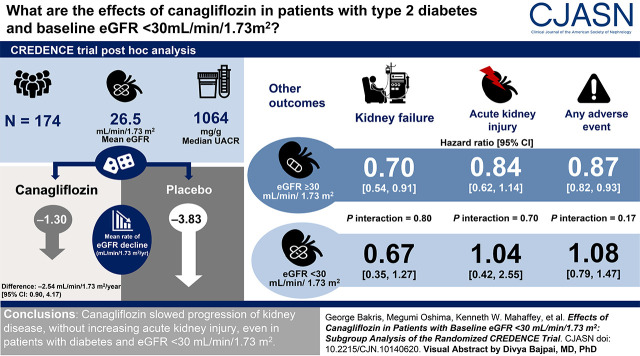
Visual Abstract
Keywords: chronic kidney disease, diabetes, diabetic nephropathy, canagliflozin
Abstract
Background and objectives
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial demonstrated that the sodium glucose cotransporter 2 (SGLT2) inhibitor canagliflozin reduced the risk of kidney failure and cardiovascular events in participants with type 2 diabetes mellitus and CKD. Little is known about the use of SGLT2 inhibitors in patients with eGFR <30 ml/min per 1.73 m2. The participants in the CREDENCE study had type 2 diabetes mellitus, a urinary albumin-creatinine ratio >300–5000 mg/g, and an eGFR of 30 to <90 ml/min per 1.73 m2 at screening. This post hoc analysis evaluated participants with eGFR <30 ml/min per 1.73 m2 at randomization.
Design, setting, participants, & measurements
Effects of eGFR slope through week 130 were analyzed using a piecewise, linear, mixed-effects model. Efficacy was analyzed in the intention-to-treat population, on the basis of Cox proportional hazard models, and safety was analyzed in the on-treatment population. At randomization (an average of 29 days after screening), 174 of 4401 (4%) participants had an eGFR <30 ml/min per 1.73 m2 (mean [SD] eGFR, 26 [3] ml/min per 1.73 m2).
Results
From weeks 3 to 130, there was a 66% difference in the mean rate of eGFR decline with canagliflozin versus placebo (mean slopes, −1.30 versus −3.83 ml/min per 1.73 m2 per year; difference, −2.54 ml/min per 1.73 m2 per year; 95% confidence interval [CI], 0.90 to 4.17). Effects of canagliflozin on kidney, cardiovascular, and mortality outcomes were consistent for those with eGFR <30 and ≥30 ml/min per 1.73 m2 (all P interaction >0.20). The estimate for kidney failure in participants with eGFR <30 ml/min per 1.73 m2 (hazard ratio, 0.67; 95% CI, 0.35 to 1.27) was similar to those with eGFR ≥30 ml/min per 1.73 m2 (hazard ratio, 0.70; 95% CI, 0.54 to 0.91; P interaction=0.80). There was no imbalance in the rate of kidney-related adverse events or AKI associated with canagliflozin between participants with eGFR <30 and ≥30 ml/min per 1.73 m2 (all P interaction >0.12).
Conclusions
This post hoc analysis suggests canagliflozin slowed progression of kidney disease, without increasing AKI, even in participants with eGFR <30 ml/min per 1.73 m2.
Introduction
Diabetes is the leading cause of kidney failure; approximately one in four adults with diabetes will develop persistent albuminuria and/or persistent declines in eGFR (1). Despite the risk of kidney failure in people with type 2 diabetes, treatment options to slow nephropathy progression are limited (2,3). Sodium glucose cotransporter 2 (SGLT2) inhibitors, which were originally developed to help control blood glucose levels in people with type 2 diabetes, have been shown to reduce the risk of cardiovascular events, including major adverse cardiovascular events (myocardial infarction, stroke, or cardiovascular death) and hospitalization for heart failure, in patients with type 2 diabetes and high cardiovascular risk in cardiovascular outcomes trials (4). Results from these trials also suggested SGLT2 inhibition slows progression to kidney failure, but the low risk of kidney disease of these study cohorts led to a small number of kidney-related events across trials (4). Until recently, there were limited data on the use of SGLT2 inhibitors in patients with compromised kidney function, and there were few treatment options for this patient population with low eGFR and high risk of developing kidney failure.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study was a dedicated kidney outcomes trial that demonstrated that the SGLT2 inhibitor canagliflozin significantly reduces the risk of kidney failure and cardiovascular events in participants with type 2 diabetes and CKD (5,6). On the basis of data from the CREDENCE trial, the US Food and Drug Administration approved canagliflozin for reducing the risk of kidney failure, doubling of serum creatinine, cardiovascular death, and hospitalization for heart failure in adults with type 2 diabetes and diabetic nephropathy with albuminuria (7). The US prescribing information for canagliflozin was also updated to allow continuation of 100 mg canagliflozin in patients already receiving therapy whose eGFR falls below 30 ml/min per 1.73 m2 with a urinary albumin-creatinine ratio (UACR) >300 mg/g until initiation of dialysis or kidney transplantation, reflecting the form of the CREDENCE trial intervention (7,8). Despite this, the potential kidney and cardiovascular benefits of canagliflozin in patients with advanced CKD (eGFR <30 ml/min per 1.73 m2) have not been reported. This article describes the efficacy and safety of canagliflozin in a post hoc subgroup analysis of CREDENCE trial participants with eGFR <30 ml/min per 1.73 m2 at randomization.
Materials and Methods
Study Design
CREDENCE (ClinicalTrials.gov identifier, NCT02065791) was a randomized, double-blind, placebo-controlled, multicenter, international trial, the details of which have been published previously (5,6). This post hoc analysis examined efficacy and safety outcomes in patients with eGFR <30 ml/min per 1.73 m2 at randomization. Although participants were enrolled in the study on the basis of an eGFR of 30 to <90 ml/min per 1.73 m2 at screening, some patients’ eGFR values changed by the time of randomization, such that the recorded eGFR measurement was <30 ml/min per 1.73 m2 by the time of randomization assessment.
Study Participants
Eligible participants were ≥30 years of age with type 2 diabetes, glycated hemoglobin (HbA1c) of 6.5%–12.0%, screening eGFR of 30 to <90 ml/min per 1.73 m2, and UACR of >300–5000 mg/g (>33.9–565.6 mg/mmol), and were receiving treatment with a stable maximum labeled or tolerated dose of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for ≥4 weeks before randomization. Exclusion criteria included nondiabetic kidney disease, type 1 diabetes mellitus, and prior treatment of kidney disease with immunosuppression or a history of KRT.
