Abstract
The American Society of Nephrology has established a new initiative, AKI!Now, with the goal of promoting excellence in the prevention and treatment of AKI by building a foundational program that transforms education and delivery of AKI care, aiming to reduce morbidity and associated mortality and to improve long-term outcomes. In this article, we describe our current efforts to improve early recognition and management involving inclusive interdisciplinary collaboration between providers, patients, and their families; discuss the ongoing need to change some of our current AKI paradigms and diagnostic methods; and provide specific recommendations to improve AKI recognition and care. In the hospital and the community, AKI is a common and increasingly frequent condition that generates risks of adverse events and high costs. Unfortunately, patients with AKI may frequently have received less than optimal quality of care. New classifications have facilitated understanding of AKI incidence and its impact on outcomes, but they are not always well aligned with AKI pathophysiology. Despite ongoing research efforts, treatments to promote or hasten kidney recovery remain ineffective. To avoid progression, the current approach to AKI emphasizes the promotion of early recognition and timely response. However, a lack of awareness of the importance of early recognition and treatment among health care team members and the heterogeneity of approaches within the health care teams assessing the patient remains a major challenge. Early identification is further complicated by differences in settings where AKI occurs (the community or the hospital), and by differences in patient populations and cultures between the intensive care unit and ward environments. To address these obstacles, we discuss the need to improve education at all levels of care and to generate specific guidance on AKI evaluation and management, including the development of a widely applicable education and an AKI management toolkit, engaging hospital administrators to incorporate AKI as a quality initiative, and raising awareness of AKI as a complication of other disease processes.
Keywords: acute kidney injury, critical care nephrology, AKI recognition, AKI awareness, AKI biomarkers, artificial intelligence, electronic alerts, AKI multidisciplinary management, AKI urinalysis, AKI pathology, incidence, goals, morbidity, kidney, hospital administrators, patient care team, quality of health care, intensive care units, longitudinal studies
Introduction
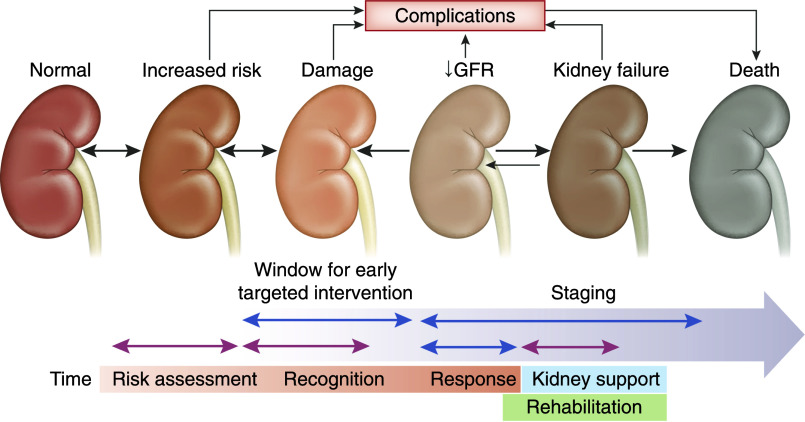
The incidence of AKI is high and increasing around the world (1–4). Standardized definitions of AKI, such as the Kidney Disease Improving Global Outcomes (KDIGO) AKI definition and staging system, have provided insight into AKI incidence and its impact on patient outcomes (5). Unfortunately, despite extensive research efforts, there are no effective treatments to promote or hasten kidney recovery. Therefore, the current approach strives to achieve the identification of populations at high risk of developing AKI, recognition of AKI in its early stages, and early response geared to prevent progression to more severe stages (Figure 1). This is especially important in the developing world, where KRT is largely unavailable or unaffordable (6). Exciting new progress in the epidemiologic and pathophysiologic understanding of AKI, as well as increasing collaborative efforts in multiple research and health care settings, are making a difference and leading to decreasing mortality, better recovery, and improved care of long-term consequences of AKI (KDIGO Controversies, submitted for publication, 2020). In this article, we discuss the current status of AKI recognition and management. We present a series of recommendations including (1) measures to improve early AKI recognition and awareness and their implementation; and (2) the use of electronic alerts, artificial intelligence, and diagnostic tools (appropriate biomarkers, urinalysis, and kidney biopsy when indicated). We also propose modifications in current paradigms such as “prerenal AKI” and “subclinical AKI.” Throughout the paper, we propose to implement a novel collaborative strategy that includes patients, their families, and all members of the health care team.
Figure 1.
Targeted approach to AKI, designed to enhance awareness and promote early recognition and intervention.
Early AKI Recognition and Awareness
Early identification of AKI has been hindered by the wide variety of clinical settings in developed and developing countries where AKI occurs and, even in a single locale, by essential differences between community- and hospital-acquired AKI. Moreover, the health care providers who first encounter patients with AKI are quite diverse, including primary care and emergency physicians, intensivists, surgeons, and nephrologists, as well as multiple other practitioners including nurses, physician extenders, rural health care providers, and pharmacists. In large areas of the world, patients with AKI are rarely or never seen by a nephrologist (7). Kidney workforce shortages are exceedingly common, particularly in low-income countries. For example, the density of nephrologists vary over 1000-fold across the globe, averaging 0.32 per million population in low-income countries and 28.52 per million population in high-income countries (8–10). Even in the developed world, the high incidence of AKI and the limited availability of nephrology subspecialists impairs expert intervention in each case. Variability in the understanding and management of AKI has limited its recognition and resulted in delayed treatment implementation (11). Therefore, a major effort is necessary to achieve early recognition and action by a multitude of heterogeneous health care professionals who may lack awareness of the problem and its management (2,12).
To fulfill that goal, education on AKI should be a core competency of the vast multitude of people involved in health care, although their required depth of knowledge may vary.
Recognizing AKI as a growing global problem (4), the International Society of Nephrology (ISN) launched the AKI 0by25 initiative in 2013, with the ambitious goal of reaching zero preventable AKI deaths worldwide by 2025 (2). Data from the Global Snapshot (12) revealed differences in recognition, management, and outcomes of AKI in different health care settings (13). A lack of awareness and a lack of access to diagnostic and therapeutic care contributed to the disparities found in the outcomes of community-acquired AKI. Education and training programs to optimize the identification and management of AKI in rural community settings in low-income countries are ongoing.
The Importance of AKI Awareness
Limited awareness of risk factors for AKI delays and/or impairs response to AKI. Thus, a health care provider may recognize AKI very late, when little can be done to manage the patient except for the provision of KRT when available. To a large extent, awareness of AKI is highly dependent on the context where AKI occurs (Figure 2). For example, the development of early oliguria postcardiac surgery raises concern for AKI, even before serum creatinine rises; the development of high fever and dehydration in the rainy season in a child in a rural community in Sub-Saharan Africa raises the possibility of AKI (13). Therefore, to promote early recognition, efforts must be made to increase awareness of the main possible determinants of AKI development in the specific context. Despite multiple commonalities, such as the impact of volume depletion or sepsis in the development of AKI, it is imperative to develop educational tools and strategies that can be easily adapted to be valid, relevant, and appropriate to the specific setting. The basic concepts are the same, but the details and risk factors must be assessed locally, along with accounting for differences in language, culture, religion, and symbology.
