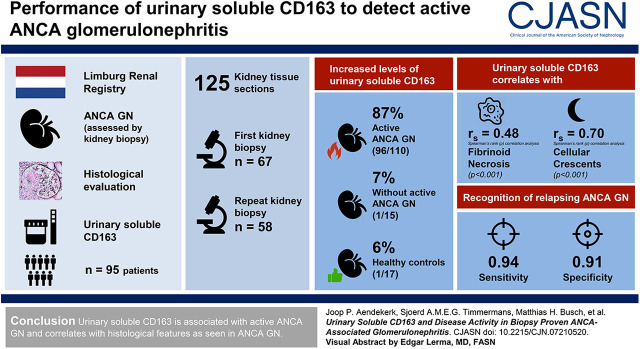
Visual Abstract
Keywords: ANCA, glomerulonephritis, vasculitis, kidney biopsy, macrophages, Acute kidney injury, kidney pathology, Biopsy
Abstract
Background and objectives
ANCA-associated GN is a common cause of rapidly progressive GN, with high relapse rates. The early recognition of an ANCA-associated GN relapse is of importance to prevent loss of kidney function. Urinary soluble CD163 has been identified as a promising marker of active ANCA-associated GN. Previous studies, however, are limited by the lack of histologic data.
Design, setting, participants, & measurements
We analyzed urinary soluble CD163 in 95 patients with ANCA-associated vasculitis who underwent a kidney biopsy. In total, 125 kidney tissue sections (first kidney biopsy, n=67; repeated biopsy, n=58) with concurrent 24-hour urine samples were studied. Correlation analyses comparing urinary soluble CD163 levels and morphologic features of ANCA-associated GN were performed using Spearman rank correlation analysis. The diagnostic performance of biomarkers to detect relapsing ANCA-associated GN was evaluated using receiver operating characteristics curve analysis.
Results
High levels of urinary soluble CD163 were found in 96 (87%) of 110 biopsies with active ANCA-associated GN compared with one (7%) of 15 biopsies without active ANCA-associated GN and one (6%) of 17 healthy controls. Urinary soluble CD163 correlated with fibrinoid necrosis (Rho=0.48, P<0.001) and cellular crescents (Rho=0.70, P<0.001) on kidney biopsy. In repeated biopsies, urinary soluble CD163’s sensitivity of 0.94 and specificity of 0.91 for the recognition of relapsing ANCA-associated GN appeared better than routine clinical measures. The presence of CD163+ cells in affected glomeruli confirmed urinary soluble CD163’s origin.
Conclusions
Urinary soluble CD163 is associated with active ANCA-associated GN and correlates with histologic features as seen in ANCA-associated GN.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2020_11_17_CJN07210520_final.mp3
Introduction
ANCA-associated vasculitis is the most prevalent small vessel vasculitis with a predilection for the respiratory tract and the kidneys (1). ANCA-associated vasculitis is the leading cause of rapidly progressive GN (2), characterized by a pauci-immune necrotizing crescentic GN (1,3), which has been linked to high morbidity, including progression to ESKD, and mortality (4,5). Patients with relapsing ANCA-associated GN (ANCA GN) are at highest risk for poor outcomes, emphasizing the need for early recognition and prompt immunosuppressive treatment of relapsing disease (6). Noninvasive biomarkers are needed because traditional laboratory measures (i.e., urinalysis and serum creatinine) can often not reliably differentiate between active disease and chronic damage, particularly in patients with persistent urinary abnormalities (7–9).
Soluble CD163, a scavenger receptor shed by M2-type macrophages upon activation (10,11), detected in urine appears to be such a promising biomarker of active ANCA GN (12–14). Macrophages promote crescent formation in experimental ANCA GN in mice (15), and CD163+ cells, indeed, have been found in patients with early ANCA GN (16). More recently, measurement of soluble CD25 (i.e., the IL-2 receptor secreted upon T cell activation) has been shown to complement urinary soluble CD163 in detecting active ANCA GN (14). Robust clinical data, however, are limited. Moreover, these markers have been studied in the context of active ANCA GN on the basis of conventional noninvasive markers of active disease rather than histologic proof (12–14). The diagnostic performance of urinary soluble CD163, either alone or in combination with soluble CD25, for the recognition of relapsing ANCA GN and during follow-up has not been evaluated.
This study was designed to determine the performance of urinary soluble CD163 to detect active ANCA GN in a large and well-defined cohort of patients with histologically proven de novo and/or relapsing ANCA GN. In addition, we performed a detailed histologic analysis of kidney biopsies to investigate whether urinary soluble CD163 levels correlate with morphologic features of active ANCA GN. Finally, we compared the performance of urinary soluble CD163, either alone or in combination with soluble CD25, and conventional markers used in clinical practice to detect relapsing ANCA GN in a large cohort of patients with repeated kidney biopsies.
Materials and Methods
Patient Cohort
Patients with ANCA-associated vasculitis, according to the Chapel Hill Consensus Conference (1), were recruited from the Limburg Renal Registry (4) between January 2016 and April 2019. Patients were included if kidney tissue sections and concurrent 24-hour urine samples were available. Patients presenting with relapsing disease were checked for previous kidney biopsies with concurrent 24-hour urine samples. Urine and serum samples were obtained at the time of kidney biopsy and, in selected patients, at the time of stable clinical remission, processed, and stored at −80°C. Clinical data were obtained from the Limburg Renal Registry and the patients’ medical records. Stable clinical remission was defined as a Birmingham Vasculitis Activity Score (17) of zero and a daily dose of prednisolone <7.5 mg. In selected patients, urine samples were available monthly for up to 6 months. Patients with no previous medical history, normal kidney function, mild urinary abnormalities (i.e., hematuria and/or proteinuria <0.5 g/d), and normal parenchyma on kidney biopsy were recruited from the Limburg Renal Registry (4) and included as healthy controls. The study was approved by the local medical ethics committee and is in accordance with the Declaration of Helsinki.