Randomization and Study Treatment
Participants were randomized to receive 100 mg oral canagliflozin daily or matching placebo. The protocol stipulated that study treatment be continued until the commencement of dialysis, receipt of a kidney transplant, occurrence of diabetic ketoacidosis, pregnancy, receipt of disallowed therapy, or study conclusion (5,6). Background treatment intensification for glycemic management and cardiovascular protection according to practice guidelines was recommended.
Outcomes
This post hoc analysis assessed the following intermediate outcomes: change from baseline in HbA1c, systolic BP, UACR, and eGFR. In addition, eGFR change was assessed and measured as the acute change in eGFR from baseline to week 3 (6), the annualized chronic change in eGFR from week 3 until the end of treatment, and the total annualized change in eGFR from baseline to week 130.
Efficacy analyses were the same as those identified in the primary study and included the effects of canagliflozin on the primary composite outcome of kidney failure, doubling of serum creatinine, or kidney or cardiovascular death (5,6). Other efficacy outcomes included the effects of canagliflozin on cardiovascular death; the composite of cardiovascular death or hospitalization for heart failure; the composite of cardiovascular death, myocardial infarction, or stroke; hospitalization for heart failure; the composite of kidney failure, doubling of serum creatinine, or kidney death; kidney failure; and the composite of dialysis, kidney transplantation, or kidney death. Safety analyses included assessments of adverse events.
Statistical Analyses
Changes in HbA1c and systolic BP over time were analyzed using a mixed-effects model for repeated measures, which included data up to week 182, assuming an unstructured covariance and adjusting for baseline value, treatment, trial visit, and interactions of treatment by visit and baseline value by visit. Due to the highly skewed distribution of UACR data, UACR was log transformed and the geometric mean of postbaseline UACR was estimated using a similar model. The geometric mean ratio was used to calculate the reduction in postrandomization UACR for canagliflozin compared with placebo.
On-treatment eGFR slope was estimated using a piecewise, linear, mixed-effects model with a knot at week 3, including the fixed effects of treatment, randomization eGFR, continuous time, and a linear spline in follow-up time, with a knot at week 3, with interactions of treatment with the time spline terms. The model also included random intercepts, initial slopes (before week 3), and long-term slopes (after week 3) to account for variation in trajectories across participants. When the full model failed to converge, a simplified model with a single random slope was used. The effects of canagliflozin on mean total slope through week 130 were computed as a weighted combination of the estimated effects on the initial and long-term slopes.
Kidney, cardiovascular, and mortality outcomes were analyzed in the intention-to-treat population, on the basis of Cox proportional hazard models in participants with randomization eGFR <30 and ≥30 ml/min per 1.73 m2. Safety outcomes were analyzed in all treated participants through 30 days after the last dose (on treatment). The interaction of treatment effects between participants with randomization eGFR <30 and ≥30 ml/min per 1.73 m2 was tested by adding eGFR category as a covariable and an interaction term of treatment by eGFR categories to the Cox model. Hazard ratios (HRs) and 95% confidence intervals (CIs) for canagliflozin versus placebo were estimated. Due to the post hoc nature of this analysis, only nominal P values were reported.
Ethics
Local institutional ethics committees approved the trial protocols at each site. All participants provided written informed consent. The trial was conducted according to the principles outlined in the Declaration of Helsinki.
Results
Patients
A total of 4401 participants were randomized to canagliflozin or placebo in the CREDENCE trial, with a median follow-up duration of 2.62 (interquartile range, 2.11–3.09) years.
At the time of screening, when eligibility for the trial on the basis of eGFR was assessed, all participants had an eGFR between 30 and <90 ml/min per 1.73 m2. However, by the time of randomization (an average of 29 days later), 174 (4%) participants had an eGFR <30 ml/min per 1.73 m2, but were still eligible for the trial as per the study protocol. The mean (SD) eGFR for these 174 participants was 35 (7) ml/min per 1.73 m2 at screening, and 26 (3) ml/min per 1.73 m2 at randomization. The distribution of eGFR values among participants with randomization eGFR <30 ml/min per 1.73 m2 is shown in Supplemental Figure 1. Median (interquartile range) time between screening and randomization measurement was comparable for patients with eGFR <30 and ≥30 ml/min per 1.73 m2 (29 [24–36] and 29 [23–36] days, respectively), with no notable differences between treatment groups. Most patients (>88%) had their randomization assessment between 3 and 8 weeks after screening. However, a higher proportion of patients with eGFR <30 ml/min per 1.73 m2 had randomization measurements >8 weeks after screening compared with patients with eGFR ≥30 ml/min per 1.73 m2 (13 of 174 [7%] versus 156 of 4226 [4%] patients; P interaction=0.01) (Supplemental Table 1).
Baseline characteristics for participants within the subgroup with eGFR <30 ml/min per 1.73 m2 were balanced between the groups randomized to canagliflozin and placebo (Table 1).
Table 1.
Baseline characteristics of participants with randomization eGFR <30 ml/min per 1.73 m2
| Characteristica | Canagliflozin (n=84) | Placebo (n=90) | Total (n=174) |
|---|---|---|---|
| Age, yr | 64 (10) | 66 (9) | 65 (10) |
| Male, n (%) | 54 (64) | 52 (58) | 106 (61) |
| Race, n (%) | |||
| White | 55 (65) | 59 (66) | 114 (66) |
| Black | 7 (8) | 2 (2) | 9 (5) |
| Asian | 14 (17) | 11 (12) | 25 (14) |
| Otherb | 8 (10) | 18 (20) | 26 (15) |
| Current smoker, n (%) | 9 (11) | 14 (16) | 23 (13) |
| History of hypertension, n (%) | 82 (98) | 89 (99) | 171 (98) |
| History of heart failure, n (%) | 9 (11) | 14 (16) | 23 (13) |
| Duration of diabetes, yr | 17.5 (9.9) | 16.6 (9.3) | 17.0 (9.6) |
| History of cardiovascular disease, n (%) | 41 (49) | 45 (50) | 86 (49) |
| History of amputation, n (%) | 10 (12) | 2 (2) | 12 (7) |
| Body mass index, kg/m2 | 32 (7) | 31 (6) | 32 (6) |
| Systolic BP, mm Hg | 138 (16) | 139 (17) | 139 (16) |
| Diastolic BP, mm Hg | 75 (9) | 76 (11) | 76 (10) |
| HbA1c, % | 8.2 (1.3) | 8.0 (1.1) | 8.1 (1.2) |
| eGFR, ml/min per 1.73 m2 | 26 (3) | 27 (3) | 26 (3) |
| UACR, mg/g, median (IQR) | 1056 (459, 2525) | 1153 (483, 2253) | 1064 (464, 2376) |
HbA1c, glycated hemoglobin; UACR, urinary albumin-creatinine ratio; IQR, interquartile range.
