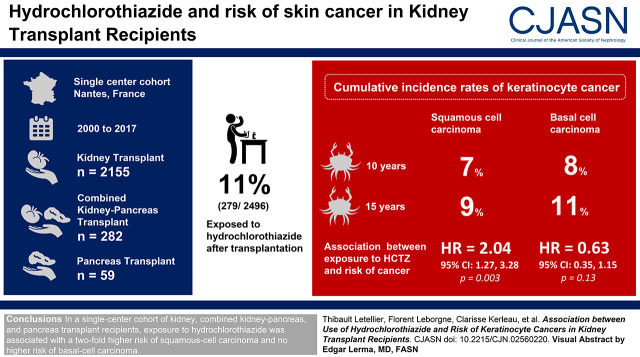
Visual Abstract
Keywords: cancer, diuretics, transplantation, kidney transplantation, skin cancer, squamous cell carcinoma, thiazide diuretics, Hydrochlorothiazide
Abstract
Background and objectives
Keratinocyte cancers, which primarily comprise squamous cell carcinomas and basal cell carcinomas, represent a major concern and potential risk for kidney transplant recipients. Hydrochlorothiazide, a diuretic widely used to treat hypertension, has been implicated in skin photosensitivity reaction. Recent studies conducted in the general population have found that hydrochlorothiazide use is associated with a higher risk of keratinocyte cancer, especially squamous cell carcinomas. High-risk groups, however, including transplant recipients were excluded from these. Our aim was to investigate whether hydrochlorothiazide use was associated with keratinocyte cancer in kidney transplant recipients on immunosuppressive therapy.
Design, setting, participants, & measurements
In a single-center cohort of kidney (n=2155), combined kidney-pancreas (n=282), and pancreas (n=59) transplant recipients from the Données Informatisées VAlidées Transplantation (DIVAT) database transplanted between 2000 and 2017 in Nantes, France, we evaluated the association between hydrochlorothiazide exposure and keratinocyte cancers. Multivariable cause-specific, time-varying Cox models were used to estimate the relationship between hydrochlorothiazide exposure and the hazard of squamous cell carcinoma and basal cell carcinoma, with hydrochlorothiazide designated as the time-dependent variable.
Results
Among the participants, 279 of 2496 (11%) were exposed to hydrochlorothiazide after the transplantation. Cumulative incidence rates of keratinocyte cancer by 10 and 15 years were 7% and 9% for squamous cell carcinomas, respectively, and 8% and 11% for basal cell carcinomas, respectively. We found a relationship between exposure to hydrochlorothiazide and the risk of squamous cell carcinomas (hazard ratio, 2.04; 95% confidence interval, 1.27 to 3.28). In contrast, we found no association between hydrochlorothiazide exposure and basal cell carcinomas (hazard ratio, 0.63; 95% confidence interval, 0.35 to 1.15).
Conclusions
In a single-center cohort of kidney, combined kidney-pancreas, and pancreas transplant recipients, exposure to hydrochlorothiazide was associated with a two-fold higher risk of squamous cell carcinoma and no higher risk of basal cell carcinoma.
Introduction
In recent decades, kidney transplant outcomes have markedly progressed. However, the use of immunosuppressive therapy, essential for preventing graft rejection, is associated with a significant adverse event profile for patients. Aside from infection, cancer is the main adverse event associated with the use of immunosuppressive agents in solid organ transplant recipients (1,2). Keratinocyte cancers, namely squamous cell carcinomas and basal cell carcinomas, account for >90% of skin cancers in transplant recipients. These typically affect more than half of patients over time, occurring 65–250 and 10–16 times, respectively, more frequently than in the general population (3,4). In addition, squamous cell carcinomas typically follow a more aggressive course in patients with transplants, resulting in significant morbidity and mortality. Aside from immunosuppressive therapy, risk factors for keratinocyte cancer reported in this population include old age, men, and fair skin type (3,4). Although cumulative sun exposure is a well-established risk factor for squamous cell carcinomas, the pattern of sun exposure associated with basal cell carcinomas is less clear (5). For some time, photosensitizing drugs have been suspected to favor the development of squamous cell carcinomas by potentiating the carcinogenic effect of ultraviolet (UV) radiation. A recent study conducted in the general population has supported an association between use of hydrochlorothiazide (HCTZ), one of those well-known photosensitizing drugs, and risk of keratinocyte cancer, especially squamous cell carcinomas (6). As the study’s focus was on the general population, high-risk groups, including transplant recipients and immunosuppressed patients, were excluded (6).
We aimed to investigate whether HCTZ use is associated with keratinocyte cancer in transplant recipients on immunosuppressive therapy independently of other known risk factors. We conducted a single-center study using a cohort of kidney, combined kidney-pancreas, and pancreas transplant recipients.
Materials and Methods
Patient Population
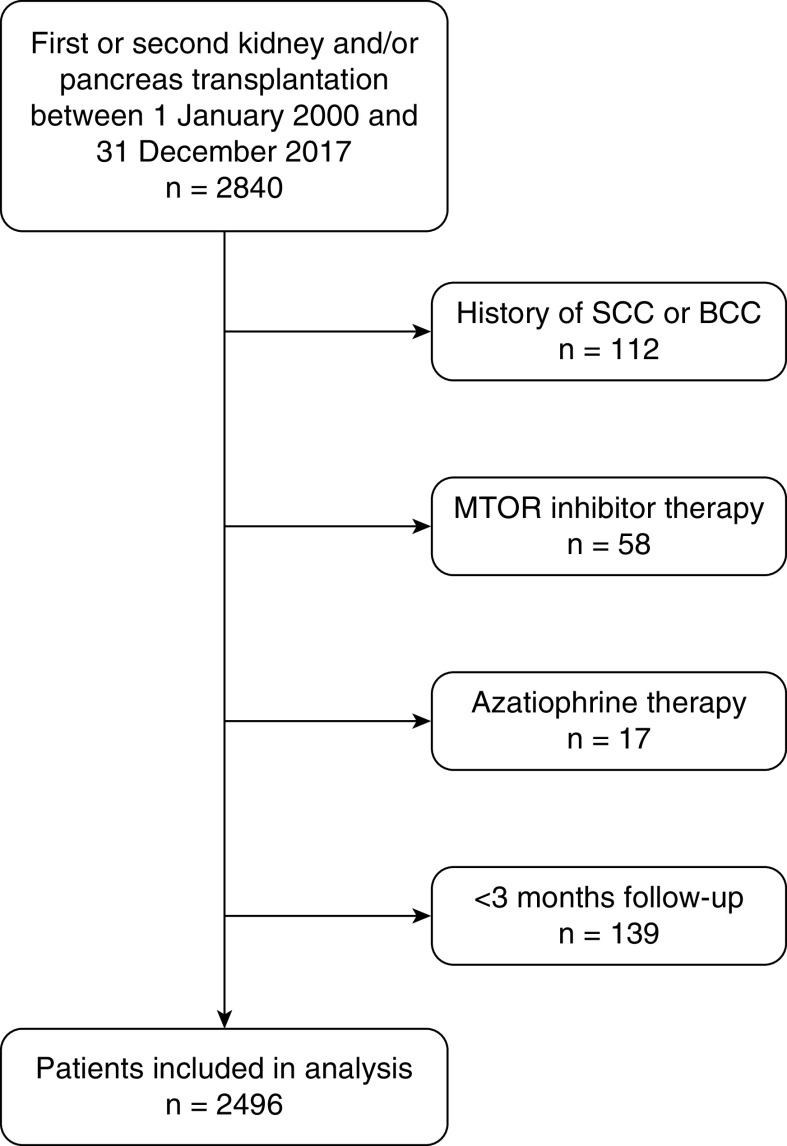
We conducted a single-center study on all consecutive adult patients transplanted with a graft that functioned for at least 3 months (kidney, pancreas, or combined kidney-pancreas) at Nantes University Hospital between January 1, 2000 and December 31, 2017. We excluded patients who had been recipients of three or more transplantations, as well as those with a prior history of basal cell carcinoma or squamous cell carcinoma. We also excluded those on initial maintenance immunosuppressive regimens that included mammalian target of rapamycin (mTOR) inhibitors or azathioprine, as these treatments have been implicated as protective and contributing factors to keratinocyte cancers, respectively (Figure 1). Patients with transplants otherwise received standard medical care (Supplemental Material).
