Abstract
Bone remodeling and regeneration are dependent on resident stem/progenitor cells with the capability to replenish mature osteoblasts and repair the skeleton. Using lineage tracing approaches, we identified a population of Dmp1+ cells that reside within cortical bone and are distinct from osteocytes. Our aims were to characterize this stromal population of transcortical perivascular cells (TPCs) in their resident niche and evaluate their osteogenic potential. To distinguish this population from osteoblasts/osteocytes, we crossed mice containing inducible DMP1CreERT2/Ai9 tomato reporter (iDMP/T) with Col2.3GFP reporter (ColGFP), a marker of osteoblasts and osteocytes. We observed iDMP/T+;ColGFP− TPCs within cortical bone following tamoxifen injection. These cells were perivascular and located within trans-cortical channels. Ex vivo bone outgrowth cultures showed TPCs migrated out of the channels onto the plate and expressed stem cell markers such as Sca1, PDGFRβ and leptin receptor. In a cortical bone transplantation model, TPCs migrate from their vascular niche within cortical bone, and contribute to new osteoblast formation and bone tube closure. Treatment with intermittent parathyroid hormone increased TPC number and differentiation. TPCs were unable to differentiated into adipocytes in the presence of rosiglitazone in vitro or in vivo. Altogether, we have identified and characterized a novel stromal lineage-restricted osteoprogenitor that is associated with trans-cortical vessels of long bones. Functionally, we have demonstrated that this population can migrate out of cortical bone channels, expand and differentiate into osteoblasts, therefore serving as a source of progenitors contributing to new bone formation.
Keywords: dentin matrix protein-1 (DMP1), cortical channel, osteoprogenitor, transplantation
Introduction
Bone development and regeneration is dependent on resident stem/progenitor cells with the capability to replenish bone cell populations and repair the skeleton. This requires multiple cell types, which are influenced by signals in the microenvironment such as growth factors and cytokines. These cues help to coordinate events such as cell migration into an injury, or cell differentiation in order to produce new bone and repair existing damage. It is generally thought that stem cells and osteoprogenitors reside in a perivascular niche [1, 2], but there is no consensus on markers to identify these cells, and the evidence suggests that there may be different cell populations involved in growth, homeostasis and healing.
Lineage tracing experiments are a useful tool to identify sub-populations within bone and track how a cell responds to stimuli such as injury. Several populations of osteoprogenitors and their localized niches have been previously examined using lineage tracing including cells expressing osterix (Osx), leptin receptor (LepR), nestin, Gremlin1, Gli1, Mx-1 and alpha-smooth muscle actin (αSMA) [3-10]. Osx-cre labels perivascular progenitors during development, osteoprogenitors, and all stages of the osteoblast lineage due to expression during osteoblast differentiation [3, 8, 11]. A large proportion of cells are Osx+ following irradiation or during fracture repair [3, 11]. αSMA labels both perivascular cells and osteoprogenitors within bone marrow and periosteum [9, 12]; notably these cells have a substantial role in fracture repair as they make up the majority of the callus [9, 13]. Nestin expressing cells are mesenchymal stem cells that can become osteoblasts, adipocytes and chondrocytes in vitro [5]. They are important in maintaining the haematopoietic niche as depletion of Nestin positive cells leads to significant reduction of haematopoietic progenitors [5]. Leptin-receptor cre marks a mesenchymal stromal progenitor cell with potential to become predominantly osteoblasts and adipocytes [4, 14, 15]. Although generated post-natally, these cells are quiescent in nature until bone injury occurs which triggers their proliferation and differentiation [4].
Dentin matrix protein 1 (DMP1) is a member of the SIBLING family of proteins, which are heavily phosphorylated integrin binding proteins that are rich in acidic amino acids. In undifferentiated osteoblasts, DMP1 in the nucleus can regulate the expression of odontoblast- and osteoblast-specific genes. Following osteoblast maturation and differentiation into osteocytes, DMP1 can become phosphorylated and is secreted into the extracellular matrix, where it has a role in matrix formation. The Dmp1 promoter has been widely utilized to study osteocyte biology. Mouse models targeting osteocytes include 8kb Dmp1-GFP [16], 10kb Dmp1-memGFP [17], 8kb and 10kb Dmp1-Cre [18, 19], 10kb Dmp1-CreERT2 (iDMP) [20] and Dmp1-DTR ablation model [21]. These models have provided a large body of valuable information on the role of osteocytes in bone biology. However, when Dmp1Cre lineage tracing is employed in combination with a very sensitive reporter strains such as Ai9 [22], its expression targets late osteoblasts and bone lining cells [23-25].
Understanding the cells involved in physiological bone turnover and bone repair is critical for developing new strategies for improving bone mass. Previous studies have identified DMP1+ cells from cortical bone with osteoprogenitor characteristics and some late osteoblast/pre-osteocyte cell lines show unexpected plasticity or bone formation capacity [26-28]. The use of the inducible Dmp1CreERT2 (iDMP) allowed us to better refine populations of cells labeled by the lineage tracing approach [20], especially in combination with an osteoblast/osteocyte marker Col2.3GFP [29]. We established the identity of a subpopulation of iDMP1+ labeled cells as perivascular and identify that this cell type is distinct from osteocytes. Using a cortical bone transplantation model, we were able to functionally assess the potential of the cells in bone regeneration.
Material and Methods
Mouse lines
All procedures were approved by the UConn Health Institutional Animal Care and Use Committee and performed in an AAALAC accredited facility. Mice were group housed in ventilated cages with a 12 h light cycle (7:00-19:00). The room temperature was maintained at 22 °C. Water and irradiated rodent chow (Teklad 2918, Invigo, Indianapolis, IN) were provided ad libitum. Transgenic mice containing inducible DMP1CreERT2 (iDMP) were crossed with Tomato reporter strain Ai9 to make (iDMP/T) and with Col2.3GFP reporter (ColGFP) as a marker of osteoblast/osteocytes generating triple transgenic mice (iDMP/T/ColGFP) [20, 22, 23, 29]. Cre recombination was induced by a single dose of tamoxifen (75g/kg of body weight; Cat #: T5648 Sigma, St. Louis MO) (Supplemental figure 1). For intravascular lectin staining, anesthetized mice were injected retro-orbitally with 100μg DyLight 649 Lycopersicon esculentum lectin (100μl volume, Cat#: DL-1178, Vector Laboratories). Animals were sacrificed after 3 minutes and bones fixed for histology. For some mice, Demeclocycline (50mg/kg; Cat #: D6140 Sigma) was injected 2 days prior to collection of bones. NSG mice (JAX stock no: 005557 [30], kind gift from Prof. David Rowe) were used as recipients for the transplantation experiments.
Preparation of cortical bone devoid of marrow, endosteum and periosteal cells
Long bones were collected from iDMP/T/ColGFP mice 2 days post tamoxifen treatment. Samples were dissected, scraped to remove periosteum and bone marrow flushed out with PBS. To ensure complete removal of bone marrow and endosteal cells, bones were centrifuged at 14000 rpm for 5 mins. These empty bone tubes were validated to be devoid of periosteal, endosteal and hematopoietic marrow cells by imaging techniques.
Immunohistochemistry
Femurs were collected from iDMP/T/ColGFP mice 2 days post tamoxifen treatment and fixed in 4% PFA for 4 days, decalcified in 14% EDTA for 7 days and switched to 30% sucrose overnight prior to embedding. Sections were cut onto Japanese Cryotape (Cryofilm 2C, Section Lab, Japan) at 25 - 50 μm in order to obtain vascular channels within cortical bone using a cryostat as described previously [31]. Following mounting and cross-linking of the sections to glass slides using Norland Optical Adhesive 61 (Norland Optical, Cranbury, NJ), sections were rehydrated, permeabilized in 0.3% Triton X-100 and blocked with 10% normal donkey serum. Primary antibody incubations were done overnight at 4 °C. Sections were incubated with secondary antibody for 1 hr at room temperature. Antibodies used are listed in Supplemental Table 1. Slides were coverslipped in 50% glycerol with DAPI (1:5000, D1306; Molecular Probes).
Light sheet microscopy
Bones from iDMP/T/ColGFP mice 2 days post tamoxifen treatment were processed using a modified FRUIT clearing method for Light Sheet Imaging Microscopy (LSM) [32]. Femurs and tibias had their diaphysis removed and bone marrow flushed with PBS. Bones were fixed in 4% PFA for 2 days and decalcified for 5 days in 5 mM EDTA. Bones were then processed for clarity by performing sequential incubations at 37°C with rotation in 24h increments in fructose containing urea as follows: 35% fructose with 8 M urea, 40% fructose with 8 M urea, 60% fructose with 6.2 M urea, 80% fructose with 4.3 M urea, 100% fructose with 1.8 M urea. All solutions contained 0.5% α-thioglycerol. The final solution had its refractive index matched to the 5x/0.16 EC Plan-Neofluar objective lens with a RI of 1.45. Bones were equilibrated in this final solution for at least 1 week prior to imaging on the Zeiss LightSheet Z.1 (Zeiss, Oberkochen, Germany). Image analysis including 3D rendering was done in Zen 2.5 lite.
