Abstract
Purpose of Review:
The pancreas is highly affected in cystic fibrosis (CF), with complications occurring early in childhood. This review highlights recent research in exocrine pancreatic function in the era of cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies and discusses how these are affecting pancreatitis and exocrine pancreatic insufficiency (EPI) in children. Additionally, new research into exocrine-endocrine interactions sheds light on how CFTR dysfunction in ductal cells may affect beta cells.
Recent Findings:
Ivacaftor has disproved the hypothesis that EPI in children with CF is irreversible. Improvements in pancreatic function have increased pancreatitis episodes in some children and reduced them in others. Imaging advances are providing complementary methods for exocrine pancreatic function testing. New research into the interplay between the exocrine and endocrine components of the pancreas are elucidating the intertwined and complex relationship between the exocrine and endocrine pancreas.
Summary:
Pancreatic complications contribute to the morbidity and mortality of children with CF. Increasing use of highly effective CFTR modulators will not only abrogate these, but will also advance our understanding of pancreatic pathophysiology in CF. New frontiers into pancreatic gene therapy and exocrine-endocrine research will help provide new therapeutic opportunities for pancreatitis, EPI, and diabetes in CF.
Keywords: CF, pancreas, pancreatitis, imaging, function
INTRODUCTION
Since Dr. Dorothy Andersen’s first description of “cystic fibrosis of the pancreas” in 1938 (1), pancreatic complications have been at the center of the gastrointestinal complications of cystic fibrosis (CF). Malnutrition secondary to exocrine pancreatic insufficiency (EPI) defined CF until the use of pancreatic enzyme replacement therapy (PERT). While PERT has been in use for over 200 years, EPI in CF remains an active area of research and clinical investigation. The permanence (or lack of permanence) of EPI has recently been called into question with new cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies. In fact, alterations in exocrine pancreatic function secondary to highly effective CFTR modulators have highlighted the precarious risk of pancreatitis in those with CF. CF patients were once thought to be protected from pancreatitis due to extreme loss of exocrine function. However, the model of pancreatitis risk for those with CFTR mutations put forward by Ooi and Durie et al. in 2011 appears to be playing out in the era of CFTR modulators (2). With this comes new investigations into how to detect and monitor pancreatic fibrosis and fat deposition as complications of recurrent pancreatitis in CF, topics set-aside long ago as foregone conclusions. Finally, new in vitro models are teaching us about the intricate interplay between the exocrine and endocrine pancreas, which may yield new insights into endocrine (and exocrine) insufficiency in CF. Basic, translational, and clinical research in the pancreatic complications of CF are evolving how we think about the pancreas in CF, deepening our understanding of the pathophysiology, but also bringing new opportunities for diagnostic and therapeutic advances.
EXOCRINE PANCREATIC INSUFFICIENCY IN CF
EPI is one of most well-known complications of CF, with CF being the most common cause for EPI. Greater than 85% of CF patients in the CF Foundation registry are noted to be EPI (3), as defined by use of PERT. While fecal elastase-1 (FE-1) testing is the most commonly used diagnostic test to identify EPI, clinicians should remember that 1) FE-1 values can fluctuate over time (4) and 2) EPI is a clinical diagnosis, not a laboratory one. CF patients may exhibit EPI, and benefit from PERT, even with FE-1 results >200 μg/g stool. In Brownell et al.’s review on “Growth and Nutrition Cystic Fibrosis,” the authors highlight that beyond pancreatic enzyme load, pancreatic enzyme efficacy must also be considered (5). Impairments in both duodenal and pancreatic bicarbonate secretion (6–8), coupled with gastric acid hypersecretion in some CF patients (9), results in an acidic intestinal luminal pH, which may impair enzyme activity and thereby contribute to malabsorption. PERT has been well-established as an effective therapy for EPI, when dosed appropriately, in malnourished patients (10) and/or those exhibiting signs/symptoms of malabsorption (11). Layer et al. undertook a meta-analysis of published studies on the effects of PERT on survival and quality of life in patients with EPI (11). They identified that, while many studies showed that PERT improves fat and protein absorption and growth in CF patients compared to placebo, there were no studies directly assessing PERT and survival, and only one examining quality of life (in chronic pancreatitis patients). Prior studies have established a positive correlation between nutrition and growth and lung function in CF patients (12), which drives our tenacious monitoring of growth and nutrition in children with CF.
