Abstract
Metabolic alkalosis is a very commonly encountered acid-base disorder that may be generated by a variety of exogenous and/or endogenous, pathophysiologic mechanisms. Multiple mechanisms are also responsible for the persistence, or maintenance, of metabolic alkalosis. Understanding these generation and maintenance mechanisms helps direct appropriate intervention and correction of this disorder. The framework utilized in this review is based on the ECF volume-centered approach popularized by Donald Seldin and Floyd Rector in the 1970s. Although many subsequent scientific discoveries have advanced our understanding of the pathophysiology of metabolic alkalosis, that framework continues to be a valuable and relatively straightforward diagnostic and therapeutic model.
Keywords: electrolytes, alkalosis, musculoskeletal abnormalities, bicarbonates
Introduction
Metabolic alkalosis is a primary acid-base disorder that increases the serum bicarbonate concentration [HCO3−] (this is usually approximated by its surrogate the venous total [CO2]) above 30 meq/L (1), causing the arterial blood [H+] to fall, i.e., the arterial blood pH increases into the alkaline range (>7.45). Metabolic alkalosis is a very common disorder, especially in ICU settings (2). The diagnostic criteria and a pathophysiologic approach to differential diagnosis and treatment on the basis of dissection of the etiology and dominant maintenance mechanisms are reviewed.
Respiratory Compensation
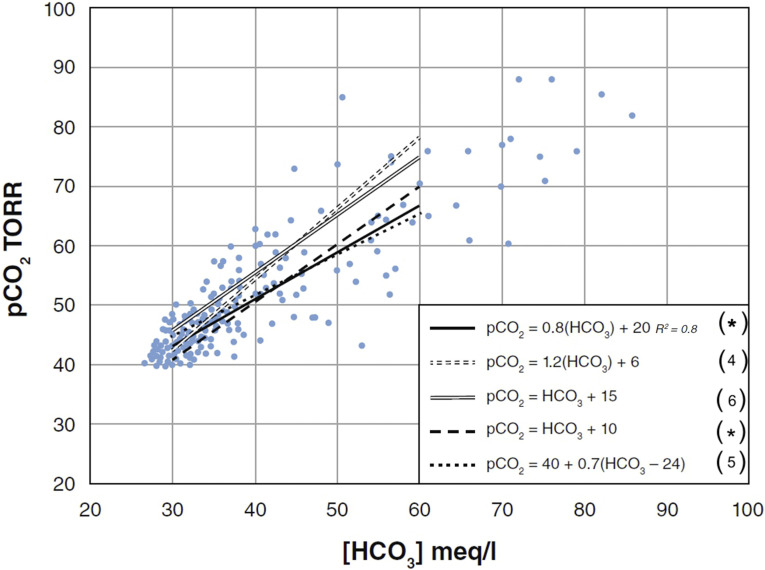
Uncomplicated metabolic alkalosis rapidly (minutes to hours) generates hypoventilatory compensation that elevates the pCO2. Compensation reduces the arterial pH, but it generally remains >7.45. Although the magnitude of the hypoventilatory response is proportional to the [HCO3−] increase, the response is very variable (Figure 1) (3–6, M.A. Fallahzadeh, et al., unpublished observations).
Figure 1.
Respiratory compensation for metabolic alkalosis. Simultaneous pCO2 and [HCO3] data points derived from a recent comprehensive literature review showing the best-fit linear regression line (*M.A. Fallahzadeh, et al., unpublished observations). Also shown are several commonly published relationship equations with adjacent references. The following very simple and easy to remember and utilize relationship: pCO2 = [HCO3]+10 (dashed line) is very similar to the best-fit regression line in the HCO3 range between 30 and 50 meq/L. If the [HCO3−] exceeds 55 mmol/L, the pCO2 may increase markedly. This is likely because of the development of a coexisting respiratory muscle weakness generated by almost inevitable severe hypokalemia. Therefore, respiratory acidosis often complicates extreme metabolic alkalosis. pCO2, partial pressure of carbon dioxide; 1 TORR, 133.32 Pascal (Pa).
Serum Chloride Concentration and Metabolic Alkalosis
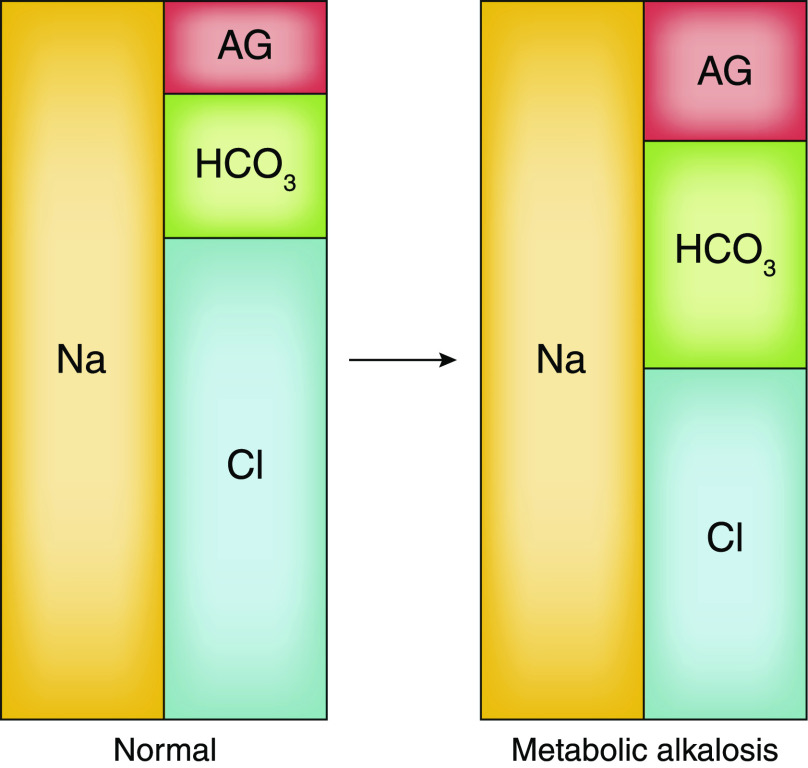
Hyper- or hypochloremia can reflect water/hydration disorders, acid/base disorders, or both. When an abnormal [Cl−] is secondary to a water/hydration disorder, there is a proportional degree of hyper- or hyponatremia. Thus the abnormal [Cl−] coexists with an abnormal [Na+] in a 1:1.4 ratio. This relationship is disrupted in acid-base disorders such as metabolic alkalosis. The elevated [HCO3−] with metabolic alkalosis is generally associated with a reciprocally reduced [Cl−] independent of [Na+]. The electrolyte profile, or Gamblegram (Figure 2), shows why the increased [HCO3−] must be accompanied by a reduction of [Cl−] (independent of [Na+]), a reduction of the [AG−], or both (7,8). In fact, metabolic alkaloses reproducibly increase the [AG−] to a small degree, mostly owing to increased negative charge density of plasma proteins (9,10). Therefore, the relative [Cl−] decrease must be even greater.