Cell isolation for flow cytometry and bone outgrowth cultures (BOCs)
Cortical bones devoid of cells as described were cut into small pieces using a razor blade and then further crushed with a metal pestle into smaller pieces. Typically, two animals were used per sample. Bone chips were then placed into 15 ml tubes and underwent 3 consecutive 60 min digestions (1 - 0.5 mg/ml Collagenase P, 2 - 5 mM EDTA in 0.1% BSA, 3 - 0.5 mg/ml Collagenase P) at 37 °C with shaking. Fractions were collected, washed and pooled after each incubation and then processed for flow cytometry staining. Similarly bone chips that were generated after a 1 hr Collagenase P digestion were placed into 1 well of a 6 well culture dish and cultured in DMEM supplemented with 10% FBS and 1X Pen Strep and cultured at 37 °C 5% CO2. Media was added on day 4 and then half media changes were done every 3 days. 14 day BOCs were imaged using AxioImager.Z1 (Zeiss) and then cells harvested for flow cytometry after a 5 min Accutase treatment (Innovative Cell Technologies, San Diego, CA, USA).
Day 7 BOCs were switched to MEMalpha media containing 10% FBS and 1x L-glutamine with osteogenic inducing supplements (50 ug/ml Ascorbic acid, 8 mM β-glycerol phosphate) or adipogenic inducing supplements (1 μM insulin and 0.5 μM rosiglitazone). Cells were differentiated for 10 days and then imaged. For adipogenic culture, cells were fixed in 10% formalin and stained with 0.3% Oil Red 0 at room temperature and then washed in water.
Flow cytometry
Single cell suspensions of cortical digests (described above) or BOCs were washed in Staining Media (SM) containing 2% Newborn Calf Serum, 1X HBSS, and 1mM Hepes and filtered through 70μm Nitex. Cells were incubated with Zombie Aqua (Cat#: 77143, Biolegend) at 1:500 for 15 min at RT, washed and then resuspended in 100 μl of antibody cocktail (pre-titrated) and stained for 45 min at 4°C in the dark. The antibody details can be found in Supplementary Table 2. Flow cytometric analysis was done on a BD-LSR II and cell sorting was done on a BD-FACS ARIA II (BD Biosciences; San Jose CA). Data was exported and then further analyzed using FlowJo software (FlowJo v10, FlowJo LLC, Ashland, Oregon, USA). Analysis of the iDMP/T+;ColGFP− and iDMP/T+;ColGFP+ populations was always done within the live, singlet, haematopoietic negative (lin−) gates (Supplemental Figure 2A-F).
For the evaluation of the effects of PTH on iDMP/T+ cells, 3-5-month old iDMP/T mice were treated with tamoxifen 2 days prior to commencement of the experiment. Mice were either treated with vehicle or intermittent PTH (PTH1-34, 40mg/kg daily Bachem) for a period of 10 days. Three mice were pooled together for each sample. Cells from the cortical digest fraction were collected and stained for surface markers.
Cortical bone transplantation model
Cortical bone tubes were prepared from femurs as described above, then cut on both ends using a Dremel 4300 (Dremel, Mount Prospect, IL, USA) to leave approximately a 5mm in length diaphyseal bone tube devoid of cancellous bone (Figure 3A). Additional cortical bone tubes were prepared and directly placed in 4% PFA to demonstrate the characteristics of the bone tubes prior to implantation (Figure 3A,B). Pairs of cortical bone tubes were subcutaneously implanted into the flanks of NSG mice (Figure 3C) and were collected at either 7, 14- or 21-days post implantation in 4% PFA (Supplemental Figure 3A). The day prior to collection the recipient NSG mice were injected with EdU (5-ethynyl-2'-deoxyuridine, 100μl of 1 mg/ml solution) (Supplemental Figure 3A). In subsequent experiments, mice were treated with intermittent PTH (PTH1-34, 40mg/kg daily; Bachem) or vehicle control to test osteogenic potential of the cells (Supplemental figure 3B) or fed rosiglitazone diet (Teklad) to induce bone marrow adipogenesis (Supplemental Figure 3C).
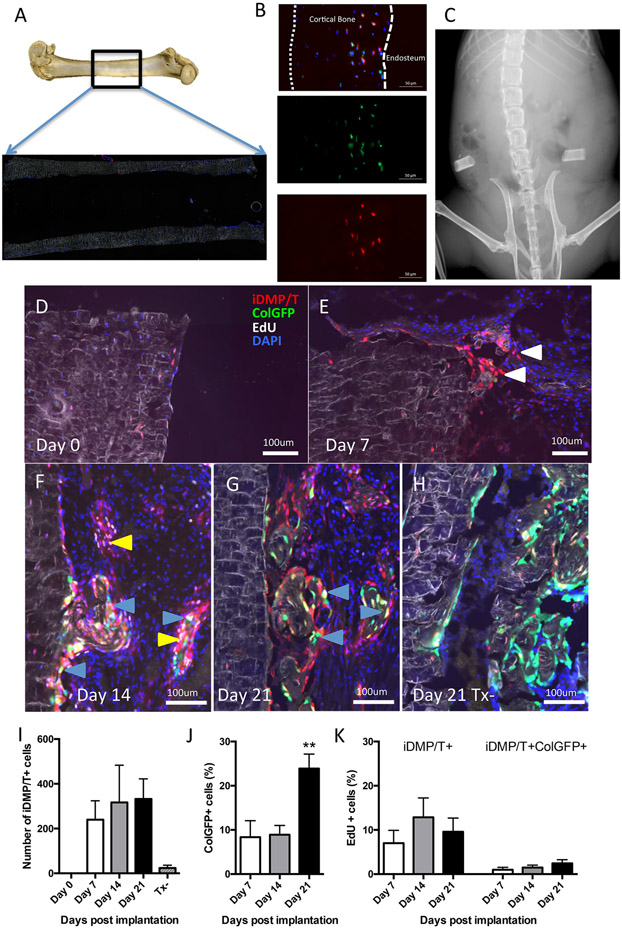
Figure 3: iDMP/T+;ColGFP− cell population expands and contributes to new bone formation in a cortical bone transplantation model.
(A) Representative picture of where cortical bone tube was harvested for transplantation, box indicates location of bone tube within the femur as a whole and a scanned section and (B) higher magnification of histology of a prepared bone tube prior to implantation showing absence of trabecular bone and absence of cells on endosteal and periosteal surfaces. Fluorescence indicates: iDMP/T (tomato); ColGFP (green) and DAPI (blue) (C) X-ray showing implantation of bone tubes. (D-H) Representative images are shown from tamoxifen-treated transplants at Day 0 (D), Day 7 (E), Day 14 (F) and Day 21 (G) and tamoxifen untreated control on Day 21 (H). White arrows indicate iDMP/T+;ColGFP− cells migrating from cortical bone. Yellow arrows indicate iDMP/T+;ColGFP− cells undergoing proliferation (as labeled by EdU). Blue arrows indicate regions where iDMP/T+ cells that have differentiated into ColGFP+ cells and are present on the surface of new bone formation. (I-K) Quantification of cells within transplantation model. Number of iDMP/T+ cells that were present in marrow cavity following transplantation (I). Proportion of iDMP/T+;ColGFP+ cells expressed as a percentage of all iDMP/T+ cells (J). iDMP/T+ cells that were EdU only or EdU/GFP+ positive as a proportion of total iDMP/T+ cells. (K). Day 0: n=4; Day 7: n=6; Day 14: n=7; and Day 21: n=7. Each column represents Mean±SEM. Statistical analyses: one-way ANOVA (I, J, K). **p<0.01.
Sectioning and staining for cortical bone tubes
Following fixation, samples were placed in 30% sucrose for at least 24 hours prior to cryoembedding (Cryomatrix, Thermo Fisher Scientific, Waltham, MA, USA). Undecalcified sections were cut onto Japanese Cryotape at 7 μm as described above. Sections were taken from the mid-section of the cortical bone transplant. Following mounting, sections were rehydrated in PBS and stained using Click-iT EdU Plus Imaging Kit (Alexa Fluor 647; Invitrogen) according to the manufacturer’s instructions or using antibodies (Supplemental Table 1). Slides were coverslipped in 50% glycerol with DAPI (1:5000, D1306; Molecular Probes). Slides were imaged using an Axioscan Z.1 (Zeiss) at 10X magnification. Supplemental figure 3D shows the individual color channels used for evaluation.
Evaluation of cell populations and characteristics of bone tubes
Following the application of standardized thresholds for each channel, the images were exported as tif files using from Zen software (Zeiss). Single channel images were converted to “8 bit colour” and used to create a composite image, using FIJI [33]. The Cell Counter plug-in was used to quantify the populations of iDMP/T+ (Tomato+) cells within the marrow cavity. Cells were only counted if they were on the bone surface or in bone marrow; cells embedded within newly formed bone were not counted, since spontaneous activation of the Cre occurs in osteocytes.