Figure 2.
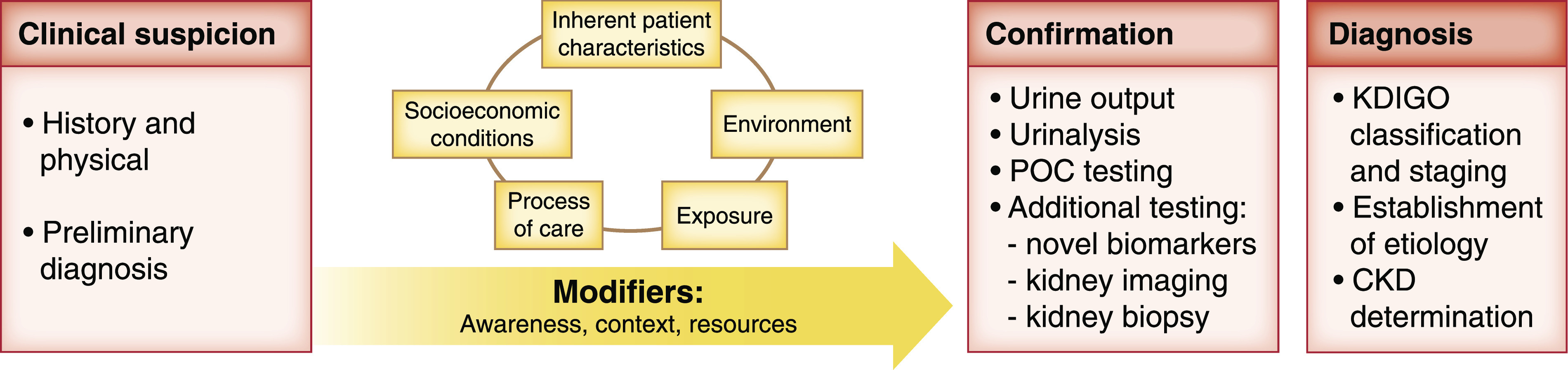
AKI awareness is key to achieve early recognition, which is highly dependent on the context and the available resources where AKI occurs. Confirmation can be achieved with basic resources such as measurement of urine output, urinalysis, and POC testing, but—where available—biomarkers, kidney imaging, and biopsy enhance the diagnostic process. POC, point of care; KDIGO, Kidney Disease Improving Global Outcomes.
The Interaction between the AKI Individual Determinants and the Context Where It Occurs
In addition to the clinical context as a determinant of AKI, each person has a variable degree of pre-existing AKI risk, determined by factors such as the individual’s genetic makeup, age, sex, pre-existing comorbidities, and immune status (Figure 3 ). Establishing this risk level is especially important when the individual is about to be exposed to interventions that can potentially cause kidney injury, such as surgery or intravascular iodinated contrast. In some contexts, risk scores can be used to assess individual risk (14–17). However, recent studies have demonstrated a large degree of heterogeneity in the sensitivity and specificity of these risk scores (18–21). Given the current difficulties in determining individual risk and how to mitigate this risk, this area is worthy of study and intervention in its own right.
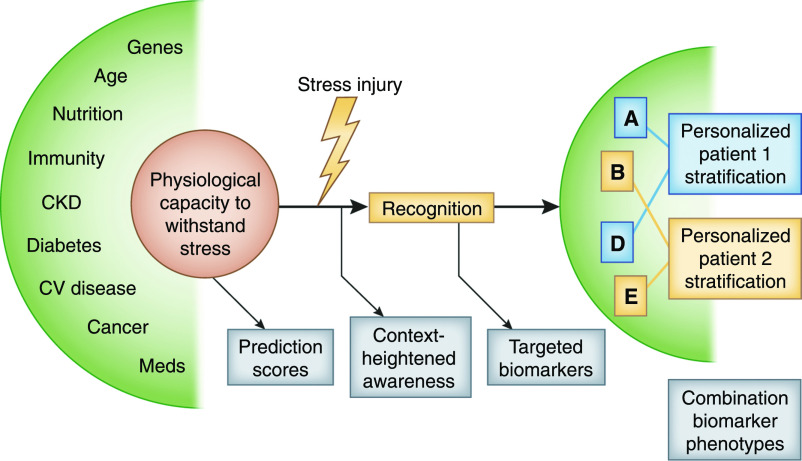
Figure 3.
Paradigms for optimal biomarker use focus on targeted biomarker testing in populations of interest. In this model, early identification of risk is enhanced by preemptive identification of patients who are high risk using prediction scores and dynamic review of the electronic health record. To further enrich the population, the Renal Angina Index (46,47) or the furosemide stress test can be used (88). By identifying higher pretest probability, the use of targeted biomarkers significantly improves diagnostic accuracy and yield. Use of targeted, multiple biomarkers to identify the type of kidney injury and to risk-stratify patients may, in the future, direct personalized treatment (58). CV, cardiovascular.
Implementing Early Recognition
One of the ways to promote recognition of subclinical or early-stage AKI in hospitals with efficient electronic health record systems is the use of electronic alerts (22,23). Automatic, continuous review of key variables such as potentially nephrotoxic exposures, changes in urine output, or increase in serum creatinine or other biomarkers may trigger an electronic alert to health care personnel, which can be used to guide further evaluation and action (20).
The use of electronic alert systems can be associated with implementation challenges, especially in the step between the production of the alert and the communication to the relevant provider; without effective communication, outcomes are unlikely to be improved (24,25) (Table 1).
Table 1.
The potential risks and benefits of electronic alerts
| Benefits | Risks | Balance |
|---|---|---|
| Decreased nephrotoxin exposure | Increased alert fatigue | Alert intrusiveness must be proportional to risk level |
| Appropriate medication choices | Need to ensure that systems are robust | Timing must be appropriate |
| Improved patient outcomes | Ensure that false-positive rates are extremely low | Mode of alerting agreed upon |
| Need to agree on what information is relayed |
Studies in the United Kingdom in the hospitalized population (26,27) have shown that optimization of the alerting sequence can improve outcomes, especially if coupled with effective care bundles or checklists. A similar application in the community is more difficult to implement.
Recently, rapid progress in the field of artificial intelligence and machine learning technologies has allowed several centers to use these technologies to predict, diagnose, and even manage different aspects of critical illnesses, including AKI. Currently, several high-precision prediction models have been developed and validated in intensive care unit and hospital settings (20,28,29). These models are able to generate a continuous AKI risk score based on the characteristics of each individual patient. Ideally, these models need to be embedded within the clinical workflow, and risk scores need to be linked with the current and developing body of knowledge, to provide real-time, patient-specific, and pathophysiology-related recommendations. The impact of these systems on the processes of care and patients’ outcomes will need to be evaluated in future studies.