Figure 2.
The three major serum electrolytes in a normal patient and a patient with metabolic alkalosis, visualized using a Gamblegram. Note, when the HCO3− concentration increases and the anion gap also increases slightly (this occurs with most forms of metabolic alkalosis), then the [Cl−] must fall and the Cl:Na ratio must fall below its normal 1:1.4 ratio.
However, an identical electrolyte pattern, increased [HCO3−] and reduced [Cl−], is also generated by compensation for chronic respiratory acidosis. Clinical assessment and arterial blood pH measurement will point toward the correct diagnosis—the blood pH is high-normal/overtly alkaline with metabolic alkalosis and low-normal/overtly acid with chronic respiratory acidosis. Venous blood pH, although less definitive than arterial, can also differentiate these disorders—add 0.03 pH units to the venous pH to approximate the arterial pH (11).
Pathogenesis: Generation and Maintenance
Seldin and Rector published a classic review, “The Generation and Maintenance of Metabolic Alkalosis,” in 1972 (12). Although many subsequent discoveries have expanded our understanding of the complex systemic neurohormonal, kidney, cellular, and paracellular mechanisms participating in the development and maintenance of this acid-base disorder, the Seldin/Rector extracellular fluid (ECF) volume-centered approach continues to be an extremely useful and relatively easy-to-understand diagnostic and therapeutic framework for the metabolic alkaloses. Some experts believe the “ECF volume-centered” approach should be replaced by a chloride-depletion model and cite experimental animal models to support their position (13–16). However, others challenge these experimental results and their interpretation (17,18). This review uses the traditional “ECF volume-centered” classification.
Normal HCO3− Reclamation, Regeneration, and Generation
Nonvolatile acids are generated by metabolism of ingested foods and oxidation of endogenous substrates. A typical Western European/American diet generates 80–100 meq/d of nonvolatile strong acids (mainly sulfuric, phosphoric, and hydrochloric). These acids release H+ that mainly reacts with HCO3− to form H2CO3, which rapidly dehydrates to CO2 and H2O. Thus, serum [HCO3−] falls and is “replaced” by the anions of the generated strong acids, i.e., Cl−, SO4−2, HPO4−2, etc. Acid-base homeostasis is restored by the kidney, which filters and secretes the acid anions mainly with Na+, then the tubules reabsorb the Na+ in exchange for H+, and finally the anions are excreted together with an equal quantity of H+, in the form of titratable acid (largely H2PO4−) and NH4+. In this way, 80–100 meq of H+ are “buffered” and excreted in the urine and 80–100 meq of HCO3− are regenerated and added back to the body fluids.
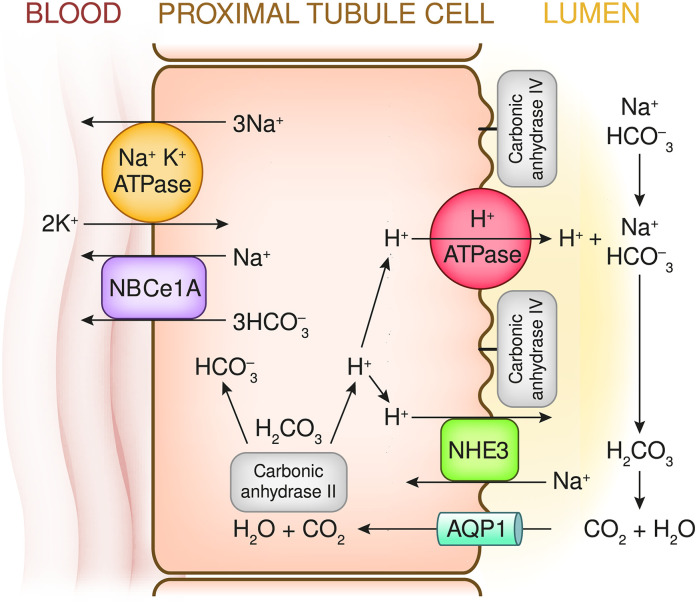
However, before the kidney can secrete/excrete the daily required load of acid and thereby regenerate the decomposed HCO3−, all the filtered HCO3− must first be reclaimed and returned to the body. About 85%–90% of the normal filtered HCO3− load (4000–4500 meq/d) is reclaimed by the proximal tubules via H+ secretion. A large fraction of proximal Na+ reabsorption occurs via the Na-H exchanger 3 (NHE3) in the luminal membrane. This exchange is energized by basolateral membrane Na-K ATPase, which reduces intracellular Na+ and generates a negative intracellular charge. This creates a strong inward (lumen into cell) electrochemical Na+ gradient. Additionally, ATP-energized H+ pumps in the lumenal membrane contribute a smaller fraction (about 30%) of proximal H+ secretion. Each secreted H+ generates a HCO3− molecule that is added to the ECF and causes the disappearance of an HCO3− molecule from the lumen. Major aspects of proximal tubule Na+ reabsorption/H+ secretion are shown in Figure 3. The 10%–15% of HCO3− that escapes proximal reclamation is reclaimed in the distal tubules/collecting ducts, as shown in Figure 4.
Figure 3.
Proximal tubule: The major proximal tubule cellular and luminal events that participate in HCO3− reclamation. Intracellular H2O combines with CO2 to generate H2CO3 that rapidly dissociates to H+ and HCO3− ions. These reactions are catalyzed by the cytoplasmic enzyme carbonic anhydrase II (CAII). Na-K ATPase in the basolateral membrane creates a steep Na+ electrochemical gradient that energizes Na+ reabsorption via the Na+-H+ antiporter exchanger (NHE3). When H+ moves into the lumen, it reacts with filtered HCO3− to form H2CO3. The H2CO3 dehydrates to H2O and CO2, a reaction catalyzed by intraluminal carbonic anhydrase IV (CAIV) (tethered to the luminal membrane). Generated CO2 flows into the cell, largely via the aquaporin 1 channel. The HCO3− ions generated within the cells move into the peritubular capillary, primarily via a Na+-3HCO3− cotransporter (the electrogenic NBCe1-A transporter; a product of the Solute Carrier Family 4 Member A4 or SLC4A4 gene). The net effect of the secretion of one H+ molecule and reabsorption of one Na+ molecule is the addition of one molecule of NaHCO3 to the extracellular fluid and the disappearance of one NaHCO3 molecule from the lumen. In addition, a smaller component of proximal tubule H+ secretion (HCO3− reclamation) is accomplished via a V-type H+-ATPase pump complex. About 85%–90% of the filtered NaHCO3 is reclaimed in the proximal tubule.