Figure 1.
Flow chart of eligibility criterion. BCC, basal cell carcinoma; mTOR, mammalian target of rapamycin; SCC, squamous cell carcinoma.
All data were extracted from the French multicenter, observational, and prospective Données Informatisées VAlidées Transplantation (DIVAT) cohort of patients with transplants (www.divat.fr). The clinical and research activities reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.” More specifically, the DIVAT cohort received Commission Nationale de l’Informatique et des Libertés Final Agreement 914184, and the Advisory Committee on Information Processing in Material Research in the Field of Health approved the use of its data for scientific research purposes.
Available Data
Complete available data are found in Supplemental Material. All patients underwent a dermatologic examination undertaken by a specialist prior to the transplant and, in accordance with guidelines regarding outpatient surveillance of kidney transplant recipients, every year after the transplant. Histologically confirmed keratinocyte cancers were entered into the database.
Regarding HCTZ exposure, we extracted the list of patients prescribed HCTZ in the post-transplant period using prescription data collected longitudinally and contemporaneously in our electronic system. We reviewed each prescription for accuracy in terms of start and stop dates (considered meaningful from 1 month of exposure) and dose. The follow-up and the collection of data ceased upon a patient’s return to dialysis or at death.
Statistical Analyses
We considered the transplant as the statistical individual. Outcomes were defined by time from transplantation to the first occurrence of a squamous cell carcinoma or basal cell carcinoma. Participants who did not have an outcome were censored at graft failure (preemptive retransplantation, return to dialysis, or death), when they were lost to follow-up, or at the end of the study, whichever came first. The cumulative incidence curves of HCTZ, squamous cell carcinoma, and basal cell carcinoma were obtained using the Aalen–Johansen estimator, using graft failures as competing events (7). The median follow-up time was estimated using the reverse Kaplan–Meier (8). Multivariable cause-specific, time-varying Cox models were used to estimate the relationship between HCTZ and the hazard of squamous cell carcinoma and basal cell carcinoma, with HCTZ designated as time-dependent variable, as is usually recommended to prevent “immortal time” bias in the analysis of treatment effect in observational studies (9,10). Graft failures were right censored (11). In our main analysis, we studied the dichotomized variable of cumulative duration since the start of HCTZ. Indeed, construing HCTZ exposure as a time-dependent variable allowed patients, initially all in the untreated group, to move and remain in the treatment group from when HCTZ was commenced. The hazard proportionality assumption was verified from the Schoenfeld residuals (12). For baseline continuous covariates, the log-linearity assumption has been checked in unadjusted analysis if the Bayesian Information Criterion was not reduced using natural spline transformation compared with the inclusion of the covariate in its natural scale. Time-dependent covariates related to immunosuppression were on the basis of 1-year time windows. Patients were categorized as exposed for post-transplantation periods, during which they received the treatment, and unexposed during other periods (13,14). On the basis of theoretical considerations and known biologic effects, we included a priori the following variables in the multivariable models: (1) baseline parameters: age, sex, retransplantation, type of transplantation, type of donor, HLA-A -B -DR mismatch ≥4, induction therapy, maintenance treatment at transplantation, and use of steroids at transplantation; and (2) time-varying covariates: rejection, maintenance treatment during follow-up (calcineurin inhibitor, mycophenolate derivatives, or mTOR inhibitor), and other malignancies. We also considered covariates that were significantly associated with the outcomes (P=0.10) in unadjusted models. We did not consider interaction term. Patients who had data missing for covariates retained in the multivariable models were excluded.
A series of sensitivity analyses was performed. First, we estimated the effect of HCTZ use on different subgroups of patients: (1) solitary kidney transplant recipients, (2) patients receiving depleting induction therapy, (3) patients receiving nondepleting induction therapy, (4) patient receiving cyclosporin at transplantation, and (5) patients receiving tacrolimus at transplantation. Second, in order to explore a potential dose-response relationship, considering that HCTZ doses remained relatively stable regardless of when it was initiated post-transplantation, we initially studied the cumulative duration of HCTZ. However, as the log-linear association between the cumulative duration of use and outcomes could not be demonstrated (likely due to the low number of patients exposed to HCTZ combined with a relatively rare occurrence of events), such analysis was impracticable. We then categorized exposure to HCTZ according to three categories. In the corresponding multivariable Cox models, the time-dependent variables associated with HCTZ use were “no use,” “mild-term use” when the cumulative HCTZ use was <2 years, and “long-term use” when it was ≥2 years.
We used R version 3.6.1 and the packages “base,” “dplyr,” “survival,” “etm,” “plotrix,” “splines,” “lattice,” “prodlim,” “forestplot,” and “ReporteRs” for all data analyses.
Results
Recipient Demographic Characteristics
We included 2496 recipients of kidney (n=2155), combined kidney-pancreas (n=282), and pancreas (n=59) transplants. Patient characteristics subdivided by HCTZ status are displayed in Table 1.
Table 1.