When available, early nephrology consultation has been shown to be beneficial and to improve the outcomes of patients with AKI (30,31). Especially in the critical care context, the role of the nephrologist as an integral, collaborative member of the AKI care team is well established (32–34). In the United States, management of AKI constitutes the majority of the hospital workload of the nephrologist (35). Nonetheless, there is a need to further develop and promote the active participation of the nephrologist as a key member of the multidisciplinary team (36). In settings where a nephrology consultant may not be physically available (e.g., rural and low-income contexts), implementation of telecommunication with reference centers has recently been shown to be effective in the context of the Pilot Project of the ISN 0by25 initiative (E. Macedo et al.: Recognition and Management of Community-Acquired AKI in Low Resource Settings–The ISN 0by25 Interventional Trial. Submitted for publication, PlosOne, 2020).
Importance of Interdisciplinary Collaboration
Strategies to recognize early AKI highlight the importance of multidisciplinary collaboration; only 7%–10% of AKI care is for primary kidney disease in isolation. Understanding the principles of detection and management must constitute a core competency among all health care team members. The Nephrotoxic Injury Negated by Just in time Action (NINJA) initiative highlights the critical role of multidisciplinary, systematic surveillance by collaborative groups including physicians, nurses, and pharmacists (37,38). In NINJA, rounding pharmacists were automatically alerted when pediatric patients were exposed to three or more nephrotoxic medications on the same day or were receiving an intravenous aminoglycoside for 3 or more days. Pharmacists would then recommend daily serum creatinine measurements to the health care teams, and report when patients develop AKI. The application of such surveillance efforts achieved a 62% reduction in AKI rates.
Recently, the use of the term “nephrotoxic” has been challenged (see KDIGO Controversies paper, in press, 2020) from multiple points of view, including that apparent “toxicity” sometimes is only an expression of physiologic effects of the drug (for example, the rise in serum creatinine when using angiotensin converting-enzyme inhibitors), whereas in other cases, it is the result of actual injury, such as the effect of aminoglycosides. Furthermore, patients and their families have occasionally shown consternation when told that physicians use “kidney-toxic” medications. No consensus for an alternative designation has yet been reached.
The role of patients and families in multidisciplinary efforts to improve outcomes from AKI is increasingly recognized. The patients’ voices must be heard, and their stories must be relayed, as this is what makes the problem real and tangible. Patient-supported initiatives are being implemented via regional or international societies such as the American Society of Nephrology (ASN), the ISN, or the National Kidney Foundation. The importance of patient participation and perspective is an integral part of the AKI!Now initiative.
Inadequacy of Serum Creatinine as an Early AKI Biomarker
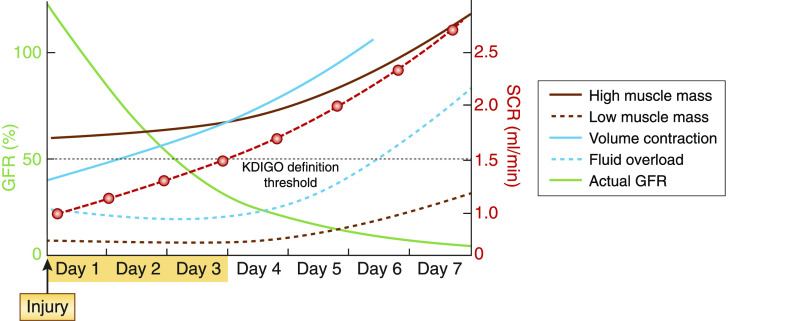
The early recognition of AKI is currently impeded by the use of elevated serum creatinine and/or change in urine output to diagnose disease; unfortunately, serum creatinine is a late, insensitive, highly confounded, nonspecific biomarker of kidney function. As shown in Figure 4, it may take as long as 72 hours postinjury to detect a rise in serum creatinine, for a number of reasons. It takes 2–3 days for serum creatinine to reach a new equilibrium postinjury. Volume expansion may lower serum creatinine and delay recognition, whereas volume contraction can induce an increase in serum concentration despite the absence of injury (39). Decreased creatinine production will blunt the rise in serum creatinine further, and children and patients with very small muscle mass have decreased production of creatinine. Acute illness (e.g., sepsis) and complications of illness (such as severe malnutrition and sarcopenia) also decrease creatinine production (40). Moreover, variation in tubular creatinine secretion and variation in the magnitude of kidney functional reserve will determine a lesser or greater degree of serum creatinine rise (41).
Figure 4.
The early recognition of AKI is currently impeded by the widespread use of serum creatinine as the main (and often the only) biomarker. Unfortunately, serum creatinine is a late, insensitive, highly confounded, nonspecific biomarker of kidney function. SCR, serum creatinine.
Importantly, under- or overestimation of GFR by serum creatinine can lead to inappropriate medication dosing, which may contribute to the poor prognosis associated with AKI (38). Overestimation of GFR may lead to overdosing and drug toxicity, whereas underestimation may lead to drug underdosing. The delayed serum creatinine rise may prolong such toxicities, and the lack of steady-state creatinine makes the use of commonly used equations such as the CKD-Epidemiology Collaboration, Modification of Diet in Renal Disease (MDRD), and Cockroft–Gault equations inappropriate for drug dosing. Furthermore, using those formulas to estimate baseline GFR leads to discordant estimations of AKI incidence depending on the formula used.
Recent studies have examined the utility of equations such as the Jelliffe and kinetic estimated GFR (KeGFR) in AKI, using well-established gold-standard methods such as technetium-99m diethylenetriaminepentaacetic acid clearance. Upcoming results of studies that utilize real-time continuous measurement of GFR with fluorescent (42–45) or other markers are eagerly awaited.
As opposed to impending or developing painful acute myocardial ischemia, where clinical symptoms can drive laboratory testing (e.g., troponin measurements), AKI is typically insidious and is rarely recognized unless the observers are alert, recognize the signs, and obtain appropriate testing. The recent development of the concept of renal angina (46,47) incorporates the paradigm of cardiac angina recognition and proposes that recognition of simple risk factors with early changes in creatinine or fluid accumulation can be used to “rule out” patients not at risk for AKI, and then direct further investigation for patients who “rule in” for renal angina.
Because of these limitations with serum creatinine and AKI detection, major investigative efforts have focused on developing sensitive and specific markers that detect early kidney injury. Biomarkers must be able to offer critical information that creatinine cannot, such as generating a signal immediately after injury, independent of volume status. Likewise, biomarkers have the potential to locate and identify the potential mechanism of kidney injury. Barasch et al. (48) has rightfully emphasized that “rather than a singular focus on serum creatinine, the coupling of causation (medical context) with sites of injury (anatomic responses) and their specific cellular responses (proteomics and transcriptomics), factoring in the extent of filtration and tubular dysfunction are the keys to advance Nephrology to a level of precision necessary to achieve diagnostic and therapeutic innovation.”
The Indispensable Urinalysis
In addition to novel biomarkers, the use of urinalysis cannot be overemphasized. Training of medical students and fellows and retraining of practicing nephrologists on the value of performing routine urinalysis goes a long way in achieving an accurate diagnosis and planning additional diagnostic measures and treatments (49–52). The diagnostic accuracy of urinalysis can be enhanced by including a microscopy score. Such scores have been shown to be able to predict patient outcomes and to distinguish between “prerenal azotemia” and acute tubular necrosis (49–51).