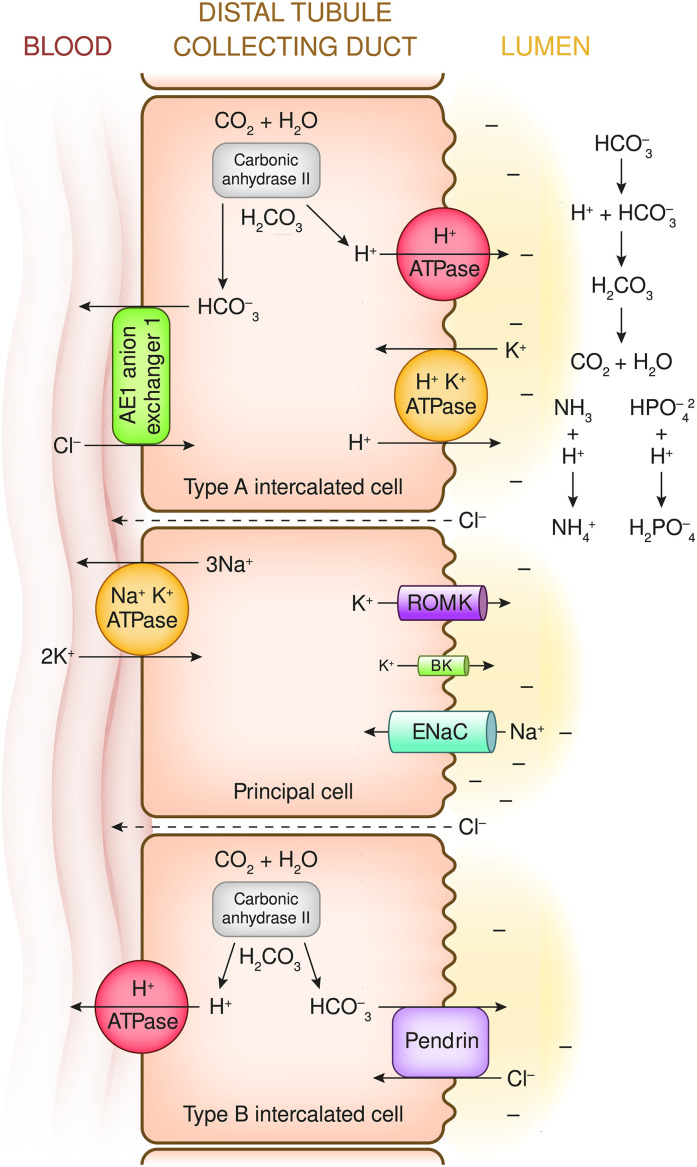
Figure 4.
Principal cells and type A and type B intercalated cells are located in the late distal convoluted tubule, the connecting tubule, and cortical collecting duct. Principal cell transport is energized primarily by electrogenic Na+-K+ ATPase pumps in the basolateral membrane. The resulting low intracellular [Na+] and negative intracellular potential difference combine to create a large gradient for Na+ to move from the lumen into these cells. Flow occurs mainly through the epithelial sodium channel (ENaC; which is composed of α, β, and γ subunits, each a product of a different gene). Inward flow of Na+ ions is more rapid than the sum of outward movement of K+ (mainly through the renal outer medullary potassium channel [ROMK]) and inward flow of Cl− (mainly via the paracellular space). Therefore, the Na+ influx generates a negative potential difference (PD of about −40 mv) in the lumen. The secretion of H+ by the type A intercalated cells initially reacts with HCO3− in the lumen, which represents HCO3− reclamation. As the luminal pH falls, additional secreted H+ combines with buffers such as HPO4−2 and NH3to generate urine titratable acid and NH4−, which creates equimolar quantitates of systemic HCO3−. See Figure 5 for a description of intercalated cell ion transport.
After HCO3− has largely disappeared from the distal tubules/collecting ducts, continued H+ secretion regenerates decomposed ECF HCO3−. If dietary and metabolic acids have produced 80–100 meq/d of nonvolatile acids, then 80–100 meq/d of H+ must be excreted by the kidneys. This regenerates the HCO3− that reacted with H+derived from nonvolatile acids and thereby decomposed or “disappeared.” Also, any HCO3− lost in stool must be regerated. As HCO3− disappears from the tubular fluid, the fluid pH falls. The minimum achievable urine pH is about 4.5, which represents only 0.03 meq/L of free [H+]. Therefore, virtually the entire excreted H+ load must be bound to urine buffers: HPO4−2 (titrated to H2PO4−) represents most of the titratable acid, and NH3 binds to H+ to form NH4+. These buffered H+ charges are electrically balanced by acid anions such as SO4− and Cl− in the urine.
Figure 4 shows the major distal kidney transport mechanisms for HCO3− reclamation, regeneration, and generation (all linked to H+ secretion) and also HCO3− secretion (by type B intercalated cells).
Metabolic Alkalosis—Source of Excess HCO3−
Usually metabolic alkalosis indicates an accumulation of “excess” HCO3−. The source of excess HCO3− can be exogenous, endogenous, or both. Exogenous sources are Na+ or K+ HCO3− salts or salts of precursors (organic anions such as lactate, acetate or citrate, which generate HCO3− when completely oxidized). These salts can be ingested/absorbed or infused (Table 1).
Table 1.
Metabolic alkalosis: Mechanisms of generation
| Ingest and absorb or infuse NaHCO3 or NaHCO3 precursors (i.e., Na acetate, Na citrate, Na gluconate) |
| Distal renal tubule HCO3 generation through enhanced H+ secretion |
| Generous delivery of NaCl (or other Na salts such as Na2SO4 or Na penicillin) to distal tubules/collecting ducts, which are actively reabsorbing Na+ |
| K+ depletion (shifting H+ into cells) |
| Remove HCl from body (vomiting/nasogastric suction/chloride-rich diarrhea) |
The two potential endogenous sources of large amounts of HCO3− are (1) the stomach and (2) the kidneys. Net endogenous HCO3− generation requires H+ removal from the body. HCO3− is generated when HCl is secreted into gastric lumen, but net HCO3− accumulation in the ECF requires the HCl to be lost externally, usually as a result of vomiting and/or suction (see section on gastric alkalosis).
Normally, kidney H+ excretion into the urine (as NH4+ and/or titratable acid) generates HCO3− to replace the quantity decomposed by nonvolatile H+ derived from dietary intake and metabolism and any HCO3− lost in alkaline stool. To the extent kidney HCO3− generation exceeds this requirement, “excess” HCO3−is generated. This generally occurs when the following conditions coexist: (1) the kidney tubules/ducts beyond the early distal tubule are avidly reabsorbing Na+ (for example, aldosterone activity is high), and (2) the delivery of salt and volume to these sites is relatively large.
Clinical examples of kidney bicarbonate overproduction are:
Primary hyperaldosteronism (especially when a high-salt diet is ingested) and other mimics of primary hyperaldosteronism (Table 2).
Loop and/or thiazide diuretics and several inherited syndromes that have diuretic like manifestations (i.e., Bartter and Gitelman syndromes).
The infusion of Na+ salts of poorly absorbed anions (PO4, SO4, penicillin, etc.) if distal tubule Na+ reabsorption is stimulated by mineralocorticoids and/or volume contraction (19,20).