Fracture study
The iDMP/T;ColGFP model was also crossed with Collagen 2.3ΔTK [34] (iDMP/T/ColGFP/ColTK) to facilitate ablation of actively proliferating osteoblasts using ganciclovir (Cytovene, Roche). GCV was administered twice a day at a dose of 8 mg/kg/day intraperitoneally for 16 days to iDMP/T/ColGFP/ColTK mice. Closed transverse tibial fractures were made in 8-week old mice following GCV treatment. Briefly, this was done by inserting a 27G needle into the medullary canal followed by a fracture being created by using a drop weight blunt guillotine device. X-rays (Faxitron LX60, Faxitron X-ray LLC, Lincolnshire, IL, USA) were used to confirm pin placement, fracture creation and to monitor fracture healing. Mice were administered 0.08mg/kg Buprenex (Buprenorphine HCl; Reckitt Benckiser Pharmaceuticals, England) every 12 hours for 48 hours following time of fracture. Bones were collected 7 days post fracture.
Statistical tests
Statistical analyses were performed using Prism 6 (GraphPad software, CA, USA). All data are expressed as means ± standard error of the mean (SEM). Differences between groups were assessed by student’s t-tests and one-way ANOVAs and followed by post hoc Tukey tests, if applicable. The statistical test used for each graph is stated in the figure legend.
Results
Presence of iDMP/T+;ColGFP− stromal cells located within trans-cortical channels
The Dmp1 promoter has been widely used as a marker of osteocytes. Within the skeleton, in addition to osteocytes, lineage tracing of iDMP cells labels late osteoblasts and bone lining cells, likely due to use of very sensitive reporter strain (Ai9) (Supplemental Figure 1A,B) [23, 35]. Transgenic mice containing inducible DMP1CreERT2 (iDMP) were crossed with Tomato reporter strain Ai9 (iDMP/T) and with Col2.3GFP reporter (ColGFP) as a marker of osteoblast/osteocytes generating triple transgenic mice (iDMP/T;ColGFP) [20, 22, 23, 29]. Activation of ColGFP occurs in this model when a cell differentiates into an osteoblast and the ColGFP remains active in osteocytes (Supplemental Figure 1C,D). Following administration of tamoxifen, we observe labeling of osteocytes in the cortical bone that are iDMP/T+ and ColGFP+, as well as osteoblasts on the bone surface (Supplemental Figure 1A,C). In the absence of tamoxifen, spontaneous activation of the cre is limited to a subset of osteocytes (Supplemental Figure 1B,D). We have observed a novel iDMP/T+;ColGFP− population present in cortical bone that is distinct from iDMP/T+;ColGFP+ osteocytes (Figure 1A-C, indicated by blue arrowhead). These iDMP/T+;ColGFP− cells are frequently found located within close proximity to trans-cortical channels (Figure 1A-C). Additionally, with lectin staining for vasculature, we observed that these cells were also perivascular (Figure 1D; Supplemental Figure 4C). Long bone imaging using clearing techniques and light sheet microscopy (LSM) identified the presence of iDMP/T+;ColGFP− cells lining channels throughout cortical bone (Figure 1E; Supplemental Figure 4C; Supplemental Movies S1 and S2).
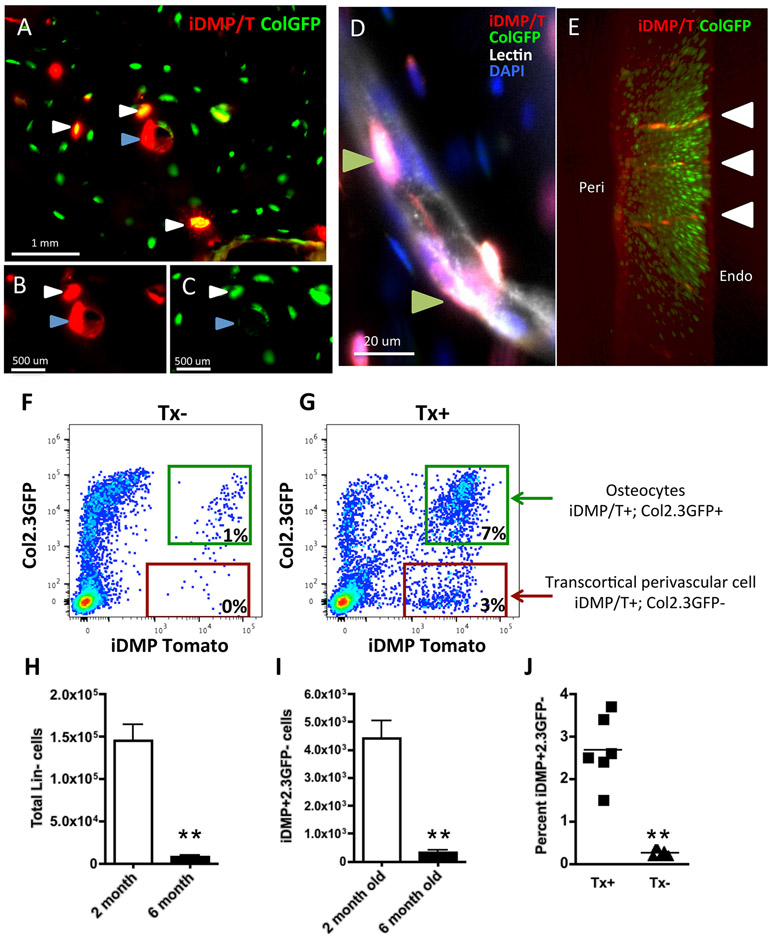
Figure 1: iDMP/T+;ColGFP− cells reside in trans-cortical channels.
(A-C) Presence of iDMP/T+ cells that are ColGFP− in cortical bone. White arrows – iDMPT/+;ColGFP+ osteocytes; Blue arrows – iDMP/T+;ColGFP− cells. (D) iDMP/T+;ColGFP− cells are associated with lectin+ vasculature (staining in white, Green arrows). (E) 3D rendering of cortical bone imaged by light sheet (5x magnification): iDMP/T+ cells – red, ColGFP – green. White arrows highlight iDMP/T+ channels. (F-H) Representative flow cytometry analyses of cortical bone digest samples, lineage negative (CD45/CD31/Ter119)− fraction, from tamoxifen untreated (F) and tamoxifen treated (G) mice. (H) Total number of lin− cells extracted from the long bones of two mice per sample. (I) Total number of iDMP/T+;ColGFP− cells isolated from bones during serial digestion. (J) Proportion of Lin− iDMP/T+;ColGFP− cells isolated from Tx+ and Tx− mice. Flow cytometry analysis for parts H-J were based on data combined from 2 independent experiments containing 6 biological replicates. Statistical analyses: non-paired Student t-test (H, I, J) **p<0.01.
Using flow cytometry, we analyzed cortical bone digests from tamoxifen treated and untreated mice (Figure 1F, G). The number of lineage negative cells (CD45/CD31/Ter119)− (Lin) released from the bone during the digestion was significantly reduced in 6-month-old mice in comparison to 2-month-old mice (Figure 1H), leading to fewer iDMP/T+;ColGFP− cells being identified in these animals (Figure 1I). We observed that this population makes up approximately 2.5% of Lin− cells from the digested cortical bone fraction (Figure 1J). This proportion was consistent in both 2 and 6-month old mice; hence the two age groups have been pooled together. We observed that this population of iDMP/T+;ColGFP− cells reside and persist within cortical bone in the mature skeleton. In the absence of tamoxifen treatment, very few Tomato+ cells are detected, and most of these are ColGFP+ osteocytes. Analysis of cell surface markers characteristic for osteoprogenitor/mesenchymal stem cells revealed that a high proportion of iDMP/T+;ColGFP− cells express CD51 (80.0 ± 2.2%) and LepR (60.0 ± 2.0%), while Sca1, PDGFRβ are expressed at lower levels, and PDGFRα very low or absent (Table 1). In contrast, iDMP/T+;ColGFP+ cells representing more mature osteoblast lineage cells within the cortical bone digest, show a decrease in expression of Sca1 0.3±0.1, and PDGFRβ1.4 ± 0.7.
Table 1: Marker expression on primary TPC (iDMP1/T+;ColGFP−) versus iDMP1/T+;ColGFP+ cells isolated directly from cortical bone .