Characteristics of 2496 transplant recipients included in the analysis according their exposure to hydrochlorothiazide during follow-up
| Characteristics | Whole Sample, n=2496 | No Hydrochlorothiazide, n=2217 | Hydrochlorothiazide, n=279 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Not Available (Missing) | Mean | SD | Not Available (Missing) | Mean | SD | Not Available (Missing) | Mean | SD | |
| Recipient age, yr | 0 | 49 | 14 | 0 | 49 | 14 | 0 | 50 | 13 |
| Not Available (Missing) | n | % | Not Available (Missing) | n | % | Not Available (Missing) | n | % | |
|---|---|---|---|---|---|---|---|---|---|
| Men | 0 | 1538 | 62 | 0 | 1353 | 61 | 0 | 185 | 66 |
| Initial nephropathy | 0 | 0 | 0 | ||||||
| Chronic tubulointerstitial nephritis/ADPKD/congenital uropathy | 962 | 39 | 880 | 40 | 82 | 29 | |||
| Diabetes | 467 | 19 | 413 | 19 | 54 | 19 | |||
| Glomerulopathy | 643 | 26 | 567 | 26 | 76 | 27 | |||
| Other | 232 | 9 | 206 | 9 | 26 | 9 | |||
| Vascular nephropathy | 192 | 8 | 151 | 7 | 41 | 15 | |||
| KRT | 46 | 41 | 5 | ||||||
| Hemodialysis | 1757 | 72 | 1544 | 71 | 213 | 78 | |||
| Peritoneal dialysis | 217 | 9 | 200 | 9 | 17 | 6 | |||
| Preemptive transplantation | 476 | 19 | 432 | 20 | 44 | 16 | |||
| Type of transplantation | 0 | 0 | 0 | ||||||
| Combined kidney-pancreas | 282 | 11 | 254 | 11 | 28 | 10 | |||
| Kidney | 2155 | 86 | 1909 | 86 | 246 | 88 | |||
| Pancreas | 59 | 2 | 54 | 2 | 5 | 2 | |||
| Retransplantation | 0 | 436 | 17 | 0 | 399 | 18 | 0 | 37 | 13 |
| Deceased donor | 0 | 2200 | 88 | 0 | 1948 | 88 | 0 | 252 | 90 |
| HLA-A -B -Dr mismatches ≥4 | 2 | 1264 | 51 | 2 | 1113 | 50 | 0 | 151 | 54 |
| Cold ischemia time ≥18 h | 21 | 1018 | 41 | 20 | 882 | 40 | 1 | 136 | 49 |
| Delayed graft function | 78 | 811 | 34 | 73 | 705 | 33 | 5 | 106 | 39 |
| Rejection | 0 | 301 | 12 | 0 | 264 | 12 | 0 | 37 | 13 |
| Other malignancies | 0 | 193 | 8 | 0 | 163 | 7 | 0 | 30 | 11 |
| Induction treatment | 0 | 0 | 0 | ||||||
| Depleting | 1219 | 49 | 1098 | 50 | 121 | 43 | |||
| Nondepleting | 1213 | 49 | 1066 | 48 | 147 | 53 | |||
| None | 64 | 3 | 53 | 2 | 11 | 4 | |||
| Calcineurin inhibitors | 0 | 0 | 0 | ||||||
| Cyclosporin | 361 | 14 | 303 | 14 | 58 | 21 | |||
| Tacrolimus | 2108 | 84 | 1892 | 85 | 216 | 77 | |||
| None | 27 | 1 | 22 | 1 | 5 | 2 | |||
| Steroid-free regimen | 0 | 482 | 19 | 0 | 416 | 19 | 0 | 66 | 24 |
| mTOR inhibitors during follow-up | 0 | 434 | 17 | 0 | 364 | 16 | 0 | 70 | 25 |
No hydrochlorothiazide (HCTZ) corresponds to patients never exposed to HCTZ during their follow-up. The HCTZ group corresponds to the other patients. ADPKD, autosomal dominant polycystic kidney disease; HLA, human leucocyte antigen; mTOR, mammalian target of rapamycin.
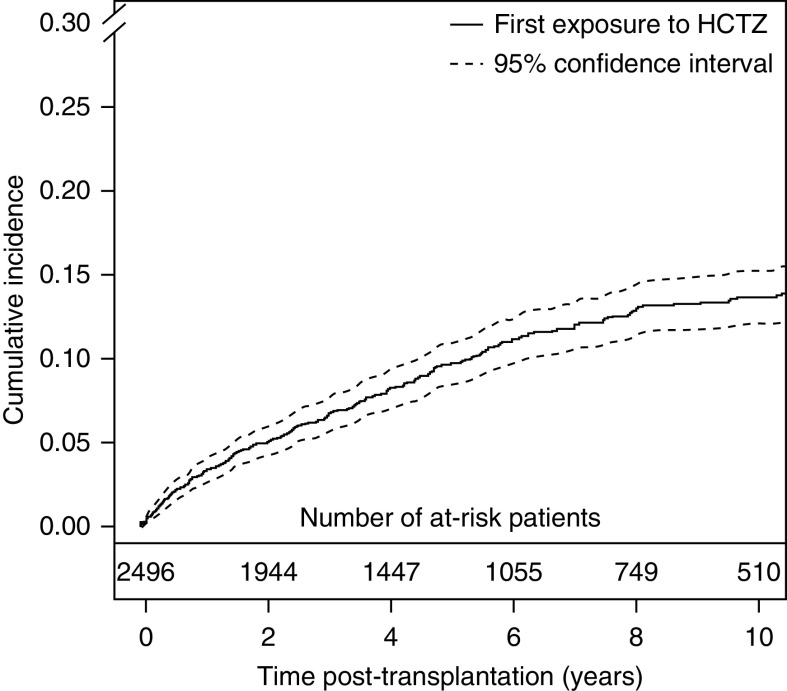
Prior to transplantation, no patient was receiving HCTZ, which was consequently started after transplantation in all 279 (11%) participants exposed at least 1 month to HCTZ during the transplantation period. Probabilities of exposure to HCTZ at 5, 10, and 15 years post-transplantation were 10% (95% confidence interval [95% CI], 8% to 11%), 14% (95% CI, 12% to 15%), and 16% (95% CI, 14% to 18%), respectively (Figure 2). Mean duration of exposure and cumulative dose of HCTZ were 2.5 years (95% CI, 2.1 to 2.8 years) and 14,436 mg (95% CI, 12,238 to 16,634 mg), respectively. Median follow-up time was 5.9 years (range from 0.2 to 18.8 years). During the follow-up, 343 deaths with a functioning graft, 439 returns to dialysis, 154 basal cell carcinomas, and 132 squamous cell carcinomas occurred.
Figure 2.
Cumulative incidence curve of hydrochlorothiazide (HCTZ). Aalen–Johansen estimator, retransplantations, returns to dialysis, and deaths are competing events.