Another endogenous source of HCO3− is the movement of K+ from within cells into the ECF. In response to hypokalemia, K+ exit is partially balanced by movement of H+ into cells and this generates ECF HCO3− (21,22). ECF [HCO3−] can also increase when the ECF volume contracts around a fixed quantity of HCO3− (23) (see section on gastric alkalosis).
Table 2.
The metabolic alkaloses
| ECF volume contracted: urine chloride concentration <20 meq/L |
| Gastric alkalosis: vomiting/nasogastric suction |
| Chloride-rich diarrhea (congenital chloridorrhea) |
| Status/postchronic hypercapnia (acute reversal of chronic respiratory acidosis) |
| Cystic fibrosis with major sweating |
| Thiazide or loop diuretics after renal tubule diuretic effect has dissipated |
| Some villous adenomas |
| ECF volume expanded: urine chloride concentration >20 meq/L |
| Primary hyperaldosteronism (unilateral adenoma/bilateral hyperplasia/glucocorticoid-sensitive hyperaldosteronism) |
| Severe Cushing syndrome (especially because of ectopic ACTH) |
| Exogenous mineralocorticoids |
| Reduced 11-β (OH) steroid dehydrogenase activity |
| Chronic licorice/carbenoxolone ingestion |
| Congenital AME syndrome (11-β HSD type 2 inactivating mutation) |
| Renin-secreting tumors |
| Some forms of congenital adrenal hyperplasia |
| 11-β hydroxylase deficiency |
| 17-α hydroxylase deficiency |
| Liddle syndrome |
| ECF volume contracted: but urine chloride concentration >20 meq/L (generally indicates a renal tubule reabsorptive defect) |
| Thiazide or loop diuretics actively working |
| Bartter syndrome (defective Na reabsorption in loop of Henle, furosemide-like lesion) |
| Gitelman syndrome (defective Na reabsorption at the thiazide-sensitive site) |
| Metabolic alkalosis: other |
| Severe potassium deficiency |
| Milk (calcium) alkali syndrome |
| NaHCO3 loads with markedly reduced GFR |
| Refeeding after fasting |
ECF, extracellular fluid; ACTH, adrenocorticotropic hormone; AME, apparent mineralocorticoid excess; HSD, hydroxysteroid dehydrogenases
Maintenance of Metabolic Alkalosis
The various mechanisms responsible for the maintanence of metabolic alkalosis are shown in Table 3. If metabolic alkalosis develops and the GFR is not markedly reduced, correction of the alkalosis should be relatively straightforward: merely excrete a large fraction of the filtered HCO3− (which should be supranormal because of the higher serum/filtered [HCO3−]). A brisk HCO3− diuresis would then reduce the [HCO3−] and restore normal acid-base status. This obviously has not occurred when metabolic alkalosis persists. Why does the kidney not excrete HCO3− and rapidly restore normal acid-base status? The answer to this question varies, depending on the underlying cause, and the precise explanations continue to be refined and debated.
Table 3.
Metabolic alkalosis: Mechanisms of maintenance
| Increased proximal renal tubule HCO3 reclamation |
| Extracellular fluid contraction |
| K+ depletion |
| Continuous or intermittent generation of new HCO3 |
| In distal kidney tubules and collecting ducts |
| Gastric HCl losses |
| Exogenous alkali |
| Reduced HCO3 filtration: reduced GFR/kidney failure |
Normal individuals ingesting up to 1000 meq/d of NaHCO3 for several weeks are able to efficiently excrete this load with a minimal increase in their serum [HCO3−] (24). Consequently, when metabolic alkalosis develops and persists despite a relatively normal GFR, this indicates the kidney is reclaiming HCO3− at a supranormal rate.
Most patients with metabolic alkalosis have developed increased proximal HCO3− reabsorption. The major stimulatory factors responsible are reduced intravascular, or effective arterial, blood volume, and hypokalemia (12). Metabolic acidosis and chronic respiratory acidosis also increase proximal tubule HCO3− reabsorption, but these disorders are not relevant to the current discussion.
Metabolic alkalosis is also usually associated with accelerated distal HCO3− reabsorption and generation. Generous distal Na+ delivery, combined with avid distal Na+ reabsorption (for example, because of high aldosterone levels), accelerates distal H+ and K+ secretion. This occurs, in part, because distal Na+ reabsorption, mainly via the epithelial sodium channel (ENaC) in principal cells, generates a lumen negative electric potential (potential difference [PD]=−40 mv), which drives paracellular and transcellular anion (mostly Cl−) reabsorption and enhances the secretion of H+ and K+. Also, many neurohormonal stimuli of distal Na+ absorption increase type A intercalated cell activity (25) (Figure 4).
Figure 5 shows three types of intercalated cells. The type B intercalated cell secretes HCO3− in exchange for Cl−, and can therefore contribute to the correction of metabolic alkalosis. However, generous distal delivery of Cl− is required to enable this cell to secrete major quantities of HCO3−. Recent studies show that intercalated cells also play an important role in NaCl reabsorption and volume regulation (25–31) (discussed in the legend for Figure 5).
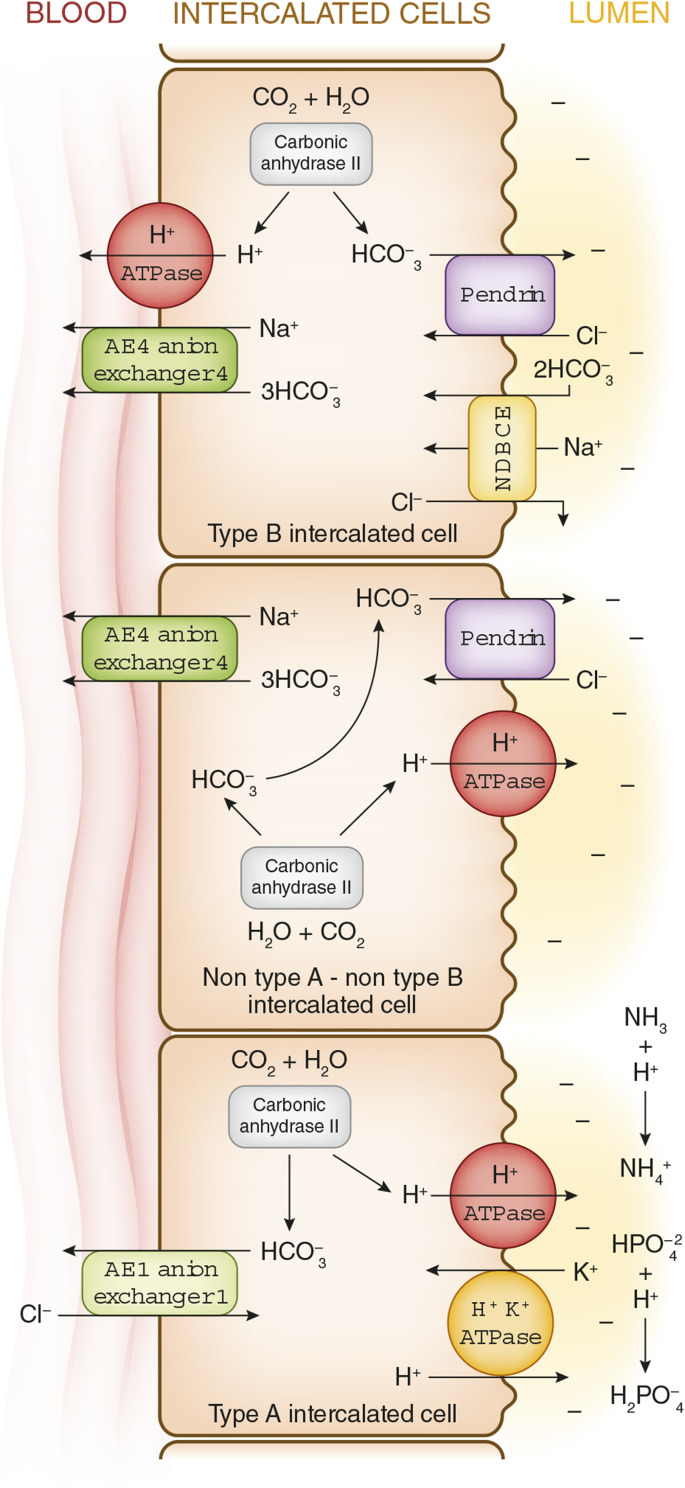
Figure 5.
