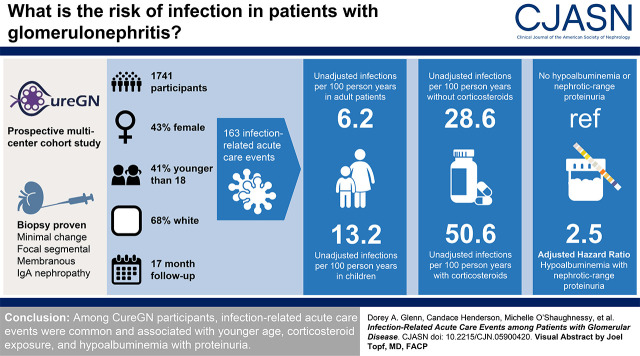
Visual Abstract
Keywords: infection, hospitalization, glomerular disease, nephrotic syndrome, kidney disease, immunosuppression, pediatric nephrology, acute care events
Abstract
Background and objectives
Infections contribute to patient morbidity and mortality in glomerular disease. We sought to describe the incidence of, and identify risk factors for, infection-related acute care events among Cure Glomerulonephropathy Network (CureGN) study participants.
Design, setting, participants, & measurements
CureGN is a prospective, multicenter, cohort study of children and adults with biopsy sample–proven minimal change disease, FSGS, membranous nephropathy, or IgA nephropathy/vasculitis. Risk factors for time to first infection-related acute care events (hospitalization or emergency department visit) were identified using multivariable Cox proportional hazards regression.
Results
Of 1741 participants (43% female, 41% <18 years, 68% White), 163 (9%) experienced infection-related acute care events over a median follow-up of 17 months (interquartile range, 9–26 months). Unadjusted incidence rates of infection-related acute care events were 13.2 and 6.2 events per 100 person-years among pediatric and adult participants, respectively. Among participants with versus without corticosteroid exposure at enrollment, unadjusted incidence rates were 50.6 and 28.6 per 100 person-years, respectively, during the first year of follow-up (adjusted hazard ratio for time to first infection, 1.31; 95% CI, 0.89 to 1.93), and 4.1 and 1.1 per 100 person-years, respectively, after 1 year of follow-up (hazard ratio, 2.99; 95% CI, 1.54 to 5.79). Hypoalbuminemia combined with nephrotic-range proteinuria (serum albumin ≤2.5 g/dl and urinary protein-creatinine ratio >3.5 mg/mg), compared with serum albumin >2.5 g/dl and urinary protein-creatinine ratio ≤3.5 mg/mg, was associated with higher risk of time to first infection (adjusted hazard ratio, 2.49; 95% CI, 1.51 to 4.12).
Conclusions
Among CureGN participants, infection-related acute care events were common and associated with younger age, corticosteroid exposure, and hypoalbuminemia with proteinuria.
Introduction
Infections are an important cause of noncardiovascular morbidity and mortality in CKD (1–4) and kidney failure (5–7). Glomerular diseases account for approximately 16% of adult (8) and 16%–24% of childhood (9–11) onset kidney failure. Patients with glomerular diseases have unique risk factors for infection, including immunosuppressive medication exposure, systemic inflammation, and high-grade proteinuria (12). Studies in patients with all-cause CKD describe a graded association of infection risk with lower eGFR and higher albuminuria (2). However, contemporary studies describing the epidemiology of infection in glomerular disease are largely lacking (13–18).
Reports from the 1960s to 1980s describe infection as the leading cause of death in children with minimal change disease (18), with annual rates of invasive bacterial infection of 1%–2% (19–22). Available data regarding the frequency and clinical spectrum of infections in adults with glomerular disease come from clinical trials (23) or single-center observational studies (14,15), which lack generalizability, or from claims-based epidemiologic studies (24), which lack detailed clinical information.
We sought to understand the epidemiology of infection-related acute care events, defined as hospitalization or emergency department (ED) visit due to infection, among adults and children with glomerular disease enrolled in the Cure Glomerulonephropathy Network (CureGN). We aimed to describe rates of, risk factors for, and characteristics of infection-related acute care events among CureGN participants, stratified by age and severity of nephrotic syndrome.
Materials and Methods
Patient Population
CureGN is a prospective, multicenter, observational, cohort study of patients with biopsy sample–proven glomerular disease (25). Eligible participants are identified and enrolled by nephrologists or study coordinators. Inclusion criteria include an initial kidney biopsy specimen showing minimal change disease, FSGS, membranous nephropathy, or IgA nephropathy/vasculitis within 5 years before enrollment. Those with kidney failure, including a kidney transplant, diabetes mellitus, SLE, HIV, active malignancy, or hepatitis B or C, at time of first kidney biopsy were excluded. This study includes participants who enrolled in CureGN between December 2014 and December 2017 and who had at least one follow-up visit. The study protocol was approved by the institutional review board at each participating center and was conducted in adherence with the Declaration of Helsinki. All participants provided written informed consent or assent, if appropriate, at enrollment.
Data Collection
Subjects were enrolled from 72 participating clinical sites in the United States, Canada, Italy, and Poland. Demographics and clinical characteristics (glomerular disease subtype, prior medication exposure, and comorbid health conditions) were collected at study enrollment. Clinical data and details of intercurrent hospitalizations or ED visits were collected approximately every 4 months at in-person or remote study visits. Biospecimens were obtained at all in-person visits, at least yearly.
Definitions
Baseline Variable Definitions.
eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration formula (26) for subjects aged ≥18 years, and the bedside Schwartz formula (27) for subjects <18 years. To account for decreased precision of estimating formulas at low serum creatinine values, eGFR was capped at 130 ml/min per 1.73 m2 (2,28). Urinary protein-creatinine ratio (UPCR) was calculated from a 24-hour urine collection, first morning void, or random spot urine. Because UPCR and serum albumin were significantly correlated (r=0.36, P<0.001) and are commonly used together to define nephrotic syndrome, these variables were combined into four categories using a UPCR cut point of 3.5 mg/mg and a serum albumin cut point of 2.5 g/dl (29). The central CureGN laboratory used a Randox RX Daytona chemistry analyzer to measure serum and urine creatinine, using the Jaffe method, and urine protein using a colorimetric method. When central measurements were not available (66% serum creatinine, 70% serum albumin, 65% UPCR), measurements from clinical site laboratories were used.
The number of comorbid conditions at enrollment was defined as the summation of hypertension, coronary artery disease, heart failure, arrhythmia, stroke, peripheral vascular disease, aortic aneurysm, valvular heart disease, celiac disease, inflammatory bowel disease, gastrointestinal bleed, cirrhosis, chronic obstructive pulmonary disease, asthma, sleep apnea, sickle cell disease, or psychiatric disease. Participants were considered exposed to tobacco if they smoked currently or within 1 year of enrollment.
Outcome: Infection-Related Acute Care Events.
Study coordinators at each clinical site collected data regarding suspected infection-related acute care events, including hospital length of stay and intensive care unit (ICU) admission, with input from local clinical investigators as needed. Two study investigators (D.A.G. and C.D.H.) manually reviewed all reported acute care events, and classified events as infection-related on the basis of International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD10) and Current Procedural Terminology discharge codes; antibiotic use (yes/no); and data from CureGN hospitalization and study visit forms. Acute care events were determined to be infection related when either the CureGN hospitalization or clinical visit forms listed an infection-related ED visit or hospitalization and when infection-related ICD10 codes were listed (Supplemental Table 1). When these variables were incongruent, the study investigators queried site coordinators to clarify the nature of the acute care event. Infections were categorized by organ system and, when possible, subtyped into bacterial, viral, or fungal subcategories. Infection-related acute care events occurring within 14 days of a prior infection of the same type or organ system were excluded, except when there was evidence that the infections were caused by different organisms. ED visits leading to hospitalization were categorized as hospitalization events.