Data are mean (SD) unless otherwise indicated.
Other includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, other, unknown, and not reported.
In the overall trial, 25% of patients in the canagliflozin group and 30% of patients in the placebo group discontinued treatment for any reason, and a total of 12% and 13% of patients in the canagliflozin and placebo groups, respectively, discontinued due to an adverse event. There were no differences in effects of canagliflozin versus placebo on discontinuation between patients with eGFR <30 and ≥30 ml/min per 1.73 m2 (P interaction >0.15; Figure 1).
Figure 1.
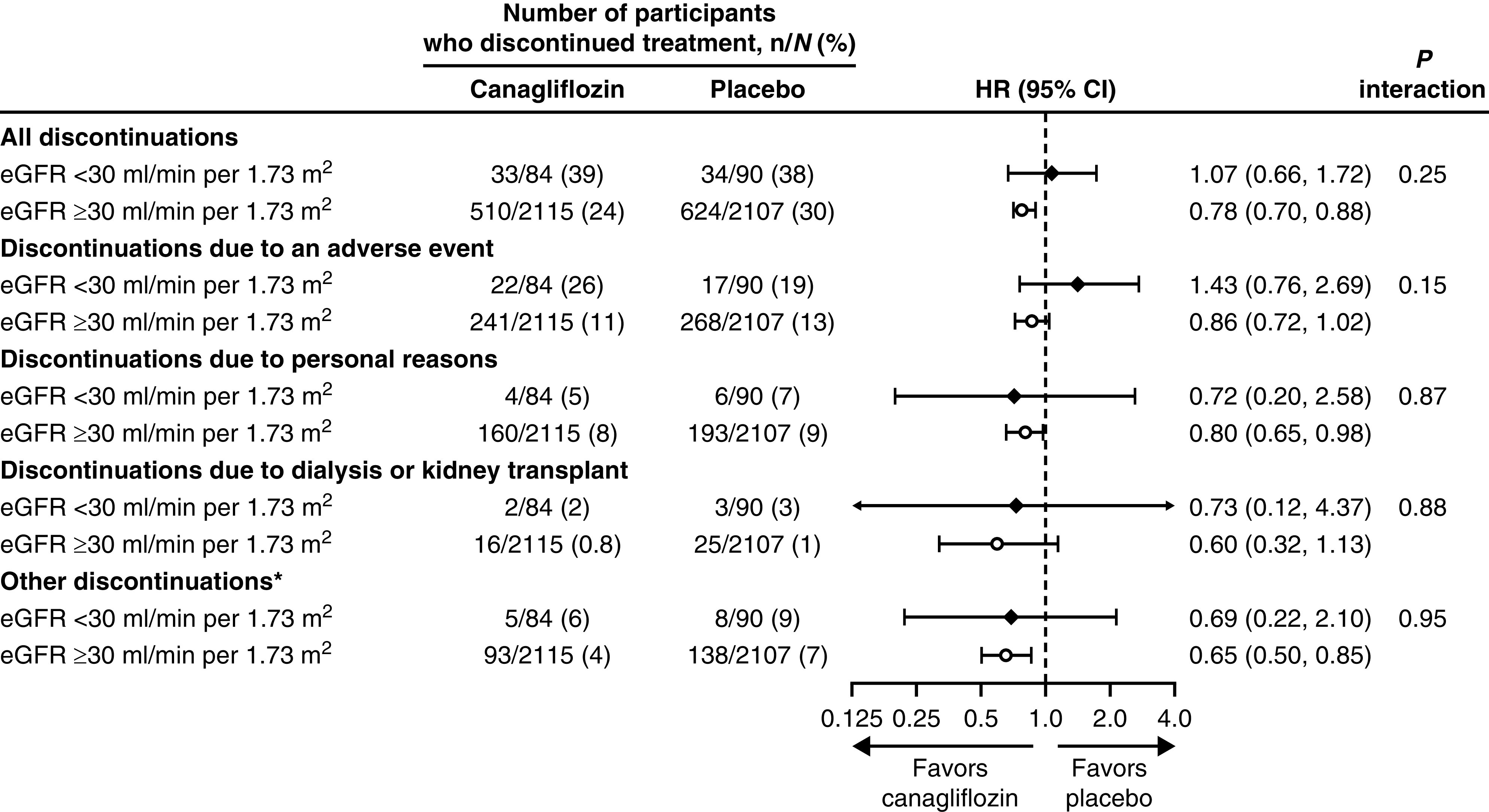
There was no difference in discontinuation of treatment at eGFR <30 ml/min per 1.73 m2 compared to eGFR >30 ml/min per 1.73 m2. *Other reasons include poor adherence, safety or tolerability, disallowed therapy, protocol violation, site closure, and other. 95% CI, 95% confidence interval; HR, hazard ratio.
Intermediate Outcomes
In the 174 patients with eGFR <30 ml/min per 1.73 m2, there was no difference between the effect of canagliflozin and placebo on HbA1c over the study (difference in least squares mean change, −0.27%; 95% CI, −0.63 to 0.09). The effect of canagliflozin on systolic BP in patients with randomization eGFR <30 ml/min per 1.73 m2 did not clearly differ compared with placebo over the study (difference in least squares mean change, −2.66 mm Hg; 95% CI, −6.18 to 0.86). In contrast, the geometric mean for UACR was 33% lower (95% CI, −49 to −10) during the study in patients treated with canagliflozin than in those in the placebo group (Figure 2A). No differences in the effects of canagliflozin on HbA1c, systolic BP, or UACR were observed between patients with eGFR <30 and ≥30 ml/min per 1.73 m2 (P for heterogeneity=0.86, 0.66, and 1.0, respectively).
Figure 2.