Exocrine Pancreatic Insufficiency and CFTR modulators
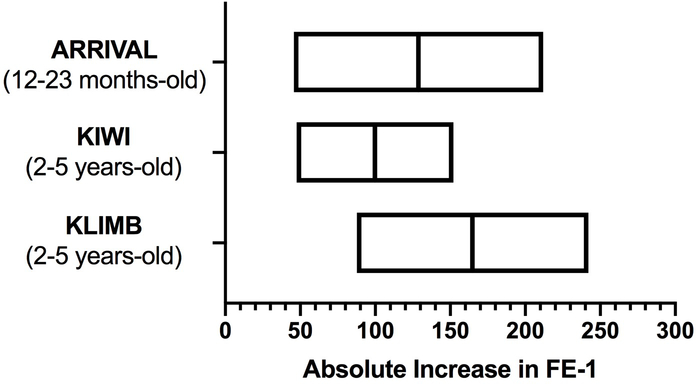
Historically, there have been no treatments to restore pancreatic function for CF patients with EPI. Clinicians have been limited to managing its aftereffects. In fact, many believed that with the very early onset of EPI in many CF patients, EPI was irreversible. Clinical studies with ivacaftor, a highly effective CFTR potentiator, have called this dogma into question. In both the ARRIVAL and KLIMB studies (13, 14), treatment of children with ivacaftor improved FE-1 measurements, some to >200 μg/g stool and others close to it. In the phase 3 single-arm ARRIVAL study, children 12–23 months (n ≥ 15) with at least one CFTR gating mutation received ivacaftor for 24 weeks. Mean ± standard deviation FE-1 increased from 182.2 ± 217.1 to 326.9 ± 152.1 μg/g stool (mean change: 164.7 ± 151.9 μg/g stool) (Figure 1). In the KLIMB study, the 84-week open label ivacaftor extension of the KIWI study, 28 children were analyzed for changes in FE-1. In the original KIWI study (24-week, single arm in children 2–5 years old), mean FE-1 increased by 99.8 ± 138.4 μg/g stool after 24 weeks of ivacaftor (15). With the KLIMB extension, at 84 weeks, the mean increase in FE-1 was 128.8 ± 428.9 μg/g stool (Figure 1) (14). Prior to initiation of ivacaftor during the KIWI study, only 6% of children had a FE-1 ≥ 200 μg/g stool (1/17), whereas after 84 weeks of ivacaftor, 35% (6/17) had FE-1 ≥ 200 μg/g stool. These studies provided some of the first data to suggest that EPI in CF may not be permanent. Of note, there was no discussion in these studies about the presence or absence of GI symptoms associated with EPI. The potential improvement of EPI on ivacaftor has also been noted in older children. Nichols et al. reviewed cases of older children on long-term ivacaftor and noted significant improvements in FE-1 (16).
Figure 1. Children treated with the CFTR potentiator ivacaftor experience an increase in exocrine pancreatic function as measured by FE-1.
Summary of data from the ARRIVAL, KIWI, and KLIMB clinical trials. The figure shows the mean (line) absolute increase in FE-1 before and after ivacaftor treatment. Box upper and lower limit lines represent 95% confidence intervals. Children in the ARRIVAL, KIWI, and KLIMB studies were treated with ivacaftor for 24, 24, and 84 weeks, respectively.
Exocrine Pancreatic Insufficiency Diagnosis by Imaging
Monitoring of exocrine pancreatic function in children using indirect and direct tests has recently been reviewed by the Pancreas Committee of the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) in discussing endoscopic pancreatic function testing (ePFT) (17). Beyond the stool, breath, and endoscopic tests discussed, new data has emerged examining exocrine pancreatic function in CF patients using ultrasound (US) and magnetic resonance imaging (MRI). Transabdominal US is the most common modality for assessing the pancreas in children given it is non-invasive, low cost, and broadly available. In examining B-mode US (i.e., grayscale US) characteristics in CF children and adults (n = 21), Engjom et al. reported that 90% of CF patients with EPI (CF-PI) (by FE-1 and bicarbonate concentration on ePFT) had pancreatic hyperechogenicity, whereas 27% of exocrine pancreatic sufficient CF (CF-PS) patients and 33% of healthy controls displayed pancreatic hyperechogenicity (18). Pfahler et al. also found increased pancreatic echogenicity in CF patients vs. healthy controls (56% vs. 7%, respectively), although they did not distinguish between pancreatic function status (19). In the former study, MRI was also performed to quantify pancreatic fat and showed that CF-PI patients had increased pancreatic fat compared to CF-PS patients (18). In a large population study in Western Germany that included abdominal MRI (SHIP: Study of Health in Pomerania), 139 adults with EPI were compared to 1319 individuals with normal pancreatic function and found that those with EPI had significantly higher pancreatic fat than healthy controls (20). In addition to B-mode US, advanced US techniques such as shear wave elastography and contrast-enhanced US have been used to examine pancreatic function in CF patients. Not surprisingly, given the increased pancreatic fat content of CF-PI patients, US shear wave elastography (which measures tissue stiffness) shows decreased values in CF-PI patients compared to healthy controls (19). In an additional study by Engjom et al., CF-PI patients displayed decreased mean capillary transit time and pancreatic blood flow compared to CF-PS and healthy patients (21).