(n=3 for Sca1, PDGFRα, PDGFRβ, CD51, and n=2 for LepR, each sample is generated from long bones from 2 animals).
| Marker | iDMP/T+;ColGFP− (%±SEM) |
iDMP/T+;ColGFP+ (%±SEM) |
|---|---|---|
| LepR+ | 60.0 ± 2.0 | 59.9 ± 1.5 |
| Sca1+ | 27.3 ± 5.4 | 0.3±0.1 |
| PDGFRα+ | 1.0 ± 0.0 | 1.1 ± 0.3 |
| PDGFRβ+ | 45.3 ± 7.1 | 1.4 ± 0.7 |
| CD51+ | 80.0 ± 2.2 | 54.8 ± 1.1 |
iDMP/T+;ColGFP− stromal cells migrate out during bone outgrowth cultures
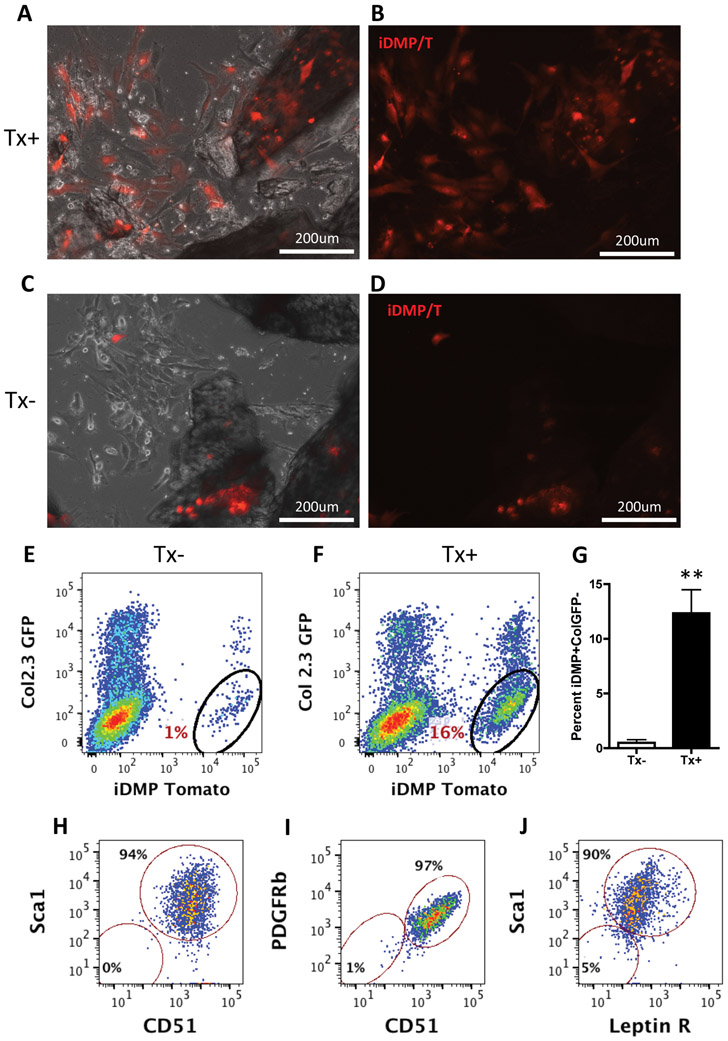
To assess these perivascular cells within cortical channels in vitro, we used bone outgrowth cultures (BOC). Mice were injected with tamoxifen, 2 days prior to culture initiation. Long bones were cleaned and cut into small pieces, then underwent enzymatic digestion to remove all surface cells. By day 6 of culture, cells had migrated out of the chips and adhered to the plate (Figure 2). In the culture derived from tamoxifen treated mice, we observed iDMP/T+ cells on the dish and on the edges of the bone chips (Fig 2A,B). In the tamoxifen untreated (Tx−) culture, we found that iDMP/T+ osteocytes were not contributing to the cell population crawling out of the bone (Figure 2C,D). Thus, since leaky cre expression is observed in 10-25% of osteocytes in the absence of tamoxifen treatment [23], the cells migrating out in these cultures appeared to be distinct from osteocytes. Two-week-old cultures were assessed for surface marker expression using flow cytometry. From tamoxifen-treated outgrowth cultures approx. 12.3±2.1% of cells were iDMP/T+;ColGFP− (Figure 2F); in comparison to 0.5±0.3% isolated from tamoxifen untreated cultures (Figure 2E). iDMP/T+;ColGFP− labeled cells from the BOC were consistently CD51+ (99%) and expressed high levels of mesenchymal stem cell markers: 94% Sca1+, 97% PDGFRβ+ and 90% LeptinR+ (Figure 2G-J). Histograms and unstained samples are shown in Supplemental figure 2G-M. BOCs were evaluated for osteogenesis and adipogenesis. Following osteogenic induction iDMP/T+;ColGFP− cells differentiate into iDMP/T+;ColGFP+ (Supplemental figure 5A). Some cells in the culture underwent adipogenesis as indicated by oil red O staining, but we did not observe any adipogenic differentiation of iDMP/T+;ColGFP− cells (Supplemental figure 5B). Thus, we have identified that iDMP/T+;ColGFP− cells are distinct from osteocytes, capable of migrating out of the bone chips, proliferating and showing lineage restricted commitment to osteogenesis.
Figure 2: iDMP/T+;ColGFP− cells crawl out during bone outgrowth cultures (BOC).
(A, B) Day 6 BOC from tamoxifen treated mice: combined phase and tomato fluorescence (C,D) Day 6 BOC from tamoxifen untreated mice (10X, magnification bar=200μm). (E) Flow cytometry from BOC cultures on day 14; iDMP/T and ColGFP expression from untreated (n=4) and (F) tamoxifen treated mice (n=6). (G) Percentage iDMP/T+;ColGFP− from BOCs. Representative dot plots showing expression of cell markers from the iDMP/T+;ColGFP− population gated in F; for: (H) Sca1 and CD51 (I) PDGFRβ and CD51 (J) and Sca1 and Leptin receptor. Cell surface markers were analyzed from two independent biological experiments. Statistical analyses: non-paired Student t-test (G) **p<0.01.
iDMP/T+;ColGFP− osteoprogenitor population, migrate, expand and contribute to new bone formation in vivo
The identification of osteoclasts within trans-cortical vessels [36], suggests that remodeling of these channels may occur. We injected mice with demeclocycline to label active bone forming surfaces a day after tamoxifen injection. We observed the presence of some bone formation occurring adjacent to iDMP/T+;ColGFP− and iDMP/T+;ColGFP+ cells within some intracortical channels (Supplemental figure 6). Testing the in vivo ability of iDMP/T+;ColGFP− cells to activate into matrix producing osteoblasts (iDMP/T+;ColGFP+) is not feasible, as endosteum is lined with iDMP/T+;ColGFP+ cells [23]. Therefore, we established an ex vivo model of bone tubes that are devoid of periosteum and endocortical osteoblasts (Figure 3A,B). Cortical diaphyseal bone tubes were implanted subcutaneously into NSG recipients (Figure 3C). When iDMP/T+;ColGFP− osteoprogenitor cells become osteoblasts they will turn on ColGFP. iDMP/T+;ColGFP− cells migrate from their vascular niche within cortical bone, contribute to bone regeneration and bone tube closure (Supplemental figure 7A). At 7 days post implantation (dpi), iDMP/T+;ColGFP− cells were present in the previously empty marrow cavity; they were elongated and stromal-like (Fig 3E). At 14 dpi, expansion of iDMP/T+;ColGFP− population was evident by EdU incorporation and some cells had differentiated into osteoblasts iDMP/T+;ColGFP+ (Fig 3F). At 21dpi, iDMP/T+;ColGFP+ cells had contributed to new bone formation (Fig 3G). In the no tamoxifen control, we observed leaky tomato reporter in osteocytes in both old and newly formed bone, but there is no evidence that osteocytes contribute to bone regeneration or formation of new osteoblasts (Fig 3H, Supplemental figure 7B).
We observed a steady progression towards bone regeneration: firstly, iDMP/T+;ColGFP− cells migrated from the cortical bone, followed by haematopoietic infiltration, differentiation of iDMP/T+;ColGFP− cells (to form iDMP/T+;ColGFP+) and new bone formation. By 7dpi, 5 out of 8 bone tubes contained dual iDMP/T+;ColGFP+ cells on the bone surface, and this proportion of ColGFP+ containing samples increased with time. New bone formation was not evident at 7dpi; 3 out of 7 tubes had new bone formation at 14dpi and all tubes had new bone at 21dpi. We quantified the total number of iDMP/T+ cells present within the marrow cavity and on the endosteal surface. By 7dpi, approximately 230 iDMP/T+ cells per section were present in the marrow cavity, and this population of cells was seen to persist at 14 and 21dpi (Fig3I). It is important to note that cells located within the new bone were not counted as it is not possible to distinguish whether the cell was an iDMP/T+;ColGFP− cell that became an osteocyte or whether spontaneous activation of the cre has occurred subsequently. Thus, at 14 and 21dpi the number of iDMP/T+ cells was underestimated. At 21dpi, there was a significant increase in the proportion of dual positive cells (iDMP/T+;ColGFP+) in comparison to 7 and 14dpi (Fig 3J). We also observed a steady rate of proliferation (approximately 8-13%) of iDMP/T+;ColGFP− cells during the time course with few iDMP/T+;ColGFP+ cells proliferating (<2.4%; Fig 3K). Additionally, we observed that the migration of iDMP/T+;ColGFP− stromal cells coincided with vascularization within the cortical bone tube transplantation model (Supplemental figure 8). At 7 dpi, we observed unorganized CD31+ staining adjacent to iDMP/T+;ColGFP− cells (Supplemental figure 8A). By 21dpi, CD31 staining showed the development of a blood vessel network (Supplemental Figure 8B), which coincided with the presence of differentiated iDMP/T+ cells (iDMP/T+;ColGFP+).