Basal Cell Carcinoma
The cumulative incidence curve for basal cell carcinoma is presented in Figure 3A. Cumulative incidence rates at 5, 10, and 15 years post-transplantation for basal cell carcinoma were 4% (95% CI, 3% to 5%), 8% (95% CI, 6% to 9%), and 11% (95% CI, 9% to 13%), respectively. Table 2 presents unadjusted analysis and the final multivariable model from which, among 2496 patients, 23 were excluded due to missing data. Multivariable Cox models were adjusted for baseline parameters (age, sex, retransplantation, type of transplantation, type of donor, HLA-A -B -DR mismatch ≥4, induction therapy, maintenance treatment at transplantation, use of steroids at transplantation, initial nephropathy, and cold ischemia time) and for time-varying covariates (rejection, maintenance treatment during follow-up [calcineurin inhibitor, mycophenolate derivatives, or mTOR inhibitor], and other malignancies). The confounder-adjusted hazard ratio (HR) associated with the HCTZ exposure was 0.63 (95% CI, 0.35 to 1.15). In sensitivity analysis of the subgroup of patients, again, no association between HCTZ use and basal cell carcinoma was found (Table 3). The adjusted HRs associated with mild-term (<2 years) and long-term use (≥2 years) of HCTZ were 0.59 (95% CI, 0.27 to 1.32) and 0.70 (95% CI, 0.30 to 1.62), respectively.
Figure 3.
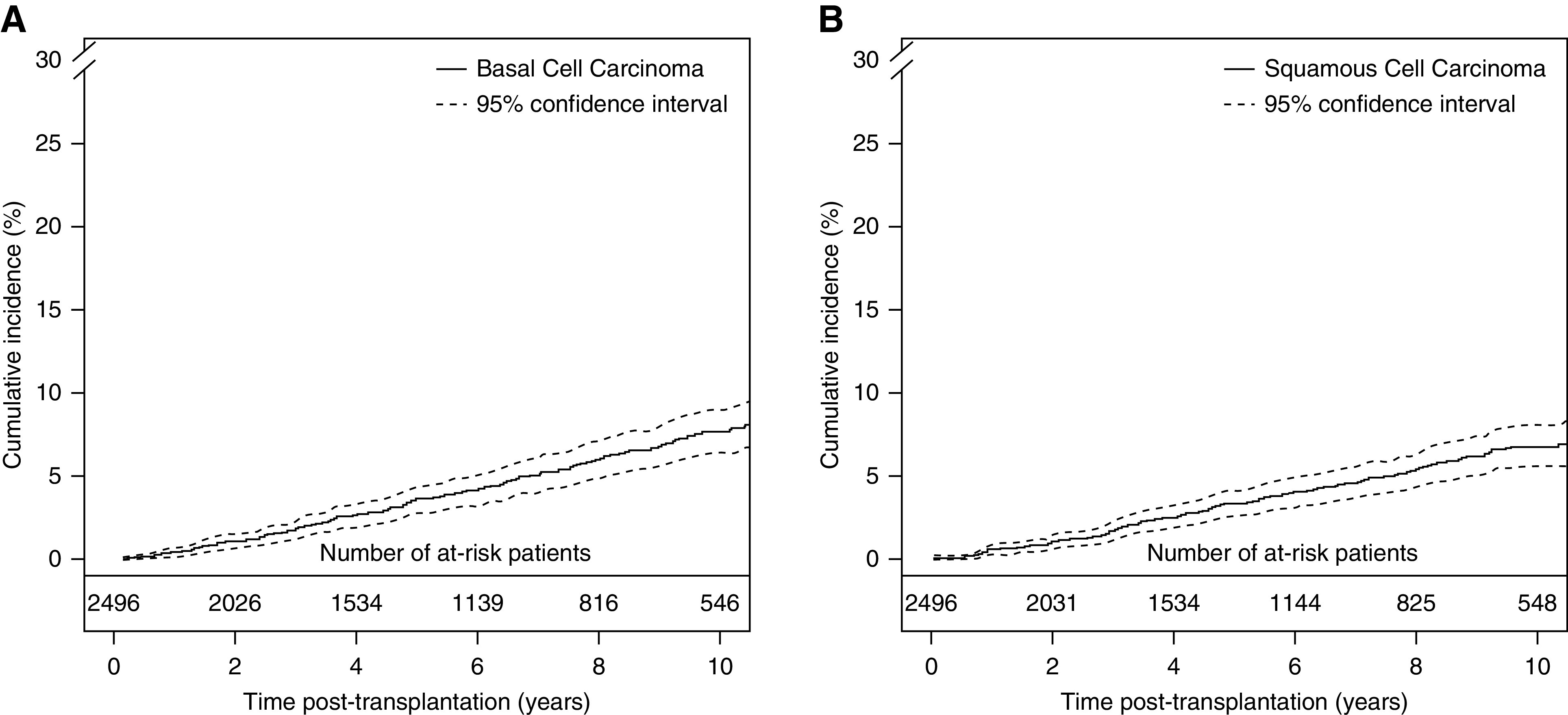
Cumulative incidence curves. (A) Basal cell carcinoma and (B) squamous cell carcinoma. Aalen–Johansen estimator, retransplantations, returns to dialysis, and deaths are competing events.
Table 2.