MRI-based assessment of exocrine pancreatic function has been of increasing interest to pancreatologists with its potential ability to provide high-fidelity cross-sectional structural information together with organ function. The most common modality to do this is secretin-magnetic resonance cholangiopancreatography (s-MRCP). In CF, s-MRCP is able to distinguish CF-PI from CF-PS and healthy controls, shows good positive correlation with FE-1 (r = 0.84), and has excellent diagnostic performance in predicting EPI in CF patients when using an intestinal volume of <70 mL at 13 minutes post-secretin (AUROC = 0.95, sensitivity = 100%, specificity = 77%) (22). Secretin-stimulated US also performs well in identifying EPI in CF patients by measuring intestinal fluid area. S-US and s-MRCP results correlate well (r = 0.79); using a post-secretin peak intestinal volume of 2.5 cm2 or area under the curve of 30 cm2 with an AUROC of 1.0 (sensitivity = 1.0, specificity = 0.96) and 0.99 (sensitivity = 1.0, specificity = 0.91) (23). Therefore, while FE-1 remains the most common modality to assess EPI in CF patients, advanced US and MRI techniques, which provide both structural and functional data, are the new frontier in the assessment of pancreatic function (Table 1). What remains to be seen is which techniques will fare the best for monitoring changes in exocrine pancreatic function in children. Identifying modalities that can distinguish subtle changes in exocrine pancreatic function will be valuable in determining clinical effectiveness of CF therapies and better understanding pancreatic physiology.
Table 1:
Comparison of imaging modalities to assess exocrine pancreatic function with FE-1 testing.
| Stool | Ultrasound | MRI | |||||
|---|---|---|---|---|---|---|---|
| FE-1 | B-Mode | Elastography | Contrast | Secretin | MRCP | s-MRCP | |
| Non-Invasive | +++ | +++ | +++ | ++ | ++ | +/− | +/− |
| Low Cost | +++ | +++ | ++ | ++ | + | − | − |
| Widely Available | +++ | +++ | ++ | + | + | ++ | + |
| Pancreatic Function | +++ | + | ++ | ++ | +++ | + | +++ |
| Pancreatic Structure | − | + | ++ | ++ | ++ | +++ | +++ |
| Data in CF | +++ | +++ | + | + | + | +++ | ++ |
Number of “+” indicates strength of characteristics for specified testing modality. “+/−” = depends on age of child and whether or not anesthesia is necessary. Abbreviations: FE-1 = fecal elastase-1, MRI = magnetic resonance imaging, MRCP = magnetic resonance cholangiopancreatolography, s-MRCP = secretin-stimulated magnetic resonance cholangiopancreatolography, CF = cystic fibrosis.
PANCREATITIS IN CF
Pancreatitis has historically been considered rare in CF due to pancreatic parenchymal loss early in infant development. However, newborn screening, advancements in genetics, and CFTR modulator therapies have all increased the proportion of CF patients with pancreatitis. Newborn screening helps to identify CF patients even before symptoms may manifest. Likewise, the introduction of expanded genetic testing and full gene sequencing within and outside of newborn screening programs have greatly increased the identification of CF patients with minimal to mild symptoms, especially those without EPI, who would have otherwise escaped diagnosis until later in life. This expansion in the relative number of CF-PS patients has increased the number of CF patients that may develop pancreatitis. Nearly a decade ago, Ooi et al. put forward a model where individuals with extreme lack of CFTR function or near-normal CFTR function are unlikely to develop pancreatitis (Figure 2) (2). However, as CFTR dysfunction decreases, but is not eliminated, individuals’ risk for developing pancreatitis increases. CFTR mutations have been implicated in pancreatitis pathogenesis for decades and additional recent papers have further supported this (24–28). Recommendations and guidelines for the management of acute pancreatitis in children have been established (29, 30) and should generally be followed for CF children with pancreatitis.
Figure 2. Pancreatitis risk model based on degree of pancreatic function.
Ooi et al. put forward a conceptual model which described that individuals with none/minimal or near-normal CFTR function are unlikely to develop pancreatitis. However, those with residual CFTR function, even if impaired, are at risk for pancreatitis. Figure reproduced from original publication with no alterations (2).
Pancreatitis and CFTR Modulators in CF
Similar to EPI, as ivacaftor use has become more widespread, case reports/series have emerged describing CF-PI patients beginning to have pancreatitis and CF patients with recurrent pancreatitis experiencing a reduction in pancreatitis episodes. Due to the time delay in highly effective CFTR modulator therapy being accessible to young children, many of these reports have been in adults, however, data from children and adolescents is increasing with greater use of these drugs in children.