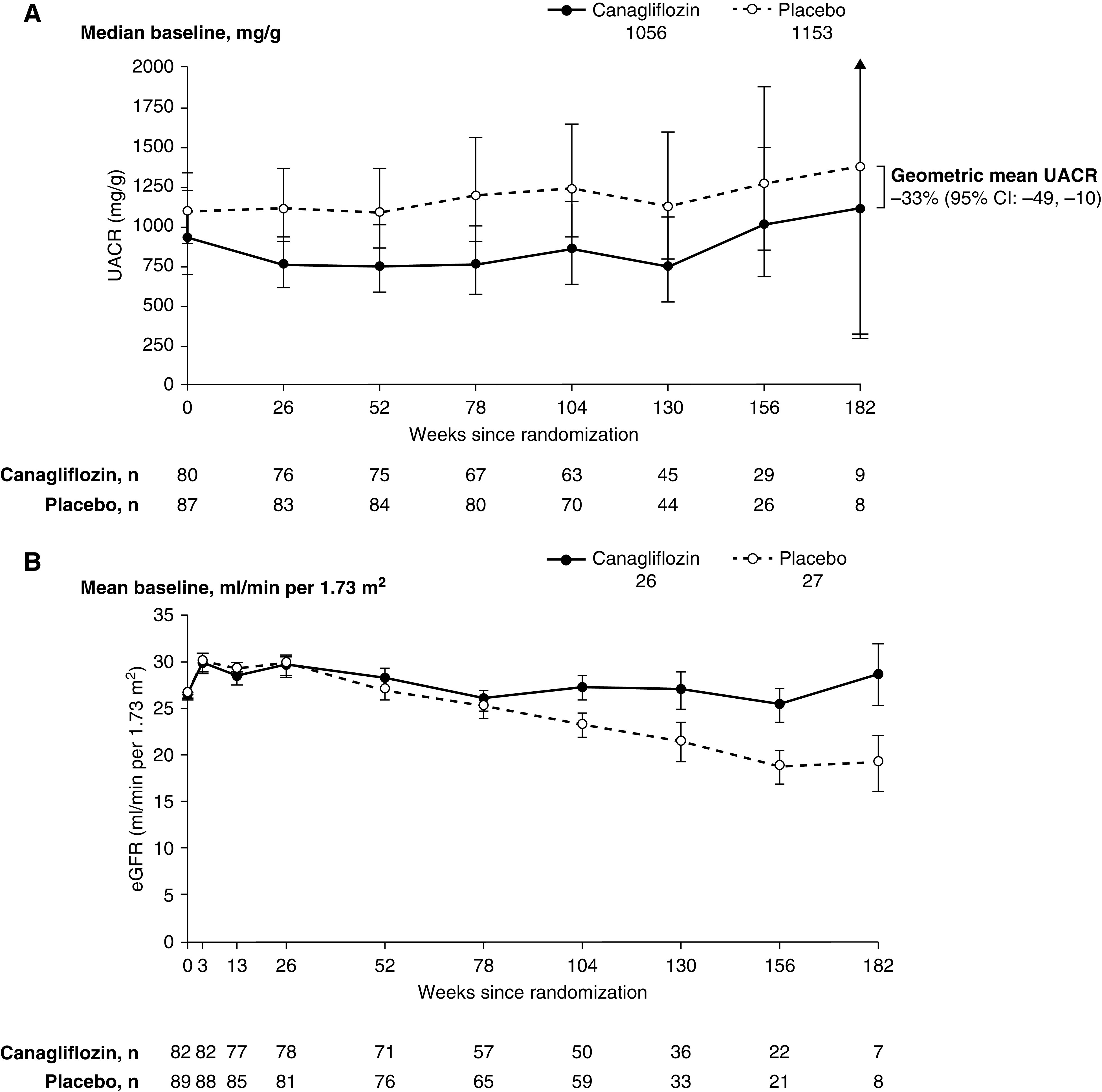
There was sustained reduction in albuminuria with a increased effect in acute GFR change with canagliflozin in participants with randomization eGFR <30 ml/min per 1.73 m2. Baseline levels of UACR (median) and eGFR (mean) are shown below the legend. Data are geometric mean ratio (95% CI) in (A). Data are mean (±SEM) in (B). UACR, urinary albumin-creatinine ratio.
Mean eGFR in participants with eGFR <30 ml/min per 1.73 m2 over the study is depicted in Figure 2B. The mean annual decline in eGFR from baseline to week 130 was slower in patients treated with canagliflozin compared with placebo (mean slopes of +0.03 versus −1.88 ml/min per 1.73 m2 per year, respectively; placebo-subtracted difference, 1.91 ml/min per 1.73 m2 per year; 95% CI, 0.18 to 3.64). The mean acute change in eGFR from baseline to week 3 was 3.26 ml/min per 1.73 m2 with canagliflozin and 4.14 ml/min per 1.73 m2 with placebo (placebo-subtracted difference, −0.88 ml/min per 1.73 m2; 95% CI, −3.16 to 1.39). From week 3 to the last measurement, there was a 66% difference in the mean rate of eGFR decline with canagliflozin compared with placebo (mean slopes of −1.30 versus −3.83 ml/min per 1.73 m2 per year, respectively; placebo-subtracted difference, 2.54 ml/min per 1.73 m2 per year; 95% CI, 0.90 to 4.17).
Kidney, Cardiovascular, and Mortality Outcomes
Despite a limited number of events in the subset of 174 participants with eGFR <30 ml/min per 1.73 m2, the point estimates for the effects of canagliflozin on most kidney, cardiovascular, and mortality outcomes were generally consistent with those seen in patients with eGFR ≥30 ml/min per 1.73 m2 (all P interaction >0.20; Figure 3). Treatment with canagliflozin reduced the risk of kidney failure in participants with eGFR ≥30 ml/min per 1.73 m2 (HR, 0.70; 95% CI, 0.54 to 0.91) and the effects were consistent in participants with eGFR <30 ml/min per 1.73 m2 (HR, 0.67; 95% CI, 0.35 to 1.27; P interaction=0.80).
Figure 3.