Kidney Tissue Sections
Formalin-fixed, paraffin-embedded, and snap-frozen kidney tissue sections were processed according to routine clinical practice (2). Kidney tissue sections with at least eight glomeruli were scored independently and blinded from patient data by two separate investigators; discrepancies were resolved by a third investigator. Kidney biopsies were classified as active ANCA GN when fibrinoid necrosis and/or (fibro-)cellular crescents were present, whereas the sole presence of fibrotic and/or sclerosed glomeruli was classified as chronic nonactive disease; active ANCA GN was classified according to the histologic classification of ANCA GN and scored for the number of normal glomeruli (Figure 1) (3,4). Also, chronicity indices were scored: that is, interstitial fibrosis and/or tubular atrophy class (i.e., zero, one, two, and three defined as chronic abnormalities in <10%, 10%–25%, 26%–50%, and >50%, respectively) and arteriosclerosis (i.e., zero and one defined as intima thickening less than media thickness and intima thickening equal to or more than media thickness, respectively) (18).
Figure 1.
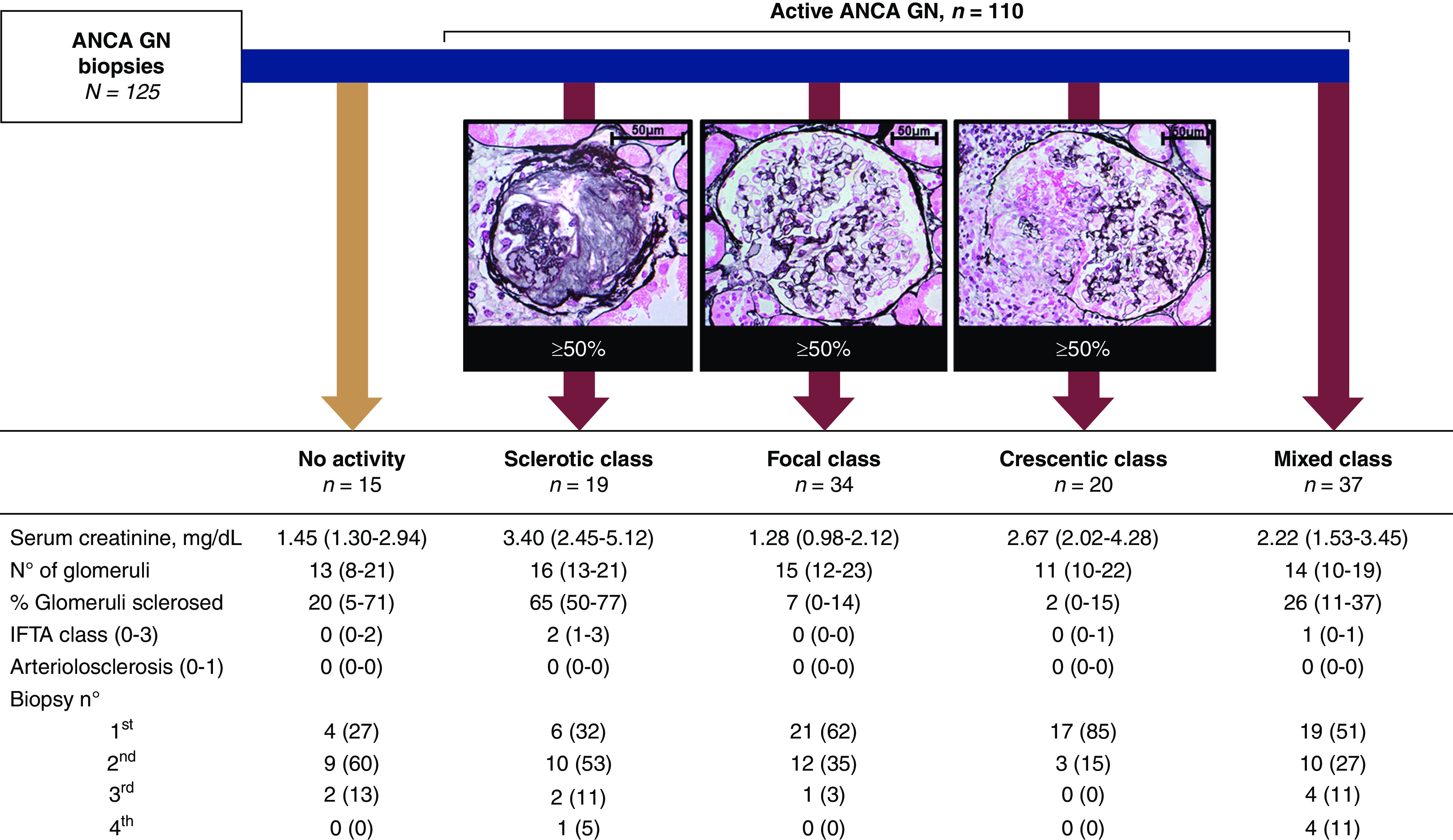
All biopsies are classified according to histologic classification of ANCA GN and presented with biopsy characteristics. Jones methenamine silver stain of representative sclerotic, normal, and crescentic glomeruli at ×630, ×400, and ×400 magnification, respectively. Data are presented as medians with interquartile ranges or numbers with percentages. ANCA GN, ANCA-associated GN; IFTA, interstitial fibrosis and/or tubular atrophy.
Formalin-fixed, paraffin-embedded sections were stained for CD163 to detect CD163+ cells after appropriate antigen retrieval. Three-micrometer sections were incubated with Tris-EDTA buffer (pH 9) at 98°C for 60 minutes and blocked with 3% H2O2. Rabbit anti-CD163 mAb (Abcam, Cambridge, United Kingdom) was diluted 1:300 in PBS and incubated for 30 minutes. EnVision anti-rabbit horseradish peroxidase (Agilent, Santa Clara, CA) was used to visualize CD163.
CD163 was semiquantitatively scored in glomeruli, the periglomerular compartment, and tubulointerstitium (Supplemental Figure 1). In the (peri-)glomerular compartments, CD163+ cells were scored as zero, one, two, and three defined as zero, one to five, six to ten, and greater than ten CD163+ cells, respectively (12). Tubulointerstitial staining was scored in five or more consecutive areas at ×200 magnification as zero, one, two, and three, defined as 0%–10%, 11%–25%, 26%–50%, and >50% CD163+ cells per area, respectively. Mean (peri-)glomerular and tubulointerstitial scores per biopsy were derived by dividing the sum of all scores by the number of glomeruli or tubulointerstitial areas scored, respectively.