Petrocheilou et al. present a case of an adolescent with CF-PI (F508del/G551D) without a prior history of acute pancreatitis who experienced restoration of pancreatic function on ivacaftor (no symptoms off PERT, only used PERT for very fatty meals). Four years after starting ivacaftor therapy, the patient developed acute epigastric abdominal pain, nausea, and elevations in both amylase (2.0x upper limit of normal) and lipase (>3.3x upper limit of normal), consistent with a diagnosis of acute pancreatitis. Following standard acute pancreatitis management, patient was discharged, and continued on ivacaftor with no subsequent pancreatitis episodes two years later (31). This case not only further emphasizes that EPI can improve with CFTR modulator therapy, but also provides a caution to CF providers. Children with CF have a multitude of reasons to experience abdominal pain (e.g., gastroesophageal reflux disease, dysmotility, constipation, small intestinal bacterial overgrowth, distal intestinal obstruction syndrome). Pancreatitis must also be considered in the differential, not only for CF-PS patients, but also for CF-PI patients on CFTR modulator therapies.
Consistent with the Ooi et al. model in Figure 2, ivacaftor can improve the frequency of recurrent pancreatitis episodes in CF patients with Class III/IV residual function mutations. Recently, several case reports describe an improvement in the number of pancreatitis episodes experienced by adults treated with ivacaftor (32–35). These reports involve a small number of patients (range 1–15) but provide consistent evidence that when CFTR modulators shift CFTR function high enough, the risk of pancreatitis can be reduced. But what about children, do these studies only apply to adults? The case series by Carrion et al. of six patients involves two children (ages 11.5 and 13.6 years-old) and reports an improvement in number of pancreatitis episodes, hospitalizations, PERT use, and weight in these children, similar to that seen in the other four adults (32). These findings support animal studies, which have shown that treatment of mouse models of chronic pancreatitis with CFTR modulators restores CFTR expression in pancreatic ducts and reduces pancreatic inflammation (36). The case report by John and Rowe (35) describing the return of pancreatitis after stopping ivacaftor not only supports the role of ivacaftor in decreasing pancreatitis episodes, but also highlights that CFTR modulator therapies are needed long-term until more permanent therapies, such as gene therapy, can be developed. Gene therapy for CF remains an active area of investigation (37–41). However, to date, there have not been any published studies directing CFTR gene correction at the pancreas. While accessibility remains a hurdle to overcome, the idea that the pancreas in CF is beyond help is no longer an impediment to this venture.
ENDOCRINE – EXOCRINE INTERACTIONS IN CF
Cystic fibrosis-related diabetes (CFRD) is a highly prevalent complication of CF, occurring in up to 55% of adult males (42). Olesen et al. identified that CFRD increases with age, occurring in 0.8% of those <10 year old, 9.7% in 10–19 years olds, 24.1% in 20–29 year-olds and 32.7% in ≥30 year olds (between 2008–2013 in Europe) (42). In a pediatric-specific epidemiology study of CFRD from 2000–2016, Perrem et al. identified a CFRD prevalence of 8.5% in children 10–18 years old in Canada (43). While CFRD prevalence is relatively low in children, using continuous glucose monitoring, Prentice et al. identified that serum glucose abnormalities are common in children as young as 2–6 years old (44).
There has long been debate on whether CFRD is a result of primary islet dysfunction or occurs secondary to inflammation from exocrine pancreatic dysfunction. Two recent studies examining CFTR expression and function in human pancreatic islets have provided convincing evidence that CFTR is almost exclusively expressed in exocrine ductal cells. Using a combination of in situ hybridization and immunohistochemistry, White et al. identified that CFTR mRNA was expressed in only 0.5% of insulin-positive cells, while CFTR protein could not be detected in any beta cells (45). Shik Mun et al. also showed that CFTR protein is expressed in ductal cells, not islets. Furthermore, in developing a “pancreas-on-a-chip,” consisting of both human exocrine ductal cells and endocrine islets, Shik Mun et al. identified that pharmacologic inhibition of CFTR function in ductal cells caused a decrease in insulin secretion from islets, likely through ductal cell to islet communication (46). Recent studies showing that endocrine and exocrine microcirculation are tightly intercalated (47) and that insulin levels affect exocrine acinar cell stress (48) provide new and compelling evidence that our historical separation of the exocrine and endocrine pancreas is flawed. Future research needs to examine the endocrine and exocrine functions of the pancreas in parallel to better understand how loss of CFTR function may affect both nutrition and diabetes in CF.
Conclusion
Pancreatic complications contribute significantly to the health care burden of many children with CF. Previously thought to be binary (CF-PI or CF-PS), in today’s spectrum of CFTR mutations, the diagnosis and monitoring of pancreatic function can be complex and, based on emerging evidence, may require consideration of both exocrine and endocrine function. FE-1 measurements are not absolute or fixed. They should be considered in the context of patient symptoms and may change due to the natural history of disease and therapeutic interventions, especially CFTR modulator therapies. The toolbox to measure pancreatic function is expanding and advanced imaging may play an increasing role. Recognition of pancreatitis in CF is important and prompt treatment should be implemented through partnerships between gastroenterologists/pancreatologists and CF providers. Now, over 80 years after Dorothy Andersen’s first description of the pancreatic manifestations of CF, pancreatic disease in CF children is once again at the forefront of CF care.