Intercalated cells: Three different intercalated cell types have been identified: type A intercalated cells, type B intercalated cells, and non–type A/non–type B intercalated cells. Each is rich in carbonic anhydrase II and is capable of generating abundant HCO3− and H+ from H2CO3. Type A intercalated cells: H+ is secreted into the lumen mainly via V-type H+ ATPase and to a smaller extent via H+-K+ ATPase. The generated cytoplasmic HCO3− moves into the peritubular capillary in exchange for Cl−, via anion exchanger 1 (AE1; a product of the Solute Carrier Family 4 Member 1 or SLC4A1 gene). This transporter is also called the “erythrocyte membrane protein band 3” in red blood cells. Type B intercalated cells: H+ is secreted into the peritubular capillary by V-type H+ ATPase in the basolateral membrane (the same proton transporter as found in type A intercalated cells, but in the opposite, or luminal, membrane). The HCO3− generated in this cell is secreted into the lumen via an anion exchanger named Pendrin (a product of the Solute Carrier Family 26 Member A4 or SLC26A4 gene) that exchanges one HCO3− for one Cl−. Pendrin is a distinct and different exchanger than the AE1 in the type A cells that is present in the basolateral membrane. A Na+/3HCO3− cotransporter AE4 (a product of the Solute Carrier Family 4 Member A4 or SLC4A4 gene) is present in the basolateral membrane. These cells also have the sodium-driven bicarbonate chloride exchanger (NDBCE; a product of the Solute Carrier Family 4 Member A8 or SLC4A8 gene) in the luminal membrane (see discussion below). Non–type A/non–type B intercalated cells: this is the third type of intercalated cell. Both a V-type H+ ATPase, pumping H+, and Pendrin, exchanging HCO3− for Cl−, coexist in the lumen membrane. The anion exchanger AE4 (a product of the Solute Carrier Family 4 Member A4 or SLC4A4 gene) is a major Na+/3 HCO3− transporter in the basolateral membrane, moving these ions into the peritubular capillary. Although intercalated cells were originally identified as major kidney acid base–regulating cells, it is now clear that these cells also play an important role in salt and volume regulation (32,33). The sodium-driven bicarbonate chloride exchanger (NDBCE) in the luminal membrane of the type B intercalated cells is an electrically neutral ion exchanger moving one Na+ and two HCO3− ions into the cell while moving one Cl− into the lumen. The net effect of two Pendrin cycles (two HCO3− ions enter the lumen in exchange for uptake of two Cl− ions) and one NDBCE cycle (two HCO3 ions and one Na+ ion enter the cell and one Cl− ion enters the lumen) has the net effect of the reabsorption on one molecule of NaCl. These acid-base and salt reabsorption interactions of intercalated cells may explain why some patients with Pendred syndrome, a genetic defect of Pendrin, develop severe salt depletion and metabolic alkalosis when treated with thiazide diuretics (34).
When the GFR is markedly reduced, metabolic acidosis usually develops. However, occasionally, metabolic alkalosis occurs and then the HCO3− load cannot be excreted because of the reduced GFR. Kidney dysfunction contributes to the maintenance of metabolic alkalosis in several syndromes, including milk-alkali or calcium-alkali syndrome (35) and bicarbonate ingestion or vomiting by patients with severe kidney dysfunction (36).
The Metabolic Alkaloses: Etiology on the Basis of ECF Volume Status
The generation, maintenance, and resolution of “classic” examples of metabolic alkalosis in each ECF volume status category are described below.
ECF Volume Contracted
Classic Example: Gastric Alkalosis.
Generation.
Gastric fluid osmolality is about 300 mosm/L with a [Cl−] of about 150 meq/L and total cations of about 150 meq/L. The [H+] typically varies between 40 and 140 meq/L, [K+] varies between 10 and 15 meq/L, and [Na+] makes up the balance (37). Secretion of HCl (via gastric type H+/K+ ATPase) into the gastric lumen generates equimolar addition of HCO3 to the ECF. Normally, gastric HCl secretion does not produce metabolic alkalosis because the acid is not lost from the body. The HCl leaves the stomach and enters the small bowel, where H+ is neutralized by HCO3− mainly secreted by the pancreas (with a smaller component from bile and intestinal epithelium). This generates CO2 and water. The secretion of HCO3− adds H+ to the body fluids. Because the quantity of HCO3− secreted into the small bowel equals the quantity of H+ delivered from the stomach, these two processes neutralize one another. However, removal of the gastric HCl from the body, by vomiting or tube suction, prevents the HCl from reaching the small bowel. Consequently, HCO3− is not secreted into the intestinal lumen so that a gastric-derived HCO3− bolus is added to the ECF and an equal quantity of Cl− is removed from the body. Later in the development of gastric alkalosis, a K+/H+ cell shift also generates additional HCO3− (described below).
Maintenance.
Initially, much of the HCO3− added to the ECF (after vomiting or gastric suction) is filtered and excreted by the kidneys largely as NaHCO3. The loss of gastric fluid combines with kidney loss of NaHCO3 and fluid to generate ECF volume contraction: GFR falls and kidney salt and fluid retention are stimulated. Distal delivery of Na+ and HCO3−, linked with secondary hyperaldosteronism, increases the fraction of HCO3− excreted as KHCO3 (40). Hypokalemia shifts K+ out of, and H+ into, cells generating ECF HCO3− (21,22). The resulting intracellular acidification of kidney tubule cells stimulates HCO3− reclamation and generation. Hypokalemia also reduces pendrin activity and type B intercalated cell density, which further limits HCO3− secretion (30,31).