Outcome Event Adjudication
To determine the validity of events recorded, we selected five study sites for local event adjudication. Adjudicators (D.A.G., L.A.G., L.M., A.B., and M.O.) were all physicians from sites with high enrollment. Both infectious and noninfectious events were identified via random sampling by the data coordinating center. Adjudicators reviewed selected events using a standardized adjudication protocol, which included determination of event type (infectious versus noninfectious), organism type (bacterial, viral, fungal), and primary infection site. Adjudicators were blinded to an event’s prior classification as infectious or noninfectious by study personnel. Events occurring outside of a CureGN study site were not adjudicated. Adjudication of a random sample of 20 (2%) infectious and noninfectious ED visits or hospitalizations resulted in high levels of agreement (κ=0.8–1.0, sensitivity 90%–100%, specificity 91%–100%) between initial infection classification and direct physician chart review.
Statistical Analyses
Continuous measures were described as mean and SD or median and interquartile range (IQR). We used the Wilcoxon rank sum test for continuous variables and the Fisher exact test for categoric variables to compare subjects ≤18 and >18 years of age. Cohen κ was used to measure agreement between infection status in CureGN data versus manual adjudication. Kaplan–Meier (KM) curves for time from enrollment to first infection-related acute care event were stratified by age categories or subgroups on the basis of UPCR and serum albumin. KM curves were compared using the log-rank test.
Risk factors for time to first infection-related acute care event were identified using unadjusted and multivariable Cox proportional hazards regression. The model included demographics (age, race, sex), immunosuppression exposure at enrollment (corticosteroids and all other immunosuppressants), glomerular disease subtype, number of comorbid conditions, smoking status, time from initial biopsy, and baseline laboratory measurements (eGFR, UPCR, and serum albumin). Observation time was censored at last study follow-up. The proportional hazards assumption was checked for all variables by plotting weighted Schoenfeld residual correlations over time and testing for a nonzero slope using linear regression. Corticosteroid exposure at enrollment violated the proportional hazard assumption in the full and pediatric-restricted models. Race and the combination of serum albumin and UPCR violated the proportional hazards assumption in the adult-restricted model. To address nonproportional hazards, final models included interaction terms between the aforementioned variables and time (time ≤1 or >1 year).
Multiple imputation was performed to handle missing data. The two variables with missing values included the combination of UPCR and serum albumin (26% missing) and eGFR (8% missing). Missing laboratory values were due to incomplete biospecimen collection. Supplemental Table 2 compares demographics and clinical characteristics of participants with complete data with those with missing UPCR and/or serum albumin measurements. The Fisher exact test was used to measure the association between corticosteroid exposure and infection-related acute care events in each group (Supplemental Table 3). Cox regression models were imputed 30 times, and the results were pooled using the Rubin rules. Joint tests were used to determine statistical differences among categoric variables. Statistical significance was defined as P<0.05, and statistical analyses were completed using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
Missing Data
Of the total cohort, 75% had complete data. Sociodemographic and clinical characteristics of participants with complete versus missing UPCR and/or serum albumin measurements were generally similar (Supplemental Table 2). However, exposure to corticosteroids was more common among those with complete data (38% versus 20%). Both groups demonstrated a significant unadjusted association between baseline corticosteroid exposure and infection-related acute care events (Supplemental Table 3).
Subject Characteristics
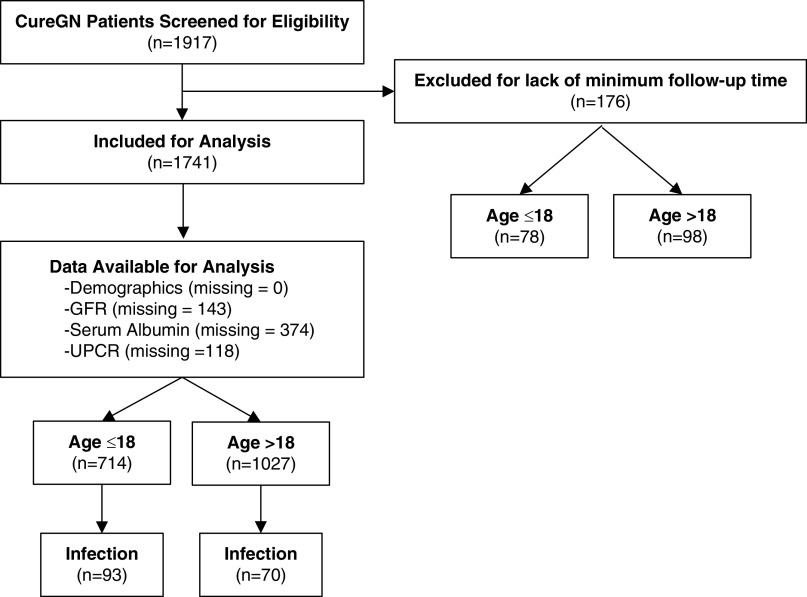
Of the 1917 total subjects enrolled in the study, 176 were excluded due to inadequate follow-up data, and the remaining 1741 were included in the study (Figure 1). Thirty-four percent of patients (n=598) were enrolled within 6 months of initial kidney biopsy, and 66% (n=1143) underwent kidney biopsy between 6 months and 5 years before study enrollment.
Figure 1.
Flow chart illustrating participants and data used for analyses of infection-related acute care events from the Cure Glomerulonephropathy Network (CureGN) study. UPCR, urinary protein-creatinine ratio.
Of the full study cohort (n=1741), 372 (21%) subjects had minimal change disease, 411 (24%) had FSGS, 329 (19%) had membranous nephropathy, and 629 (36%) had IgA nephropathy/vasculitis. At baseline, 19% of the cohort was ≤10 years, 22% were 11–18 years, 47% were 19–60 years, and 12% were >60 years. Females accounted for 43% (n=741) of the cohort; 15% (n=268) of the cohort was Black. Baseline characteristics stratified by age ≤18 or >18 years of age are shown in Table 1. Differences between the strata included glomerular disease subtype (minimal change disease more common in children and membranous nephropathy more common in adults), greater immunosuppression exposure in children, and lower eGFR and higher proteinuria in adults. Race, sex, serum albumin, and follow-up time did not differ significantly between those ≤18 and those >18 years of age.
Table 1.