Key Points.
Exocrine pancreatic insufficiency in children with CF is not fixed and may improve, or even resolve, in the presence of highly effective CFTR modulator therapy.
Continued advancements in US and MR imaging tools may provide both structural and functional assessment of the pancreas in patients with CF.
CF children should be monitored for pancreatitis when presenting with abdominal pain, even if thought to be exocrine pancreatic insufficient. CF patients with recurrent pancreatitis may improve on highly effective CFTR modulators.
Endocrine dysfunction may occur early on in childhood in CF.
Endocrine and exocrine functions of the pancreas are tightly interlinked through multi-faceted anatomical and functional communications and should be assessed in parallel.
Acknowledgments
Financial Support and Sponsorship: Dr. Sellers is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K08-DK120939), CF Foundation (SELLER16L0), North American Pediatric Gastroenterology, Hepatology, and Nutrition Foundation, and Stanford University.
Footnotes
Conflicts of Interest: Dr. Sellers is a member of the NASPGHAN Pancreas Committee. None of the financial support listed above contributed to or influenced the content of this manuscript.
References
- 1.Andersen DH. Cystic Fibrosis of the Pancreas and its Relation to Celiac Disease: A Clinical and Pathological Study. Am J Dis Child. 1938;56(2):344–99. [Google Scholar]
- 2.Ooi CY, Dorfman R, Cipolli M, Gonska T, Castellani C, Keenan K, et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011;140(1):153–61. [DOI] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation Patient Registry 2016 Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2017. [Google Scholar]
- 4.O’Sullivan BP, Baker D, Leung KG, Reed G, Baker SS, Borowitz D. Evolution of pancreatic function during the first year in infants with cystic fibrosis. J Pediatr. 2013;162(4):808–12 e1. [DOI] [PubMed] [Google Scholar]
- 5.Brownell JN, Bashaw H, Stallings VA. Growth and Nutrition in Cystic Fibrosis. Semin Respir Crit Care Med. 2019;40(6):775–91.* This review discusses the various mechanisms whereby growth and nutrition can be impaired in CF.
- 6.Pratha VS, Hogan DL, Martensson BA, Bernard J, Zhou R, Isenberg JI. Identification of transport abnormalities in duodenal mucosa and duodenal enterocytes from patients with cystic fibrosis. Gastroenterology. 2000;118(6):1051–60. [DOI] [PubMed] [Google Scholar]
- 7.Zoppi G, Shmerling DH, Gaburro D, Prader A. The electrolyte and protein contents and outputs in duodenal juice after pancreozymin and secretin stimulation in normal children and in patients with cystic fibrosis. Acta Paediatr Scand. 1970;59(6):692–6. [DOI] [PubMed] [Google Scholar]
- 8.Gelfond D, Ma C, Semler J, Borowitz D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci. 2013;58(8):2275–81. [DOI] [PubMed] [Google Scholar]
- 9.Cox KL, Isenberg JN, Ament ME. Gastric acid hypersecretion in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1982;1(4):559–65. [DOI] [PubMed] [Google Scholar]
- 10.Guven B, Demir Mis M, Karaman K, Sahin Yasar A. Effectivity of Pancreatic Enzyme Replacement Therapy in Malnourished Children. J Pediatr Gastroenterol Nutr. 2020. [DOI] [PubMed] [Google Scholar]
- 11.Layer P, Kashirskaya N, Gubergrits N. Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency. World journal of gastroenterology : WJG. 2019;25(20):2430–41.* Most commonly research on PERT examines effectiveness of fat absorption and/or weight gain, however, this paper examines the effect of PERT on mortality and quality of life.
- 12.Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142(6):624–30. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld M, Wainwright CE, Higgins M, Wang LT, McKee C, Campbell D, et al. Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study. Lancet Respir Med. 2018;6(7):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfeld M, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. An open-label extension study of ivacaftor in children with CF and a CFTR gating mutation initiating treatment at age 2–5years (KLIMB). Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2019;18(6):838–43.** This open-label extension of the KIWI study examines the effects of extended use of ivacaftor (84 weeks) in CF children with gating mutations on liver function tests, sweat chloride, weight/body mass index, and FE-1 measurements. Results indicated that FE-1 improvements on ivacaftr were sustained over the duration of the study.
- 15.Davies JC, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4(2):107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols AL, Davies JC, Jones D, Carr SB. Restoration of exocrine pancreatic function in older children with cystic fibrosis on ivacaftor. Paediatr Respir Rev. 2020.* A case series showing that similar to young children, exocrine pancreatic insufficiency can be restored in older children with CF by ivacaftor.
