Abstract
BACKGROUND:
ABO compatibility can affect platelet transfusion safety and efficacy, and ABO-incompatible (ABOi) platelets likely increases the risks of transfusion reactions though the magnitude of this risk is unclear.
STUDY DESIGN AND METHODS:
Data collected on all platelet transfusions administered over 36+ months were classified based on patient and product ABO blood group type and merged with a data set that included all transfusion reactions reported during that period. The transfusion reaction rates among various subsets was calculated.
RESULTS:
In patients greater than 1 year of age, the transfusion reaction rate in the ABO-compatible (ABO-identical) platelet group was 1.0%, while the ABOi platelet group had an elevated reaction rate of 1.7%. The increased reaction rate for ABOi platelets held true even if the analysis were limited to Centers for Disease Control and Prevention/National Healthcare Safety Network qualifying reactions or just allergic or febrile nonhemolytic reactions. The increased reaction rate with ABOi platelets was independent of unit age. Surprisingly, major-incompatible transfusions (A/B antigen incompatible) had the highest rate of reactions, at 2.0%. During the study period, three acute hemolytic reactions were reported out of 2522 plasma-incompatible platelet transfusions (0.12%).
CONCLUSIONS:
Our results find that compatible platelet transfusions have the lowest rate of transfusion reactions. While hemolytic reactions were observed with plasma-incompatible transfusions, the rate was low. Transfusion of ABO antigen-incompatible platelets had the highest rate of transfusion reactions and resulted in a transfusion reaction rate 1.5 to 2 times that of ABO compatible transfusions.
Similar to red blood cells (RBCs), ABO blood group antigens are expressed on the surface of platelets, possibly allowing ABO compatibility to affect platelet transfusion outcomes. In contrast to RBCs, most apheresis platelets also contain substantial amounts of plasma, which may contain anti-A or anti-B antibodies that could also lead to complications following transfusion of ABO-incompatible platelets. For the purposes of this study, ABO-compatible describes a donor-recipient pair with the identical ABO type while ABO-incompatible (ABOi) describes a donor-recipient pair with different ABO types. The latter term encompasses the following three subcategories of incompatibility: ABO minor-incompatible (donor plasma containing antibodies against ABO blood group antigens on recipient RBC and platelets), ABO major-incompatible (recipient plasma containing antibodies against ABO blood group antigens on donor RBC and platelets), and ABO bidirectional-incompatible (donor and recipient plasma both containing antibodies against ABO blood group antigens on each other’s RBC and platelets).
ABO-compatible platelet transfusions are generally considered optimal, but providing ABO-compatible platelets to all patients is often considered impractical or impossible and would result in increased product wastage.1,2 Previous studies have shown that ABO-compatible platelets provide a moderately higher platelet increment than ABOi platelets.3 ABO-identical platelets have also been shown to reduce the incidence of platelet refractoriness and HLA antibody production in patients undergoing treatment for leukemia or Hodgkin’s lymphoma.4 Regarding transfusion reactions, multiple case reports have described hemolytic reactions following the transfusion of ABO minor-incompatible platelets.5,6 Nonetheless, ABOi platelet transfusions remain common, and while the potential risks are generally recognized, the relative risk (or odds ratio) of transfusion reactions in the setting of ABOi platelet transfusions is unknown.1,7 The primary purpose of this study was to determine the rate of transfusion reactions with ABOi platelet transfusions compared to ABO-compatible platelets and determine what types of reactions are observed with ABOi platelet transfusions. Finally, the type of incompatibility was examined to determine if this impacted the transfusion reaction rate or not.