Measurement of Soluble CD163 and CD25
Soluble CD163 and soluble CD25 were assessed by using commercially available ELISAs (DY1607 and DY223, respectively; both from R&D Systems, Minneapolis, MN). Urine (1:4) and serum (1:2) were diluted in test medium (i.e., 1% BSA and PBS); results above the maximum value were titrated. Urinary soluble CD163 and CD25 were corrected for creatinine excretion and depicted in nanograms per millimole; serum soluble CD25 was depicted in nanograms per milliliter.
Routine Markers of Active Antineutrophil Cytoplasmic Antibody–Associated Glomerulonephritis
Routine markers of active ANCA GN were AKI (i.e., >30% increase in serum creatinine and/or >25% decline in eGFR) (17), new-onset hematuria (i.e., ≥1+ on urine dipstick analysis or ten or more red blood cells per high-power field within 6 months preceding a biopsy, succeeding two or more consecutive negative assessments), proteinuria at the time of biopsy, and increased C-reactive protein (i.e., >10 mg/L).
Statistical Analyses
Statistical analyses were performed using SPSS 25.0 (IBM, Armonk, NY) and GraphPad version 6.0 (GraphPad Software, San Diego, CA). Categorical variables were expressed as numbers (percentages) and compared by using the chi-squared or Fisher exact test as appropriate. Continuous variables are presented as mean ± SD or median (interquartile range) as appropriate. Continuous variables were compared by using the independent samples t test or the Mann–Whitney U test as appropriate. Paired variables were compared using the Wilcoxon signed-rank test. For the comparison of three or more groups, the Kruskal–Wallis test with post hoc analysis using the Dunn multiple comparisons test adjusted by the Bonferroni correction for multiple testing was used. Correlations were assessed using Spearman rank (Rho) correlation analysis. Receiver operating characteristics curves were created for the predefined biomarkers, and optimal cutoff values were assessed for proteinuria, urinary soluble CD163, and serum and urinary soluble CD25 using the Youden index (19). A P value of 0.05 was considered significant.
Results
Patient Cohort
We included 95 patients with ANCA-associated vasculitis, including 55 (58%) with granulomatosis and polyangiitis, 37 (39%) with microscopic polyangiitis, and three (3%) with eosinophilic granulomatosis with polyangiitis (Table 1). Myeloperoxidase ANCA and proteinase-3 ANCA were detected in 47 (50%) and 45 (47%) patients; one (1%) patient had both myeloperoxidase ANCA and proteinase-3 ANCA detected, whereas two (2%) patients had no ANCA serum reactivity. In total, 125 kidney biopsies were analyzed, including 67 (54%) first biopsies and 58 (46%) repeated kidney biopsies (i.e., second biopsy, n=44; third biopsy, n=9; and fourth biopsy, n=5) (Supplemental Figures 2 and 3). Active ANCA GN was diagnosed on 110 (88%) kidney biopsies (Figure 1). Patient characteristics at the time of kidney biopsy are depicted in Table 2.
Table 1.
Patient characteristics
| Characteristics | Patients, n=95 | Healthy Controls, n=17 |
|---|---|---|
| Men/women | 62/33 | 5/12 |
| Granulomatosis with polyangiitis, % | 55 (58) | |
| Microscopic polyangiitis, % | 37 (39) | |
| Eosinophilic granulomatosis with polyangiitis, % | 3 (3) | |
| MPO-ANCA, % | 47 (50) | |
| PR3-ANCA, % | 45 (47) | |
| MPO-/PR3-ANCA, % | 1 (1) | |
| No ANCA, % | 2 (2) |
Data are presented as numbers (%). MPO, myeloperoxidase; PR3, proteinase-3.
Table 2.
Patient characteristics at the time of kidney biopsy
| Characteristics | First Biopsy, n=67 | Second Biopsy, n=44 | Third Biopsy, n=9 | Fourth Biopsy, n=5 |
|---|---|---|---|---|
| Active ANCA GN | 63 (94) | 35 (80) | 7 (78) | 5 (100) |
| Extrarenal involvement | 52 (78) | 22 (50) | 3 (34) | 3 (60) |
| Maintenance treatmenta | 16 (36) | 4 (44) | 1 (20) | |
| Serum creatinine, mg/dl | 1.95 (1.23–3.62) | 2.22 (1.43–3.81) | 3.40 (2.14–4.03) | 1.64 (1.26–3.37) |
| AKI, n/N | 18/43 | 5/9 | 3/5 | |
| Proteinuria, g/d | 0.92 (0.26–2.16) | 0.73 (0.39–2.05) | 1.72 (0.93–3.90) | 0.71 (0.30–1.55) |
| Hematuria, n/N | 61/66 | 40/43 | 8/9 | 5/5 |
| C-reactive protein, mg/L | 50 (6–116) | 14 (2–60) | 16 (4–50) | 61 (21–138) |
| Urinary soluble CD163, ng/mmol | 524 (92–1957) | 381 (15–794) | 527 (178–1040) | 169 (37–861) |
| Serum soluble CD25, ng/L | 1303 (785–1986) | 987 (736–1599) | 1114 (740–2091) | 556 (352–2871) |
| Urinary soluble CD25, ng/mmol | 242 (134–422) | 131 (71–236) | 137 (24–311) | 217 (115–617) |
Data are presented as medians (interquartile ranges) or numbers (%), unless stated otherwise. ANCA GN, ANCA-associated GN.
Any form of immunosuppressive therapy ≤6 months prior to biopsy.
Urinary Soluble CD163 and Active Disease
Elevated levels of urinary soluble CD163 (>30 ng/mmol) were found in 96 (87%) of 110 patients with active ANCA GN compared with one (7%) of 15 patients with no active disease on kidney biopsy and one (6%) of 17 healthy controls (Figure 2A). The highest levels of urinary soluble CD163 were found in the crescentic class (Figure 2B). Also, urinary soluble CD163 correlated with morphologic features of active ANCA GN (i.e., capillary breaks, fibrinoid necrosis, and/or crescent formation), whereas an inverse correlation was found with the percentage of normal glomeruli (Figure 3). Neither interstitial fibrosis and/or tubular atrophy class nor the extent of arteriolosclerosis affected urinary soluble CD163 (Supplemental Figure 4).