Results of the unadjusted and multivariable cause-specific, time-dependent Cox models studying the risk of basal cell carcinoma (n=2473)
| Exposure | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | |
| HTCZ exposure | 0.79 | [0.45 to 1.40] | 0.63 | [0.35 to 1.15] |
| Recipient age | 1.05 | [1.04 to 1.07] | 1.05 | [1.04 to 1.07] |
| Men | 1.39 | [0.99 to 1.95] | 1.52 | [1.04 to 2.23] |
| Initial nephropathy | ||||
| Chronic tubulointerstitial nephritis/ADPKD/congenital uropathy (versus glomerulopathy) | 1.22 | [0.83 to 1.77] | 1.25 | [0.84 to 1.86] |
| Diabetes (versus glomerulopathy) | 0.53 | [0.29 to 0.96] | 0.72 | [0.31 to 1.63] |
| Other (versus glomerulopathy) | 0.81 | [0.42 to 1.57] | 0.65 | [0.33 to 1.28] |
| Vascular nephropathy (versus glomerulopathy) | 1.94 | [1.09 to 3.46] | 1.07 | [0.57 to 2.01] |
| KRT | — | — | ||
| Hemodialysis (versus preemptive transplantation) | 0.96 | [0.63 to 1.47] | — | — |
| Peritoneal dialysis (versus preemptive transplantation) | 1.51 | [0.80 to 2.86] | — | — |
| Kidney transplantation | 2.93 | [1.44 to 5.94] | 1.80 | [0.60 to 5.40] |
| Retransplantation | 1.09 | [0.73 to 1.64] | 1.04 | [0.59 to 1.81] |
| Deceased donor | 1.03 | [0.62 to 1.71] | 0.97 | [0.54 to 1.72] |
| HLA-A -B -Dr mismatches ≥4 | 0.86 | [0.63 to 1.19] | 0.87 | [0.62 to 1.24] |
| Cold ischemia time ≥18 h | 0.74 | [0.54 to 1.02] | 0.56 | [0.39 to 0.80] |
| Delayed graft function | 0.96 | [0.68 to 1.36] | — | — |
| Rejection | 0.71 | [0.36 to 1.40] | 0.89 | [0.45 to 1.73] |
| Other malignancies | 0.89 | [0.39 to 2.04] | 0.38 | [0.16 to 0.94] |
| Induction treatment | ||||
| Nondepleting (versus depleting) | 1.11 | [0.80 to 1.53] | 0.79 | [0.51 to 1.23] |
| None (versus depleting) | 1.09 | [0.45 to 2.60] | 0.99 | [0.41 to 2.43] |
| Calcineurin inhibitors | ||||
| Cyclosporin (versus tacrolimus) | 1.13 | [0.79 to 1.61] | 1.31 | [0.88 to 1.93] |
| None (versus tacrolimus) | 0.67 | [0.16 to 2.76] | 0.68 | [0.14 to 3.26] |
| Steroid-free regimen | 1.03 | [0.71 to 1.49] | 0.98 | [0.66 to 1.46] |
| Calcineurin inhibitors during follow-up | 0.46 | [0.32 to 0.67] | 0.49 | [0.28 to 0.85] |
| mTOR inhibitors during follow-up | 1.87 | [1.19 to 2.92] | 1.01 | [0.54 to 1.88] |
| Mycophenolate derivatives during follow-up | 0.68 | [0.46 to 0.99] | 0.88 | [0.58 to 1.34] |
HCTZ, hydrochlorothiazide; ADPKD, autosomal dominant polycystic kidney disease; —, not available; HLA, human leucocyte antigen; mTOR, mammalian target of rapamycin.
Table 3.
Results of the sensitivity analyses
| Sub-group | Basal Cell Carcinoma | Squamous Cell Carcinoma | ||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | |
| Solitary kidney transplantation | 0.67 | [0.37 to 1.23] | 2.10 | [1.29 to 3.42] |
| Depleting induction | 0.43 | [0.17 to 1.13] | 1.98 | [0.91 to 4.33] |
| Nondepleting induction | 0.78 | [0.34 to 1.77] | 1.94 | [0.95 to 3.98] |
| Cyclosporin at transplantation | 0.25 | [0.04 to 1.63] | 2.26 | [0.49 to 10.48] |
| Tacrolimus at transplantation | 0.87 | [0.46 to 1.64] | 2.18 | [1.30 to 3.64] |
Squamous Cell Carcinoma
The cumulative incidence curve of squamous cell carcinoma is presented in Figure 3B. Cumulative incidence rates at 5, 10, and 15 years post-transplantation for squamous cell carcinoma were 3% (95% CI, 3% to 4%), 7% (95% CI, 6% to 8%), and 9% (95% CI, 8% to 11%), respectively. Table 4 presents unadjusted analysis and the final multivariable model from which, among 2496 patients, 80 were excluded due to missing data. Multivariable Cox models were adjusted for baseline parameters (age, sex, retransplantation, type of transplantation, type of donor, HLA-A -B -DR mismatch ≥4, induction therapy, maintenance treatment at transplantation, use of steroids at transplantation, initial nephropathy, cold ischemia time, and delayed graft function) and for time-varying covariates (rejection, maintenance treatment during follow-up [calcineurin inhibitor, mycophenolate derivatives, or mTOR inhibitor], and other malignancies). The confounder-adjusted HR was 2.04 (95% CI, 1.27 to 3.28), indicating a two-fold higher risk of squamous cell carcinoma from time of exposure. We did not identify a significant dependence of the excess hazard with the time post-transplantation. As displayed in Table 3, sensitivity analysis maintained this association even when analysis was restricted to solitary kidney transplant recipients (HR, 2.10; 95% CI, 1.29 to 3.42). When we considered patients according to their baseline immunosuppression, the confounder-adjusted HRs were 1.94 (95% CI, 0.95 to 3.98) and 1.98 (95% CI, 0.91 to 4.33) for those on nondepleting induction agents and depleting induction agents, respectively, and the confounder-adjusted HRs were 2.26 (95% CI, 0.49 to 10.48) and 2.18 (95% CI, 1.30 to 3.64) for those on cyclosporin and tacrolimus, respectively, as calcineurin inhibitor drugs. To further explore a potential dose-response relationship, we performed a sensitivity analysis considering the exposure to HCTZ according to three categories. The adjusted HRs associated with a mild-term use (<2 years) and a long-term use (≥2 years) when compared with no use were 1.99 (95% CI, 1.08 to 3.68) and 2.12 (95% CI, 1.14 to 3.94), respectively.
Table 4.
Results of the unadjusted and multivariable cause-specific, time-dependent Cox models studying the risk of squamous cell carcinoma (n=2416)
| Exposure | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | |
| HTCZ exposure | 2.36 | [1.53 to 3.65] | 2.04 | [1.27 to 3.28] |
| Recipient age | 1.07 | [1.05 to 1.08] | 1.06 | [1.04 to 1.07] |
| Men | 2.10 | [1.40 to 3.13] | 2.22 | [1.45 to 3.39] |
| Initial nephropathy | ||||
| Chronic tubulointerstitial nephritis/ADPKD/congenital uropathy (versus glomerulopathy) | 0.75 | [0.49 to 1.16] | 0.86 | [0.56 to 1.33] |
| Diabetes (versus glomerulopathy) | 0.53 | [0.29 to 0.97] | 0.61 | [0.26 to 1.40] |
| Other (versus glomerulopathy) | 1.43 | [0.83 to 2.46] | 1.01 | [0.53 to 1.90] |
| Vascular nephropathy (versus glomerulopathy) | 1.62 | [0.88 to 2.99] | 0.73 | [0.38 to 1.42] |
| KRT | — | — | ||
| Hemodialysis (versus preemptive transplantation) | 1.54 | [0.91 to 2.61] | — | — |
| Peritoneal dialysis (versus preemptive transplantation) | 1.42 | [0.63 to 3.23] | — | — |
| Kidney transplantation | 2.49 | [1.22 to 5.06] | 0.68 | [0.19 to 2.36] |
| Retransplantation | 1.99 | [1.36 to 2.90] | 2.41 | [1.16 to 4.99] |
| Deceased donor | 2.79 | [1.23 to 6.34] | 1.65 | [0.67 to 4.05] |
| HLA-A -B -Dr mismatches ≥4 | 0.90 | [0.64 to 1.26] | 0.96 | [0.65 to 1.40] |
| Cold ischemia time ≥18 h | 1.60 | [1.13 to 2.29] | 1.32 | [0.88 to 1.98] |
| Delayed graft function | 1.49 | [1.05 to 2.11] | 0.96 | [0.66 to 1.40] |
| Rejection | 0.17 | [0.04 to 0.71] | 0.24 | [0.06 to 1.01] |
| Other malignancies | 0.56 | [0.17 to 1.81] | 0.19 | [0.05 to 0.66] |
| Induction treatment | ||||
| Nondepleting (versus depleting) | 1.04 | [0.74 to 1.48] | 1.19 | [0.61 to 2.35] |
| None (versus depleting) | 0.77 | [0.28 to 2.12] | 0.97 | [0.30 to 3.18] |
| Calcineurin inhibitors | ||||
| Cyclosporin (versus tacrolimus) | 0.48 | [0.29 to 0.80] | 0.67 | [0.39 to 1.16] |
| None (versus tacrolimus) | 0.32 | [0.04 to 2.42] | 0.25 | [0.03 to 1.90] |
| Steroid-free regimen | 0.96 | [0.64 to 1.43] | 1.05 | [0.66 to 1.68] |
| Calcineurin inhibitors during follow-up | 0.39 | [0.26 to 0.59] | 0.29 | [0.16 to 0.52] |
| mTOR inhibitors during follow-up | 0.93 | [0.49 to 1.76] | 0.30 | [0.13 to 0.68] |
| Mycophenolate derivatives during follow-up | 0.47 | [0.32 to 0.70] | 0.64 | [0.41 to 1.01] |
HCTZ, hydrochlorothiazide; ADPKD, autosomal dominant polycystic kidney disease; —, not available; HLA, human leucocyte antigen; mTOR, mammalian target of rapamycin.