- 17.Patel N, Sellers ZM, Grover A, Liu QY, Maqbool A, Morinville VD, et al. Endoscopic Pancreatic Function Testing (ePFT) in Children: a Position Paper from the NASPGHAN Pancreas Committee. J Pediatr Gastroenterol Nutr. 2020;In press.* This Position Paper describes the use of endoscopic pancreatic function testing (ePFT) in children and provides a sample protocol on how to perform ePFT in children.
- 18.Engjom T, Kavaliauskiene G, Tjora E, Erchinger F, Wathle G, Laerum BN, et al. Sonographic pancreas echogenicity in cystic fibrosis compared to exocrine pancreatic function and pancreas fat content at Dixon-MRI. PloS one. 2018;13(7):e0201019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfahler MHC, Kratzer W, Leichsenring M, Graeter T, Schmidt SA, Wendlik I, et al. Point shear wave elastography of the pancreas in patients with cystic fibrosis: a comparison with healthy controls. Abdom Radiol (NY). 2018;43(9):2384–90. [DOI] [PubMed] [Google Scholar]
- 20.Kromrey ML, Friedrich N, Hoffmann RT, Bulow R, Volzke H, Weiss FU, et al. Pancreatic Steatosis Is Associated With Impaired Exocrine Pancreatic Function. Invest Radiol. 2019;54(7):403–8. [DOI] [PubMed] [Google Scholar]
- 21.Engjom T, Nylund K, Erchinger F, Stangeland M, Laerum BN, Mezl M, et al. Contrast-enhanced ultrasonography of the pancreas shows impaired perfusion in pancreas insufficient cystic fibrosis patients. BMC Med Imaging. 2018;18(1):14.** This study used contrast-enhanced US to examine the perfusion of the pancreas in CF patients in order to determine if this technique can be used to evaluate pancreatic function. They identified longer mean capillary transit-time, lower blood blow and blood volume in CF-PI patients compared to CF-PS patients and non-CF healthy controls.
- 22.Engjom T, Tjora E, Erchinger F, Madzak A, Dimcevski G, Frokjaer JB, et al. Secretin-Stimulated Magnetic Resonance Imaging Reveals Variable Diagnostic Accuracy According to Etiology in Pancreatic Disease. Pancreas. 2020;49(3):361–7.** This study evaluated the ability to determine exocrine pancreatic function by measuring intestinal fluid volume from secretin-MRCP. S-MRCP measurements were compared from CF patients, chronic pancreatitis patients, and healthy controls. EPI was defined by FE-1 values and pancreatic bicarbonate concentration obtained by ePFT. The authors report different accuracy in identifying EPI between CF and CP, with s-MRCP being most accurate in CF.
- 23.Engjom T, Tjora E, Wathle G, Erchinger F, Laerum BN, Gilja OH, et al. Secretin-stimulated ultrasound estimation of pancreatic secretion in cystic fibrosis validated by magnetic resonance imaging. Eur Radiol. 2018;28(4):1495–503.* The authors compare pancreatic volume output between s-US and s-MRCP in CF patients and found that s-US has comparable results to s-MRCP for the estimation of exocrine pancreatic function in CF patients.
- 24.Nabi Z, Talukdar R, Venkata R, Aslam M, Shava U, Reddy DN. Genetic Evaluation of Children with Idiopathic Recurrent Acute Pancreatitis. Dig Dis Sci. 2020. [DOI] [PubMed] [Google Scholar]
- 25.Baldwin C, Zerofsky M, Sathe M, Troendle DM, Perito ER. Acute Recurrent and Chronic Pancreatitis as Initial Manifestations of Cystic Fibrosis and Cystic Fibrosis Transmembrane Conductance Regulator-Related Disorders. Pancreas. 2019;48(7):888–93.** Retrospective review of children and young adults with acute recurrent/chronic pancreatitis in patients with CF, CF-related metabolic syndrome, and CFTR-related disorder. Half of the subjects had at least one other CF manifestation (e.g. sinusities, nasal polyps, pneumonia). This study highlights the importance of extensive CFTR genetic and functional testing in patients with acute recurrent/chronic pancreatitis.
- 26.Abu-El-Haija M, Valencia CA, Hornung L, Youssef N, Thompson T, Barasa NW, et al. Genetic variants in acute, acute recurrent and chronic pancreatitis affect the progression of disease in children. Pancreatology. 2019;19(4):535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iso M, Suzuki M, Yanagi K, Minowa K, Sakurai Y, Nakano S, et al. The CFTR gene variants in Japanese children with idiopathic pancreatitis. Hum Genome Var. 2019;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou WB, Tang XY, Zhou DZ, Qian YY, Hu LH, Yu FF, et al. SPINK1, PRSS1, CTRC, and CFTR Genotypes Influence Disease Onset and Clinical Outcomes in Chronic Pancreatitis. Clinical and translational gastroenterology. 2018;9(11):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-El-Haija M, Kumar S, Quiros JA, Balakrishnan K, Barth B, Bitton S, et al. Management of Acute Pancreatitis in the Pediatric Population: A Clinical Report From the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee. J Pediatr Gastroenterol Nutr. 2018;66(1):159–76.** This Clinical Report from the NASPGHAN Committee provides expert recommendations and guidelines on the management of acute pancreatitis in children. Generally, these principles should be applied to children with CF presenting with acute (or acute on chronic) pancreatitis.