Figure 2.
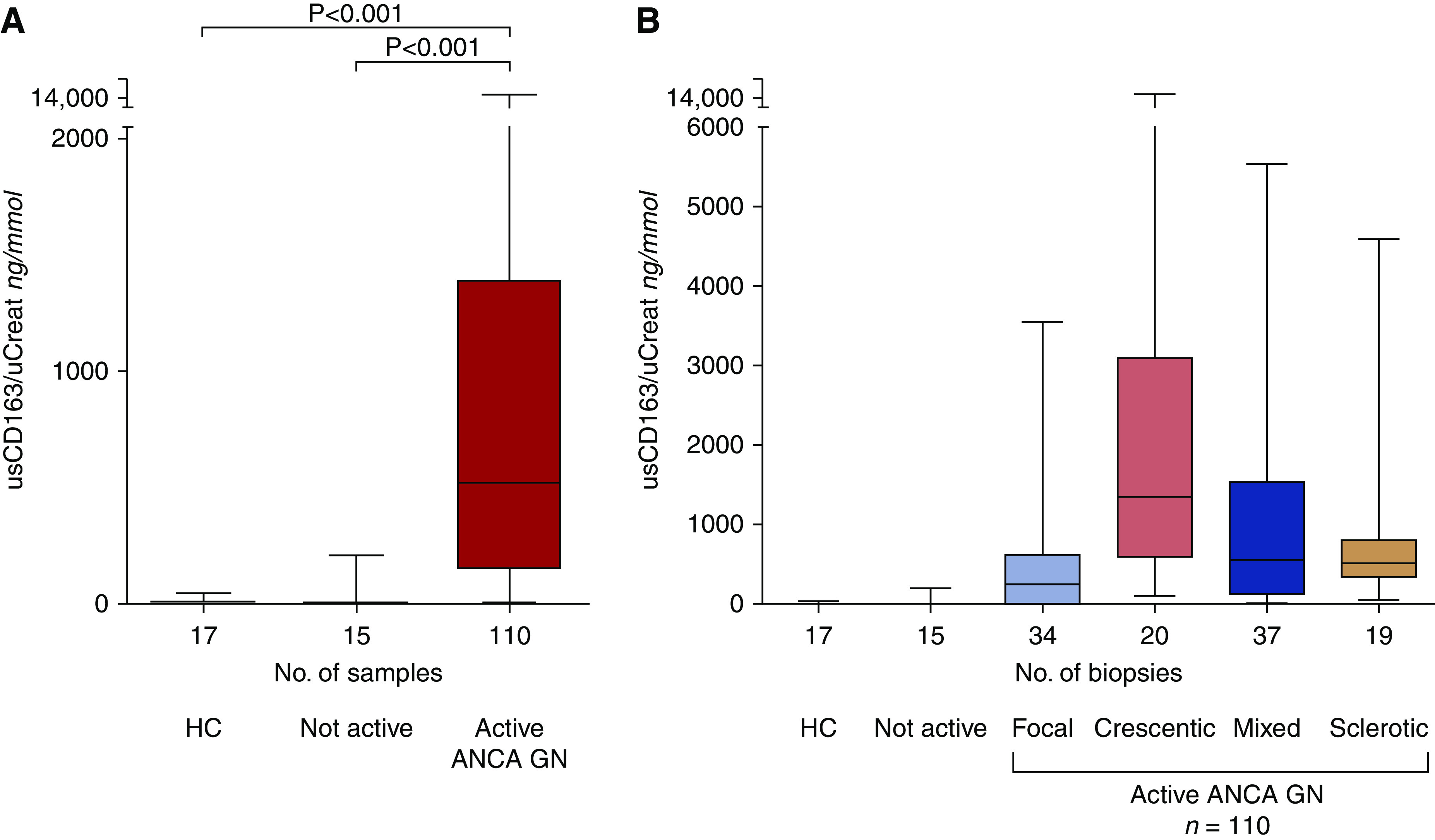
Levels of urinary soluble CD163 (usCD163) corrected for urinary creatinine (uCreat) levels (nanograms per millimole) are increased in active ANCA-associated GN. (A) Patients with ANCA-associated GN (ANCA GN) active disease had higher usCD163 levels compared with patients without active ANCA GN (n=15) or healthy controls (HCs; n=17). (B) usCD163 levels divided according to the ANCA GN classification. Data are presented as medians with interquartile ranges (box).
Figure 3.
