induces sustained bladder hyperalgesia. Physiol. Behav 2015; 139: 541–8. [DOI] [PubMed] [Google Scholar]
- 135.Wang Z, Chang HH, Gao Y et al. Effects of water avoidance stress on peripheral and central responses during bladder filling in the rat: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. PLoS One 2017; 12: e0182976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao Y, Zhang R, Chang HH, Rodriguez LV. The role of C-fibers in the development of chronic psychological stress induced enhanced bladder sensations and nociceptive responses: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. Neurourol. Urodyn 2018; 37: 673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matos R, Serrao P, Rodriguez L, Birder LA, Cruz F, Charrua A. The water avoidance stress induces bladder pain due to a prolonged alpha1A adrenoceptor stimulation. Naunyn Schmiedebergs Arch. Pharmacol. 2017; 390: 839–44. [DOI] [PubMed] [Google Scholar]
- 138.Dias B, Serrao P, Cruz F, Charrua A. Effect of water avoidance stress on serum and urinary NGF levels in rats: diagnostic and therapeutic implications for BPS/IC patients. Sci. Rep 2019; 9: 14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pierce AN, Di Silvestro ER, Eller OC, Wang R, Ryals JM, Christianson JA. Urinary bladder hypersensitivity and dysfunction in female mice following early life and adult stress. Brain Res. 2016; 1639: 58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chiu CD, Lee MH, Chen WC, Ho HL, Wu HC. Alexithymia and anesthetic bladder capacity in interstitial cystitis/bladder pain syndrome. J. Psychosom. Res 2017; 100: 15–21. [DOI] [PubMed] [Google Scholar]
- 141.Kullmann FA, McDonnell BM, Wolf-Johnston AS et al. Stress-induced autonomic dysregulation of mitochondrial function in the rat urothelium. Neurourol. Urodyn 2019; 38: 572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pinto R, Lopes T, Costa D et al. Ulcerative and nonulcerative forms of bladder pain syndrome/interstitial cystitis do not differ in symptom intensity or response to onabotulinum toxin A. Urology 2014; 83: 1030–4. [DOI] [PubMed] [Google Scholar]
- 143.Doiron RC, Tolls V, Irvine-Bird K, Kelly KL, Nickel JC. Clinical phenotyping does not differentiate hunner lesion subtype of interstitial cystitis/bladder pain syndrome: a relook at the role of cystoscopy. J. Urol 2016; 196: 1136–40. [DOI] [PubMed] [Google Scholar]
- 144.Van Moh F, Vetter J, Lai HH. Comparison of urologic and non-urologic presentation in interstitial cystitis/bladder pain syndrome patients with and without Hunner lesions. Neurourol. Urodyn 2018; 37: 2911–8. [DOI] [PubMed] [Google Scholar]