“Prerenal” and “Subclinical” AKI
“Subclinical AKI,” recently defined as an elevation in levels of kidney damage biomarkers with no change in functional biomarkers, and therefore not fulfilling the conventional criteria for AKI, describes a subgroup of patients with an increased risk of poor outcomes (including mortality and need for dialysis), when compared with non-AKI patients (53,54). For example, in the context of volume expansion, a patient may not have evidence of AKI based on our current functional markers due to dilution and/or decreased creatinine production, but may have evidence of kidney injury based on the presence of kidney damage biomarkers. This represents subclinical AKI. In contrast, a patient with decompensated heart failure treated with diuretics may experience an increase in serum creatinine, which may actually be associated with better outcomes by unloading tissue edema. Such patients may not require cessation of diuretics (or volume re-expansion) if kidney damage biomarkers are not elevated (55). There is a need to re-examine the current use of the term “prerenal AKI” and its implications. The term is widely used and implies that in “prerenal AKI,” the decrease in GFR is due to insufficient kidney perfusion and, consequently, that additional volume expansion will be of benefit. Unfortunately, not all forms of what is called “prerenal” AKI respond to volume expansion and, in some cases (decompensated heart failure, abdominal compartment syndrome), the clinical status may be exacerbated by additional volume expansion. The controversies regarding “subclinical” and “prerenal” AKI were a topic of interest that will be further commented on in the upcoming report of the recent KDIGO Controversies in AKI meeting. It is apparent that an updated definition of clinical AKI and a nomenclature that incorporates biomarkers may be necessary (56,57). Unfortunately, the application of a more complex nomenclature incorporating biomarkers that may not be widely available may be difficult to generalize in resource-limited settings.
What Newer Biomarkers Have to Offer
Recent evidence suggests that traditional methods to detect AKI may be leveraged by newer, more sophisticated analytic tools capable of prediction and identification: risk stratification, novel AKI biomarker panels, and clinical information systems (58).
A biomarker panel would not necessarily replace serum creatinine but provide greater diagnostic granularity and phenotyping on the presence and nature of injury (59). Recently, Bhatraju et al. (60) emphasized that one of the reasons for the lack of safe and effective pharmacologic interventions may be heterogeneity within the AKI population, which hampers the identification of specific pathophysiologic pathways and therapeutic targets. They tested whether AKI subphenotypes had prognostic and therapeutic implications by identifying and analyzing two molecularly distinct responses to vasopressin therapy. Such subphenotypes could improve risk prognostication and may be useful for predictive enrichment in clinical trials.
In addition to their use for diagnosis, novel AKI biomarkers may also probe into the underlying mechanisms of injury and thereby shed new light on the field (61–64). For instance, the development of cell-cycle arrest biomarkers highlights the importance of cell regeneration in AKI recovery and may suggest future novel therapeutics (65–68).
Furthermore, biomarkers may be helpful in distinguishing the cause of AKI. Moledina et al. (69) have recently demonstrated that urine biomarkers TNF-a and IL-9 may differentiate acute interstitial nephritis from other kidney diseases, such as acute tubular necrosis, glomerular diseases, and diabetic kidney disease. In another example, Belcher et al. (70) demonstrated that kidney injury biomarkers were higher in patients with cirrhosis who developed acute tubular necrosis compared with patients with prerenal azotemia or hepatorenal syndrome. Likewise, a markedly low fractional excretion of sodium (<0.2) was more specific for hepatorenal syndrome. In contrast, low levels of biomarkers can guide the continuation of diuretic therapy and renin-angiotensin-aldosterone system inhibitors for optimal decongestion and management of acute decompensation of heart failure (71,72).
Parenthetically, newer findings could potentially help us understand the mechanisms of “organ cross-talk” during AKI, including the mechanisms that lead to neurologic and cardiac injury (73), and novel developments in the fields of transcriptomics and urine metabolomics have enabled the identification of cell-specific kidney response pathways to injury (48).
Despite this progress, the adoption of biomarkers in clinical practice has been slow. Multiple reasons may be at play, including the well-known inertia of practitioners to introduce newer discoveries into practice, but other issues such as cost considerations, reimbursement, and a lack of evidence that the use of biomarkers changes outcomes are likely contributing.
The problem is further compounded by the absence of a clinically available gold standard of GFR measurement. The use of inaccurate reference standards makes the assessment of the operating characteristics and performance of newer biomarkers problematic (74) because errors in the reference test can bias the assessment of the new test being evaluated (48).
While biomarker developers necessarily focus in depth on the exact context where the biomarker performs best, general practitioners need a simpler, more practical paradigm that leads to wide adoption and a better understanding of the new tool (75). Education on the best scenario where the yield of the biomarker is highest will go a long way in promoting wider adoption.
Cost is a significant challenge, but more appropriate use, and practitioner understanding of the importance of pretest probability determining when the application of the test is appropriate, may help decrease the impact of this expense, and the significant cost of severe and nonrecovering AKI must be entered into the equation.
The recovery phase of AKI needs to be better understood. Post-AKI readmission rates and AKI relapse are very high, and progression toward CKD and ESKD are a significant risk to the quality of life and survival. Efforts to identify how better to predict and promote recovery are currently studied intensely (76–78).
More Kidney Biopsies Are Necessary
Finally, the need to perform more kidney biopsies in patients with AKI must be emphasized. Our limited understanding of the histopathology of the AKI syndrome impairs progress in our insight of disease mechanisms, classifications, and treatments. Similar to what happens with CKD, where a stage in the disease is misunderstood as a diagnosis (“this patient has CKD4”), the widespread use of AKI KDIGO stages has led to a misunderstanding of their significance (79). Studies have shown a concerning lack of correlation between the KDIGO stage, the presumed mechanism, and histopathology (81). A significant portion of poor AKI outcomes may be attributable to a lack of clinical comprehension of what is the disease, leading to the application of late or inappropriate treatments (48,81–84). The ongoing National Institutes of Health (NIH) Kidney Precision Medicine Project (https://kpmp.org) is currently gathering kidney biopsy tissue from patients with AKI, aiming to identify critical cells, pathways, and targets for novel therapies, as well as provide important information on the correlation between AKI staging and the underlying histopathology. These results will likely shed light on when tissue diagnosis is necessary and lead to newer therapeutic discoveries.
Recommendations
An action agenda should incorporate a strategy with multiple components, which will serve to increase awareness and early recognition, as other AKI research items should focus on further understanding AKI physiopathology.
We propose to include the following items (Figure 5): First, improvement in AKI education is essential at both under- and postgraduate levels for all health care professionals, emphasizing the importance of identifying patients at risk of AKI, as well as highlighting that AKI education should be considered a core competency at all levels. Thus, the United Kingdom’s Academy of Royal Medical Colleges has published a core competency for AKI, providing a pragmatic approach relevant to each health care professional as described by the National Institutes of Health and Clinical Excellence (85,86).
Figure 5.