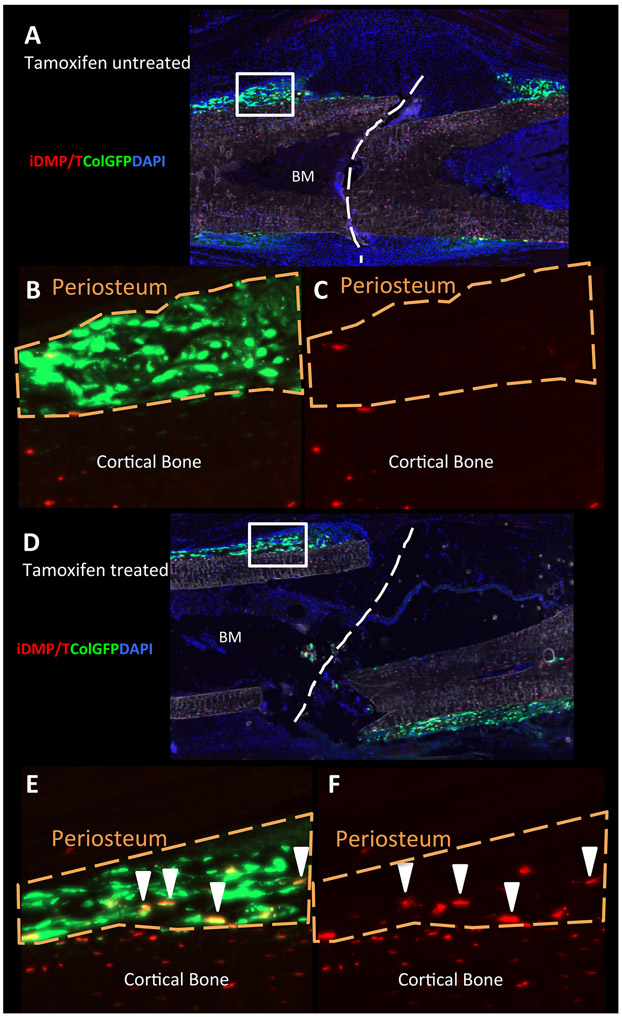
We also evaluated whether these iDMP/T+;ColGFP− osteoprogenitors could contribute to fracture repair. To eliminate contribution of mature osteoblasts we used an osteoblast selective ablation, a Col2.3TK model [34]. iDMP/T/ColGFP/ColTK mice were treated with GCV for 16 days prior to fracture to selectively ablate ColGFP+ osteoblasts, in order to specifically evaluate the contribution of iDMP/T+;ColGFP− cells to fracture repair at 7 days post fracture (dpf). We have utilized the same Col1a1 2.3kb promoter to achieve selective targeting of ColGFP expressing cells. In tamoxifen untreated fractured bone, we did not observe any contribution of iDMP/T+ to Col2.3GFP osteoblasts within newly formed periosteum. We identified a small number of iDMP/T+ cells on the periosteal surface, stromal in appearance that differentiated into osteoblasts and were iDMP/T+;ColGFP+ (Figure 4, arrows). Although this shows some contribution of TPCs to fracture healing, most of the callus does not arise from iDMP/T+;ColGFP− cells. This is in contrast to “bone tube” samples devoid of bone marrow and periosteum, a model in which these iDMP/T+;ColGFP− cells significantly contribute to endosteal bone formation, clearly demonstrating their potential and their role in bone regeneration is context dependent.
Figure 4: Few iDMP/T+ cells contribute to fracture repair.
(A) Fracture callus from an untreated control (no tamoxifen) mouse at 7dpf, inset denotes location of (B) and (C). Tomato and GFP channels are shown in (B); Tomato only in (C). (D) Fracture callus from tamoxifen-treated mouse at 7dpf, inset denotes location of (E) and (F). Tomato and GFP channels are shown in (E); Tomato only in (F). White arrows show the presence of iDMP/T+;ColGFP positive cells in the newly forming fracture callus.
iDMP/T+;ColGFP− osteoprogenitors respond to PTH treatment
Intermittent parathyroid hormone (PTH) is also known to remodel vessels and induces perivascular cells to subsequently migrate to bone surfaces [15]; it is also a strong inducer of bone formation. We wanted to test whether the iDMP/T+;ColGFP− cortical progenitor cells respond to intermittent PTH treatment and whether this would influence bone regeneration. Two approaches were utilized; an evaluation of expansion of iDMP/T+;ColGFP− in vivo by PTH and evaluation of the effects of PTH using bone tube transplants.
Following 10 days of PTH treatment we observed an expansion of iDMP/T+GFP− and iDMP/T+GFP+ populations within the cortical bone. This reflects on the known mechanism of action of PTH by expanding the transcortical osteoprogenitor cell population (iDMP/T+GFP−) and subsequently resulting in increased mature osteoblast numbers (iDMP/T+GFP+) (Supplemental Figure 9).
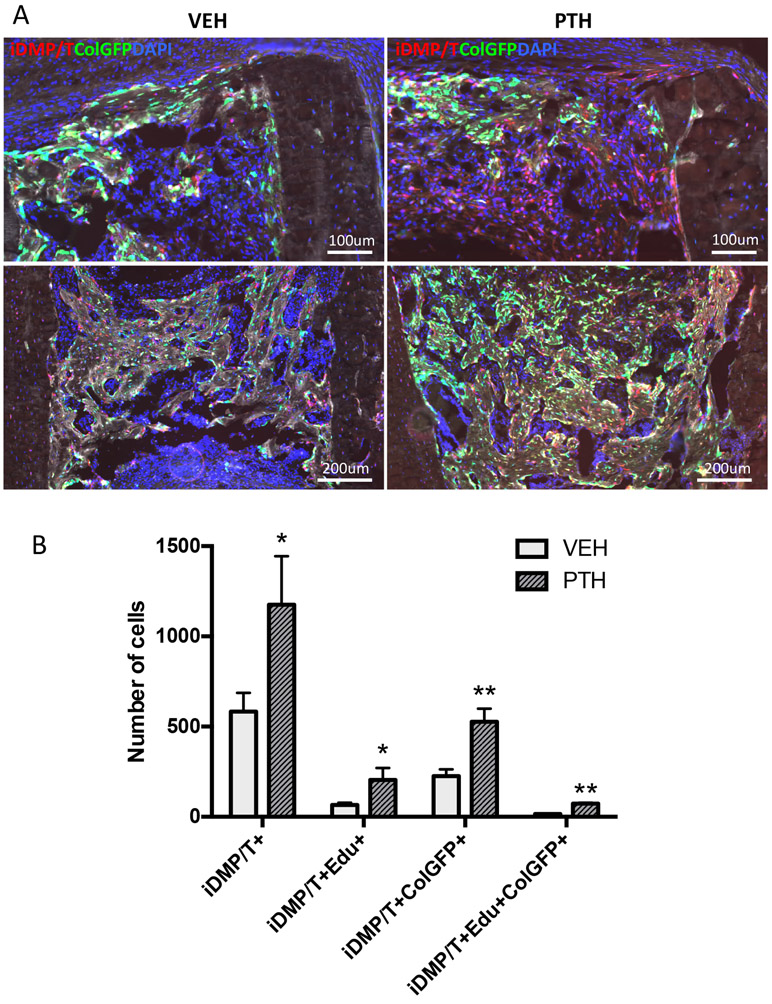
In another study we used bone tube transplants, and starting at 3 dpi, PTH was administered subcutaneously (Supplemental figure 3B). We observed a greater number of iDMP/T+ cells in the bone marrow cavity in the PTH treated group, and also noticed that there were increases in the amount of iDMP/T+;ColGFP+ cells in the PTH group relative to the vehicle (VEH) treated control (Figure 5A). We observed a 2-3x increase in the number of iDMP/T+;ColGFP− cells within the bone tube (Figure 5B). Also in the PTH treated group, significant increases in the number of proliferating iDMP/T+ (iDMP/T+ and EdU+) cells, differentiated (iDMP/T+/ColGFP+ cells and triple positive (iDMP/T+;ColGFP+ and EdU+) cells, relative to the VEH control (Figure 5B). Since PTH is known to push cells towards an osteoblastic and away from an adipocytic fate [14], we also assessed the potential of the iDMP/T+;ColGFP− cells to form adipocytes in response to rosiglitazone treatment (ROSI). While we observed adipocytes in our transplantation model, they were not derived from iDMP/T+;ColGFP− cells (Supplemental Figure 10). Thus, we have identified perivascular iDMP/T+;ColGFP− in cortical channels that respond to PTH and have osteoblast-lineage restricted potential.