- 30.Sellers ZM, Dike C, Zhang KY, Giefer MJ, Uc A, Abu-El-Haija M. A Unified Treatment Algorithm and Admission Order Set for Pediatric Acute Pancreatitis. J Pediatr Gastroenterol Nutr. 2019;68(6):e109–e11.* This Letter to the Editor supplements reference 29 by providing a treatment algorithm and admission order set based on the principles set forward in the NASPGHAN Clinical Report and the expertise among pancreatologists at several tertiary care pediatric centers.
- 31.Petrocheilou A, Kaditis AG, Loukou I. Pancreatitis in A Patient with Cystic Fibrosis Taking Ivacaftor. Children (Basel). 2020;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrion A, Borowitz DS, Freedman SD, Siracusa CM, Goralski JL, Hadjiliadis D, et al. Reduction of Recurrence Risk of Pancreatitis in Cystic Fibrosis With Ivacaftor: Case Series. J Pediatr Gastroenterol Nutr. 2018;66(3):451–4.** This retrospective review examined the incidence of pancreatitis, hosptializations, opiate use, weight, FE-1, and PERT use before and after ivacaftor therapy. Two out of six subjects were children. None of the patients had recurrent of pancreatitis or hospitalization after beginning ivacaftor.
- 33.Kounis I, Levy P, Rebours V. Ivacaftor CFTR Potentiator Therapy is Efficient for Pancreatic Manifestations in Cystic Fibrosis. Am J Gastroenterol. 2018;113(7):1058–9. [DOI] [PubMed] [Google Scholar]
- 34.Akshintala VS, Kamal A, Faghih M, Cutting GR, Cebotaru L, West NE, et al. Cystic fibrosis transmembrane conductance regulator modulators reduce the risk of recurrent acute pancreatitis among adult patients with pancreas sufficient cystic fibrosis. Pancreatology. 2019;19(8):1023–6. [DOI] [PubMed] [Google Scholar]
- 35.Johns JD, Rowe SM. The effect of CFTR modulators on a cystic fibrosis patient presenting with recurrent pancreatitis in the absence of respiratory symptoms: a case report. BMC Gastroenterol. 2019;19(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng M, Szymczak M, Ahuja M, Zheng C, Yin H, Swaim W, et al. Restoration of CFTR Activity in Ducts Rescues Acinar Cell Function and Reduces Inflammation in Pancreatic and Salivary Glands of Mice. Gastroenterology. 2017;153(4):1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erwood S, Laselva O, Bily TMI, Brewer RA, Rutherford AH, Bear CE, et al. Allele-Specific Prevention of Nonsense-Mediated Decay in Cystic Fibrosis Using Homology-Independent Genome Editing. Mol Ther Methods Clin Dev. 2020;17:1118–28.** There are currently no FDA-approved drugs for the treatment of nonsense CFTR mutations in CF. The authors used CRISPR/Cas9 to introduce a deletion in the downstream genic region following the premature stop codon. This resulted in increased expression of W1282X protein and CFTR channel activity in human bronchial epithelial cells. Although these increases were modest, this approach represents a new strategy towards a genetic therapy for those CF patients with nonsense mutations.
- 38.Suzuki S, Crane AM, Anirudhan V, Barilla C, Matthias N, Randell SH, et al. Highly Efficient Gene Editing of Cystic Fibrosis Patient-Derived Airway Basal Cells Results in Functional CFTR Correction. Mol Ther. 2020.** The authors used zinc-finger nucleases to correct CFTR mutations through two strategies, 1) correction of the specific F508del mutation, and 2) insertion of a partial cDNA in order to provide more generalizable correction to other CFTR mutations. Using zinc-finger nuclease mRNA and AAV delivery of ds-DNA to primary human bronchial epithelial cells, the authors showed correction of 31% of F508del alleles resulting in restoration of CFTR protein and 40% of non-CF short-circuit current. The authors were able to achieve similar gene correction rates with insertion of partial CFTR cDNA (in intron 8) in F508del homozygous and G542X/R785X airway cells, although the latter had less restoration of CFTR current. This research complements that done by others (references 37, 39 and 40) using TALEN and CRISPR/Cas9 to provide additional CFTR gene editing tools for future therapeutics.