Baseline characteristics of 1741 participants in the Cure Glomerulonephropathy Network study
| Sociodemographic and Clinical Characteristics | n (%) or Median (IQR)a | ||
|---|---|---|---|
| Total (n=1741) | Age ≤18 (n=714) | Age >18 (n=1027) | |
| Age, yr | |||
| <10 | 334 (19) | 334 (47) | 0 (0) |
| 11–18 | 380 (22) | 380 (53) | 0 (0) |
| 19–60 | 318 (47) | 0 (0) | 813 (79) |
| >60 | 214 (12) | 0 (0) | 214 (21) |
| Female sex | 741 (43) | 296 (41) | 445 (43) |
| Race | |||
| White | 1177 (68) | 486 (68) | 691 (67) |
| Black | 268 (15) | 119 (17) | 149 (15) |
| Other | 296 (17) | 109 (15) | 187 (18) |
| Glomerular disease type | |||
| Minimal change disease | 372 (21) | 247 (35) | 125 (12) |
| FSGS | 411 (24) | 162 (23) | 249 (24) |
| Membranous nephropathy | 329 (19) | 39 (5) | 290 (28) |
| IgA nephropathy/vasculitis | 629 (36) | 266 (37) | 363 (35) |
| Corticosteroids | 586 (34) | 292 (41) | 294 (29) |
| Other immunosuppressionb | 593 (34) | 327 (46) | 266 (26) |
| eGFRc | |||
| <60 ml/min per 1.73 m2 | 482 (30) | 49 (8) | 433 (46) |
| 60–90 ml/min per 1.73 m2 | 400 (25) | 143 (22) | 257 (27) |
| >90 ml/min per 1.73 m2 | 716 (45) | 461 (71) | 255 (27) |
| Missing | 143 (8) | 61 (9) | 82 (8) |
| UPCRc | |||
| <0.2 mg/mg | 423 (25) | 252 (37) | 171 (17) |
| 0.2–1 mg/mg | 453 (27) | 189 (27) | 264 (27) |
| 1–3.5 mg/mg | 414 (25) | 115 (17) | 299 (30) |
| >3.5 mg/mg | 381 (23) | 132 (19) | 249 (25) |
| Missing | 70 (4) | 26 (4) | 44 (4) |
| Serum albumin, g/dl | 3.8 (3.1–4.2) | 3.8 (3.0–4.2) | 3.8 (3.1–4.1) |
| Missing | 374 (22) | 139 (20) | 235 (23) |
| Serum albumin (g/dl) and UPCR (mg/mg)c | |||
| Albumin >2.5 and UPCR >3.5 | 915 (70) | 394 (71) | 521 (69) |
| Albumin ≤2.5 and UPCR ≤3.5 | 210 (16) | 63 (11) | 147 (19) |
| Albumin ≤2.5 and UPCR >3.5 | 71 (5) | 45 (8) | 26 (3) |
| Albumin >2.5 and UPCR ≤3.5 | 121 (9) | 55 (10) | 66 (9) |
| Missing | 424 (24) | 157 (22) | 267 (26) |
| Tobacco exposure | 430 (25) | 18 (3) | 412 (40) |
| Number of comorbidities | |||
| 0 | 631 (36) | 388 (54) | 243 (24) |
| 1–3 | 996 (57) | 314 (44) | 682 (66) |
| >3 | 114 (7) | 12 (2) | 102 (10) |
| Time from kidney biopsy to enrollment (mo) | 12.2 (3.9–31.8) | 10.9 (2.9–28.8) | 13.3 (4.7–34.5) |
IQR, interquartile range; UPCR, urinary protein-creatinine ratio.
Values for categoric variables are presented as count (percentage), and values for continuous variables are presented as median (interquartile range).
Other immunosuppressant medications include cyclosporine, oral and intravenous cyclophosphamide, tacrolimus, rituximab, mycophenolate, and azathioprine.
Percentage of those with nonmissing data.
Infections Leading to Acute Care Events
Over a median follow-up of 17 months, 163 CureGN participants (9%) developed 227 infections requiring a hospitalization or ED visit, with 25% (n=40) of these participants experiencing more than one event. Frequency and characteristics of infection-related acute care events stratified by age and baseline corticosteroid exposure are presented in Tables 2 and 3. Infections accounted for 22% of all hospitalizations and 24% of all ED visits. Median hospital length of stay was 4 days (IQR, 2–7), and admission to the ICU was required in 8% of infection-related hospitalizations. Among all infections, 106 (47%) were bacterial, 80 (35%) were viral, four (2%) were fungal, and no organism type was identified for 37 (16%).
Table 2.
Frequency and characteristics of acute care events (hospitalizations and emergency department visits) among 1741 Cure Glomerulonephropathy Network participants
| Acute Care Event | Age Group | Baseline Steroid Exposure | Overall (n=1741) | ||
|---|---|---|---|---|---|
| ≤18 yr (n=714) | >18 yr (n=1027) | Yes (n=586) | No (n=1155) | ||
| Infection-related eventsa | |||||
| Total | 134 | 93 | 129 | 98 | 227 |
| Hospitalizations, n (%) | 74 (55) | 59 (63) | 73 (57) | 60 (61) | 133 (59) |
| Emergency department visits, n (%) | 60 (45) | 34 (37) | 56 (43) | 38 (39) | 94 (41) |
| Patients with ≥1 infection-related event, n (%) | 93 (13) | 70 (7) | 98 (13) | 65 (7) | 163 (9) |
| Infection-related events per subject, n (%)a,b | |||||
| 1 | 71 (76) | 52 (74) | 76 (78) | 47 (72) | 123 (75) |
| 2 | 12 (13) | 14 (20) | 14 (14) | 12 (19) | 26 (16) |
| ≥3 | 10 (11) | 4 (6) | 8 (8) | 6 (9) | 14 (9) |
| Infection-related acute care events per 100 person-yr (95% CI)a | 13.2 (11.2 to 15.7) | 6.2 (2.1 to 7.6) | 11.2 (9.4 to 13.3) | 7.2 (5.9 to 8.8) | 9.0 (7.9 to 10.3) |
| Length of stay (d), median (IQR)c | 4 (2–6) | 4 (3–7) | 4 (2–7) | 4 (2–6) | 4 (2–7) |
| ICU stay due to infection, n (%)c | 8 (11) | 2 (3) | 6 (5) | 4 (4) | 10 (8) |
95% CI, 95% confidence interval; IQR, interquartile range; ICU, intensive care unit.
Inclusive of multiple infections within individuals.
Of number of subjects with ≥1 infection-related acute care event.
Of hospitalized infection-related acute care events only.
Table 3.
Descriptors of infection-related acute care events (hospitalizations and emergency department visits) by organ system and organism type among 1741 participants in the Cure Glomerulonephropathy Network study
| Infection Site and Type | Age Group, n (%) | Baseline Steroid Exposure, n (%) | Overall, n (%) (n=1741) | ||
|---|---|---|---|---|---|
| ≤18 yr (n=714) | >18 yr (n=1027) | Yes (n=586) | No (n=1155) | ||
| Primary site of infectiona | |||||
| Total infections | 134 (100) | 93 (100) | 129 (100) | 98 (100) | 227 (100) |
| Pulmonary/lower respiratory tract | 29 (22) | 27 (29) | 34 (26) | 22 (23) | 56 (25) |
| Upper respiratory tract/throat | 37 (28) | 11 (12) | 28 (22) | 20 (20) | 48 (21) |
| Gastrointestinal | 13 (10) | 13 (14) | 17 (13) | 9 (9) | 26 (11) |
| Multisystem infection | 19 (14) | 7 (8) | 13 (10) | 13 (13) | 26 (11) |
| Skin and soft tissue | 11 (8) | 12 (13) | 13 (10) | 10 (10) | 23 (10) |
| Genitourinary | 8 (6) | 11 (12) | 6 (5) | 13 (13) | 20 (9) |
| Bacteremia and septicemia | 8 (6) | 8 (9) | 10 (8) | 6 (6) | 16 (7) |
| Ear and nose | 6 (4) | 1 (1) | 4 (3) | 3 (3) | 7 (3) |
| Bone and joint | 1 (1) | 1 (1) | 2 (2) | 0 (0) | 2 (1) |
| Eye | 2 (1) | 0 (0) | 1 (1) | 1 (1) | 2 (1) |
| Central nervous system | 0 (0) | 2 (2) | 1 (1) | 1 (1) | 2 (1) |
| Organism typea | |||||
| Bacterial | 56 (42) | 50 (54) | 63 (49) | 43 (44) | 106 (47) |
| Viral | 53 (40) | 27 (29) | 45 (35) | 35 (36) | 80 (35) |
| Fungal | 1 (1) | 3 (3) | 2 (2) | 2 (2) | 4 (2) |
| Unknown | 24 (18) | 13 (14) | 19 (5) | 18 (18) | 37 (16) |
Infections occurring within 14 d of a previous infection of the same type or organ system were considered a single event.