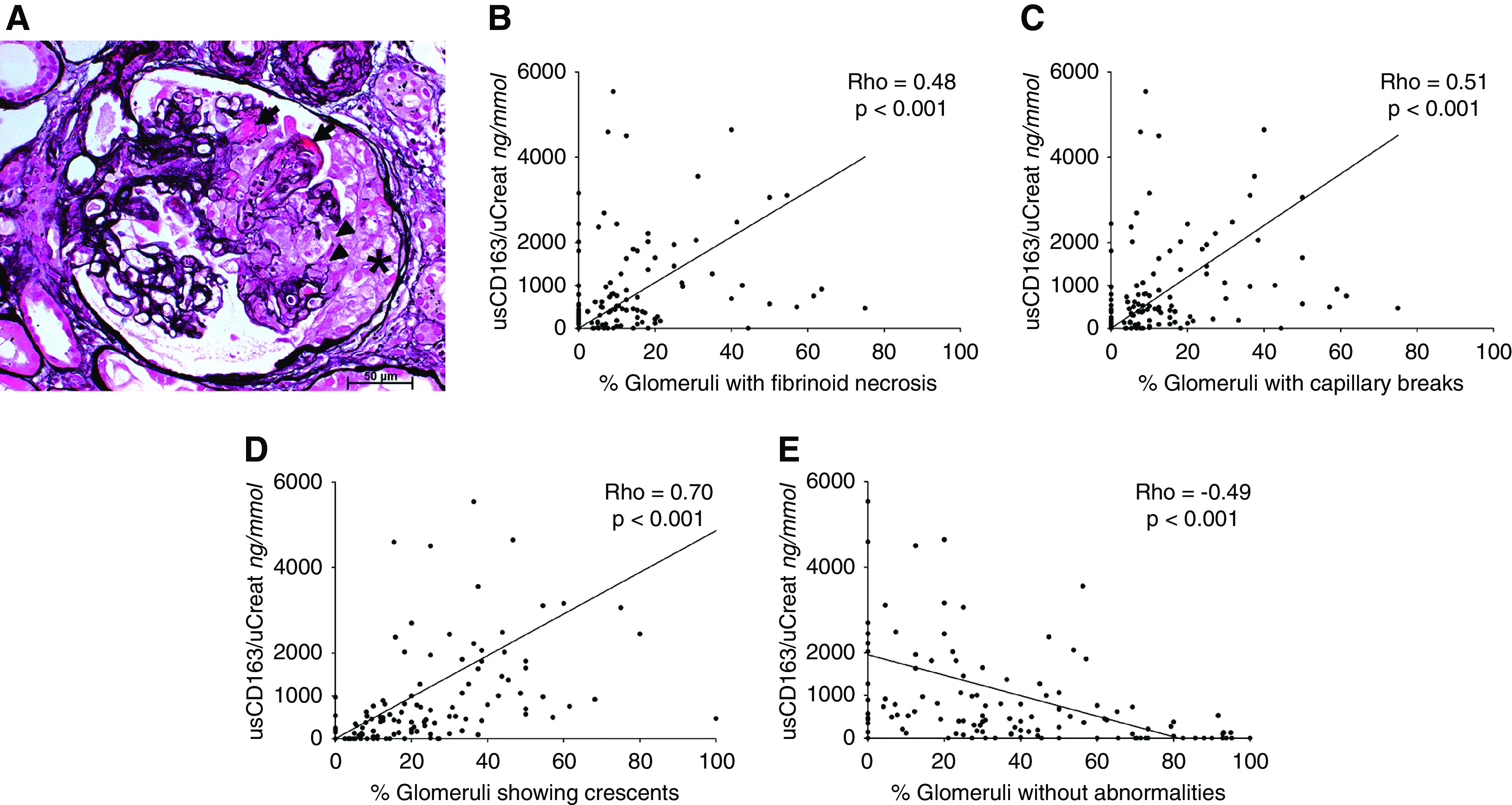
Urinary soluble CD163 (usCD163) correlated with histologic features of active ANCA-associated GN (ANCA GN). (A) Active ANCA GN lesions characterized by fibrinoid necrosis (arrows), capillary breaks (arrowheads), and cellular crescentic lesions (asterisk; Jones methenamine silver stain). Magnification, ×400. (B–D) Correlation between usCD163 and the percentage of glomeruli showing (B) capillary breaks, (C) fibrinoid necrosis, and (D) cellular crescents. (E) The percentage of normal glomeruli correlated inversely with usCD163 levels. Of note, three values of usCD163 (all >9000 ng/mmol) included in the correlation analysis are not included in (B–E) to improve visibility and decrease overlap of individual scatter points at lower usCD163 levels (i.e., <1000 ng/mmol). These values (i.e., >9000 ng/mmol) did not affect the significance of the findings. uCreat, urinary creatinine.
At the time of stable clinical remission (n=38), a significant decline in urinary soluble CD163 was found (Supplemental Figure 5). Five patients with active ANCA GN collected urinary samples every month after the initiation of remission induction treatment and showed a steady decline of urinary soluble CD163, which became undetectable within 3 months (Supplemental Figure 6).
To study whether the sensitivity of detecting active ANCA GN could be increased compared with using urinary soluble CD163 alone, we tested serum and urinary soluble CD25. Fourteen (13%) of 110 patients with active ANCA GN had normal levels of urinary soluble CD163. Four of them had elevated levels of both serum and urinary soluble CD25. Thus, 99 (90%) of 110 patients with active ANCA GN had elevated levels of either urinary soluble CD163 or both serum and urinary soluble CD25.
CD163+ Cells in Antineutrophil Cytoplasmic Antibody–Associated Glomerulonephritis
Forty-seven kidney tissue sections from 28 patients (first biopsy, n=22; repeated biopsy, n=25) (Supplemental Figure 7) were stained and scored for CD163 to assess the in vivo counterparts of our findings. Forty biopsies showed active ANCA GN (i.e., focal class, n=12; crescentic class, n=8; mixed class, n=13; and sclerotic class, n=7), whereas seven biopsies showed no disease activity. CD163+ cells were abundantly present in affected glomeruli but not in glomeruli from patients with no active ANCA GN (Figure 4, A and B). Minimal staining for CD163, however, was found within the capillaries of some unaffected glomeruli (Figure 4C). Also, CD163+ cells were found in the periglomerular area of affected and sclerosed glomeruli (Figure 4D). Tubulointerstitial staining of CD163+ cells was present in patients with ANCA-associated vasculitis and active ANCA GN as well as in those patients without GN (Figure 4E).
Figure 4.