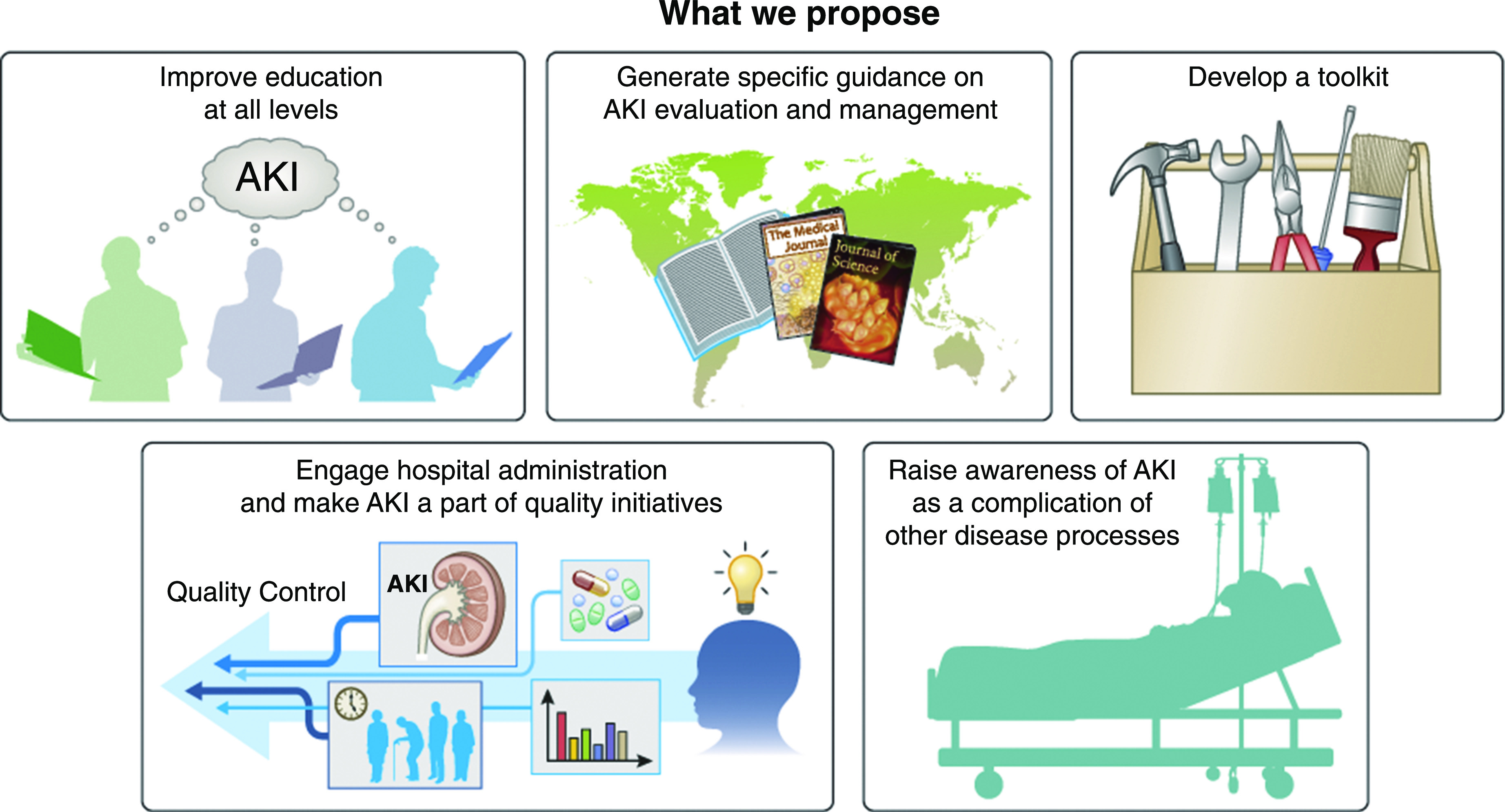
Proposed recommendations.
Efforts by the 0by25 ISN initiative have led to the adaptation of similar education instruments to the rural community context in Nepal, Bolivia, and Malawi, with excellent results (E. Macedo et al.: Recognition and Management of Community-Acquired AKI in Low Resource Settings–The ISN 0by25 Interventional Trial. Submitted for publication, PlosOne, 2020).
Recognizing this requisite, the AKI!Now initiative recently conducted an Expert Forum and a Landscape Analysis (manuscript in preparation) to identify, catalog, and describe available data; assess other AKI initiatives; and conduct a needs assessment, which will be used to optimize interventions based on recognized need. In collaboration with ICREON, a digital innovation agency, the AKI!Now initiative is developing a live, web-based, continuously updated, and self-maintained “clinical collection compendium” of all relevant ASN publications, journals, and educational activities including Kidney Week, Kidney News Online, and NephSAP, which will soon be made easily accessible worldwide. The database will be flexible and modifiable by users and will greatly facilitate wide access to known and developing knowledge. In addition, the online ASN Communities (https://community.asn-online.org/) represent a worldwide, expert-moderated forum where all aspects of AKI and kidney disease are freely discussed.
Second, physicians must be provided with specific guidance for evaluating and managing patients with AKI. The AKI Clinical Practice Guidelines from KDIGO has developed a strong platform for this purpose, but some aspects of AKI are now controversial in light of new data (5) and will soon be updated. The 0by25 Global Snapshot has also developed specific guidelines that were developed and adapted to specific community contexts and demonstrated usefulness. Those guidelines are widely available.
An AKI toolkit must be designed to be used globally, including a checklist of simple measures to be rapidly implemented to reduce risk and injury. These checklists must be specific to individual settings and patients, taking into account the very diverse environments where AKI occurs: the community, the general hospital ward, and the intensive care unit in high- and low-income countries, as well as the community and the hospital in low-middle income countries.
Importantly, patients who have suffered an episode of AKI and their families commonly do not receive appropriate information and often do not know the patient has suffered AKI. Information must be provided regarding the causes and effects of the episode and, crucially, the need for short- and long-term follow-up. There is a strong case to be made for increasing health literacy within the general population and the role of kidneys in health. Initiatives such as the “Think Kidneys Sick Day Guidance” (http://wwwthinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf) are appealing but their application has not yet been fully proven in practice.
Third, hospital administrators and quality control personnel should consider AKI as a core competency and should be made aware of the evidence through the same education paradigms, including AKI as a core measure of general medical care and as a key factor that determines outcomes. Hospitals should be encouraged to adopt audit measures in the care of patients with AKI, as proposed by National Confidential Enquiry into Patient Outcome and Death and other guideline bodies (87). Hospital-acquired AKI episodes, their progression, and short- and long-term outcomes should be considered benchmarks for hospital care of acutely ill patients, utilizing all of the early diagnosis tools discussed above.
Around the world, the upfront health utilization, the morbidity and mortality risk associated with populations, and the subsequent impact upon long-term health are poorly recognized and should be seen as factors determining policy and goals of budget allocation.
Fourth, AKI awareness must be raised as a complication of other disease processes. The close relationship and common disease pathways between AKI, sepsis, and multiple organ failure generate challenges and opportunities to understand and incorporate AKI into the context of the patient as a whole individual and to create new areas of discovery. Similarly, this relationship is relevant in areas where endemic conditions such as severe malaria, gastroenteritis, and leptospirosis are associated with high AKI-associated morbidity and mortality, and whenever sex and income disparities play a significant role in determining patient outcomes.
Conclusion
We expect that the application of our current recommendations aiming to improve awareness, early recognition, and prompt management of AKI, utilizing a strongly collaborative approach and the latest advances in AKI diagnosis, will significantly contribute to decrease the severity of the condition and lead to a reduction in its acute and chronic consequences.