ECF contraction generates proximal and distal Na+ reabsorption and HCO3− reclamation. Distally, Na+ reabsorption increases H+ and K+ secretion. K+ depletion also increases H+ secretion by type A intercalated cells via both H+ ATPase and H/K ATPase pumps (Figures 3 and 4), and increases kidney NH3 generation and excretion (38,39).
During the maintenance phase, the urine electrolyte pattern fluctuates. Generally, filtered Na+, K+, HCO3−, Cl−, and water are avidly reabsorbed, generating concentrated, electrolyte-poor, and relatively acid (denoted as “paradoxical aciduria” because metabolic alkalosis exists) urine. However, intermittently (for example, immediately after loss of a large bolus of gastric fluid), the serum [HCO3−] acutely increases, and for a period of time, the larger load of filtered HCO3− cannot be completely reclaimed despite the multiple stimulatory factors described previously. When this occurs, the urine [HCO3−], [Na+], [K+], and pH all temporarily increase. Subsequently, the serum [HCO3−] declines, the ECF volume contracts further, and filtered NaHCO3 is completely reclaimed. Urine electrolyte concentrations and pH again fall. However, it is important to note that throughout these cyclic variations, the urine [Cl−] remains low. Thus, a low urine [Cl−] (<20 meq/L) generally indicates the kidney’s ongoing response to reduced ECF volume or intra-arterial blood volume.
Resolution.
The factors responsible for kidney HCO3− retention are reversed by adequate ECF volume expansion (NaCl infusion) and KCl repletion. Adequate restoration of ECF volume is signified by rising urine [Cl−] and the development of a NaHCO3 diuresis. K+ replacement moves K+ into, and H+ out of, cells into the ECF, simultaneously reducing the [HCO3−] and increasing intracellular pH. The urine electrolyte profiles discussed above presume relatively intact kidney tubule function and the absence of diuretic activity.
The term “contraction alkalosis” has been used to describe several different disorders in which the ECF “contracts” around a relatively fixed quantity of HCO3−. Although gastric alkalosis is an ECF volume-“contracted” condition and the ECF contraction contributes importantly to both its pathogenesis and maintenance, the major cause of the blood [HCO3−] increase is not shrinkage of the ECF per se, but rather generation of HCO3− owing to gastric HCl loss and cellular H+-K+ shifts (21–23,40). Conversely, although expansion of the ECF with NaCl does dilute the ECF [HCO3−] to a small degree, its correcting action in these patients is mainly a result of kidney HCO3− excretion.
Reducing or stopping the loss of gastric HCl is of course critical for reversing the process at its initiation point. However, if the gastric fluid losses cannot be stopped, then reducing the gastric fluid [HCl] concentration with an H2 blocker or proton pump inhibitor can be helpful (41).
Table 2 lists the most common forms of ECF volume-contracted metabolic alkalosis. The urine [Cl−] is typically reduced to <20 meq/L.
ECF Volume Expanded
Classic Example: Primary Mineralocorticoid Excess Syndromes.
Primary hyperaldosteronism is a condition of autonomous, or inappropriately upregulated, aldosterone secretion. This generates ECF volume expansion, hypertension, hypokalemia, and metabolic alkalosis. A unilateral adrenal adenoma secreting aldosterone is the prototypical cause of this disorder, but many conditions can mimic the electrolyte and acid-base pathophysiology of primary hyperaldosteronism (Table 2).
ECF Volume Regulation of Renin and Aldosterone (Normal Physiology).
The reabsorption of Na+ in late distal tubules/collecting ducts is mainly accomplished by principal cells (Figure 4) through ENaC pores in the luminal membranes. When these pores are open, the electrochemical gradient favors reabsorption of Na+. This generates a lumen negative electrical potential (−40 mv) that drives both chloride reabsorption and secretion of K+ and H+.
ECF volume contraction in normal individuals reduces the GFR and sharply increases the reabsorption of NaCl and NaHCO3 in the proximal tubules. Volume contraction also generates secondary hyperaldosteronism (high aldosterone activity driven by high renin and angiotensin II activity). The result of these coordinated actions is that the potent aldosterone-driven stimulus to reabsorb Na+ in the distal/collecting tubules is coordinated with increased proximal reabsorption, which sharply reduces distal salt and water delivery. Consequently, reduced Na+ delivery to aldosterone-sensitive sites blunts the magnitude of Na+ reabsorption and the indirectly linked secretion of K+ and H+. The opposite occurs in response to ECF volume expansion in normal individuals—the GFR increases, proximal salt and water reabsorption fall, and generous distal salt and water delivery ensues. Simultaneously, renin angiotensin II and aldosterone levels fall. Now despite generous distal salt and water delivery, low aldosterone levels downmodulate distal Na+ reabsorption, and indirectly linked K+ and H+ secretion. This describes the normal reciprocal physiologic balance that exists between the magnitude of distal delivery of salt and water and neuro-hormonal stimulation of distal Na+ reabsorption and K+ and H+ secretion. This exquisite reciprocal balance is disrupted by autonomous aldosterone secretion (12,42).
Generation.
Autonomous hyperaldosteronism increases distal Na+ reabsorption, expanding the ECF. This expansion raises the GFR and reduces proximal tubule salt and water reabsorption. Generous distal salt and water delivery ensue. This results in inappropriately high aldosterone activity combining with generous distal tubule salt and water delivery. This combination represents pathophysiology and high rates of Na+ reabsorption, and indirectly linked K+ and H+ secretion ensue. Excretion of K+ and H+ exceeds physiologic requirements, generating hypokalemia and metabolic alkalosis. The development of hypokalemia and K+ depletion contribute importantly to HCO3− generation when K+ shifts out of cells in exchange for H+ (22), and kidney H+ secretion and ammonia excretion increases. Additionally, hypokalemia increases proximal tubule HCO3− reclaimation and H+/K+ ATPase activity in type A intercalated cells (25,27).
Maintenance Phase.
Usually, expansion of the ECF reduces proximal salt reabsorption and HCO3− reclamation and simultaneously reduces renin, angiotensin II, and aldosterone levels. Consequently, distal Na+ reabsorption and K+ and H+ secretion remain modest despite high delivery rates. However, when autonomous aldosterone secretion combines with generous distal salt delivery, inappropriately high levels of distal Na+ reabsorption and K+ and H+ excretion develop. As hypokalemia and K+ depletion ensue, they contribute importantly to both additional HCO3− generation and kidney HCO3− reclamation via systemic K+/H+ cell shifts and acidification of kidney tubule cells. Hypokalemia also increases H+/K+-ATPase activity in type A intercalated cells (Figures 4 and 5) (25,27). Furthermore, aldosterone also increases salt reabsorption via a sequence of pendrin-related events (Figure 5) (28). During the maintenance phase of autonomous hyperaldosteronism, the urine electrolytes reflect the patient’s salt intake. Thus, the urine [Cl−] will generally be >20 meq/L.