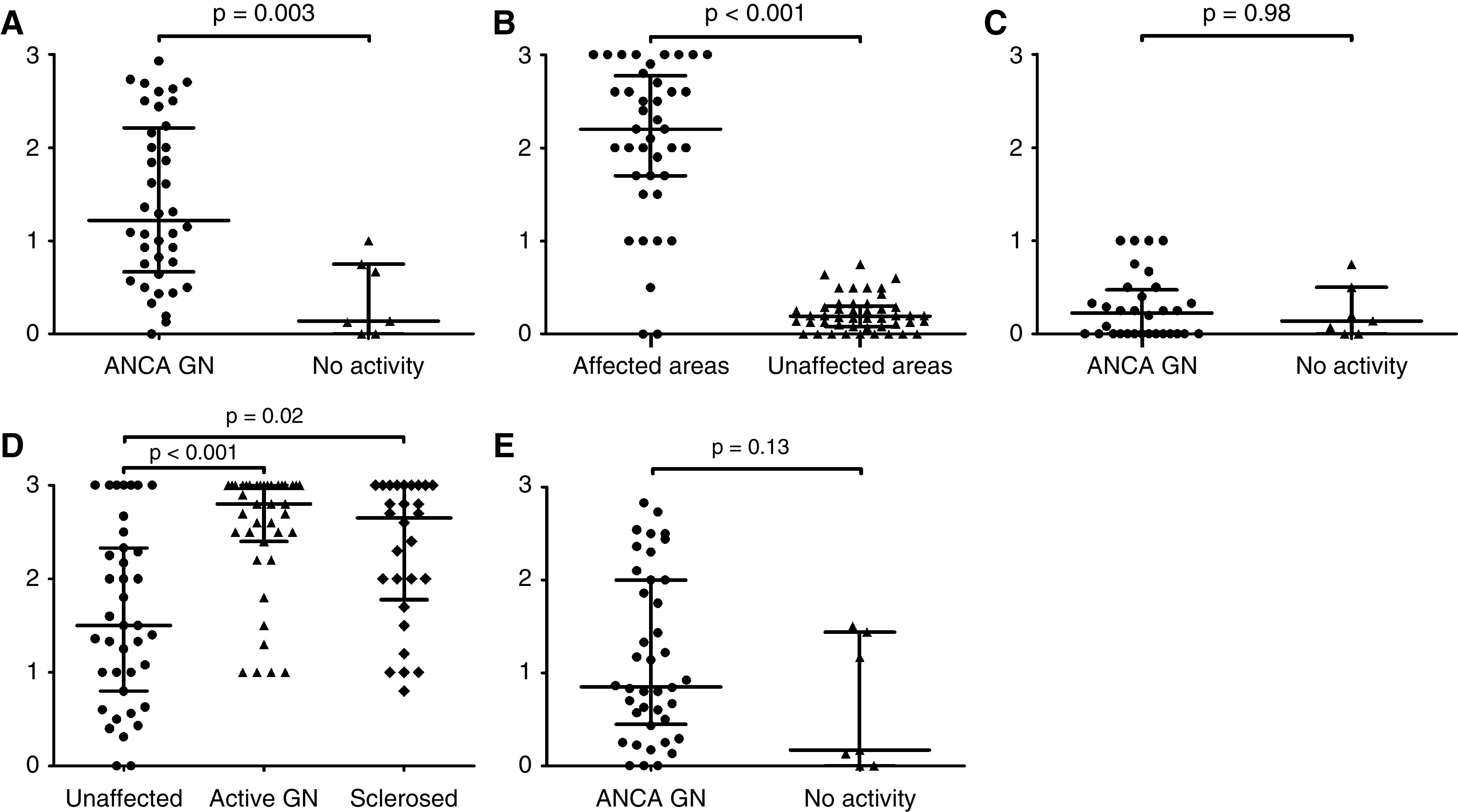
Biopsies were stained for CD163+cells in active ANCA-associated GN (ANCA GN; n=40; i.e., focal [n=12], crescentic [n=8], mixed [n=13], and sclerotic [n=7] classes) and biopsies showing no activity (n=7). CD163+ cell staining in (A) glomeruli of active ANCA GN versus biopsies with no ANCA GN activity, (B) affected versus unaffected areas of glomeruli, (C) unaffected glomeruli in active ANCA GN versus biopsies with no ANCA GN activity, (D) periglomerular areas of unaffected versus active versus sclerosed glomeruli, and (E) tubulointerstitial areas of active ANCA GN versus biopsies with no ANCA GN activity. Each point represents the mean score of CD163+ cell staining per biopsy. Data are presented as medians with interquartile ranges.
Urinary Soluble CD163 and Routine Markers of Active Antineutrophil Cytoplasmic Antibody–Associated Glomerulonephritis
The diagnostic performance of urinary soluble CD163 and conventional noninvasive markers to detect relapsing ANCA GN was assessed in 58 repeated kidney biopsies (Supplemental Figure 3) from patients with suspected relapsing ANCA GN (active ANCA GN, n=47; no activity, n=11). The optimal cutoff was calculated using a receiver operating characteristics analysis. Urinary soluble CD163, at a cutoff of 30 ng/mmol, had a sensitivity of 0.94 and a specificity of 0.91, outperforming conventional markers (i.e., new-onset hematuria, AKI, proteinuria [cutoff of 0.60 g/d], and C-reactive protein >10 mg/L) (Table 3). Of note, urinary soluble CD163’s sensitivity was higher than the combination of AKI with new-onset hematuria and/or proteinuria (94% versus 70%, respectively). Furthermore, the combined use of urinary soluble CD163 with serum and urinary soluble CD25 did not improve the performance to diagnose relapsing ANCA GN (Table 3).
Table 3.
Diagnostic performance of urinary soluble CD163, serum and urinary soluble CD25, and conventional markers for detecting relapsing ANCA-associated GN
| Markers | Cutoff | Samples: Active ANCA-Associated GN/All Available | Area under the Curve [95% Confidence Interval] | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Positive Likelihood Ratio Weighted for Prevalence | Negative Likelihood Ratio Weighted for Prevalence |
|---|---|---|---|---|---|---|---|---|---|
| AKI | 47/57 | 0.66 [0.48 to 0.83] | 0.51 | 0.80 | 0.92 | 0.26 | 12.02 | 2.89 | |
| New-onset hematuria | 45/55 | 0.58 [0.38 to 0.78] | 0.67 | 0.50 | 0.86 | 0.25 | 6.02 | 2.97 | |
| Proteinuria | 0.60 g/d | 47/58 | 0.78 [0.64 to 0.91] | 0.68 | 0.82 | 0.94 | 0.38 | 16.11 | 1.66 |
| AKI with hematuria and/or proteinuria | 47/57 | 0.75 [0.59 to 0.92] | 0.70 | 0.80 | 0.94 | 0.36 | 16.50 | 1.77 | |
| C-reactive protein >10 mg/L | 47/57 | 0.62 [0.43 to 0.81] | 0.64 | 0.60 | 0.88 | 0.26 | 7.54 | 2.83 | |
| Urinary soluble CD163 | 30 ng/mmol | 47/58 | 0.94 [0.88 to 1.00] | 0.94 | 0.91 | 0.98 | 0.78 | 44.53 | 0.28 |
| Serum soluble CD25 | 1350 ng/L | 46/57 | 0.61 [0.42 to 0.79] | 0.35 | 0.91 | 0.94 | 0.25 | 16.58 | 3.05 |
| Urinary soluble CD25 | 210 ng/mmol | 47/58 | 0.50 [0.31 to 0.69] | 0.32 | 0.82 | 0.88 | 0.22 | 7.43 | 3.47 |
| Urinary soluble CD163 with serum and urinary soluble CD25 | 46/57 | 0.92 [0.82 to 1.00] | 0.94 | 0.91 | 0.98 | 0.78 | 43.67 | 0.28 |
In total, 58 patients suspect of having relapsing ANCA-associated GN were used.