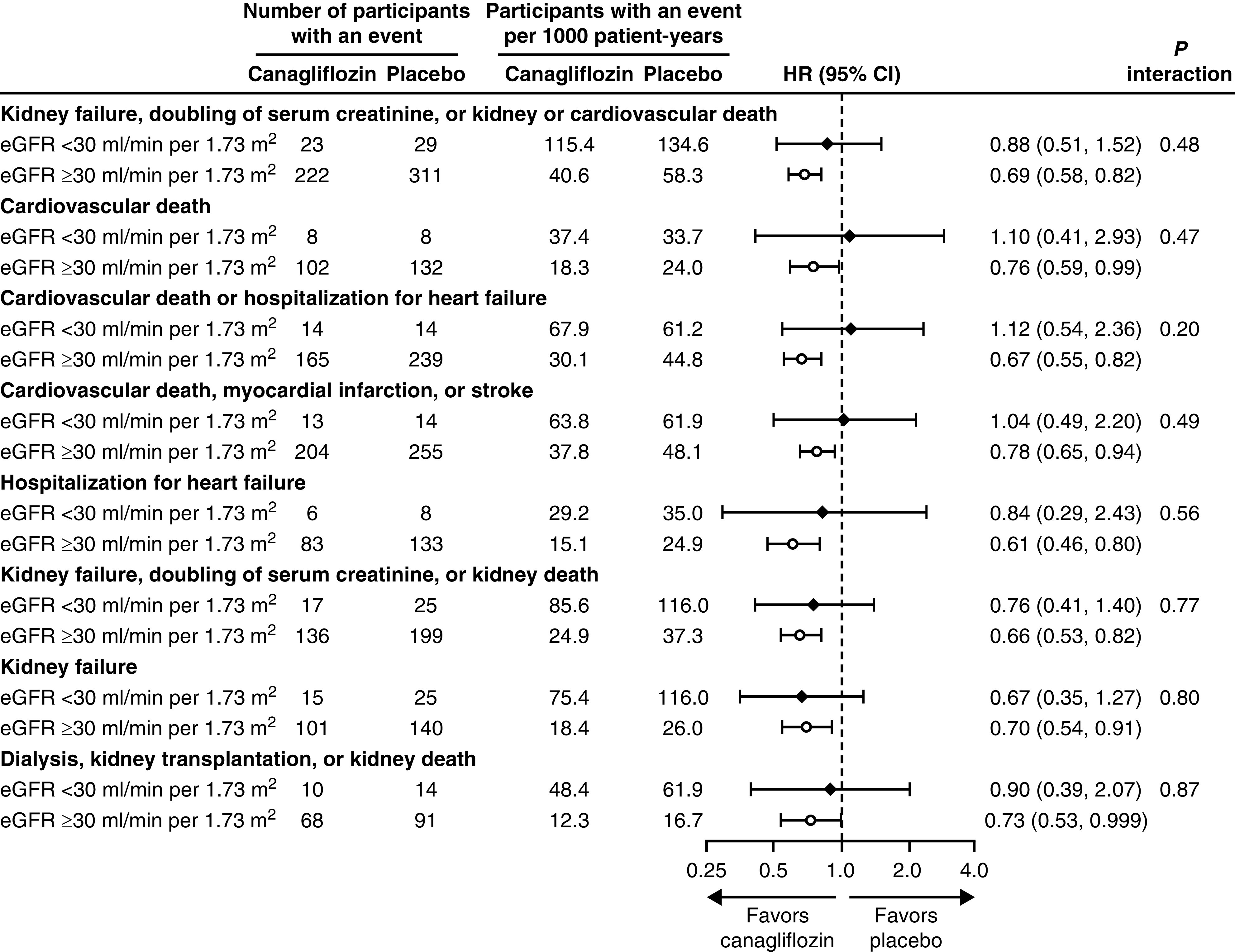
There was no difference in cardiovascular or kidney outcomes between those with eGFR <30 ml/min per 1.73 m2 compared to those with eGFR >30 ml/min per 1.73 m2.
Safety
Within the subgroup of patients with eGFR <30 ml/min per 1.73 m2, those treated with canagliflozin had similar rates of adverse events, serious adverse events, hyperkalemia, and hypoglycemia compared with those treated with placebo. There was also no imbalance in the rate of kidney-related adverse events or AKI events with canagliflozin compared with placebo in this subgroup. In addition, there was no evidence of heterogeneity in the occurrence of adverse events between patients with eGFR <30 and ≥30 ml/min per 1.73 m2 (all P interaction >0.12; Figure 4). The incidence of serious adverse events during on- and off-treatment periods is shown in Supplemental Table 2. Among patients with eGFR <30 ml/min per 1.73 m2, the number of participants with a fracture (four in each group) or amputation (three with canagliflozin and one with placebo) was low between groups.
Figure 4.
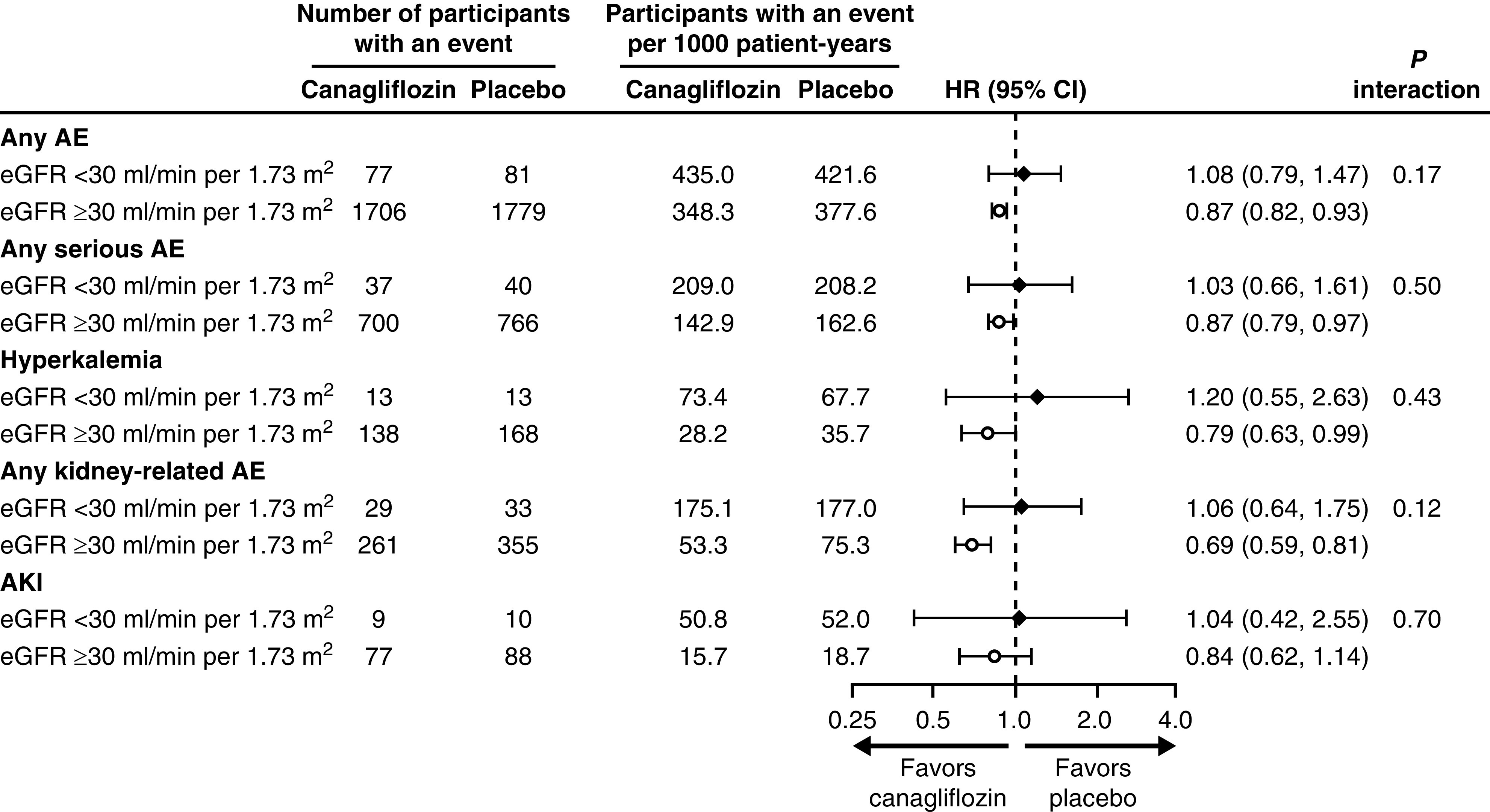
There was no difference in adverse events between those with eGFR <30 ml/min per 1.73 m2 and >30 ml/min per 1.73 m2. AE, adverse event.