Figure 5: iDMP/T+;ColGFP− cells respond to PTH treatment within the cortical bone transplantation model.
(A) Representative merged images from vehicle and PTH groups contain DAPI (blue), iDMP/T+ (tomato) and ColGFP (green). (B) Evaluation of cell identities within cortical bone tube transplants. Each column represents Mean±SEM. VEH: n=6; PTH: n=4. Statistical analyses: student’s t-tests (C, D). *p<0.05, **p<0.01.
Discussion
We have identified and characterized the iDMP/T+;ColGFP− osteoprogenitor as part of the trans-cortical vessels (TCV) niche. In mice, trans-cortical vessels are associated with CD31+ vessels and nerves [36, 37]. A recent study has shown the CX3CR1+ osteoclasts were associated with these TCVs and were involved in resorbing bone for TCV formation [36]. Our data shows the presence of trans-cortical perivascular cells (TPCs) that can participate in coordination of TCV remodeling, particularly by serving as a source of osteoblasts.
Notably, mice do not have Haversian systems like larger organisms, however, these trans-cortical vessels are similar as they contain arteries, veins and nerves, and thus act to facilitate the diffusion of molecules and signals to osteocytes and other cells within cortical bone. Intracortical remodeling is known to occur, however the specific osteoprogenitors involved have not been identified. Vhranas et al. demonstrated that PTH increases intracortical remodeling in ovariectomized rabbits, and thus the resident stromal component within cortical bone appears PTH-responsive [38]. The TPC subset characterized in the current study also shows evidence of PTH-responsiveness.
Commonly used as a marker for osteocytes, the Dmp1 promoter, when combined with a sensitive reporter like Ai9 has been demonstrated to label various non-bone cell types including cells within kidney, brain and the intestine [35, 39]. In addition, we and others have shown that Dmp1-cre also labels osteoblasts and bone lining cells on the endosteal surface [23, 35]. Our previous work utilized DMPCre, a constitutively active model that indicated potential for de-differentiation of osteocytes [26]. However, the availability of the inducible promoter allowed for better lineage tracing and for identification of a different cell type other than osteocytes localized on the bone surface and within cortical bone.
Although, the stem/progenitor ability has long been reserved for the cells within bone marrow, it is now recognized that the majority of MSCs and osteoprogenitors in the bone marrow reside close to the endosteal surface and require enzymatic digest to release [40-42]. Using a combination of GFP directed actual transgene expression to identify osteoblasts and osteocytes in combination with inducible Dmp1CreERT2 (iDMP) we were able to tease out a novel population of cells. Here, we have identified the osteoprogenitor population that resides in the TCV niche within cortical bone. We have shown that the iDMP/T+;ColGFP− cells are rare (2-3% of Lin− digested cortical bone fraction), can migrate and proliferate and selectively differentiate into osteoblasts contributing to new bone formation. Upon activation and egress from cortical bone, this population continues to be perivascular associating with CD31+ vessels and are PTH-responsive. We have evaluated the mesenchymal progenitor potential of this population by assessing adipogenesis. Rosiglitasone was not able to induce adipogenesis of iDMP/T+;ColGFP− cells in vitro or in vivo indicating the selective osteoprogenitor capability of these cells [26, 35]. At this point, it is unclear whether iDMP/T+;ColGFP− can self-renew, but their lineage restricted commitment to osteogenesis support the notion that they do not represent a mesenchymal stem cell. Additional work is required to establish whether a similar TPC population exists in larger animals and the potential role it has in intracortical remodeling.
Vascularization is an important process that supports bone development and repair. Osteoblast precursor cells have been observed to wrap around blood vessels during development and following fracture [3]. During development, osteoblast precursors and blood vessels co-invade cartilage; these two populations appear to be dependent on each other as inhibition of either vascular invasion [43, 44] or perichondral osteoblastogenesis [45-49] impaired the formation of the primary ossification center. iDMP/T+;ColGFP− stromal cells have close proximity to lectin+ and CD31+ vessels. However, the iDMP/T+;ColGFP− cells are located within cortical bone and thus differ from other perivascular osteoprogenitors, which are commonly described as being in the bone marrow [15]. As shown in our previous study, iDMP/T+ cells from bone marrow are distinct from bone marrow MSCs based on their functional characteristics [23]. Inducible DMP1cre (iDMP) labels <0.2% of cells within the bone marrow. The majority of these cells express CD45 and these cells do not have CFU-F potential, extended culturing of these cells does not show their expansion, thus indicating that iDMP/T+ cells from bone marrow are a population distinct from bone marrow MSCs [23].
We also show that these iDMP/T+;ColGFP− cells have the ability to migrate, expand and differentiate into osteoblasts in our cortical bone transplantation model, the successful extravasation of these cells from cortical bone coincided with successful vascularization of the bone tubes. It is unclear at this stage whether there is interdependence between the iDMP/T+;ColGFP− stromal cells and vascularization, and whether impediment of vascularization or ablation of iDMP/T+;ColGFP− stromal cells would impair the bone regeneration observed in the cortical bone tube transplantation model.
By identifying and understanding the properties of these TPCs, we may be able to gain insight into TCV and cortical bone remodeling and identify potential mechanisms to improve bone mass. This study has some important limitations, in particular the lack of specificity of the iDMP labeling which makes it challenging to specifically track the TCV channel osteoprogenitors. For future studies, more specific markers are required in order to understand the activity of these cells under more physiological conditions. Our work defines transcortical perivascular cells as a novel population of cells within bone providing further evidence for the presence of different classes of stem/progenitor cells capable of osteogenesis.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Supplementary Material
Supplemental Movie S1: LSM Z-stacks of tamoxifen treated iDMP/T;ColGFP femur. Tamoxifen treated iDMP/T;ColGFP tissue cleared femur imaged using Light Sheet Microscopy (LSM) with a 5x objective lens. Z stacks were obtained every 1.47 uM for a total of 182 uM depth into the cortical bone. iDMP/T – tomato; ColGFP – green.
Supplemental Movie S2: LSM Z-stacks of untreated iDMP/T;ColGFP femur. Untreated iDMP/T;ColGFP tissue cleared femur imaged using Light Sheet Microscopy (LSM). Z stacks were obtained every 1.47 uM for a total of 315 uM depth into the cortical bone. iDMP/T – tomato; ColGFP – green.
Supplemental figure 1: iDMP/T;ColGFP model showing osteocytes labeled within cortical bone and the presence of iDMPT/+ cells on bone surface following tamoxifen administration. (A, C, E) Representative image of cortical bone from tamoxifen iDMP/T;ColGFP mouse. (B, D, F) Representative image of cortical bone from non-tamoxifen treated iDMP/T;ColGFP mouse. Dotted line denotes bone surface. Individual colour channels are shown: Tomato (A, B); GFP (C, D) and DAPI (E, F). White arrows highlight iDMP/T+ osteoblasts present on the bone surface.
Supplemental figure 2: Gating strategy for flow cytometry analysis. (A) Cell size based on side scatter area (SSC-A) and forward scatter area (FSC-A). (B) Singlets based on forward scatter width (FSC-W) and height (FSC-H). (C) Singlets based on side scatter width (SSC-W) and height (SSC-H). (D) Exclusion of dead cells. (E) Exclusion of haematopoeitic and vascular cells based on CD45/CD31/Ter119 staining. (F) Gating of iDMP/T+;ColGFP− cells. Each of these steps was done consecutively to isolate iDMP/T+;ColGFP− cells. (G-J) Histograms of iDMP/T+;ColGFP− cells (red) with unstained control (grey) from BOCs: (G) CD51, (H) Sca1, (I) PDGFRβ and (J) Leptin receptor. (K-M) Plots of the unstained control with gates from figure 1: (K) Sca1 and CD51, (L) PDGFRβ and CD51 and (M) Sca1 and Leptin receptor. Images are representative from 1 experiment; 2 independent experiments were performed.
Supplemental figure 3: Experimental design and fluorescent analysis of cortical bone tubes (A) Experimental design for cortical bone tube time course. Experimental design for cortical bone tubes with PTH treatment (B) and (C) rosiglitazone treatments. (D) Example of individual fluorescent proteins and EdU staining from bone tube sections used for evaluation in cortical bone transplantation model. DAPI, tomato, DIC, GFP channel, EdU staining – AF647 channel and merge of all channels.
Supplemental figure 4: Lower magnification and individual channels from image shown in Figure 1D and 3D light sheet rendering of iDMP/T;ColGFP femur. (A) Lower magnification of Figure 1D showing two lectin stained vascular channels (white) within cortical bone. (B) Individual channels from Figure 1D. White- lectin stained vasculature, Tomato- iDMP/T, Green- ColGFP, Blue- DAPI. White arrows highlight iDMP/T+;ColGFP− perivascular cells. (C) 3D rendering of flushed femur (bone marrow cavity empty) tissue cleared and imaged by light sheet (5X magnification): iDMP/T+ cells – red, ColGFP – green. Blue arrows highlight iDMP/T+ channels within cortical bone.