The AKI!Now initiative will continue to disseminate the results of ongoing research and practice, and help establish a link between the health care team members, the patients, and their families, to achieve that goal jointly.
Disclosures
A. Agarwal is President of ASN and a member of the Steering Committee for AKI!Now. A. Agarwal reports receiving grants from Baxter, during the conduct of the study; personal fees from Akebia Therapeutics and Angion; personal fees and other from Goldilocks Therapeutics, Inc.; and personal fees from Dynamed and Reata Pharmaceuticals; outside the submitted work. S.L. Goldstein reports receiving personal fees from Baxter Healthcare, BioPorto Inc., CHF Solutions, Fresenius, MediBeacon, and Medtronic, outside the submitted work. K.D. Liu reports receiving personal fees from Biomerieux, Durect, Potrero Med, Quark, Theravance, and UpToDate; and other from the American Thoracic Society, Amgen, AstraZeneca, Baxter, and the National Policy Forum on Critical Care and Acute Renal Failure; outside the submitted work. M.D. Okusa reports receiving personal fees from UpToDate, outside the submitted work. C.R. Parikh reports receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute; and other from Akebia Therapeutics, Inc., Genfit Biopharmaceutical Company, and Renaltix AI; outside the submitted work. A. Vijayan is a speaker for Sanofi-Aventis and a consultant for NxStage and Boerhinger-Ingelheim. All remaining authors have nothing to disclose.
Funding
ASN has received a Baxter Healthcare Corporation unrestricted educational grant to support the AKI!Now ASN initiative.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS: Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14: 607–625, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, Cruz D, Jaber B, Lameire NH, Lombardi R, Lewington A, Feehally J, Finkelstein F, Levin N, Pannu N, Thomas B, Aronoff-Spencer E, Remuzzi G: International society of nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 385: 2616–2643, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Ostermann M, Cerdá J: The burden of acute kidney injury and related financial issues. Contrib Nephrol 193: 100–112, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology: World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S: Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 6.Cerdá J, Mohan S, Garcia-Garcia G, Jha V, Samavedam S, Gowrishankar S, Bagga A, Chakravarthi R, Mehta R: Acute kidney injury recognition in low- and middle-income countries. Kidney Int Rep 2: 530–543, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jha V, Arici M, Collins AJ, Garcia-Garcia G, Hemmelgarn BR, Jafar TH, Pecoits-Filho R, Sola L, Swanepoel CR, Tchokhonelidze I, Wang AY, Kasiske BL, Wheeler DC, Spasovski G; Conference Participants: Understanding kidney care needs and implementation strategies in low- and middle-income countries: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int 90: 1164–1174, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Bello AK, Levin A, Tonelli M, Okpechi IG, Feehally J, Harris D, Jindal K, Salako BL, Rateb A, Osman MA, Qarni B, Saad S, Lunney M, Wiebe N, Ye F, Johnson DW: Assessment of global kidney health care status. JAMA 317: 1864–1881, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris DCH, Davies SJ, Finkelstein FO, Jha V, Donner JA, Abraham G, Bello AK, Caskey FJ, Garcia GG, Harden P, Hemmelgarn B, Johnson DW, Levin NW, Luyckx VA, Martin DE, McCulloch MI, Moosa MR, O’Connell PJ, Okpechi IG, Pecoits Filho R, Shah KD, Sola L, Swanepoel C, Tonelli M, Twahir A, van Biesen W, Varghese C, Yang CW, Zuniga C; Working Groups of the International Society of Nephrology’s 2nd Global Kidney Health Summit: Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int 95: S1–S33, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, Fox CS, Gansevoort RT, Heerspink HJL, Jardine M, Kasiske B, Köttgen A, Kretzler M, Levey AS, Luyckx VA, Mehta R, Moe O, Obrador G, Pannu N, Parikh CR, Perkovic V, Pollock C, Stenvinkel P, Tuttle KR, Wheeler DC, Eckardt KU; ISN Global Kidney Health Summit participants: Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 390: 1888–1917, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Olowu WA, Niang A, Osafo C, Ashuntantang G, Arogundade FA, Porter J, Naicker S, Luyckx VA: Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: A systematic review. Lancet Glob Health 4: e242–e250, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Mehta RL, Burdmann EA, Cerdá J, Feehally J, Finkelstein F, García-García G, Godin M, Jha V, Lameire NH, Levin NW, Lewington A, Lombardi R, Macedo E, Rocco M, Aronoff-Spencer E, Tonelli M, Zhang J, Remuzzi G: Recognition and management of acute kidney injury in the international society of nephrology 0by25 global Snapshot: A multinational cross-sectional study. Lancet 387: 2017–2025, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Macedo E, Cerdá J, Hingorani S, Hou J, Bagga A, Burdmann EA, Rocco V M, Mehta L R: Recognition and management of acute kidney injury in children: The ISN 0by25 global snapshot study. PLoS One 13: e0196586, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G: A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J Am Coll Cardiol 44: 1393–1399, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Srisawat N, Kulvichit W, Mahamitra N, Hurst C, Praditpornsilpa K, Lumlertgul N, Chuasuwan A, Trongtrakul K, Tasnarong A, Champunot R, Bhurayanontachai R, Kongwibulwut M, Chatkaew P, Oranrigsupak P, Sukmark T, Panaput T, Laohacharoenyot N, Surasit K, Keobounma T, Khositrangsikun K, Suwattanasilpa U, Pattharanitima P, Santithisadeekorn P, Wanitchanont A, Peerapornrattana S, Loaveeravat P, Leelahavanichkul A, Tiranathanagul K, Kerr SJ, Tungsanga K, Eiam-Ong S, Sitprija V, Kellum JA: The epidemiology and characteristics of acute kidney injury in the Southeast Asia intensive care unit: A prospective multicentre study [published online ahead of print May 10, 2019]. Nephrol Dial Transplant doi: 10.1093/ndt/gfz087 [DOI] [PubMed] [Google Scholar]
- 17.Park S, Cho H, Park S, Lee S, Kim K, Yoon HJ, Park J, Choi Y, Lee S, Kim JH, Kim S, Chin HJ, Kim DK, Joo KW, Kim YS, Lee H: Simple postoperative AKI risk (SPARK) classification before noncardiac surgery: A prediction index development study with external validation. J Am Soc Nephrol 30: 170–181, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie S, Feng Z, Tang L, Wang X, He Y, Fang J, Li S, Yang Y, Mao H, Jiao J, Liu W, Cao N, Wang W, Sun J, Shao F, Li W, He Q, Jiang H, Lin H, Fu P, Zhang X, Liu Y, Wu Y, Xi C, Liang M, Qu Z, Zhu J, Wu G, Zheng Y, Na Y, Li Y, Li W, Cai G, Chen X: Risk factor analysis for AKI including laboratory indicators: A nationwide multicenter study of hospitalized patients. Kidney Blood Press Res 42: 761–773, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Feng F, Li M, Chang X, Wei B, Dong C: Development of a risk stratification-based model for prediction of acute kidney injury in critically ill patients. Medicine (Baltimore) 98: e16867, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiofolo C, Chbat N, Ghosh E, Eshelman L, Kashani K: Automated continuous acute kidney injury prediction and surveillance: A random forest model. Mayo Clin Proc 94: 783–792, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Adhikari L, Ozrazgat-Baslanti T, Ruppert M, Madushani RWMA, Paliwal S, Hashemighouchani H, Zheng F, Tao M, Lopes JM, Li X, Rashidi P, Bihorac A: Improved predictive models for acute kidney injury with IDEA: Intraoperative data embedded analytics. PLoS One 14: e0214904, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutherland SM, Chawla LS, Kane-Gill SL, Hsu RK, Kramer AA, Goldstein SL, Kellum JA, Ronco C, Bagshaw SM; 15 ADQI Consensus Group: Utilizing electronic health records to predict acute kidney injury risk and outcomes: Workgroup statements from the 15(th) ADQI consensus conference. Can J Kidney Health Dis 3: 11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin M, Wilson FP: Utility of electronic medical record alerts to prevent drug nephrotoxicity. Clin J Am Soc Nephrol 14: 115–123, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, Feldman HI, Fernandez H, Gitelman Y, Lin J, Negoianu D, Parikh CR, Reese PP, Urbani R, Fuchs B: Automated, electronic alerts for acute kidney injury: A single-blind, parallel-group, randomised controlled trial. Lancet 385: 1966–1974, 2015. 25726515 [Google Scholar]