Discussion
In this post hoc analysis of participants in the CREDENCE trial with eGFR <30 ml/min per 1.73 m2 at randomization, treatment with canagliflozin reduced albuminuria and the rate of eGFR decline compared with placebo. The effects of canagliflozin on kidney, cardiovascular, and mortality outcomes in participants with eGFR <30 ml/min per 1.73 m2 appeared to be consistent with those seen in participants with eGFR ≥30 ml/min per 1.73 m2 at randomization. There was no detectable increase in harmful effects, including kidney-related adverse events and AKI, with canagliflozin compared with placebo in participants with eGFR <30 ml/min per 1.73 m2. These results support the use and continuation of SGLT2 inhibitor treatment, even in patients with eGFR <30 ml/min per 1.73 m2, until the commencement of maintenance dialysis or receipt of a kidney transplant, and clinicians should consider this when discussing treatment options for patients with low eGFR.
Canagliflozin slowed the rate of eGFR decline in this subgroup of patients with eGFR <30 ml/min per 1.73 m2. Unlike the overall population, there was no detectable initial acute drop in eGFR with canagliflozin in this subgroup, which is consistent with results of a subgroup analysis of patients with stage 3b and 4 CKD from another SGLT2 inhibitor study (9). There is no clear evidence that a reduction in intraglomerular pressure occurs at this stage of nephropathy with these agents (10), although recent data demonstrate BP reduction is similar in this advanced stage to that observed in early stages of nephropathy (11). Other factors, such as BP reduction, may have also contributed to the slowing of CKD. In addition, the requirement that the screening eGFR had to exceed 30 ml/min per 1.73 m2 suggests some of the randomization eGFR values that were <30 ml/min per 1.73 m2 probably resulted from random variation in patients whose typical eGFR was >30 ml/min per 1.73 m2. Thus, an additional consideration is reversion to the mean because this cohort did have eGFR values just >30 ml/min per 1.73 m2 at screening and <30 ml/min per 1.73 m2 at randomization. Furthermore, most participants had eGFR levels between 20 and 30 ml/min per 1.73 m2, so these results may not be generalizable to people with even lower eGFR levels.
Studies in animal models demonstrate SGLT2 inhibition results in sympathetic inhibition of kidney nerve function, and this may account for the glucose-independent effect of SGLT2 inhibition on BP (12). Therefore, the 3–4 mm Hg reduction in BP that results from SGLT2 inhibition may contribute, in some part, to renoprotection, similar to what was observed in the captopril trial, where a 4 mm Hg difference in BP contributed to slowed progression of diabetic nephropathy (13). The aforementioned denervation hypothesis is also consistent with results from the Dapagliflozin and Prevention of Adverse-Outcomes in Heart Failure trial, which demonstrated the SGLT2 inhibitor dapagliflozin was beneficial in people without diabetes (14). The denervation hypothesis also aligns with the beneficial effects of canagliflozin observed in the subset of patients with eGFR <30 ml/min per 1.73 m2, in whom some kidney and cardiovascular benefits are seen and are glucose independent. Additionally, the heterogeneity of effects on HbA1c, but not on other efficacy outcomes between eGFR subgroups (30 to <45, 45 to <60, and 60 to <90 ml/min per 1.73 m2) further supports a glucose-independent mechanism of kidney and cardiovascular protection (8). Although other potential mechanisms for renoprotection are actively being studied (11,15–17), it is clear the albuminuria reduction seen in this subgroup was similar to the overall population, and this is a well-accepted surrogate for renoprotection (18).
The conclusions that can be drawn from this nonprespecified-subgroup, post hoc analysis should be interpreted cautiously due to the limited statistical precision to robustly assess these outcomes due to the small sample size of this participant group.
The recently terminated Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) trial (19) and Effects of Dapagliflozin in Nondiabetic Patients With Proteinuria (DIAMOND) trial (20) recruited participants with eGFR down to 25 ml/min per 1.73 m2, whereas the Study of Heart and Kidney Protection with Empagliflozin (EMPA-Kidney) trial (ClinicalTrials.gov Identifier: NCT03594110) includes those with an eGFR down to 20 ml/min per 1.73 m2. Once available, data from these trials will provide additional insight into the effect of SGLT2 inhibitors in people with lower initial eGFR levels. Until the DIAMOND study is finished, we can say that dapagliflozin was safe and effective in people with eGFR down to 25 ml/min per 1.73 m2 recruited for DAPA-CKD, and the consistent benefit of canagliflozin in the overall CREDENCE population and in patients with eGFR <30 ml/min per 1.73 m2 at randomization suggests there is no reason to discontinue treatment until the commencement of maintenance dialysis or receipt of a kidney transplant, as stipulated in the CREDENCE protocol. Although there may be similar renoprotective effects in people with eGFR <30 ml/min per 1.73 m2, we would not recommend initiating treatment with an SGLT2 inhibitor in people with eGFR <30 ml/min per 1.73 m2 until results of the other pending studies are available.