Recovery Phase.
Successful resection of an adrenal aldosterone secreting adenoma generally reverses the entire syndrome. However, if hypertension has existed for a long period of time, it may persist because of structural vascular pathology. In lieu of surgery, drugs that block the action of aldosterone can be very helpful. The physical and biochemical manifestations of primary hyperaldosteronism can also be ameliorated by ingestion of a very-low-salt diet, which reduces distal salt delivery, blunting H+ and K+ loss. Conversely, the physical findings and electrolyte abnormalities are exacerbated by a high-salt diet (42,43). Analagously, other mineralocorticoid excess syndromes and mineralocorticoid excess-like syndromes can sometimes be reversed or cured at their source and/or treated by blocking downstream pathophysiology.
ECF Volume Contracted: Diuretic and Diuretic-Like Etiologies
Classic Example: Thiazide and/or Loop Diuretics.
Thiazide and/or loop diuretics very frequently generate hypokalemia and metabolic alkalosis. Despite development of a relatively contracted ECF, or effective arterial blood volume, the generation and maintenance mechanisms of this condition has many similarities to the ECF volume-expanded condition of primary hyperaldosteronism (42). That is because increased distal salt and volume delivery (due to the diuretics) combine with activation of the renin angiotensin II-aldosterone axis.
Generation.
Inhibition of the Na/K/2Cl cotransporter (NKCC2) in the thick limb of Henle by loop diuretics and/or inhibition of the neutral Na/Cl cotransporter (NCC) in the diluting segment by thiazide diuretics increases NaCl and volume delivery to more distal sites. Diuretics also generally increase renin, angiotensin II, and aldosterone levels, generating a state of secondary hyperaldosteronism. In the absence of diuretics, secondary hyperaldosteronism is typically associated with reduced distal salt and volume delivery, which limits the magnitude of distal Na+ reabsorption (and thereby H+ and K+ secretion). However, diuretics generate a state of secondary hyperaldosteronism linked with generous distal tubule Na+ and volume delivery. Therefore, enhanced distal Na+ reabsorption via principal cell ENaCs occurs together with generous distal Na+ and volume delivery, accelerating distal H+ and K+ secretion and generating metabolic alkalosis and hypokalemia. Hypokalemia also generates additional ECF HCO3− via cellular H+/K+ exchange, which also stimulates distal H+ secretion. During periods of diuretic activity, urine [Na+] and [Cl−] are both high. However, diuretic action is generally intermittent, and periods of diuretic activity cycle with periods of inactivity and recovery. During the “off-diuretic” phases, avid kidney salt reabsorption markedly reduces distal NaCl delivery, limiting principal cell Na+ reabsorption and distal K+ and H+ secretion. Now, urine [Cl−] and [Na+] fall to low levels, reflecting the relative ECF-contracted state. Thus, the urine [Cl−] and [Na+] cycle up and down depending on the level of diuretic activity. In contrast, diuretic-mimicking disorders such as Bartter and Gitelman syndromes are characterized by persistent, high urine [Cl−] because they never develop an “off-diuretic-like” period.
Maintenance.
Again, many similarities to the maintenance mechanisms described for primary hyperaldosteronism exist. Hypokalemia increases both proximal and distal tubule H+ and NH4+ secretion. ECF and/or effective intra-arterial volume is reduced, generating a neurohormonal cascade that increases proximal tubule NaCl and HCO3− reclamation. Periods of diuretic activity deliver salt and volume to distal segments that are responding to hyperaldosteronism. These generation and maintenance phases cycle as diuretic activity waxes and wanes.
Recovery Phase.
Stopping the diuretic markedly reduces distal delivery of salt and volume so that HCO3− generation ceases. However, metabolic alkalosis will not resolve unless potent stimuli that accelerate proximal and distal salt reabsorption can be reduced or eliminated. Therefore, if diuretics were initiated to treat avid salt retention generated by heart failure or cirrhosis, the metabolic alkalosis generally persists until the underlying disorder can be ameliorated. Reversing hypokalemia and K+ depletion is also important. If the clinical situation mandates continuing diuretics despite severe metabolic alkalosis, the addition of potassium sparing (triamterene, spironolactone, etc.) or “acidifying” (acetazolamide) diuretics can be helpful. Note, however, that acetazolamide often generates marked K+ wasting, so aggressive K+ supplementation is generally required (44).
All three types of intercalated cells located in the distal tubule/collecting ducts not only play a major role in acid/base regulation, but also participate in volume regulation and NaCl balance (32,33). These cells may be especially important in moderating the development of metabolic alkalosis in patients receiving thiazide diuretics (34) (Figure 5).
Diagnostic Approach.
When the cause of metabolic alkalosis is not readily apparent from the history and physical examination, then it is very helpful to categorize the disorder on the basis of the patient’s kidney function and volume status. If the GFR is markedly reduced and major acidic gastrointestinal fluid losses do not exist, a source of exogenous bicarbonate loading should be sought. If the GFR is not markedly reduced, then carefully assess volume status with history, physical examination, and a spot urine [Cl−] measurement. A urine [Cl−] <20 meq/L is consistent with a reduced ECF or effective intra-arterial volume, whereas a urine [Cl−] >20 meq/L suggests an expanded state. Consider the diagnoses in Table 2. However, recognize that diuretic-generated metabolic alkaloses are characterized by cyclic changes in urine [Cl−] as the diuretic effect waxes and wanes. In general, widely varying urine [Cl−] changes indicate diuretic use (which some patients may deny).
Metabolic alkalosis is a very common disorder. This brief review provides a diagnostic and therapeutic framework using an ECF volume-oriented physiologic approach to the generation, maintenance, and resolution of this disorder. Space limitations preclude in-depth discussion of many fascinating clinical metabolic alkalosis syndromes and a number of recent physiologic and pathophysiologic discoveries that enhance our understanding of this disorder.