MATERIALS AND METHODS
Study overview
This retrospective study was approved by the institutional review board (IRB 201811791) at our large academic medical center, which encompasses a Level 1 trauma center, solid-organ transplantation, hematopoietic stem cell transplantation, and a pediatric hospital with a neonatal intensive care unit. Electronic medical records (EMRs; EPIC Systems Corporation) and blood bank laboratory information system (LIS; Haemonetics) data at our institution were reviewed from October 2015 (when the LIS was implemented) through November 21, 2018. All platelets transfused during this period were leukoreduced apheresis components, and anti-A or anti-B titers were not performed. In May 2018, Verax testing was implemented, and a small fraction of platelets were issued on Day 6 or 7 after that date. Similar to other institutions,1 ABO-compatible platelets are prioritized for infants and ABO-identical units are the first choice for all patients when inventory allows. The blood bank policy prioritizes giving ABO-identical platelets when other factors are equivalent. This policy prioritizes giving antigen-incompatible units (e.g., AB to A) versus plasma-incompatible units (e.g., O to A). Despite this policy, ABOi platelets were commonly provided largely based on inventory and platelet expiration dates. All transfusion reactions reported to the blood bank were systematically evaluated, and hard copies of the laboratory investigations were available for review. Electronic reports from the LIS and EMR were merged and used to compile data on all platelet transfusions and all transfusion reactions during the study. Our policy is to prioritize giving neonates ABO-compatible platelets, and the compatibility percentage in children less than 1 year of age was much higher (77%) than in other age groups. In addition, reaction rates in children less than 1 year of age was quite low, with only one reaction reported in 873 transfusions. For these reasons, transfusion occurring in patients less than 1 year of age were excluded from the analysis. An overview of the study design is shown in Fig. 1.
Fig. 1.

Study design and data acquisition. This schematic summarizes how the ABO compatibility for all platelet transfusions between October 2015 and November 2018 was obtained and then merged with transfusion reaction data that allowed the determination of reaction rates for platelet transfusions during this time frame.
Compatibility of transfused platelets
The first data set was generated from a custom issue/return product report from the LIS, which included all the blood products issued by the blood bank, along with their final disposition (returned, transfused, wasted). The report also provided each patient’s medical record number (MRN). This report indicated that 14,338 platelet units were transfused during the study to 2826 unique patients. ABO blood group types of the platelet unit and the patient were merged into the spreadsheet from two different sources. Patient ABO types and date of birth were obtained via a report from the EMR, which includes all ABO types reported during the time of the study. Duplicates sharing both the same MRN and same ABO type were removed, and then all MRNs with one unique ABO type were merged into the platelet transfused report. Patients with more than one unique ABO blood type recorded during the study period were identified, most of whom had undergone an ABOi hematopoietic stem cell transplant. Patients with more than one unique ABO type were entered into the report manually, based on their ABO type at the time of each transfusion. In a few cases, platelets were transfused between dates when the ABO type of the patient changed. In those instances, the patient ABO type was listed as unknown for that transfusion, and the ABO compatibility of the platelet transfusion was also listed as unknown. Rarely, no ABO type was available or performed (such as with emergency-released blood products), and the compatibility of these transfusions was also listed as unknown. In all, just 19 of the 14,338 platelet transfusions fell into this category of unknown compatibility. None of these 19 transfusions were associated with a transfusion reaction. The ABO types of the transfused platelet units were obtained from a separate LIS report. These data were exported into a spreadsheet and then merged with the platelet transfusion data described above.
Compatibility of platelet transfusions can be classified by a variety of schemes. For this study, we used the classification scheme illustrated in Fig. 2. Only ABO-identical transfusions were classified as compatible. Plasma-incompatible transfusions were classified as minor incompatible, while A/B antigen-incompatible transfusions were classified as major incompatible. Transfusions that were incompatible for both plasma and A/B antigens were classified as bidirectional incompatible (A into B or B into A).
Fig. 2.

Compatibility chart used for this study. This chart shows how the 14,319 platelet transfusions between October 2015 and November 2018 into patients with known ABO type at the time of the transfusion were classified. Compatible (Comp) is defined as ABO identical. Major incompatible (Major) includes transfusion where the product contains A or B antigens not found in the patient. Minor incompatible (Minor) includes transfusions where the product contains anti-A or anti-B antibodies that are incompatible with the patient’s ABO type. Bidirectional (Bidir) means the transfusion contains both incompatible antigens and incompatible antibodies.