Disclosures
All of the authors received research support and consulting fees from Janssen in relation to their roles on the steering committee of the CREDENCE trial. R. Agarwal reports serving as associate editor of the American Journal of Nephrology and Nephrology Dialysis Transplantation; receiving personal fees for serving on a steering committee and speaker fees from Janssen, during the conduct of the study; and receiving research funding from GlaxoSmithKline and personal fees from Akebia, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, ER Squibb and Sons, Fresenius, Gilead, GlaxoSmithKline, Ironwood Pharmaceuticals, Johnson & Johnson, Opko, Otsuka, Reata, Relypsa, Sanofi, and Takeda, outside the submitted work. G. Bakris reports serving as a consultant for Alnylam, AstraZeneca, Ionis, KBP Biosciences, Merck, Novo Nordisk, and Relypsa; serving as editor of American Journal of Nephrology and Nephrology, editor-in-chief of UpToDate, and nephrology and hypertension section editor of UpToDate; receiving research funding paid to the University of Chicago for serving as the principal investigator on national clinical trials for Bayer, Janssen, and Novo Nordisk; serving as associate editor of Diabetes Care, Hypertension Research, and Nephrology Dialysis Transplantation; serving on a steering committee for Janssen, with monies going to University of Chicago Medicine, during the conduct of the study; and serving on a steering committee for Vascular Dynamics. C. Cannon reports receiving consulting fees from Aegerion, Alnylam, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Corvidia, Eisai, Eli Lilly, GlaxoSmithKline, Innovent, Kowa, Merck, Pfizer, Regeneron, and Sanofi; research funding from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Janssen, Merck, and Takeda; and personal fees for serving on a steering committee and speaker fees from Janssen, during the conduct of the study. G. Capuano is a full-time employee of Janssen Research & Development, LLC. D. Charytan has served on data safety and monitoring boards for Allena Pharmaceuticals and AstraZeneca; consulted for Amgen, AstraZeneca, Daiichi Sankyo, Douglas & London, Eli Lilly, Fresenius, Gilead, GlaxoSmithKline, Medtronic/Covidien, Merck, Novo Nordisk, and Zoll; received research support from Amgen and Medtronic; received fees paid by Janssen Pharmaceuticals to the Baim Institute for work on the CREDENCE trial steering committee and as a scientific lead; and received salary support from the Baim Institute for this work through October 2018. After that time, he received consulting fees from Baim. He has also served on a clinical endpoint committee for Merck and PLC Medical. D. de Zeeuw has served on steering committees and/or as a speaker for AbbVie and Janssen; has served on data safety and monitoring committees for Bayer; has served on advisory boards and/or as a speaker for Bayer, Boehringer Ingelheim, Fresenius, Mundipharma, and Mitsubishi Tanabe; and reports receiving personal fees for serving on a steering committee and speaker fees from Janssen, during the conduct of the study. R. Edwards is a full-time employee of Janssen Research & Development, LLC. T. Greene reports receiving personal fees for serving on a steering committee and speaker fees from Janssen, during the conduct of the study; and receiving research support from AstraZeneca and Boehringer Ingelheim, and consulting fees from Durect, Janssen, and Pfizer, outside the submitted work. M. Jardine has served on advisory boards sponsored by Akebia, Baxter, Boehringer Ingelheim, Merck Sharp & Dohme, and Vifor; is responsible for research projects that have received unrestricted funding from Amgen, Baxter, Eli Lilly, and Merck Sharp & Dohme; has spoken at scientific meetings sponsored by Amgen and Janssen, with any consultancy, honoraria, or travel support paid to her institution; serves on a steering committee sponsored by CSL; and reports receiving personal fees for serving on a steering committee and speaker fees from Janssen, during the conduct of the study. She is supported by a Medical Research Future Fund Next Generation Clinical Researchers Program Career Development Fellowship. H. Heerspink reports receiving personal fees for serving on a steering committee and speaker fees from Janssen, during the conduct of the study; consulting fees from AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Merck, and Mitsubishi Tanabe; and research support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen, outside the submitted work. A. Levin reports serving as a scientific advisor to AstraZeneca, Boehringer Ingelheim, and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); receiving personal fees for serving on a steering committee and speaker fees from Janssen, during the conduct of the study; receiving fees for her time as CREDENCE National Coordinator from Janssen, directed to her academic team; serving as a data safety and monitoring board member for Kidney Precision Medicine, NIDDK, and the University of Washington Kidney Research Institute Scientific Advisory Committee; and research funding from the Canadian Institute of Health Research and Kidney Foundation of Canada, outside the submitted work. K. Mahaffey reports receiving consulting or other services (speaker fees for continuing medical education events only) fees from Abbott, Ablynx, Anthos, AstraZeneca, Baim Institute, Boehringer Ingelheim, CSL Behring, Elsevier, GlaxoSmithKline, Intermountain Health, Johnson & Johnson, MedErgy, Medscape, Mitsubishi, Mount Sinai, Mundipharma, MyoKardia, the National Institutes of Health (NIH), Novartis, Novo Nordisk, Portola, Radiometer, Regeneron, SmartMedics, Springer Publishing, Theravance, and the University of California, San Francisco; grants from Afferent, Amgen, Apple Inc., AstraZeneca, Cardiva Medical, Inc., Daiichi, Ferring, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, NIH, Novartis, Sanofi, St. Jude, and Tenax; and research grants and personal fees from Janssen, during the conduct of the study; and grants and personal fees from Amgen, AstraZeneca, Johnson & Johnson (Janssen), NIH, Novartis, and Sanofi, outside the submitted work. B. Neal’s institution has received consultancy, honoraria, or travel support for contributions he has made to advisory boards and/or the continuing medical education programs of Abbott, Janssen, Novartis, Pfizer, Roche, and Servier; he is supported by an Australian National Health and Medical Research Council Principal Research Fellowship; holds a research grant for this study from Janssen; and has held research grants for other large-scale cardiovascular outcome trials from Merck Schering-Plough, Roche, and Servier. R. Oh is a full-time employee of Janssen Research & Development, LLC. M. Oshima reports receiving personal fees for serving on a steering committee and speaker fees from Janssen, during the conduct of the study; and grants from the Japan Society for the Promotion of Science Program for Fostering Globally Talented Researchers, outside the submitted work. V. Perkovic reports receiving personal fees for serving on a steering committee and advisory boards and speaking at scientific meetings, during the conduct of the study. He also reports receiving fees for advisory boards, steering committee roles, or scientific presentations from AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol Myers Squibb, Boehringer Ingelheim, Dimerix, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, PharmaLink, Relypsa, Retrophin, Sanofi, Servier, Vifor, and Tricida; and a Senior Research Fellowship and Program Grant from the Australian National Health and Medical Research Council. C. Pollock reports receiving fees paid to steering committee members and travel to steering committee meetings, and funds to attend meetings to present the data from the broader CREDENCE study from Johnson & Johnson/Janssen Cilag, during the conduct of the study; and honoraria for serving on advisory boards and as a speaker for AstraZeneca, Boehringer Ingelheim/Eli Lilly, and Merck Sharp & Dohme; personal fees from Eli Lily for serving as an Australian advisory board member; and personal fees from Novartis, for serving as a speaker, outside the submitted work. N. Rosenthal is a full-time employee of Janssen Research & Development, LLC. D. Wheeler has received fees for advisory boards, steering committee roles, or scientific presentations from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Mitsubishi, Mundipharma, Napp, Ono Pharma, Tricida, and Vifor Fresenius; and fees and travel funding from Janssen for his role as a member of the CREDENCE steering committee. H. Zhang reports receiving personal fees for serving on a steering committee, speaker fees, and consulting fees from Janssen. B. Zinman reports receiving personal fees for serving on a steering committee and speaker fees from Janssen, during the conduct of the study; consulting fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; and grants from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk, outside the submitted work.