- 39.Fleischer A, Vallejo-Diez S, Martin-Fernandez JM, Sanchez-Gilabert A, Castresana M, Del Pozo A, et al. iPSC-Derived Intestinal Organoids from Cystic Fibrosis Patients Acquire CFTR Activity upon TALEN-Mediated Repair of the p.F508del Mutation. Mol Ther Methods Clin Dev. 2020;17:858–70.* The authors used transcription activator-like effector nuclease (TALEN)-mediated homologous recombination to correct F508del mutations in patient-derived iPSCs. Unlike that done in references 38 and 40, this method required selection of edited clones prior to the generation of epithelial cells. Editing effect on CFTR function was verified by the forskolin-induced swelling assay using intestinal organoids derived from edited and non-edited iPSCs.
- 40.Vaidyanathan S, Salahudeen AA, Sellers ZM, Bravo DT, Choi SS, Batish A, et al. High-Efficiency, Selection-free Gene Repair in Airway Stem Cells from Cystic Fibrosis Patients Rescues CFTR Function in Differentiated Epithelia. Cell Stem Cell. 2020;26(2):161–71 e4.** This study used CRISPR/Cas9 and AAV delivery of ds-DNA for homologous recombination repair of F508del alleles in nasal and bronchial cultures from CF and non-CF patients. We achieved correction of the F508del mutation in ~40% of alleles. This resulted in restoration of CFTR function in differentiated airway epithelial cell monolayers to ~31% of non-CF patients.
- 41.Maule G, Casini A, Montagna C, Ramalho AS, De Boeck K, Debyser Z, et al. Allele specific repair of splicing mutations in cystic fibrosis through AsCas12a genome editing. Nat Commun. 2019;10(1):3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olesen HV, Drevinek P, Gulmans VA, Hatziagorou E, Jung A, Mei-Zahav M, et al. Cystic fibrosis related diabetes in Europe: Prevalence, risk factors and outcome; Olesen et al. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2019.** A retrospective review of the European CF Patient Registry from 2008–2015 to examine the prevalence of CFRD in children and adults in 2015. Established an overal prevalence of 21.6% across all age groups, but identified variable prevalence with lowest in the <10 year-old group and highest in the ≥30 year-old group.
- 43.Perrem L, Stanojevic S, Solomon M, Carpenter S, Ratjen F. Incidence and risk factors of paediatric cystic fibrosis-related diabetes. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2019;18(6):874–8.* This retrospective study evaluates the incidence of CFRD in children (ages 10–18 years old) listed in the Canadian CF Registry from 2000 to 2016. Their findings are similar to that found in the reference 42.
- 44.Prentice BJ, Ooi CY, Verge CF, Hameed S, Widger J. Glucose abnormalities detected by continuous glucose monitoring are common in young children with Cystic Fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2020.* The authors use continuous glucose monitoring in children <10 years old to identify that glucose abnormalities are present in young childen, ages 2–6 years old. While the diagnosis of CFRD is low in this age group, pathogenesis of diabetes may be occurring early in life.
- 45.White MG, Maheshwari RR, Anderson SJ, Berlinguer-Palmini R, Jones C, Richardson SJ, et al. In Situ Analysis Reveals That CFTR Is Expressed in Only a Small Minority of beta-Cells in Normal Adult Human Pancreas. J Clin Endocrinol Metab. 2020;105(5).* Used a combination of immunohistochemistry and in situ hybridization to examine the localization of CFTR protein or mRNA, respectively, in human and ferret beta and ductal cells. Identified that CFTR expression is confined to ductal cells.
- 46.Shik Mun K, Arora K, Huang Y, Yang F, Yarlagadda S, Ramananda Y, et al. Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat Commun. 2019;10(1):3124.** This study took a unique approach to studying the interactions between the exocrine and endocrine pancreas. The authors developed a two-channel chip where they grew human pancreatic ductal cells on one side of a porous membrane and human islets on the other side. Samples were obtained from patients undergoing total pancreatectomy islet autotransplantation. By manipulating conditions on either the ductal or islet side they could study the effect on the opposite cell type (which was not directly manipulated).
- 47.Dybala MP, Kuznetsov A, Motobu M, Hendren-Santiago BK, Philipson LH, Chervonsky AV, et al. Integrated Pancreatic Blood Flow: Bi-Directional Microcirculation Between Endocrine and Exocrine Pancreas. Diabetes. 2020.** This paper examines the micro-circulation of mouse and human islets. Flow analysis shows that islets and exocrine components share an intertwined network of capillaries. Using directional flow analysis the authors describe the blood flow patterns into and out of islets and how this interfaces with components of the exocrine pancreas. Multiple models of islet blood flow are described.
- 48.Yatchenko Y, Horwitz A, Birk R. Endocrine and exocrine pancreas pathologies crosstalk: Insulin regulates the unfolded protein response in pancreatic exocrine acinar cells. Exp Cell Res. 2019;375(2):28–35.* This study examined the effect of insulin concentrations on ER stress unfolded protein response on acinar cells. They found that when acinar cells were exposed to high levels of insulin, a host of proteins associated with the ER stress unfolded protein response were upregulated, suggesting that insulin levels may alter the health of acinar cells.