Discussion
Accurate biomarkers for early recognition of active ANCA GN are paramount to optimize treatment and prevent long-term complications (4–6). We demonstrate that high levels of urinary soluble CD163 reflect active ANCA GN in a large and well-defined cohort of patients with biopsy-proven ANCA-associated vasculitis both at the time of first presentation and at relapse. Urinary soluble CD163’s sensitivity and specificity to detect relapsing ANCA GN were 0.94 and 0.91, respectively, outperforming conventional markers of active disease. Serum and urinary soluble CD25 did not contribute to urinary soluble CD163’s performance in relapsing ANCA GN. The abundance of CD163+ cells in affected glomeruli confirmed the in vivo counterpart. Urinary soluble CD163 normalized after remission induction treatment and remained low at the time of stable clinical remission. Urinary soluble CD163 as a marker of disease activity during follow-up can improve the detection of subtle levels of activity in relapsing ANCA GN, which can be missed by conventional clinical markers. Assessing urinary soluble CD163 during follow-up of ANCA GN could help guide treatment decisions and, consequently, may affect prognosis. Our findings may also help to lower the number of unnecessary repeated kidney biopsies.
The glomerular presence of CD163+ cells (that is, M2-type macrophages) has been demonstrated in early experimental ANCA-associated vasculitis and is associated with excretion of CD163 in urine (12). We found high levels of urinary soluble CD163 in patients with active ANCA GN but not in patients with ANCA-associated vasculitis without kidney involvement at the time of sampling, corroborating findings from previous studies (12–14). These previous studies, however, are limited by the lack of histologic proof, hampering firm conclusions. We show that levels of urinary soluble CD163 correlate with morphologic features of active ANCA GN but do not distinguish between ANCA GN classes.
CD163 has a molecule mass of 130 kD and cannot be filtered through the normal glomerulus; thus, a loss of glomerular integrity is needed to detect urinary soluble CD163. We found that glomerular margination of CD163+ cells can be seen in early stages of ANCA GN, characterized by capillary breaks and fibrinoid necrosis, confirming previous findings (16). Urinary soluble CD163, indeed, reflects the presence of CD163+ cells in affected glomeruli. These findings indicate that urinary soluble CD163 may already be detectable early in the disease course. CD163+ cells were also found in sclerotic glomeruli and the tubulointerstitium, either in the presence of active ANCA GN or not, reflecting their role in tissue repair and fibrosis (20). Of note, CD163+ cells in sclerotic areas did not affect levels of urinary soluble CD163.
It is noteworthy that urinary soluble CD163 is not specific for active ANCA GN as glomerulonephritides not related to ANCA, such as antiglomerular basement membrane disease and proliferative and/or membranous lupus nephritis, can present with high levels of urinary soluble CD163 (12,21,22). Thus, the kidney biopsy remains the standard to diagnose a first episode of ANCA GN. We, however, are the first to demonstrate that urinary soluble CD163 can be used as a marker of active ANCA GN during follow-up, with a better diagnostic performance compared with conventional markers. It is, therefore, particularly beneficial in patients with persistent urinary abnormalities and/or CKD.
We used a lower cutoff value of urinary soluble CD163 compared with previous studies (i.e., 30 versus 300–350 ng/mmol), with a higher sensitivity (12,14). This can be explained by our study design comparing urinary soluble CD163 with the closest thing to a gold standard (that is, histologic proof of active ANCA GN), preventing “false-positive” patients when using conventional markers. Patients with ANCA-associated vasculitis and either persistent or new-onset urinary abnormalities, indeed, can have a variety of histologic findings not linked to active ANCA GN (7). Furthermore, we used 24-hour urine samples, which more reliably reflect daily urinary soluble CD163 excretion.
We provide preliminary insight in urinary soluble CD163’s response to treatment. Urinary soluble CD163 levels decreased during remission induction treatment and normalized within 3 months, suggesting a rapid resolution of glomerular inflammation. Moreover, urinary soluble CD163 remained low at the time of stable clinical remission. At present, it remains to be established whether various treatment regimens affect the dynamics of urinary soluble CD163 differently. Normalization of routine markers took much longer as compared with urinary soluble CD163. This probably reflects slowly resolving structural damage to the kidneys.
In line with Dekkema et al. (14), we found that measuring serum and urinary soluble CD25 can facilitate the recognition of patients with active ANCA GN who present with normal levels of urinary soluble CD163 at the time of first presentation. No added value, however, was observed during follow-up.
Future prospective studies are needed to confirm the performance of urinary soluble CD163, with or without serum and urinary soluble CD25, as a marker for activity in relapsing ANCA GN. In these studies, discrepancies between these novel markers and conventional markers (e.g., AKI, proteinuria, and new-onset hematuria) should ideally be resolved by performing a kidney biopsy to differentiate activity from chronicity and to gain crucial insight into how these markers could be applied in clinical practice.
Taken together, urinary soluble CD163 is associated with active ANCA GN. Urinary soluble CD163 correlates with early morphologic features of active ANCA GN and seems to be a robust marker of relapsing ANCA GN.
Disclosures
All authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We gratefully thank Nele Bijnens, Henk van Rie, and Ruud Theunissen (Laboratory of Clinical Immunology, Maastricht University Medical Center, Maastricht, The Netherlands) for their technical assistance. Furthermore, we gratefully thank Myrurgia Abdul-Hamid (Department of Pathology, Maastricht University Medical Center, Maastricht, The Netherlands) and Dr. Agnieszka Smolinska (Department of Pharmacology and Toxicology, Maastricht University, Maastricht, The Netherlands). Part of this study was presented at the American Society of Nephrology Kidney Week 2018 in San Diego, California and at the 19th International Vasculitis and ANCA Workshop 2019 in Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: M. Christiaans, T. Fung, M. Gelens, J. Kooman, K. Leunissen, E. Litjens, F. van der Sande, E. Duijnhoven, S. Boorsma, J. Huitema, J. Wirtz, F. de Heer, M. Krekels, F. Stifft, G. Verseput, N. ter Braak, L. Frenken, and S. Gaertner
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07210520/-/DCSupplemental.