The incidence rate of infection-related acute care events per 100 person-years among those exposed to corticosteroids was 50.6 events (95% confidence interval [95% CI], 39.1 to 65.6) during the first year of study follow-up and 4.1 events (95% CI, 2.8 to 6.0) after 1 year of study follow-up (Table 4). In unadjusted analyses, corticosteroid exposure versus nonexposure was associated with a higher risk of time to first infection (hazard ratio [HR], 2.20; 95% CI, 1.62 to 2.99). Because this exposure violated proportional hazards, an interaction term between the corticosteroid exposure and follow-up time (time ≤1 or >1 year) was constructed. The unadjusted risk of infection-related acute care events was 1.81-fold higher (95% CI, 1.27 to 2.58) in the first year of study follow-up and 4.05-fold higher (95% CI, 2.14 to 7.67) after 1 year of study follow-up in those exposed versus not exposed to corticosteroids at enrollment.
Table 4.
Risk factors for time to first infection-related acute care event (hospitalization or emergency department visit) in unadjusted and multivariable adjusted Cox regression analysis among 1741 Cure Glomerulonephropathy Network participants
| Predictors | Infections (n)a | Unadjusted | Multivariable Model (imputed) | ||
|---|---|---|---|---|---|
| IR (95% CI) per 100 person-yra | HR (95% CI) | HR (95% CI) | P Valueb | ||
| Age (yr) | <0.001 | ||||
| ≤10 | 61 | 14.4 (11.2 to 18.5) | 2.69 (1.55 to 4.66) | 4.62 (2.11 to 10.08) | |
| 11–18 | 32 | 6.5 (4.6 to 9.2) | 1.21 (0.67 to 2.21) | 2.22 (1.04 to 4.72) | |
| 19–60 | 54 | 4.7 (3.6 to 6.2) | 0.91 (0.52 to 1.58) | 1.25 (0.68 to 2.27) | |
| >60 | 16 | 5.2 (3.2 to 8.5) | Ref | Ref | |
| Sex | 0.29 | ||||
| Male | 88 | 6.4 (5.2 to 7.9) | 0.85 (0.62 to 1.15) | 0.84 (0.61 to 1.16) | |
| Female | 75 | 7.6 (6.0 to 9.5) | Ref | Ref | |
| Race | 0.22 | ||||
| Black | 35 | 11.1 (7.9 to 15.4) | 1.73 (1.18 to 2.54) | 1.44 (0.95 to 2.17) | |
| Other | 26 | 6.7 (4.5 to 9.8) | 1.07 (0.70 to 1.65) | 1.14 (0.74 to 1.77) | |
| White | 102 | 6.2 (5.1 to 7.5) | Ref | Ref | |
| Glomerular disease type | 0.95 | ||||
| Minimal change disease | 47 | 9.8 (7.3 to 13.0) | 1.77 (1.19 to 2.63) | 0.90 (0.56 to 1.44) | |
| FSGS | 41 | 7.9 (5.8 to 10.7) | 1.41 (0.93 to 2.13) | 0.89 (0.56 to 1.40) | |
| Membranous nephropathy | 25 | 5.6 (3.8 to 8.3) | 1.02 (0.63 to 1.65) | 0.86 (0.49 to 1.50) | |
| IgA nephropathy/vasculitis | 50 | 5.5 (4.1 to 7.2) | Ref | Ref | |
| eGFR at enrollment, per 10 ml/min per 1.73 m2 | — | — | 1.02 (0.97 to 1.07) | 0.96 (0.90 to 10.2) | 0.18 |
| Corticosteroid use at enrollmentc | |||||
| Yes | 83 | 11.1 (9.0 to 13.8) | — | — | |
| No | 80 | 4.9 (4.0 to 6.2) | — | — | |
| Time (≤1 yr)×steroid | 0.16 | ||||
| Yes | 57 | 50.6 (39.1 to 65.6) | 1.81 (1.27 to 2.58) | 1.31 (0.89 to 1.93) | |
| No | 65 | 28.6 (22.4 to 36.5) | Ref | Ref | |
| Time (>1 yr)×steroid | 0.001 | ||||
| Yes | 26 | 4.1 (2.8 to 6.0) | 4.05 (2.14 to 7.67) | 2.99 (1.54 to 5.79) | |
| No | 15 | 1.1 (0.7 to 1.8) | Ref | Ref | |
| Other immunosuppressiond | 0.10 | ||||
| Yes | 77 | 9.8 (7.9 to 12.3) | 1.80 (1.32 to 2.44) | 1.34 (0.94 to 1.91) | |
| No | 86 | 5.4 (4.4 to 6.7) | Ref | Ref | |
| Serum albumin (g/dl)/UPCR (mg/mg) | 0.001 | ||||
| Albumin >2.5 and UPCR >3.5 | 27 | 10.9 (7.5 to 15.9) | 1.88 (1.21 to 2.93) | 1.81 (1.15 to 2.86) | |
| Albumin ≤2.5 and UPCR ≤3.5 | 13 | 13.0 (7.5 to 22.4) | 2.29 (1.27 to 4.14) | 1.83 (1.00 to 3.36) | |
| Albumin ≤2.5 and UPCR >3.5 | 23 | 16.4 (10.9 to 24.7) | 2.82 (1.76 to 4.51) | 2.49 (1.51 to 4.12) | |
| Albumin >2.5 and UPCR ≤3.5 | 72 | 5.6 (4.5 to 7.1) | Ref | Ref | |
| No. of comorbiditiese | — | — | 1.11 (1.00 to 1.22) | 1.22 (1.09 to 1.36) | <0.001 |
| Smoking at enrollment | 0.26 | ||||
| Yes | 36 | 6.1 (4.4 to 8.4) | 0.85 (0.59 to 1.23) | 1.30 (0.82 to 2.06) | |
| No | 127 | 7.2 (6.0 to 8.5) | Ref | Ref | |
| Time from biopsy to enrollment | 0.37 | ||||
| ≤12 mo | 90 | 8.3 (6.7 to 10.2) | 1.39 (1.02 to 1.89) | 1.17 (0.83 to 1.63) | |
| >12 mo | 73 | 5.7 (4.6 to 7.2) | Ref | Ref | |
95% CI, 95% confidence interval; IR, incidence rate; HR, hazard ratio; Ref, reference; UPCR, urinary protein-creatinine ratio.
First infections only.
P values were calculated using joint tests.
Corticosteroid exposure at enrollment (yes/no) violated the proportional hazard assumption (HR, 2.20; 95% CI, 1.62 to 2.99), so a follow-up time interaction model was developed: no exposure (referent) versus exposure and ≤1-yr follow-up versus exposure and >1-yr follow-up.
Other immunosuppressant medications include cyclosporine, oral and intravenous cyclophosphamide, tacrolimus, rituximab, mycophenolate, and azathioprine.
Comorbidities included hypertension, coronary artery disease, aortic aneurysm, valvular heart disease, heart failure, peripheral vascular disease, stroke, asthma, chronic obstructive pulmonary disorder, sickle cell disease, inflammatory bowel disease, cirrhosis, gastrointestinal bleed, HIV, hepatitis B or C, sleep apnea, cancer, or psychiatric diagnosis.