Discussion
In our cohort of transplant recipients, we found that, following exposure to HCTZ post-transplantation, patients had a significantly higher risk of developing squamous cell carcinoma (HR, 2.04; 95% CI, 1.27 to 3.28), regardless of the period of that exposure. By contrast, we have not found an association between HCTZ exposure and the development of basal cell carcinoma.
The association between HCTZ exposure and the incidence of squamous cell carcinoma was also found when the analysis was restricted to solitary kidney transplant recipients.
Our findings were made possible due to the availability and use of prospective data on the exposure (i.e., HTCZ) and outcome variable (i.e., keratinocyte cancer) over an extended length of time, combined with a multivariable model that incorporated both time-dependent exposure to HCTZ and confounders (at baseline and some time dependent [13]) that were not eliminated in the exclusion process (i.e., patients on an mTOR inhibitor or azathioprine at baseline).
More than 60 years ago, HCTZ became the first available oral diuretic, and owing to its favorable safety profile, it demonstrated a clear benefit in the treatment of hypertension (15). However, as soon as it was introduced, severe skin photosensitivity reactions were reported (16,17). It is well established that a number of medications defined as photosensitizing potentiate the erythema reaction to UV light and, through a photocarcinogenic effect, bring about a higher risk of squamous cell carcinoma (18). For instance, azathioprine is well known to exacerbate skin photosensitivity and is associated with a higher incidence of squamous cell carcinoma (19,20).
In 2013, on the basis of limited human evidence (21–23), the International Agency for Research on Cancer highlighted HCTZ as a possible carcinogen (2B). Since then, nationwide patient-control studies in Denmark have unearthed a strong association between HCTZ use and squamous cell carcinoma (6,24). These results were replicated in the United Kingdom (25), leading to multiple national health agencies publishing safety alerts regarding its use in 2018 (26,27). More recently, no association was demonstrated between HCTZ and skin cancer in an Asian population, highlighting the likely importance of baseline photosensitivity to UV as a contributing factor (28). A strength of these studies was their ability to demonstrate a dose-response relationship between HCTZ exposure and risk of keratinocyte cancer. In keeping with a lesser role for cumulative UV exposure (5), only very long periods of HCTZ exposure have been associated with the development of basal cell carcinoma.
To investigate a potential dose-response relationship in our population, we considered the exposure to HCTZ according to three categories. Results for HRs for both mild-term use (<2 years) and long-term use (≥2 years) were comparable. In other words, we observed an association between HCTZ exposure and squamous cell carcinoma as soon as a mild-term exposure occurred and no dose-response relationship. In the general population, this association has only been demonstrated in those with prolonged exposure to HCTZ (>3 years) with a demonstrable dose-response relationship. The risk we have demonstrated in our study is comparable with that reported following 6 years of exposure in the original study. These discrepancies could be related to a potentially additive and interactive effect that HCTZ has on a group of patients intrinsically made vulnerable to squamous cell carcinoma through their exposure to immunosuppressive agents. Thus, the absence of a dose-response relationship in our cohort could be, on the one hand, superseded by the effect of immunosuppressants and on the other hand, due to an inadequate follow-up interval compared with the baseline study conducted in the general population (6). These are only suppositions, and larger studies in terms of both numbers and length of follow-up are required to investigate them.
Squamous cell carcinoma in solid organ transplant recipients occurs as a result of complex interactions between immune-mediated mechanisms of tumor promotion (mainly human papilloma virus activation and reduced tumor surveillance) combined with UV-induced carcinogenic effects (4). Thus, the emergence of possibly negative synergistic effects with the addition of photosensitizing drugs such as HCTZ, which are suspected to enhance UV-induced DNA damage, is unsurprising (29). Similarly, several studies conducted in lung transplant recipients report that voriconazole exposure, used in the prophylaxis and treatment of invasive fungal infection and also implicated as a photosensitizing drug (30), is an independent risk factor for the development of squamous cell carcinoma (31–33).
Importantly, we were able to highlight the negative effects of using HCTZ in a population mainly treated with immunosuppressants belonging to the modern era, namely tacrolimus and mycophenolate mofetil. Indeed, as stated above, direct UV-induced carcinogenic effects are well documented with azathioprine, the maintenance therapy historically used in kidney transplantation, but such effects have not been established for cyclosporin, tacrolimus, and mycophenolate mofetil (20,34). With improvements in surveillance, this likely explains why the risk of squamous cell carcinoma in solid organ transplant recipients has drastically decreased in recent decades from 65 to 250 times (3) compared with the general population to 20 times (35,36). In sensitivity analysis, we observed an HR slightly higher with the use of depleting versus nondepleting induction agents, as has been previously reported (37).