Supplemental Figure 1. IHC CD163 and semiquantitative scoring in glomeruli, the periglomerular compartment, and tubulointerstitium.
Supplemental Figure 2. Flow chart of all included patients and available biopsies.
Supplemental Figure 3. Flow chart of the patients, biopsies, and data used per analysis.
Supplemental Figure 4. Urinary soluble CD163 levels and IFTA class and extent of arteriolosclerosis.
Supplemental Figure 5. Levels of urinary soluble CD163 were lower at the time of stable remission.
Supplemental Figure 6. Dynamics of urinary soluble CD163 and conventional markers during remission induction treatment of five patients with ANCA GN.
Supplemental Figure 7. Flow chart of included patients and available biopsies used for CD163 staining.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 65: 1–11, 2013. [DOI] [PubMed] [Google Scholar]
- 2.van Paassen P, van Breda Vriesman PJ, van Rie H, Tervaert JW: Signs and symptoms of thin basement membrane nephropathy: A prospective regional study on primary glomerular disease-The Limburg Renal Registry. Kidney Int 66: 909–913, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Hilhorst M, Wilde B, van Breda Vriesman P, van Paassen P, Cohen Tervaert JW; Limburg Renal Registry : Estimating renal survival using the ANCA-associated GN classification. J Am Soc Nephrol 24: 1371–1375, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moiseev S, Novikov P, Jayne D, Mukhin N: End-stage renal disease in ANCA-associated vasculitis. Nephrol Dial Transplant 32: 248–253, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Slot MC, Tervaert JW, Franssen CF, Stegeman CA: Renal survival and prognostic factors in patients with PR3-ANCA associated vasculitis with renal involvement. Kidney Int 63: 670–677, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Geetha D, Seo P, Ellis C, Kuperman M, Levine SM: Persistent or new onset microscopic hematuria in patients with small vessel vasculitis in remission: Findings on renal biopsy. J Rheumatol 39: 1413–1417, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Rhee RL, Davis JC, Ding L, Fervenza FC, Hoffman GS, Kallenberg CGM, Langford CA, McCune WJ, Monach PA, Seo P, Spiera R, St Clair EW, Specks U, Stone JH, Merkel PA: The utility of urinalysis in determining the risk of renal relapse in ANCA-associated vasculitis. Clin J Am Soc Nephrol 13: 251–257, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rovin BH, Caster DJ, Cattran DC, Gibson KL, Hogan JJ, Moeller MJ, Roccatello D, Cheung M, Wheeler DC, Winkelmayer WC, Floege J; Conference Participants : Management and treatment of glomerular diseases (part 2): Conclusions from a Kidney Disease Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 95: 281–295, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK: Identification of the haemoglobin scavenger receptor. Nature 409: 198–201, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Etzerodt A, Moestrup SK: CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal 18: 2352–2363, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Reilly VP, Wong L, Kennedy C, Elliot LA, O’Meachair S, Coughlan AM, O’Brien EC, Ryan MM, Sandoval D, Connolly E, Dekkema GJ, Lau J, Abdulahad WH, Sanders JS, Heeringa P, Buckley C, O’Brien C, Finn S, Cohen CD, Lindemeyer MT, Hickey FB, O’Hara PV, Feighery C, Moran SM, Mellotte G, Clarkson MR, Dorman AJ, Murray PT, Little MA: Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 27: 2906–2916, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran SM, Monach PA, Zgaga L, Cuthbertson D, Carette S, Khalidi NA, Koening CL, Langford CA, McAlear CA, Moreland L, Pagnoux C, Seo P, Specks U, Sreih A, Wyse J, Ytterberg SR, Merkel PA, Little MA; Vasculitis Clinical Research Consortium : Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 35: 283–291, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekkema GJ, Abdulahad WH, Bijma T, Moran SM, Ryan L, Little MA, Stegeman CA, Heeringa P, Sanders JF: Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoantibody-associated vasculitis: A cohort study. Nephrol Dial Transplant 34: 234–242, 2019. [DOI] [PubMed] [Google Scholar]
- 15.Rousselle A, Kettritz R, Schreiber A: Monocytes promote crescent formation in anti-myeloperoxidase antibody-induced glomerulonephritis. Am J Pathol 187: 1908–1915, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, David MZ, Hyjek E, Chang A, Meehan SM: M2 macrophage infiltrates in the early stages of ANCA-associated pauci-immune necrotizing GN. Clin J Am Soc Nephrol 10: 54–62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, Flossmann O, Hall C, Hollywood J, Jayne D, Jones R, Lanyon P, Muir A, Scott D, Young L, Luqmani RA: Modification and validation of the Birmingham vasculitis activity score (version 3). Ann Rheum Dis 68: 1827–1832, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Sethi S, D’Agati VD, Nast CC, Fogo AB, De Vriese AS, Markowitz GS, Glassock RJ, Fervenza FC, Seshan SV, Rule A, Racusen LC, Radhakrishnan J, Winearls CG, Appel GB, Bajema IM, Chang A, Colvin RB, Cook HT, Hariharan S, Herrera Hernandez LP, Kambham N, Mengel M, Nath KA, Rennke HG, Ronco P, Rovin BH, Haas M: A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int 91: 787–789, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Youden WJ: Index for rating diagnostic tests. Cancer 3: 32–35, 1950. [DOI] [PubMed] [Google Scholar]
- 20.Wynn TA, Vannella KM: Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun PP, Zhou XJ, Su JQ, Wang C, Yu XJ, Su T, Liu G, Wang SX, Nie J, Yang L: Urine macrophages reflect kidney macrophage content during acute tubular interstitial and glomerular injury. Clin Immunol 205: 65–74, 2019. [DOI] [PubMed] [Google Scholar]
- 22.Endo N, Tsuboi N, Furuhashi K, Shi Y, Du Q, Abe T, Hori M, Imaizumi T, Kim H, Katsuno T, Ozaki T, Kosugi T, Matsuo S, Maruyama S: Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis. Nephrol Dial Transplant 31: 2023–2033, 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.