Pediatric Cohort
Over a median follow-up time of 16 months (IQR, 9–25), a total of 537 all-cause acute care events occurred among pediatric CureGN participants (n=714), of which 25% (n=134) were infection related (Table 2). The incidence rate of all infection-related acute care events (including first and recurrent infections) among pediatric participants was 13.2 per 100 person-years. Length of stay for infection-related hospitalizations was 4 days (IQR, 2–6), and 11% of those hospitalizations (n=8) required ICU admission. The most common sites of infection were the upper (28%) and lower (22%) respiratory tract (Table 3). Among all infections, 42% were bacterial, 40% were viral, and 1% were fungal.
Adult Cohort
Over a median follow-up time of 17 months (IQR, 9–26), a total of 507 all-cause acute care events occurred among adult CureGN participants (n=1027), of which 18% (n=93) were infection related. The incidence rate of all infection-related acute care events (including first and recurrent infections) among adult participants was 6.2 per 100 person-years. Length of stay for infection-related hospitalizations was 4 days (IQR, 3–7). Pulmonary and lower respiratory tract infections accounted for 29% of infections among adult participants (Table 3). Among all infections, 54% were bacterial, 29% were viral, and 3% were fungal.
Multivariable Models
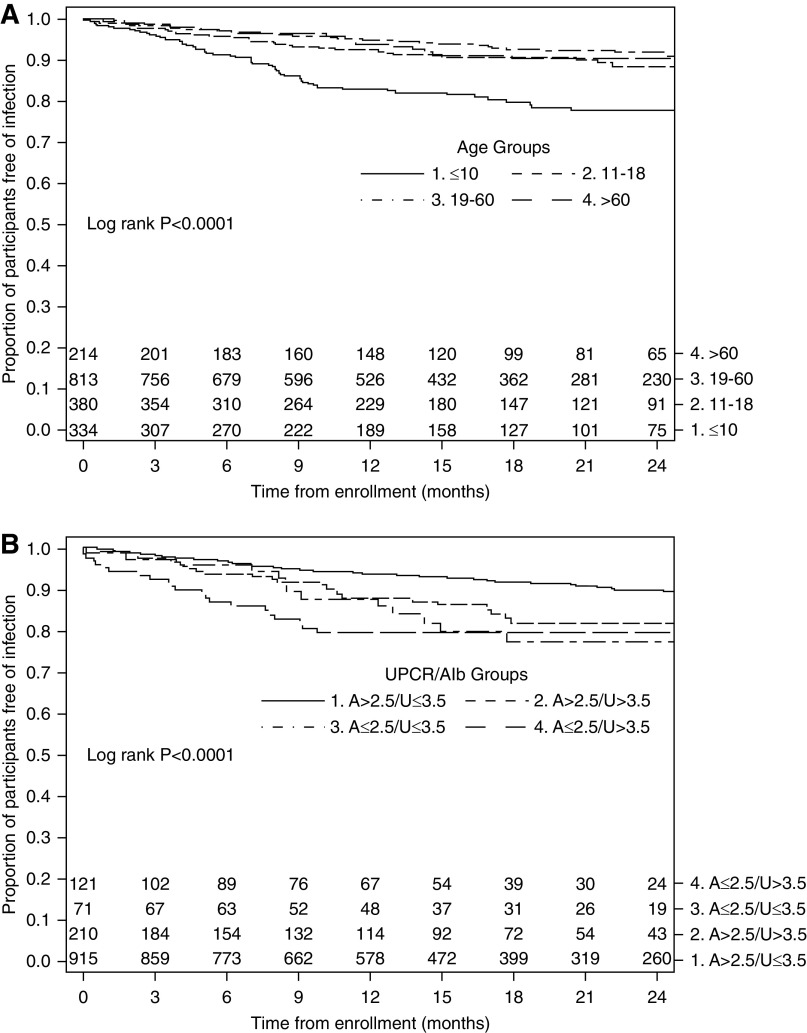
Table 4 summarizes unadjusted incidence rates and factors associated with time to first infection-related acute care event in multivariable Cox regression analysis for a cohort including both adult and pediatric participants. In the model, baseline steroid exposure with follow-up time >1 year, younger age, the presence of hypoalbuminemia (<2.5 g/dl) combined with nephrotic-range proteinuria, and number of comorbid health conditions were associated with time to first infection-related acute care event. Corticosteroid exposure at enrollment was associated with nearly a three-fold higher risk of time to first infection-related acute care event (HR, 2.99; 95% CI, 1.54 to 5.79) after 1 year of follow-up, and a trend toward a higher risk of time to first infection-related acute care event during the first year of follow-up (HR, 1.31; 95% CI, 0.89 to 1.93). Each additional comorbidity was associated with time to first infection (HR, 1.22; 95 CI, 1.09 to 1.36). Compared with those >60 years of age, patients ≤10 years had a 4.62-fold higher risk (95% CI, 2.11 to 10.08) and those 11–18 years had a 2.22-fold higher risk (95% CI, 1.04 to 4.72) of first infection-related acute care event. The combination of hypoalbuminemia and nephrotic-range proteinuria at baseline was associated with a 2.49-fold higher risk of first infection (95% CI, 1.51 to 4.12). Figure 2, A and B, illustrates the strength of these latter two variables in KM survival curves that plot the relationship of (1) age and (2) hypoalbuminemia (<2.5 g/dl), combined with nephrotic-range proteinuria and time to first infection-related acute care event.
Figure 2.
Time to first infection-related acute care event stratified by age and UPCR/albumin category. A, serum albumin; U, urine protein-creatinine ratio.
Supplemental Tables 4 and 5 summarize multivariable Cox regression models restricted to pediatric and adult participants, respectively, and demonstrate that baseline corticosteroid exposure and the combination of hypoalbuminemia and nephrotic-range proteinuria are associated with time to first infection-related acute care event in these subgroups.
Discussion
CureGN represents one of the largest observational cohort studies of adults and children with biopsy sample–proven glomerular disease assembled to date. In our analysis, younger age, the combination of hypoalbuminemia and nephrotic-range proteinuria, a higher number of health comorbidities, and corticosteroid exposure were independently associated with time to first infection-related acute care event. We describe an overall incidence rate of infection-related hospitalization or ED visit (including first and recurrent infections) of nine per 100 person-years, which is approximately 6.5 times higher than the rate of infection-related hospitalization in the general population in the United States (30). Physiologic mechanisms thought to underlie the higher infection risk in glomerular disease include edema, urinary loss of Ig and complement components, immune cell dysregulation, and exposure to immunosuppressive medications (12,31–33). Infectious risk may additionally be mediated by both disease- and treatment-related factors, including altered T-cell function, ineffective opsonization, suboptimal response to vaccination, and malnutrition (34).
Direct comparisons to infection-related incidence rates in prior studies are limited by variation in study design, study population, outcome definitions, and statistical measures. Among heterogeneous populations of patients with all-cause CKD or kidney failure, ANCA vasculitis, SLE, or IgA nephropathy, the incidence of infections considered severe or requiring hospitalization range from two to 24 infections per 100 person-years (Figure 3) (2,3,14,23,24,35). The distribution of infections by organism type (bacterial, viral, fungal) in our study is fairly aligned with previous studies in patients with kidney and autoimmune diseases, with bacterial infections comprising roughly half of severe infections (35). As in our study, pulmonary/lower respiratory infections were the most frequent infection type across other kidney diseases (2,3,36).