Funding
The CREDENCE study was sponsored by Janssen Research & Development.
Supplementary Material
Acknowledgments
We thank all participants, investigators, and trial teams for their participation in the trial.
The CREDENCE study was sponsored by Janssen Research & Development, LLC, and was conducted collaboratively by the sponsor, an academic-led steering committee, and an academic research organization, George Clinical. Analyses were performed by George Clinical and independently confirmed by the sponsor. Medical writing support was provided by Dr. Elizabeth Meucci, of MedErgy, and was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
All authors provided input through the development of this manuscript and approved the final version for submission.
Because Dr. David M. Charytan is an associate editor of CJASN, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Are SGLT2 Inhibitors Safe and Effective in Advanced Diabetic Kidney Disease?” on pages 1694–1695.
Data Sharing Statement
Data from this study will be made available in the public domain via the Yale University Open Data Access Project (http://yoda.yale.edu/) once the product and relevant indication studied have been approved by regulators in the United States and the European Union and the study has been completed for 18 months.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10140620/-/DCSupplemental.
Supplemental Table 1. Time between screening and randomization measurements.
Supplemental Table 2. Serious adverse events during on- and off-treatment.
Supplemental Figure 1. Distribution of eGFR at randomization in participants with randomization eGFR <30 ml/min per 1.73 m2.
References
- 1.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH: Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 316: 602–610, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS: SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393: 31–39, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, Bull S, Cannon CP, Charytan DM, de Zeeuw D, Edwards R, Greene T, Heerspink HJL, Levin A, Pollock C, Wheeler DC, Xie J, Zhang H, Zinman B, Desai M, Perkovic V; CREDENCE study investigators : The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 46: 462–472, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Janssen Pharmaceuticals: INVOKANA (canagliflozin) tablets, for oral use [package insert]. Titusville, NJ, 2019
- 8.Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, Bajaj HS, Bull S, Cannon CP, Charytan DM, de Zeeuw D, Luca di Tanna G, Greene T, Heerspink HJL, Levin A, Neal B, Pollock C, Qiu R, Sun T, Wheeler DC, Zhang H, Zinman B, Rosenthal N, Perkovic V; CREDENCE Study Investigators: Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: A secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol 31: 1128–1139, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dekkers CCJ, Wheeler DC, Sjöström CD, Stefansson BV, Cain V, Heerspink HJL: Effects of the sodium-glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and stages 3b-4 chronic kidney disease. Nephrol Dial Transplant 33: 2005–2011, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picken M, Long J, Williamson GA, Polichnowski AJ: Progression of chronic kidney disease after acute kidney injury: Role of self-perpetuating versus hemodynamic-induced fibrosis. Hypertension 68: 921–928, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sternlicht H, Bakris GL: Blood pressure lowering and sodium-glucose co-transporter 2 inhibitors (SGLT2is): More than osmotic diuresis. Curr Hypertens Rep 21: 12, 2019. [DOI] [PubMed] [Google Scholar]
- 12.Herat LY, Magno AL, Rudnicka C, Hricova J, Carnagarin R, Ward NC, Arcambal A, Kiuchi MG, Head GA, Schlaich MP, Matthews VB: SGLT2 inhibitor–induced sympathoinhibition: A novel mechanism for cardiorenal protection. JACC Basic Transl Sci 5: 169–179, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 14.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators: Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995–2008, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Yaribeygi H, Katsiki N, Butler AE, Sahebkar A: Effects of antidiabetic drugs on NLRP3 inflammasome activity, with a focus on diabetic kidneys. Drug Discov Today 24: 256–262, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Woods TC, Satou R, Miyata K, Katsurada A, Dugas CM, Klingenberg NC, Fonseca VA, Navar LG: Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol 49: 331–342, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyachi Y, Tsuchiya K, Shiba K, Mori K, Komiya C, Ogasawara N, Ogawa Y: A reduced M1-like/M2-like ratio of macrophages in healthy adipose tissue expansion during SGLT2 inhibition. Sci Rep 8: 16113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, Grams ME, Greene T, Tighiouart H, Matsushita K, Ballew SH, Sang Y, Vonesh E, Ying J, Manley T, de Zeeuw D, Eckardt KU, Levin A, Perkovic V, Zhang L, Willis K: Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: A scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 75: 84–104, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto R, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020. [DOI] [PubMed] [Google Scholar]
- 20.Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Gafor AHA, Greasley PJ, Laverman GD, Lim SK, Di Tanna GL, Reich HN, Vervloet MG, Wong MG, Gansevoort RT, Heerspink HJL; DIAMOND Investigators : Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): A randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 8: 582–593, 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.