Supplemental Figure 5: In vitro potential of iDMP/T;ColGFP− cells
(A-B) Day 7 bone out-growth chip cultures from tamoxifen untreated and treated animals were differentiated in osteogenic inducing media (A) or adipogenic inducing media (B) for 10 days. iDMP/T+;ColGFP− cells differentiate into osteoblasts as detected by co-localization of iDMP/T and Col2.3GFP signal following osteogenic induction (A) 4x images for Brightfield, iDMP/T (red) ColGFP (green). Oil-red-O stained adipocytes are not labeled by iDMP/T indicative of lack of adipogenic potential B) 10x images of Oil Red O (orange, arrowheads), iDMP (red, arrows) and ColGFP (green, arrows).
Supplemental figure 6: Demeclocycline labeling is evident in trans-cortical channel associated with both iDMP/T and ColGFP labeling. (A) Merged image of cortical bone: demeclocycline (blue), ColGFP (green) and iDMP/T (tomato). BM – bone marrow. Individual images of each channel: (B) Blue- Demeclocycline, (C) Green− ColGFP and (D) Tomato− iDMP/T. White arrows indicate areas of new bone formation in proximity to iDMP/T+ cells.
Supplemental figure 7: Full-length cortical bone tube transplantation demonstrating the difference between tamoxifen treated and untreated tubes. (A) Overview of tamoxifen treated bone tube. (B) Presence of iDMP/T+ cells on bone surface of tamoxifen treated bone tube. (C,D) show tomato and GFP fluorescence respectively as single images. (E) Overview of tamoxifen untreated bone tube. (F) Presence of spontaneous cre activation only in osteocytes of bone tube. (G,H) show tomato and GFP fluorescence respectively as single images.
Supplemental figure 8: Migration of iDMP/T+;ColGFP− cells coincided with CD31+ vessel vascularization within the cortical bone tube transplantation model. (A-B) CD31+ vascularization and iDMP/T+ cells are associated with each other in bone tube model. (A) 7dpi disorganization of CD31+ cells adjacent to migrating iDMP/T+;ColGFP− cells and (B) 21 dpi, some iDMP/T+;ColGFP− cells have differentiated (iDMP/T+;ColGFP+), white arrows indicate organization of CD31+ cells into vascular networks. Merged images contain DAPI (blue), CD31 (cyan), iDMP/T+ (tomato) and ColGFP (green).
Supplemental Figure 9: iDMP/T+;ColGFP− cells within the cortical bone are increased in response to intermittent PTH. (A,B) Representative flow cytometry analyses of Lin− cortical bone digest cells in vehicle (A) and PTH treated mice (B). (C) Percentage of Lin− iDMP/T+;ColGFP− cells isolated from vehicle and PTH treated mice. (D) Percentage of Lin− iDMP/T+;ColGFP− cells isolated from vehicle and PTH treated mice. Total of 2 independent biological samples were used within each treatment group.
Supplemental Figure 10: iDMP/T+;ColGFP− cells do not form adipocytes during rosiglitazone-induced adipogenesis. (A) Representative image from cortical bone tube that had been treated at Day 4 post-transplantation with rosiglitazone for 17 days. iDMP/T – tomato; ColGFP – green, Perilipin – white. Inset from (A): (B) Merged, (C) Perilipin staining (AF647), (D) ColGFP (green) and (E) iDMP/T (tomato).
Significance statement.
We identified a novel population of osteoprogenitors that reside in transcortical channels and are perivascular, hereafter termed transcortical perivascular cells (TPCs). While mesenchymal stem cells and osteoprogenitors have been associated with vasculature within bone marrow; this is the first report of an osteoprogenitor specific to cortical bone that may serve as a source of osteoblasts for intracortical remodeling. To specifically examine TPCs from cortical bone, we developed a novel ex vivo cortical bone transplantation model, in which we observed that cells within cortical bone migrate out of the transplant and begin to reconstitute the bone marrow cavity and form new bone.
Acknowledgments
We would like to thank Erin Wilkie for technical assistance in weaning and genotyping the transgenic mice. We would like to thank Dr. Iman Al-Naggar for the FRUIT protocol that was adapted for light sheet imaging of bone samples, Susan Staurovsky for assistance with light sheet imaging, and Dr. Evan Jellison for flow cytometry assistance. We would like to thank Prof. David Rowe for the provision of NSG mice.
Supported by: This work has been supported by NIH/NIDDK 112374 to CJR and RB, and NIH/NIAMS grants AR055607, AR070813 and Regenerative Medicine Research Fund (RMRF) grant 16-RMB-UCHC-10 to I.K.
Footnotes
Conflict of Interest Statement
All of the authors have nothing to disclose.
References
- 1.Crisan M, Yap S, Casteilla L et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 2.Sacchetti B, Funari A, Michienzi S et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. [DOI] [PubMed] [Google Scholar]
- 3.Maes C, Kobayashi T, Selig MK et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou BO, Yue R, Murphy MM et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendez-Ferrer S, Michurina TV, Ferraro F et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worthley DL, Churchill M, Compton JT et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, He G, Lee WC et al. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat Commun. 2017;8:2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park D, Spencer JA, Koh BI et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grcevic D, Pejda S, Matthews BG et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012;30:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debnath S, Yallowitz AR, McCormick J et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizoguchi T, Pinho S, Ahmed J et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalajzic Z, Li H, Wang LP et al. Use of an alpha-smooth muscle actin GFP reporter to identify an osteoprogenitor population. Bone. 2008;43:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews BG, Grcevic D, Wang L et al. Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. J Bone Miner Res. 2014;29:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Arai A, Udagawa N et al. Parathyroid Hormone Shifts Cell Fate of a Leptin Receptor-Marked Stromal Population from Adipogenic to Osteoblastic Lineage. J Bone Miner Res. 2019. [DOI] [PubMed] [Google Scholar]
- 15.Caire R, Roche B, Picot T et al. Parathyroid Hormone Remodels Bone Transitional Vessels and the Leptin Receptor-Positive Pericyte Network in Mice. J Bone Miner Res. 2019. [DOI] [PubMed] [Google Scholar]
- 16.Kalajzic I, Braut A, Guo D et al. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35:74–82. [DOI] [PubMed] [Google Scholar]
- 17.Kamel-ElSayed SA, Tiede-Lewis LM, Lu Y et al. Novel approaches for two and three dimensional multiplexed imaging of osteocytes. Bone. 2015;76:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y, Xie Y, Zhang S et al. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320–325. [DOI] [PubMed] [Google Scholar]
- 19.Bivi N, Condon KW, Allen MR et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res. 2012;27:374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell WF Jr., Barry KJ, Tulum I et al. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol. 2011;209:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato M, Asada N, Kawano Y et al. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab. 2013;18:749–758. [DOI] [PubMed] [Google Scholar]
- 22.Madisen L, Zwingman TA, Sunkin SM et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matic I, Matthews BG, Wang X et al. Quiescent Bone Lining Cells Are a Major Source of Osteoblasts During Adulthood. Stem Cells. 2016;34:2930–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SW, Pajevic PD, Selig M et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. 2012;27:2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalajzic I, Matthews BG, Torreggiani E et al. In vitro and in vivo approaches to study osteocyte biology. Bone. 2013;54:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torreggiani E, Matthews BG, Pejda S et al. Preosteocytes/osteocytes have the potential to dedifferentiate becoming a source of osteoblasts. PLoS One. 2013;8:e75204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang D, Gronthos S, Isenmann S et al. The Late Osteoblast/Preosteocyte Cell Line MLO-A5 Displays Mesenchymal Lineage Plasticity In Vitro and In Vivo. Stem Cells Int. 2019;2019:9838167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K, Le L, Chun BM et al. A Novel Osteogenic Cell Line That Differentiates Into GFP-Tagged Osteocytes and Forms Mineral With a Bone-Like Lacunocanalicular Structure. J Bone Miner Res. 2019;34:979–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalajzic I, Kalajzic Z, Kaliterna M et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17:15–25. [DOI] [PubMed] [Google Scholar]
- 30.Shultz LD, Lyons BL, Burzenski LM et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. [DOI] [PubMed] [Google Scholar]
- 31.Dyment NA, Jiang X, Chen L et al. High-Throughput, Multi-Image Cryohistology of Mineralized Tissues. J Vis Exp. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou B, Zhang D, Zhao S et al. Scalable and DiI-compatible optical clearance of the mammalian brain. Front Neuroanat. 2015;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schindelin J, Arganda-Carreras I, Frise E et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visnjic D, Kalajzic I, Gronowicz G et al. Conditional ablation of the osteoblast lineage in Col2.3deltatk transgenic mice. J Bone Miner Res. 2001;16:2222–2231. [DOI] [PubMed] [Google Scholar]
- 35.Lim J, Burclaff J, He G et al. Unintended targeting of Dmp1-Cre reveals a critical role for Bmpr1a signaling in the gastrointestinal mesenchyme of adult mice. Bone Res. 2017;5:16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grüneboom A, Hawwari I, Weidner D et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nature Metabolism. 2019;1:236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chartier SR, Mitchell SAT, Majuta LA et al. The Changing Sensory and Sympathetic Innervation of the Young, Adult and Aging Mouse Femur. Neuroscience. 2018;387:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vrahnas C, Buenzli PR, Pearson TA et al. Differing Effects of Parathyroid Hormone, Alendronate, and Odanacatib on Bone Formation and on the Mineralization Process in Intracortical and Endocortical Bone of Ovariectomized Rabbits. Calcif Tissue Int. 2018;103:625–637. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Link DC. Targeting of Mesenchymal Stromal Cells by Cre-Recombinase Transgenes Commonly Used to Target Osteoblast Lineage Cells. J Bone Miner Res. 2016;31:2001–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siclari VA, Zhu J, Akiyama K et al. Mesenchymal progenitors residing close to the bone surface are functionally distinct from those in the central bone marrow. Bone. 2013;53:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morikawa S, Mabuchi Y, Kubota Y et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu H, Guo ZK, Jiang XX et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–560. [DOI] [PubMed] [Google Scholar]
- 43.Colnot C, Lu C, Hu D et al. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol. 2004;269:55–69. [DOI] [PubMed] [Google Scholar]
- 44.Maes C, Carmeliet P, Moermans K et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73. [DOI] [PubMed] [Google Scholar]
- 45.Komori T, Yagi H, Nomura S et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. [DOI] [PubMed] [Google Scholar]
- 46.Otto F, Thornell AP, Crompton T et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. [DOI] [PubMed] [Google Scholar]
- 47.Colnot C, de la Fuente L, Huang S et al. Indian hedgehog synchronizes skeletal angiogenesis and perichondrial maturation with cartilage development. Development. 2005;132:1057–1067. [DOI] [PubMed] [Google Scholar]
- 48.Nakashima K, Zhou X, Kunkel G et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. [DOI] [PubMed] [Google Scholar]
- 49.Hill TP, Spater D, Taketo MM et al. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie S1: LSM Z-stacks of tamoxifen treated iDMP/T;ColGFP femur. Tamoxifen treated iDMP/T;ColGFP tissue cleared femur imaged using Light Sheet Microscopy (LSM) with a 5x objective lens. Z stacks were obtained every 1.47 uM for a total of 182 uM depth into the cortical bone. iDMP/T – tomato; ColGFP – green.