Figure 3.
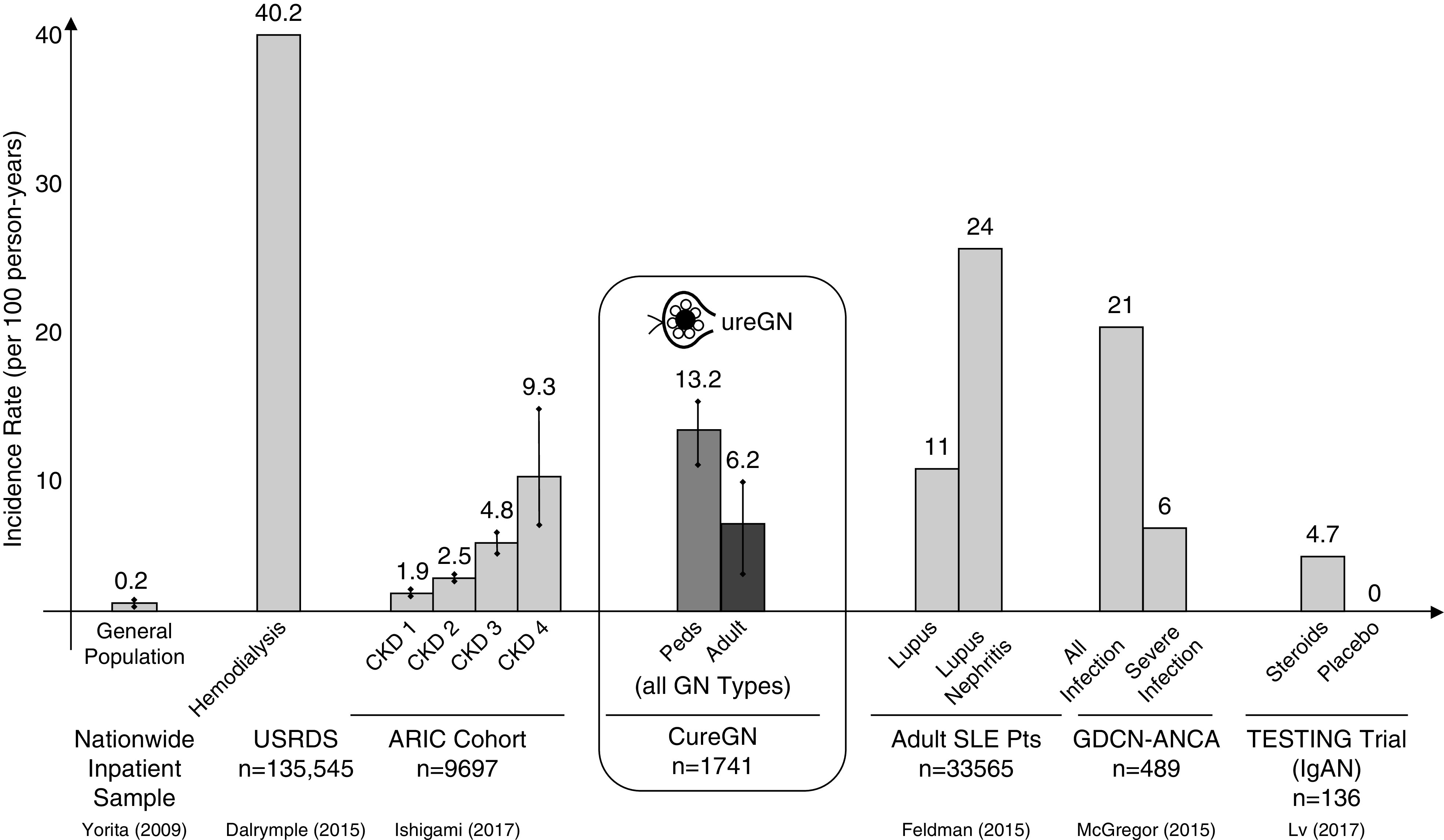
Rates of infectious events per 100 person-years in the CureGN cohort relative to rates from published studies in patients with CKD, GN, and the general population. When appropriate, incidence rates were calculated from reported cumulative incidence rates using the formula CI=1–e(−IR×T). IgAN, IgA nephropathy; Peds, pediatric patients; Pts, patients; USRDS, United States Renal Data System; CI, cummulative incidence; IR, incidence rate; T, time; ARIC, Atherosclerosis Risk in Communities Study; GDCN, Glomerular Disease Collaborative Network.
Our results highlight the substantial burden of infections in pediatric patients with glomerular disease. Pneumonia, sepsis, and peritonitis due to Streptococcus pneumonia have historically been among the most common infections in children with nephrotic syndrome (37–41). Although the distribution of infections in more contemporary cohorts (36) is roughly similar to our findings, a direct comparison is challenging due to the broader definition of acute care events in our study. Peritonitis remains an infection of particular concern because it is estimated to occur in 2%–6% of children with nephrotic syndrome (41) and carries a mortality risk of 1%–5% (18). In our cohort, peritonitis accounted for 8% of serious bacterial infections among pediatric participants (data not shown).
Infection-related acute care events were particularly burdensome in the youngest age group of <10 years, a finding observed in previous studies of children with nephrotic syndrome (13,36). In the absence of a control group, we are unable to determine whether this finding is due to a proportionately higher rate of infections in younger children overall versus a disproportionately higher infection rate in children compared with adults with glomerular disease, or differences in the tendency to seek care for children versus adults with suspected infection.
Among adult patients with minimal change disease, recent studies demonstrate a substantial burden of infections requiring hospitalization, ranging from 13% to 14% (42,43). General population studies of older adults have similarly found a higher risk of infection among patients with CKD with versus without proteinuria at much lower levels (>0.3 mg/mg) (2). Our finding that the combination of nephrotic-range proteinuria and hypoalbuminemia was associated with a higher risk for infection is supportive of the long-held belief that urinary loses of Ig and complement, independent of any concurrent immunosuppression exposure, predispose to infection.
Corticosteroid exposure at enrollment was an independent risk factor for infection in our analysis. Although we found a three-fold greater risk of infection-related acute care events in those exposed to corticosteroids after the first year of enrollment, the absolute risk of infection was far greater within the first year. Additionally, the risk difference between those exposed and those unexposed to corticosteroids was greater in the first year after study enrollment, suggesting that both steroid exposure and shorter-term follow-up were risk factors for time to first infection. Potential explanations for this finding include more aggressive initial steroid therapy and remission of disease activity over time. Conversely, the higher infection risk of initial corticosteroids after 1 year may be due to ongoing corticosteroid exposure and need for additional immunosuppressant therapy if there is no response to corticosteroids. There is wide variability in the clinical approach to corticosteroid use in patients with glomerular diseases, and the dose and duration of therapy that optimally balances benefit and risk is rarely known. Future studies should examine the relationship between steroid dosage and duration on infection risk in this population because even low dosages (<5 mg/d) have been associated with higher infection risk in other autoimmune conditions (44). Pragmatic trials are needed to better understand the minimal effective dose and duration of corticosteroids to achieve clinical goals or establish the efficacy of steroid-free protocols altogether, such as recently published for adults with minimal change disease (45).
The combination of hypoalbuminemia and nephrotic-range proteinuria was also a risk factor for time to first infection in our cohort. This finding has implications for future efforts at infection-risk mitigation because interventions might target patients in relapse or those with nephrotic-range proteinuria/hypoalbuminemia. This is an issue of particular relevance given that serious infections have affected the success of clinical trials in patients with glomerular disease (23). eGFR was not associated with time to first infection, likely due to the relatively preserved kidney function in the cohort.