Our observational study suffers from several limitations. First, it uses data that were not purposefully collected to answer the specific research question. Using data collected from a single center, the number of events detected was also limited. Furthermore, we lacked information regarding two important risk factors for keratinocyte cancer: UV exposure and skin phenotype (of note, most of the western France population is of fair skin type). However, there is no reason to assume there is a relationship between these risk factors and the use of HTCZ, which may not be considered as confounders. Second, HTCZ use prior to first transplantation would have been an important factor to consider, but this information was not collected in our database. Third, it would have been interesting to analyze each patient from the point of their first transplantation and then during subsequent dialysis and retransplantation periods. However, because follow-up data were not available for patients returning to dialysis, we considered the graft as the statistical individual by censoring the return to dialysis without keratinocyte cancers. Among consequences for retransplantations, prior exposure to HCTZ and other immunosuppressive drugs was not available. To tentatively consider the treatment exposures during previous transplantation or dialysis periods, we included in multivariable models the transplantation rank as a proxy. Finally, other unobserved confounding factors, such as other time-dependent drug exposures, cannot be excluded, making it difficult to establish firm causality.
Our results must be cautiously balanced with the risk related to poorly managed hypertension. Indeed, the overall prevalence of hypertension in kidney transplant recipients is up to 85% and is associated with shortened allograft survival and higher cardiovascular morbidity and mortality (38). Thiazide diuretics, including HCTZ, are recommended as a first-line treatment option for hypertension in the general population (39), and they seem efficient and safe for kidney transplant recipients (40,41). In addition to usual mechanisms, hypertension in kidney transplant recipients results from the use of immunosuppressive medications. For instance, calcineurin inhibitor–induced hypertension is related to kidney vasoconstriction, and it is efficiently counteracted by the use of calcium-channel blockers (42). Moreover, because sodium retention occurs due to activation of the thiazide-sensitive sodium chloride cotransporter by tacrolimus (43), HCTZ could be especially effective for this group of patients. Of note, in the Danish study, other antihypertensive drugs, including the usual alternative to HCTZ, bendroflumethiazide, another thiazide-type diuretic, and indapamide, a thiazide-like diuretic (chlorthalidone, was not studied), were not associated with higher risks of skin cancer and could therefore be considered as safe alternatives.
In summary, our study suggests an association between HCTZ exposure and the development of squamous cell carcinoma in kidney transplant recipients with fair skin type. Broader studies will be required to confirm these results. Physicians should carefully evaluate the risks and benefits of HCTZ use, especially in kidney transplant recipients with known nonmodifiable risk factors, such as fair skin, men, and old age.
Disclosures
J. Dantal reports receiving research funding from Novartis. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
J. Dantal, F. Leborgne, T. Letellier, and S. Ville conceived and/or designed the study; A. Gaultier, C. Kerleau, and T. Letellier acquired data; J. Dantal, A. Gaultier, F. Leborgne, T. Letellier, and S. Ville played an important role in interpreting the results; J. Dantal, F. Leborgne, T. Letellier, and S. Ville drafted or revised the manuscript; and J. Dantal, F. Leborgne, T. Letellier, S. Ville, and members of the Divat Consortium approved the final version.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: Divat Consortium, Gilles Blancho, Julien Branchereau, Diego Cantarovich, Agnès Chapelet, Clément Deltombe, Fadi Fakhouri, Lucile Figueres, Claire Garandeau, Magali Giral, Caroline Gourraud-Vercel, Maryvonne Hourmant, Lola Jacquemont, Sabine Le Bot, and Aurélie Meurette
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02560220/-/DCSupplemental.
Supplemental Material. Methods.
References
- 1.Au E, Wong G, Chapman JR: Cancer in kidney transplant recipients. Nat Rev Nephrol 14: 508–520, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Rosales BM, De La Mata N, Vajdic CM, Kelly PJ, Wyburn K, Webster AC: Cancer mortality in kidney transplant recipients: An Australian and New Zealand population-based cohort study, 1980-2013. Int J Cancer 146: 2703–2711, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Euvrard S, Kanitakis J, Claudy A: Skin cancers after organ transplantation. N Engl J Med 348: 1681–1691, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Mittal A, Colegio OR: Skin cancers in organ transplant recipients. Am J Transplant 17: 2509–2530, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Madan V, Lear JT, Szeimies R-M: Non-melanoma skin cancer. Lancet 375: 673–685, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Pedersen SA, Gaist D, Schmidt SAJ, Hölmich LR, Friis S, Pottegård A: Hydrochlorothiazide use and risk of nonmelanoma skin cancer: A nationwide case-control study from Denmark. J Am Acad Dermatol 78: 673–681.e9, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Aalen OO, Johansen S: An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Stat 5: 141–150, 1978 [Google Scholar]
- 8.Schemper M, Smith TL: A note on quantifying follow-up in studies of failure time. Control Clin Trials 17: 343–346, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Shariff SZ, Cuerden MS, Jain AK, Garg AX: The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol 19: 841–843, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Weinhandl ED, Gilbertson DT, Collins AJ, St Peter WL: Issues regarding ‘immortal time’ in the analysis of the treatment effects in observational studies. Kidney Int 81: 341–350, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Andersen PK, Geskus RB, de Witte T, Putter H: Competing risks in epidemiology: Possibilities and pitfalls. Int J Epidemiol 41: 861–870, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenfeld D: Partial residuals for the proportional hazards regression model. Biometrika 69: 239–241, 1982 [Google Scholar]
- 13.Xie D, Yang W, Jepson C, Roy J, Hsu JY, Shou H, Anderson AH, Landis JR, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Statistical methods for modeling time-updated exposures in cohort studies of chronic kidney disease. Clin J Am Soc Nephrol 12: 1892–1899, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ: Survival analysis: Time-dependent effects and time-varying risk factors. Kidney Int 74: 994–997, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Moser M, Feig PU: Fifty years of thiazide diuretic therapy for hypertension. Arch Intern Med 169: 1851–1856, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Harber LC, Lashinsky AM, Baer RL: Photosensitivity due to chlorothiazide and hydrochlorothiazide. N Engl J Med 261: 1378–1381, 1959 [DOI] [PubMed] [Google Scholar]
- 17.Harber LC, Lashinsky AM, Baer RL: Skin manifestations of photosensitivity due to chlorothiazide and hydrochlorothiazide. J Invest Dermatol 33: 83–84, 1959 [DOI] [PubMed] [Google Scholar]