- 25.Hoste EA, Kashani K, Gibney N, Wilson FP, Ronco C, Goldstein SL, Kellum JA, Bagshaw SM; 15 ADQI Consensus Group: Impact of electronic-alerting of acute kidney injury: Workgroup statements from the 15(th) ADQI consensus conference. Can J Kidney Health Dis 3: 10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrasekar T, Sharma A, Tennent L, Wong C, Chamberlain P, Abraham KA: A whole system approach to improving mortality associated with acute kidney injury. QJM 110: 657–666, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW: The epidemiology of hospitalised acute kidney injury not requiring dialysis in England from 1998 to 2013: Retrospective analysis of hospital episode statistics. Int J Clin Pract 70: 330–339, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyner JL, Carey KA, Edelson DP, Churpek MM: The development of a machine learning inpatient acute kidney injury prediction model. Crit Care Med 46: 1070–1077, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Tomašev N, Glorot X, Rae JW, Zielinski M, Askham H, Saraiva A, Mottram A, Meyer C, Ravuri S, Protsyuk I, Connell A, Hughes CO, Karthikesalingam A, Cornebise J, Montgomery H, Rees G, Laing C, Baker CR, Peterson K, Reeves R, Hassabis D, King D, Suleyman M, Back T, Nielson C, Ledsam JR, Mohamed S: A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 572: 116–119, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta RL, McDonald B, Gabbai F, Pahl M, Farkas A, Pascual MT, Zhuang S, Kaplan RM, Chertow GM: Nephrology consultation in acute renal failure: does timing matter? Am J Med 113: 456–461, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Endre ZH: The role of nephrologist in the intensive care unit. Blood Purif 43: 78–81, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Askenazi DJ, Heung M, Connor MJ Jr., Basu RK, Cerdá J, Doi K, Koyner JL, Bihorac A, Golestaneh L, Vijayan A, Okusa MD, Faubel S; American Society of Nephrology Acute Kidney Injury Advisory Group: Optimal role of the nephrologist in the intensive care unit. Blood Purif 43: 68–77, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darmon M, Ostermann M, Cerda J, Dimopoulos MA, Forni L, Hoste E, Legrand M, Lerolle N, Rondeau E, Schneider A, Souweine B, Schetz M: Diagnostic work-up and specific causes of acute kidney injury. Intensive Care Med 43: 829–840, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Ronco C, Bellomo R: Critical care nephrology: The time has come. Nephrol Dial Transplant 13: 264–267, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Koyner JL, Cerdá J, Goldstein SL, Jaber BL, Liu KD, Shea JA, Faubel S; Acute Kidney Injury Advisory Group of the American Society of Nephrology: The daily burden of acute kidney injury: A survey of U.S. nephrologists on world kidney day. Am J Kidney Dis 64: 394–401, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Cerdá J, Tolwani A, Palevsky P: Challenges of performing renal replacement therapy in the intensive care unit - The nephrologist perspective. Clin Nephrol 90: 11–17, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Goldstein SL: Nephrotoxicities. F1000 Res 6: 55, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostermann M, Chawla LS, Forni LG, Kane-Gill SL, Kellum JA, Koyner J, Murray PT, Ronco C, Goldstein SL; ADQI 16 workgroup: Drug management in acute kidney disease - Report of the acute disease quality initiative XVI meeting. Br J Clin Pharmacol 84: 396–403, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, Wright P, Peterson MW, Rock P, Hyzy RC, Anzueto A, Truwit JD; National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network: Acute kidney injury in patients with acute lung injury: Impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med 39: 2665–2671, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerdá J, Cerdá M, Kilcullen P, Prendergast J: In severe acute kidney injury, a higher serum creatinine is paradoxically associated with better patient survival. Nephrol Dial Transplant 22: 2781–2784, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Sharma A, Mucino MJ, Ronco C: Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 127: 94–100, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Wang E, Meier DJ, Sandoval RM, Von Hendy-Willson VE, Pressler BM, Bunch RM, Alloosh M, Sturek MS, Schwartz GJ, Molitoris BA: A portable fiberoptic ratiometric fluorescence analyzer provides rapid point-of-care determination of glomerular filtration rate in large animals. Kidney Int 81: 112–117, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molitoris BA, Reilly ES: Quantifying glomerular filtration rates in acute kidney injury: A requirement for translational success. Semin Nephrol 36: 31–41, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molitoris BA: Measuring glomerular filtration rate in the intensive care unit: No substitutes please. Crit Care 17: 181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solomon R, Goldstein S: Real-time measurement of glomerular filtration rate. Curr Opin Crit Care 23: 470–474, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Basu RK, Kaddourah A, Goldstein SL; AWARE Study Investigators: Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: A multicentre, multinational, prospective observational study. Lancet Child Adolesc Health 2: 112–120, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein SL, Chawla LS: Renal angina. Clin J Am Soc Nephrol 5: 943–949, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Barasch J, Zager R, Bonventre JV: Acute kidney injury: A problem of definition. Lancet 389: 779–781, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavanaugh C, Perazella MA: Urine sediment examination in the diagnosis and management of kidney disease: Core curriculum 2019. Am J Kidney Dis 73: 258–272, 2019 [DOI] [PubMed] [Google Scholar]
- 50.Perazella MA: The urine sediment as a biomarker of kidney disease. Am J Kidney Dis 66: 748–755, 2015 [DOI] [PubMed] [Google Scholar]
- 51.Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR: Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 3: 1615–1619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Claure-Del Granado R, Macedo E, Mehta RL: Urine microscopy in acute kidney injury: Time for a change. Am J Kidney Dis 57: 657–660, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Haase M, Kellum JA, Ronco C: Subclinical AKI--An emerging syndrome with important consequences. Nat Rev Nephrol 8: 735–739, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM, Shlipak MG, Parikh CR; TRIBE-AKI Consortium: Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 25: 1063–1071, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH, Testani JM: Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: Insights from the DOSE trial. J Card Fail 22: 753–760, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA, Chawla LS, Cruz D, Ince C, Okusa MD; ADQI 10 workgroup: Potential use of biomarkers in acute kidney injury: Report and summary of recommendations from the 10th acute dialysis quality initiative consensus conference. Kidney Int 85: 513–521, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teo SH, Endre ZH: Biomarkers in acute kidney injury (AKI). Best Pract Res Clin Anaesthesiol 31: 331–344, 2017 [DOI] [PubMed] [Google Scholar]