This study does have several limitations. Although there is an existing literature with which to compare rates of infection, the lack of a control group limits our ability to estimate the risk of infection attributable to glomerular disease. Our study did not account for infections managed at home or by primary care physicians. Nonetheless, the most severe infections and those necessitating significant health care utilization were captured. It was unexpected that tobacco exposure was not a risk factor for infection; this may have been due to lack of data on duration and intensity of exposure. The frequency of infections before enrollment, especially among prevalent participants, was not available. Additionally, corticosteroid dosage and influenza and pneumococcal vaccination status were not available for our analysis. Strengths of our study include its large size, diverse patient population, standardized data collection, and the breadth of captured data. Moreover, our adjudication protocol used randomization of events and blinded physician review to validate outcomes.
Our study highlights that infections leading to acute care events are frequently experienced by children and adults with glomerular disease. Traditional or pragmatic trials of infection-prevention strategies, including antimicrobial prophylaxis and vaccination, among patients with glomerular disease receiving immunosuppression and/or those with nephrotic syndrome are urgently needed. Future studies should investigate predictors for the development of multiple infections in patients with glomerular disease and consider targeting infection-prevention interventions toward those at highest risk, for example those ≤18 years of age with additional risk factors such as nephrotic-range proteinuria, hypoalbuminemia, multiple comorbidities, or prolonged corticosteroid exposure.
Disclosures
K. Gibson reports clinical trial contracts with Aurinia and Retrophin, and honorarium received as a member of the Reata CKD Advisory Board. She is a working group member for the Kidney Disease Improving Global Outcomes GN guidelines writing group. L. Greenbaum has received grant support from Advicenne, Alexion, Apellis, Reata, and Vertex; received consulting fees from Abbvie, Advicenne, Akebia, Alexion, Leadiant, Otsuka, Ra Pharma, and Vifor; and has served on the data safety monitoring committee for studies sponsored by Alnylam, Relypsa, Retrophin, and University of California, San Diego. L. Mariani discloses participation on the CKD Advisory Board for Reata Pharmaceuticals. She received research funding from Boehringer Ingelheim, outside the submitted work. A. Mottl has clinical trial contracts with Aurinia, Boehringer Ingelheim, Calliditas, Duke Clinical Research Institute, and Pfizer; has been a member of the scientific advisory board for AstraZeneca; and is a consultant to Bayer. All remaining authors have nothing to disclose.
Funding
Funding for the CureGN Consortium is provided by National Institute of Diabetes and Digestive and Kidney Diseases grants U24DK100845 (formerly UM1DK100845), U01DK100846 (formerly UM1DK100846), U01DK100876 (formerly UM1DK100876), U01DK100866 (formerly UM1DK100866), and U01DK100867 (formerly UM1DK100867). Patient recruitment is supported by the NephCure Foundation. Dates of funding for the first phase of CureGN was September 16, 2013 to May 31, 2019.
Supplementary Material
Acknowledgments
The CureGN Consortium members, from within the four Participating Clinical Center networks and the Data Coordinating Center, are acknowledged by the authors as collaborators (not coauthors) on this manuscript. The list of collaborators and their affiliations are provided in the Supplemental Material.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05900420/-/DCSupplemental.
Supplemental Material. CureGN Consortium collaborators.
Supplemental Table 1. Infection-related ICD10 codes identified among 1741 CureGN participants.
Supplemental Table 2. Sociodemographic and clinical characteristics of CureGN participants at study enrollment, stratified by complete versus missing UPCR and/or serum albumin data.
Supplemental Table 3. Table testing the association between corticosteroids and number of infection-related acute care events by whether complete versus missing UPCR-albumin data (using Fischer exact test).
Supplemental Table 4. Risk factors for time to first infection-related acute care event (hospitalization or ED visit) in unadjusted and multivariable adjusted Cox regression analysis among (n=714) CureGN participants age ≤18.
Supplemental Table 5. Risk factors for time to first infection-related acute care event (hospitalization or ED visit) in unadjusted and multivariable adjusted Cox regression analysis among (n=1027) CureGN participants age >18.
References
- 1.James MT, Quan H, Tonelli M, Manns BJ, Faris P, Laupland KB, Hemmelgarn BR; Alberta Kidney Disease Network : CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 54: 24–32, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Ishigami J, Grams ME, Chang AR, Carrero JJ, Coresh J, Matsushita K: CKD and risk for hospitalization with infection: The Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 69: 752–761, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalrymple LS, Katz R, Kestenbaum B, de Boer IH, Fried L, Sarnak MJ, Shlipak MG: The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis 59: 356–363, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishigami J, Matsushita K: Clinical epidemiology of infectious disease among patients with chronic kidney disease. Clin Exp Nephrol 23: 437–447, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lofaro D, Vogelzang JL, van Stralen KJ, Jager KJ, Groothoff JW: Infection-related hospitalizations over 30 years of follow-up in patients starting renal replacement therapy at pediatric age. Pediatr Nephrol 31: 315–323, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalrymple LS, Johansen KL, Chertow GM, Cheng SC, Grimes B, Gold EB, Kaysen GA: Infection-related hospitalizations in older patients with ESRD. Am J Kidney Dis 56: 522–530, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allon M, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Depner TA, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ; HEMO Study Group : The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrol Dial Transplant 20: 1180–1186, 2005. [DOI] [PubMed] [Google Scholar]
- 8.United States Renal Data System (USRDS): 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016. Available at: https://www.usrds.org/annual-data-report/previous-adrs/. Accessed September 25, 2020 [Google Scholar]
- 9.European Society for Paediatric Nephrology/European Renal Association–European Dialysis and Transplant Association (ESPN/ERA-EDTA): ESPN/ERA-EDTA Registry (annual report), 2016. Available at: https://www.espn-reg.org/files/AR2014_Final.pdf. Accessed September 25, 2020
- 10.National American Pediatric Renal Trials and Collaborative Studies The EMMES Corporation : North American Pediatric Renal Transplant Cooperative Study 2008 Annual Report, 2008. Available at: https://www.naprtcs.org/system/files/2008_Annual_CKD_Report.pdf. Accessed February 1, 2020
- 11.Lewis MA, Shaw J, Sinha MD, Adalat S, Hussain F, Castledine C, van Schalkwyk D, Inward C: UK Renal Registry 12th annual report (December 2009): Chapter 14: Demography of the UK paediatric renal replacement therapy population in 2008. Nephron Clin Pract 115: c279–c288, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Kopp JB, Anders H-J, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, Romagnani P: Podocytopathies. Nat Rev Dis Primers 6: 68, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfakeekh K, Azar M, Sowailmi BA, Alsulaiman S, Makdob SA, Omair A, Albanyan E, Bawazeer MS: Immunosuppressive burden and risk factors of infection in primary childhood nephrotic syndrome. J Infect Public Health 12: 90–94, 2019. [DOI] [PubMed] [Google Scholar]