- 18.Stern RS: Photocarcinogenicity of drugs. Toxicol Lett 102-103: 389–392, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Vos M, Plasmeijer EI, van Bemmel BC, van der Bij W, Klaver NS, Erasmus ME, de Bock GH, Verschuuren EAM, Rácz E: Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant 37: 853–859, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Hofbauer GFL, Attard NR, Harwood CA, McGregor JM, Dziunycz P, Iotzova-Weiss G, Straub G, Meyer R, Kamenisch Y, Berneburg M, French LE, Wüthrich RP, Karran P, Serra AL: Reversal of UVA skin photosensitivity and DNA damage in kidney transplant recipients by replacing azathioprine. Am J Transplant 12: 218–225, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Jensen AØ, Thomsen HF, Engebjerg MC, Olesen AB, Sørensen HT, Karagas MR: Use of photosensitising diuretics and risk of skin cancer: A population-based case-control study. Br J Cancer 99: 1522–1528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries E, Trakatelli M, Kalabalikis D, Ferrandiz L, Ruiz-de-Casas A, Moreno-Ramirez D, Sotiriadis D, Ioannides D, Aquilina S, Apap C, Micallef R, Scerri L, Ulrich M, Pitkänen S, Saksela O, Altsitsiadis E, Hinrichs B, Magnoni C, Fiorentini C, Majewski S, Ranki A, Stockfleth E, Proby C; EPIDERM Group: Known and potential new risk factors for skin cancer in European populations: A multicentre case-control study. Br J Dermatol 167[Suppl 2]: 1–13, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Robinson SN, Zens MS, Perry AE, Spencer SK, Duell EJ, Karagas MR: Photosensitizing agents and the risk of non-melanoma skin cancer: A population-based case-control study. J Invest Dermatol 133: 1950–1955, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pottegård A, Hallas J, Olesen M, Svendsen MT, Habel LA, Friedman GD, Friis S: Hydrochlorothiazide use is strongly associated with risk of lip cancer. J Intern Med 282: 322–331, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Morales DR, Pacurariu A, Slattery J, Kurz X: Association between hydrochlorothiazide exposure and different incident skin, lip and oral cavity cancers: A series of population-based nested case-control studies. Br J Clin Pharmacol 86: 1336–1345, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agence nationale de sécurité du médicament et des produits de santé: Hydrochlorothiazide - Risque de cancer de la peau non-mélanome (carcinome basocellulaire, carcinome épidermoïde) - Lettre aux professionnels de santé, 2018. Available at: https://www.ansm.sante.fr/S-informer/Informations-de-securite-Lettres-aux-professionnels-de-sante/Hydrochlorothiazide-Risque-de-cancer-de-la-peau-non-melanome-carcinome-basocellulaire-carcinome-epidermoide-Lettre-aux-professionnels-de-sante. Accessed May 20, 2020
- 27.United Kingdom Government: Hydrochlorothiazide: Risk of non-melanoma skin cancer, particularly in long-term use, 2018. Available at: https://www.gov.uk/drug-safety-update/hydrochlorothiazide-risk-of-non-melanoma-skin-cancer-particularly-in-long-term-use. Accessed May 20, 2020
- 28.Pottegård A, Pedersen SA, Schmidt SAJ, Lee C-N, Hsu C-K, Liao T-C, Shao S-C, Lai EC-C: Use of hydrochlorothiazide and risk of skin cancer: A nationwide Taiwanese case-control study. Br J Cancer 121: 973–978, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunisada M, Masaki T, Ono R, Morinaga H, Nakano E, Yogianti F, Okunishi K, Sugiyama H, Nishigori C: Hydrochlorothiazide enhances UVA-induced DNA damage. Photochem Photobiol 89: 649–654, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Haylett AK, Felton S, Denning DW, Rhodes LE: Voriconazole-induced photosensitivity: Photobiological assessment of a case series of 12 patients. Br J Dermatol 168: 179–185, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Feist A, Lee R, Osborne S, Lane J, Yung G: Increased incidence of cutaneous squamous cell carcinoma in lung transplant recipients taking long-term voriconazole. J Heart Lung Transplant 31: 1177–1181, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Singer JP, Boker A, Metchnikoff C, Binstock M, Boettger R, Golden JA, Glidden DV, Arron ST: High cumulative dose exposure to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J Heart Lung Transplant 31: 694–699, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vadnerkar A, Nguyen MH, Mitsani D, Crespo M, Pilewski J, Toyoda Y, Bermudez C, Kwak EJ, Silveira FP, Clancy CJ: Voriconazole exposure and geographic location are independent risk factors for squamous cell carcinoma of the skin among lung transplant recipients. J Heart Lung Transplant 29: 1240–1244, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Voskamp P, Bodmann CA, Koehl GE, Tensen CP, Bavinck JNB, Willemze R, Geissler EK, de Gruijl FR: No acceleration of UV-induced skin carcinogenesis from evenly spread dietary intake of cyclosporine in contrast to oral bolus dosages. Transplantation 96: 871–876, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Rizvi SMH, Aagnes B, Holdaas H, Gude E, Boberg KM, Bjørtuft Ø, Helsing P, Leivestad T, Møller B, Gjersvik P: Long-term change in the risk of skin cancer after organ transplantation: A population-based nationwide cohort study. JAMA Dermatol 153: 1270–1277, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menzies S, O’Leary E, Callaghan G, Galligan M, Deady S, Gadallah B, Lenane P, Lally A, Houlihan DD, Morris PG, Sexton DJ, McCormick PA, Egan JJ, O’Neill JP, Conlon PJ, Moloney FJ: Declining incidence of keratinocyte carcinoma in organ transplant recipients. Br J Dermatol 181: 983–991, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, Leichtman AB, Held PJ, Port FK: Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant 4: 87–93, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Weir MR, Burgess ED, Cooper JE, Fenves AZ, Goldsmith D, McKay D, Mehrotra A, Mitsnefes MM, Sica DA, Taler SJ: Assessment and management of hypertension in transplant patients. J Am Soc Nephrol 26: 1248–1260, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement D, Coca A, De Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen S, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder R, Shlyakhto E, Tsioufis K, Aboyans V, Desormais I; Authors/Task Force members : 2018 practice guidelines for the management of arterial hypertension of the European society of hypertension and the European society of cardiology: ESH/ESC task force for the management of arterial hypertension [published correction appears in J Hypertens 37: 456, 2019 10.1097/HJH.0000000000002026]. J Hypertens 36: 2284–2309, 2018. [DOI] [PubMed] [Google Scholar]
- 40.Taber DJ, Srinivas TM, Pilch NA, Meadows HB, Fleming JN, McGillicuddy JW, Bratton CF, Thomas B, Chavin KD, Baliga PK, Egede LE: Are thiazide diuretics safe and effective antihypertensive therapy in kidney transplant recipients? Am J Nephrol 38: 285–291, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Moes AD, Hesselink DA, van den Meiracker AH, Zietse R, Hoorn EJ: Chlorthalidone versus amlodipine for hypertension in kidney transplant recipients treated with tacrolimus: A randomized crossover trial. Am J Kidney Dis 69: 796–804, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Kuypers DRJ, Neumayer HH, Fritsche L, Budde K, Rodicio JL, Vanrenterghem Y; Lacidipine Study Group: Calcium channel blockade and preservation of renal graft function in cyclosporine-treated recipients: A prospective randomized placebo-controlled 2-year study. Transplantation 78: 1204–1211, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Hoorn EJ, Walsh SB, McCormick JA, Fürstenberg A, Yang C-L, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, Unwin RJ, Ellison DH: The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17: 1304–1309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.