- 58.Basu RK, Gist K, Wheeler DS: Improving acute kidney injury diagnostics using predictive analytics. Curr Opin Crit Care 21: 473–478, 2015 [DOI] [PubMed] [Google Scholar]
- 59.Belcher JM, Parikh CR: Is it time to evolve past the prerenal azotemia versus acute tubular necrosis classification? Clin J Am Soc Nephrol 6: 2332–2334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhatraju PK, Zelnick LR, Herting J, Katz R, Mikacenic C, Kosamo S, Morrell ED, Robinson-Cohen C, Calfee CS, Christie JD, Liu KD, Matthay MA, Hahn WO, Dmyterko V, Slivinski NSJ, Russell JA, Walley KR, Christiani DC, Liles WC, Himmelfarb J, Wurfel MM: Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am J Respir Crit Care Med 199: 863–872, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wasung ME, Chawla LS, Madero M: Biomarkers of renal function, which and when? Clin Chim Acta 438: 350–357, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Parikh CR, Devarajan P: New biomarkers of acute kidney injury. Crit Care Med 36[4 Suppl]: S159–S165, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Agarwal A, Dong Z, Harris R, Murray P, Parikh SM, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative XIII Working Group: Cellular and molecular mechanisms of AKI. J Am Soc Nephrol 27: 1288–1299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kashani K, Cheungpasitporn W, Ronco C: Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin Chem Lab Med 55: 1074–1089, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Bonventre JV: Maladaptive proximal tubule repair: Cell cycle arrest. Nephron Clin Pract 127: 61–64, 2014 [DOI] [PubMed] [Google Scholar]
- 66.Kellum JA, Chawla LS: Cell-cycle arrest and acute kidney injury: the light and the dark sides. Nephrol Dial Transplant 31: 16–22, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, Humphreys BD, Koyner JL, Liu KD, Mour G, Nolin TD, Bihorac A; American Society of Nephrology Acute Kidney Injury Advisory Group: Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis 68: 19–28, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu KD, Vijayan A, Rosner MH, Shi J, Chawla LS, Kellum JA: Clinical adjudication in acute kidney injury studies: Findings from the pivotal TIMP-2*IGFBP7 biomarker study. Nephrol Dial Transplant 31: 1641–1646, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moledina DG, Wilson FP, Pober JS, Perazella MA, Singh N, Luciano RL, Obeid W, Lin H, Kuperman M, Moeckel GW, Kashgarian M, Cantley LG, Parikh CR: Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight 4: 127456, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belcher JM, Garcia-Tsao G, Sanyal AJ, Thiessen-Philbrook H, Peixoto AJ, Perazella MA, Ansari N, Lim J, Coca SG, Parikh CR; TRIBE-AKI Consortium: Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin J Am Soc Nephrol 9: 1857–1867, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dupont M, Shrestha K, Singh D, Awad A, Kovach C, Scarcipino M, Maroo AP, Tang WH: Lack of significant renal tubular injury despite acute kidney injury in acute decompensated heart failure. Eur J Heart Fail 14: 597–604, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, Felker GM, Hernandez AF, O’Connor CM, Sabbisetti VS, Bonventre JV, Wilson FP, Coca SG, Testani JM: Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation 137: 2016–2028, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Awad AS, Okusa MD: Distant organ injury following acute kidney injury. Am J Physiol Renal Physiol 293: F28–F29, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Emerson SC, Waikar SS, Fuentes C, Bonventre JV, Betensky RA: Biomarker validation with an imperfect reference: Issues and bounds. Stat Methods Med Res 27: 2933–2945, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cerdá J: A biomarker able to predict acute kidney injury before it occurs? Lancet 394: 448–450, 2019 [DOI] [PubMed] [Google Scholar]
- 76.Cerdá J, Liu KD, Cruz DN, Jaber BL, Koyner JL, Heung M, Okusa MD, Faubel S; American Society of Nephrology AKI Advisory Group: Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol 10: 1859–1867, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettilä V, Prowle JR, Schetz M, Joannidis M: Renal recovery after acute kidney injury. Intensive Care Med 43: 855–866, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stanski NL, Stenson EK, Cvijanovich NZ, Weiss SL, Fitzgerald JC, Bigham MT, Jain PN, Schwarz A, Lutfi R, Nowak J, Allen GL, Thomas NJ, Grunwell JR, Baines T, Quasney M, Haileselassie B, Wong HR: PERSEVERE biomarkers predict severe acute kidney injury and renal recovery in pediatric septic shock [published online ahead of print on January 9, 2020]. Am J Respir Crit Care Med doi: 10.1164/rccm.201911-2187OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kiryluk K, Bomback AS, Cheng YL, Xu K, Camara PG, Rabadan R, Sims PA, Barasch J: Precision medicine for acute kidney injury (AKI): Redefining AKI by agnostic kidney tissue interrogation and genetics. Semin Nephrol 38: 40–51, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chu R, Li C, Wang S, Zou W, Liu G, Yang L: Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin J Am Soc Nephrol 9: 1175–1182, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moledina DG, Cheung B, Kukova L, Luciano RL, Peixoto AJ, Wilson FP, Alfano S, Parikh CR: A survey of patient attitudes toward participation in biopsy-based kidney research. Kidney Int Rep 3: 412–416, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moledina DG, Hall IE, Thiessen-Philbrook H, Reese PP, Weng FL, Schröppel B, Doshi MD, Wilson FP, Coca SG, Parikh CR: Performance of serum creatinine and kidney injury biomarkers for diagnosing histologic acute tubular injury. Am J Kidney Dis 70: 807–816, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moledina DG, Luciano RL, Kukova L, Chan L, Saha A, Nadkarni G, Alfano S, Wilson FP, Perazella MA, Parikh CR: Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol 13: 1633–1640, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moledina DG, Parikh CR: Phenotyping of acute kidney injury: Beyond serum creatinine. Semin Nephrol 38: 3–11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewington AJ, Cerdá J, Mehta RL: Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int 84: 457–467, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.National Institute for Health and Care Excellence: Acute kidney injury: prevention, detection and management (NICE guideline NG148), 2019. Available at: https://www.nice.org.uk/guidance/ng148. Accessed April 1, 2020
- 87.Stewart JA: Adding insult to injury: Care of patients with acute kidney injury. Br J Hosp Med (Lond) 70: 372–373, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Koyner JL, Davison DL, Brasha-Mitchell E, Chalikonda DM, Arthur JM, Shaw AD, Tumlin JA, Trevino SA, Bennett MR, Kimmel PL, Seneff MG, Chawla LS: Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc Nephrol 26: 2023–2031, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]