- 14.McGregor JG, Hogan SL, Hu Y, Jennette CE, Falk RJ, Nachman PH: Glucocorticoids and relapse and infection rates in anti-neutrophil cytoplasmic antibody disease. Clin J Am Soc Nephrol 7: 240–247, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGregor JG, Negrete-Lopez R, Poulton CJ, Kidd JM, Katsanos SL, Goetz L, Hu Y, Nachman PH, Falk RJ, Hogan SL: Adverse events and infectious burden, microbes and temporal outline from immunosuppressive therapy in antineutrophil cytoplasmic antibody-associated vasculitis with native renal function. Nephrol Dial Transplant 30[Suppl 1]: i171–i181, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senguttuvan P, Ravanan K, Prabhu N, Tamilarasi V: Infections encountered in childhood nephrotics in a pediatric renal unit. Indian J Nephrol 14: 85–88, 2004 [Google Scholar]
- 17.Gulati S, Kher V, Gupta A, Arora P, Rai PK, Sharma RK: Spectrum of infections in Indian children with nephrotic syndrome. Pediatr Nephrol 9: 431–434, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Report of the International Study of Kidney Disease in Children: Minimal change nephrotic syndrome in children: Deaths during the first 5 to 15 years’ observation. Pediatrics 73: 497–501, 1984. [PubMed] [Google Scholar]
- 19.Pittet LF, Posfay-Barbe KM, Chehade H, Rudin C, Wilhelm-Bals A, Rodriguez M, Siegrist CA, Parvex P: Optimizing seroprotection against pneumococcus in children with nephrotic syndrome using the 13-valent pneumococcal conjugate vaccine. Vaccine 34: 4948–4954, 2016. [DOI] [PubMed] [Google Scholar]
- 20.McIntyre P, Craig JC: Prevention of serious bacterial infection in children with nephrotic syndrome. J Paediatr Child Health 34: 314–317, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Trompeter RS, Lloyd BW, Hicks J, White RHR, Cameron JS: Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1: 368–370, 1985. [DOI] [PubMed] [Google Scholar]
- 22.Wilfert CM, Katz SL: Etiology of bacterial sepsis in nephrotic children 1963-1967. Pediatrics 42: 840–843, 1968. [PubMed] [Google Scholar]
- 23.Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, Monaghan H, Zhao M, Barbour S, Reich H, Cattran D, Glassock R, Levin A, Wheeler D, Woodward M, Billot L, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Wang HY, Perkovic V; TESTING Study Group : Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: The TESTING randomized clinical trial. JAMA 318: 432–442, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, Costenbader KH: Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol 67: 1577–1585, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariani LH, Bomback AS, Canetta PA, Flessner MF, Helmuth M, Hladunewich MA, Hogan JJ, Kiryluk K, Nachman PH, Nast CC, Rheault MN, Rizk DV, Trachtman H, Wenderfer SE, Bowers C, Hill-Callahan P, Marasa M, Poulton CJ, Revell A, Vento S, Barisoni L, Cattran D, D’Agati V, Jennette JC, Klein JB, Laurin LP, Twombley K, Falk RJ, Gharavi AG, Gillespie BW, Gipson DS, Greenbaum LA, Holzman LB, Kretzler M, Robinson B, Smoyer WE, Guay-Woodford LM; CureGN Consortium : CureGN study rationale, design, and methods: Establishing a large prospective observational study of glomerular disease. Am J Kidney Dis 73: 218–229, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delanaye P, Schaeffner E, Ebert N, Cavalier E, Mariat C, Krzesinski JM, Moranne O: Normal reference values for glomerular filtration rate: What do we really know? Nephrol Dial Transplant 27: 2664–2672, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Kidney Disease Improving Global Outcomes : KDIGO Clinical Practice Guideline for Glomerulonephritis. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf. Accessed January 7, 2020
- 30.Christensen KLY, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB: Infectious disease hospitalizations in the United States. Clin Infect Dis 49: 1025–1035, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Eddy AA, Symons JM: Nephrotic syndrome in childhood. Lancet 362: 629–639, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Ogi M, Yokoyama H, Tomosugi N, Hisada Y, Ohta S, Takaeda M, Wada T, Naito T, Ikeda K, Goshima S, Takasawa K, Kobayashi K: Risk factors for infection and immunoglobulin replacement therapy in adult nephrotic syndrome. Am J Kidney Dis 24: 427–436, 1994. [DOI] [PubMed] [Google Scholar]
- 33.McCaffrey J, Lennon R, Webb NJA: The non-immunosuppressive management of childhood nephrotic syndrome. Pediatr Nephrol 31: 1383–1402, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalrymple LS, Go AS: Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol 3: 1487–1493, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Migita K, Sasaki Y, Ishizuka N, Arai T, Kiyokawa T, Suematsu E, Yoshimura M, Kawabe Y, Matsumura R, Akagawa S, Mori S, Shirai M, Watanabe Y, Minami N, Soga T, Owan I, Ohshima S, Yoshizawa S, Matsui T, Tohma S, Bito S: Glucocorticoid therapy and the risk of infection in patients with newly diagnosed autoimmune disease. Medicine (Baltimore) 92: 285–293, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei C-C, Yu I-W, Lin H-W, Tsai AC: Occurrence of infection among children with nephrotic syndrome during hospitalizations. Nephrology (Carlton) 17: 681–688, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Gorensek MJ, Lebel MH, Nelson JD: Peritonitis in children with nephrotic syndrome. Pediatrics 81: 849–856, 1988. [PubMed] [Google Scholar]
- 38.Krensky AM, Ingelfinger JR, Grupe WE: Peritonitis in childhood nephrotic syndrome: 1970–1980. Am J Dis Child 136: 732–736, 1982. [DOI] [PubMed] [Google Scholar]
- 39.Liponski I, Cochat P, Gagnadoux MF, Parchoux B, Niaudet P, David L, Broyer M: [Bacterial complications of nephrotic syndrome in children]. Presse Med 24: 19–22, 1995. [PubMed] [Google Scholar]
- 40.Hsu K, Pelton S, Karumuri S, Heisey-Grove D, Klein J; Massachusetts Department of Public Health Epidemiologists : Population-based surveillance for childhood invasive pneumococcal disease in the era of conjugate vaccine. Pediatr Infect Dis J 24: 17–23, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Feinstein EI, Chesney RW, Zelikovic I: Peritonitis in childhood renal disease. Am J Nephrol 8: 147–165, 1988. [DOI] [PubMed] [Google Scholar]
- 42.Fenton A, Smith SW, Hewins P: Adult minimal-change disease: Observational data from a UK centre on patient characteristics, therapies, and outcomes. BMC Nephrol 19: 207, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinzawa M, Yamamoto R, Nagasawa Y, Oseto S, Mori D, Tomida K, Hayashi T, Izumi M, Fukunaga M, Yamauchi A, Tsubakihara Y, Rakugi H, Isaka Y: Age and prediction of remission and relapse of proteinuria and corticosteroid-related adverse events in adult-onset minimal-change disease: A retrospective cohort study. Clin Exp Nephrol 17: 839–847, 2013. [DOI] [PubMed] [Google Scholar]
- 44.George M, Baker J, Winthrop K, Wu Q, Chen L, Xie F, Yun F, Curtis J: Risk of serious infection with long-term use of low-dose glucocorticoids in patients with rheumatoid arthritis. Presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting, Atlanta, GA, November 8–13, 2019
- 45.Medjeral-Thomas NR, Lawrence C, Condon M, Sood B, Warwicker P, Brown H, Pattison J, Bhandari S, Barratt J, Turner N, Cook HT, Levy JB, Lightstone L, Pusey C, Galliford J, Cairns TD, Griffith M: Randomized, controlled trial of tacrolimus and prednisolone monotherapy for adults with de novo minimal change disease: A multicenter, randomized, controlled trial [published correction appears in Clin J Am Soc Nephrol 15: 1027, 2020 10.2215/CJN.06290420]. Clin J Am Soc Nephrol 15: 209–218, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.