Disclosures
The author has nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kraut JA, Lew V, Madias NE: Re-evaluation of total CO2 concentration in apparently healthy younger adults. Am J Nephrol 48: 15–20, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maehle K, Haug B, Flaatten H, Nielsen E: Metabolic alkalosis is the most common acid-base disorder in ICU patients. Critical Care 28[14]: 420–420, 2014. 10.1186/cc13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javaheri S, Kazemi H: Metabolic alkalosis and hypoventilation in humans. Am Rev Respir Dis 136: 1011–1016, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Feldman M, Alvarez NM, Trevino M, Weinstein GL: Respiratory compensation to a primary metabolic alkalosis in humans. Clin Nephrol 78: 365–369, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Adrogué HJ, Madias NE: Secondary responses to altered acid-base status: The rules of engagement. J Am Soc Nephrol 21: 920–923, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Hamm LL, DuBose, TD. Chapter 16. In: Brenner and Rector’s The Kidney, 11th Ed., edited by Yu A, Chertow G, Luyckx V, Marsden P, Skorecki K, Taal M, Philadelphia, PA, Elsevier, 2019, pp 496–536 [Google Scholar]
- 7.Emmett M, Narins RG: Clinical use of the anion gap. Medicine (Baltimore) 56: 38–54, 1977. [PubMed] [Google Scholar]
- 8.Emmett M, Seldin DW: Evaluation of acid-base disorders from plasma composition In: The Regulation of Acid-Base Balance, edited by Seldin DW, Giebisch G, New York, Raven Press, 1989, pp 213–263 [Google Scholar]
- 9.Madias NE, Ayus JC, Adrogué HJ: Increased anion gap in metabolic alkalosis: The role of plasma-protein equivalency. N Engl J Med 300: 1421–1423, 1979 [DOI] [PubMed] [Google Scholar]
- 10.Adrogué HJ, Brensilver J, Madias NE: Changes in the plasma anion gap during chronic metabolic acid-base disturbances. Am J Physiol 235: F291–F297, 1978 [DOI] [PubMed] [Google Scholar]
- 11.Byrne AL, Bennett M, Chatterji R, Symons R, Pace NL, Thomas PS: Peripheral venous and arterial blood gas analysis in adults: Are they comparable? A systematic review and meta-analysis. Respirology 19: 168–175, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Seldin DW, Rector FC Jr.: Symposium on acid-base homeostasis. The generation and maintenance of metabolic alkalosis. Kidney Int 1: 306–321, 1972 [DOI] [PubMed] [Google Scholar]
- 13.Luke RG, Galla JH: It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol 23: 204–207, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen RA, Julian BA, Dubovsky EV, Galla JH, Luke RG: On the mechanism by which chloride corrects metabolic alkalosis in man. Am J Med 84: 449–458, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Galla JH, Bonduris DN, Luke RG: Effects of chloride and extracellular fluid volume on bicarbonate reabsorption along the nephron in metabolic alkalosis in the rat. Reassessment of the classical hypothesis of the pathogenesis of metabolic alkalosis. J Clin Invest 80: 41–50, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galla JH, Bonduris DN, Luke RG: Correction of acute chloride-depletion alkalosis in the rat without volume expansion. Am J Physiol 244: F217–F221, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Norris SH, Kurtzman NA: Does chloride play an independent role in the pathogenesis of metabolic alkalosis? Semin Nephrol 8: 101–108, 1988 [PubMed] [Google Scholar]
- 18.Garella S, Cohen JJ, Northrup TE: Chloride-depletion metabolic alkalosis induces ECF volume depletion via internal fluid shifts in nephrectomized dogs. Eur J Clin Invest 21: 273–279, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Stinebaugh B, Miller RB, Relman AS: The influence of non-reabsorbable anions on acid excretion. Clin Sci 36: 53–65, 1969. [PubMed] [Google Scholar]
- 20.Zietse R, Zoutendijk R, Hoorn EJ: Fluid, electrolyte and acid-base disorders associated with antibiotic therapy. Nat Rev Nephrol 5: 193–202, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Halperin ML, Scheich A: Should we continue to recommend that a deficit of KCl be treated with NaCl? A fresh look at chloride-depletion metabolic alkalosis. Nephron 67: 263–269, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Aronson PS, Giebisch G: Effects of pH on potassium: New explanations for old observations. J Am Soc Nephrol 22: 1981–1989, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garella S, Chang BS, Kahn SI: Dilution acidosis and contraction alkalosis: Review of a concept. Kidney Int 5: 279–283, 1975 [DOI] [PubMed] [Google Scholar]
- 24.Van Goidsenhoven GM, Gray OV, Price AV, Sanderson PH: The effect of prolonged administration of large doses of sodium bicarbonate in man. Clin Sci 13: 383–401, 1954. [PubMed] [Google Scholar]
- 25.Roy A, Al-bataineh MM, Pastor-Soler NM: Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol 10: 305–324, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R: Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wall SM: Recent advances in our understanding of intercalated cells. Curr OpinNephrol Hypertens 14: 480–484, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Soleimani M: SLC26 Cl-/HCO3- exchangers in the kidney: Roles in health and disease. Kidney Int 84: 657–666, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wall SM, Weinstein AM: Cortical distal nephron Cl(−) transport in volume homeostasis and blood pressure regulation. Am J Physiol Renal Physiol 305: F427–F438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham TD, Verlander JW, Wang Y, Romero CA, Yue Q, Chen C, Thumova M, Eaton DC, Lazo-Fernandez Y, Wall SM: Aldosterone regulates pendrin and epithelial sodium channel activity through intercalated cell mineralocorticoid receptor-dependent and -independent mechanisms over a wide range in serum potassium. J Am Soc Nephrol 31: 483–499, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP: Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62: 2109–2117, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Kleyman TR, Satlin LM, Hallows KR: Opening lines of communication in the distal nephron. J Clin Invest 123: 4139–4141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soleimani M: The multiple roles of pendrin in the kidney. Nephrol Dial Transplant 30: 1257–1266, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pela I, Bigozzi M, Bianchi B: Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol 69: 450–453, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Patel AM, Goldfarb S: Got calcium? Welcome to the calcium-alkali syndrome. J Am Soc Nephrol 21: 1440–1443, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Huber L, Gennari FJ: Severe metabolic alkalosis in a hemodialysis patient. Am J Kidney Dis 58: 144–149, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Riddel MJ, Strong JA, Cameron D: The electrolyte concentration of human gastric secretion. Q J Exp Physiol 65: 1–11, 1960 [Google Scholar]
- 38.Wall SM: Mechanisms of NH4+ and NH3 transport during hypokalemia. Acta Physiol Scand 179: 325–330, 2003 [DOI] [PubMed] [Google Scholar]
- 39.DuBose TD Jr., Good DW: Effects of chronic Cl depletion alkalosis on proximal tubule transport and renal production of ammonium. Am J Physiol 269: F508–F514, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Kassirer JP, Schwartz WB: The response of normal man to selective depletion of hydrochloric acid. Factors in the genesis of persistent gastric alkalosis. Am J Med 40: 10–18, 1966. [DOI] [PubMed] [Google Scholar]
- 41.Eiro M, Katoh T, Watanabe T: Use of a proton-pump inhibitor for metabolic disturbances associated with anorexia nervosa. N Engl J Med 346: 140, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Stowasser M, Gordon RD: Primary aldosteronism: Changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev 96: 1327–1384, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Harrington JT, Hulter HN, Cohen JJ, Madias NE: Mineralocorticoid-stimulated renal acidification: The critical role of dietary sodium. Kidney Int 30: 43–48, 1986. [DOI] [PubMed] [Google Scholar]
- 44.Ellison DH: Clinical pharmacology in Diuretic use. Clin J Am Soc Nephrol 14: 1248–1257, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]