Supplemental Movie S2: LSM Z-stacks of untreated iDMP/T;ColGFP femur. Untreated iDMP/T;ColGFP tissue cleared femur imaged using Light Sheet Microscopy (LSM). Z stacks were obtained every 1.47 uM for a total of 315 uM depth into the cortical bone. iDMP/T – tomato; ColGFP – green.
Supplemental figure 1: iDMP/T;ColGFP model showing osteocytes labeled within cortical bone and the presence of iDMPT/+ cells on bone surface following tamoxifen administration. (A, C, E) Representative image of cortical bone from tamoxifen iDMP/T;ColGFP mouse. (B, D, F) Representative image of cortical bone from non-tamoxifen treated iDMP/T;ColGFP mouse. Dotted line denotes bone surface. Individual colour channels are shown: Tomato (A, B); GFP (C, D) and DAPI (E, F). White arrows highlight iDMP/T+ osteoblasts present on the bone surface.
Supplemental figure 2: Gating strategy for flow cytometry analysis. (A) Cell size based on side scatter area (SSC-A) and forward scatter area (FSC-A). (B) Singlets based on forward scatter width (FSC-W) and height (FSC-H). (C) Singlets based on side scatter width (SSC-W) and height (SSC-H). (D) Exclusion of dead cells. (E) Exclusion of haematopoeitic and vascular cells based on CD45/CD31/Ter119 staining. (F) Gating of iDMP/T+;ColGFP− cells. Each of these steps was done consecutively to isolate iDMP/T+;ColGFP− cells. (G-J) Histograms of iDMP/T+;ColGFP− cells (red) with unstained control (grey) from BOCs: (G) CD51, (H) Sca1, (I) PDGFRβ and (J) Leptin receptor. (K-M) Plots of the unstained control with gates from figure 1: (K) Sca1 and CD51, (L) PDGFRβ and CD51 and (M) Sca1 and Leptin receptor. Images are representative from 1 experiment; 2 independent experiments were performed.
Supplemental figure 3: Experimental design and fluorescent analysis of cortical bone tubes (A) Experimental design for cortical bone tube time course. Experimental design for cortical bone tubes with PTH treatment (B) and (C) rosiglitazone treatments. (D) Example of individual fluorescent proteins and EdU staining from bone tube sections used for evaluation in cortical bone transplantation model. DAPI, tomato, DIC, GFP channel, EdU staining – AF647 channel and merge of all channels.
Supplemental figure 4: Lower magnification and individual channels from image shown in Figure 1D and 3D light sheet rendering of iDMP/T;ColGFP femur. (A) Lower magnification of Figure 1D showing two lectin stained vascular channels (white) within cortical bone. (B) Individual channels from Figure 1D. White- lectin stained vasculature, Tomato- iDMP/T, Green- ColGFP, Blue- DAPI. White arrows highlight iDMP/T+;ColGFP− perivascular cells. (C) 3D rendering of flushed femur (bone marrow cavity empty) tissue cleared and imaged by light sheet (5X magnification): iDMP/T+ cells – red, ColGFP – green. Blue arrows highlight iDMP/T+ channels within cortical bone.
Supplemental Figure 5: In vitro potential of iDMP/T;ColGFP− cells
(A-B) Day 7 bone out-growth chip cultures from tamoxifen untreated and treated animals were differentiated in osteogenic inducing media (A) or adipogenic inducing media (B) for 10 days. iDMP/T+;ColGFP− cells differentiate into osteoblasts as detected by co-localization of iDMP/T and Col2.3GFP signal following osteogenic induction (A) 4x images for Brightfield, iDMP/T (red) ColGFP (green). Oil-red-O stained adipocytes are not labeled by iDMP/T indicative of lack of adipogenic potential B) 10x images of Oil Red O (orange, arrowheads), iDMP (red, arrows) and ColGFP (green, arrows).
Supplemental figure 6: Demeclocycline labeling is evident in trans-cortical channel associated with both iDMP/T and ColGFP labeling. (A) Merged image of cortical bone: demeclocycline (blue), ColGFP (green) and iDMP/T (tomato). BM – bone marrow. Individual images of each channel: (B) Blue- Demeclocycline, (C) Green− ColGFP and (D) Tomato− iDMP/T. White arrows indicate areas of new bone formation in proximity to iDMP/T+ cells.
Supplemental figure 7: Full-length cortical bone tube transplantation demonstrating the difference between tamoxifen treated and untreated tubes. (A) Overview of tamoxifen treated bone tube. (B) Presence of iDMP/T+ cells on bone surface of tamoxifen treated bone tube. (C,D) show tomato and GFP fluorescence respectively as single images. (E) Overview of tamoxifen untreated bone tube. (F) Presence of spontaneous cre activation only in osteocytes of bone tube. (G,H) show tomato and GFP fluorescence respectively as single images.
Supplemental figure 8: Migration of iDMP/T+;ColGFP− cells coincided with CD31+ vessel vascularization within the cortical bone tube transplantation model. (A-B) CD31+ vascularization and iDMP/T+ cells are associated with each other in bone tube model. (A) 7dpi disorganization of CD31+ cells adjacent to migrating iDMP/T+;ColGFP− cells and (B) 21 dpi, some iDMP/T+;ColGFP− cells have differentiated (iDMP/T+;ColGFP+), white arrows indicate organization of CD31+ cells into vascular networks. Merged images contain DAPI (blue), CD31 (cyan), iDMP/T+ (tomato) and ColGFP (green).
Supplemental Figure 9: iDMP/T+;ColGFP− cells within the cortical bone are increased in response to intermittent PTH. (A,B) Representative flow cytometry analyses of Lin− cortical bone digest cells in vehicle (A) and PTH treated mice (B). (C) Percentage of Lin− iDMP/T+;ColGFP− cells isolated from vehicle and PTH treated mice. (D) Percentage of Lin− iDMP/T+;ColGFP− cells isolated from vehicle and PTH treated mice. Total of 2 independent biological samples were used within each treatment group.
Supplemental Figure 10: iDMP/T+;ColGFP− cells do not form adipocytes during rosiglitazone-induced adipogenesis. (A) Representative image from cortical bone tube that had been treated at Day 4 post-transplantation with rosiglitazone for 17 days. iDMP/T – tomato; ColGFP – green, Perilipin – white. Inset from (A): (B) Merged, (C) Perilipin staining (AF647), (D) ColGFP (green) and (E) iDMP/T (tomato).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.