Transfusion reactions
The other major data set used in this study included all suspected transfusion reactions reported to the blood bank during the study period. Per protocol at our institution, when a suspected transfusion reaction is ordered, a patient blood sample is drawn and sent to the blood bank to repeat ABO-Rh blood typing and perform a direct antiglobulin test. A posttransfusion specimen of the patient’s plasma is also examined for evidence of hemolysis. Suspected transfusion reactions are classified by physicians on the transfusion medicine service following the Centers for Disease Control and Prevention (CDC) and National Healthcare Safety Network (NHSN) guidelines for classification of transfusion reactions.8 These results were manually entered into a spreadsheet from paper files maintained in the blood bank for every reaction. A total of 476 transfusion reactions were reported during the study period, 165 of which involved a single platelet transfusion. Twelve of the 476 transfusion reactions involved multiple blood products, including at least one platelet transfusion. A review of those reactions by two of the authors (CMK and NM) clearly implicated a single platelet product in 3 of the 12 transfusions. In total, 168 transfusion reactions were attributed to platelet transfusions in 132 unique patients, and 167 of these reactions were to subjects greater than 1 year of age and were included in the analysis. These transfusion reactions were categorized as follows: acute hemolytic (AHTR; N = 3), allergic (N = 72), febrile nonhemolytic (FNHTR; N = 51), transfusion-associated circulatory overload (TACO; N = 1), transfusion-associated dyspnea (TAD; N = 4), transfusion-related acute lung injury (TRALI; N = 1). A total of 35 reactions did not meet criteria for a specific transfusion reaction as defined by the CDC/NHSN guidelines and were classified as unknown/unrelated. Reactions were not routinely graded for severity, and this information was not included in the initial data acquisition. As allergic reactions are often clinically mild and the CDC/NHSN has stopped tracking minor allergic reactions, the severity of allergic reactions was determined by one of the authors (CMK). Reactions were determined to be severe following CDC/NHSN criteria, and severe reactions generally involved symptoms of laryngeal edema, dyspnea, or oxygen desaturation. A total of 18.1% of the allergic reactions were classified as severe.
Platelet unit age
The expiration date of the transfused platelet units was obtained from the LIS report, which also included ABO type. This date was used to calculate the age of the unit at the time it was issued from the blood bank based on a 5-day expiration date for nearly all platelets transfused during this time. For units that had a modified expiration date due to preparation of aliquots from the unit, washing, or rapid bacterial testing, the age of the unit was calculated using the collection date of the unit that was available within the LIS.
Statistical analysis
We estimated that about 33% of platelet transfusions would be ABOi and that transfusion reaction rates would be about 1%. Using a power calculator for dichotomous endpoints, we estimated that we would need to review at least 5000 platelet transfusions to detect an increase in transfusion reaction rates to 2%. However, we designed the study to be as large as possible in the time frame indicated to allow detection of relatively modest changes in reaction rates and allow for subset analysis based on specific type of incompatibility and type of reaction. Transfusion reaction rates for the compatible versus the ABOi transfusions were compared using the chi-square test.9 This test compares the “expected” number of events for that group to the actual number of events observed. This test was only applied when the “expected” number of events for each group was at least five.10 The transfusion reaction rate for the compatible platelet transfusions was used to determine the expected number of events within each group. P values less than 0.05 were considered statistically significant. Univariate logistic regression analysis was conducted to determine the odds ratio (and 95% confidence interval) for transfusion reactions, CDC/NHSN qualifying reactions, FNHTRs and allergic reactions with platelet transfusion compatibility, patient age, patient ABO type, unit ABO type, and sex. These analyses were performed with computer software (SAS, version 9.4, SAS Institute). If the 95% confidence interval did not overlap with 1.0, the results were considered statistically significant.
RESULTS
Platelet transfusion compatibility rates
During the study period, 13,447 platelet transfusions to patients greater than 1 year of age could be classified for ABO compatibility. Of these, 61.7% of platelet transfusions were compatible (ABO identical), 19.7% were major incompatible, 14.1% were minor incompatible, and 4.5% were bidirectional incompatible. These results are similar to reported rates at other institutions.1,2 When compatibility rates were evaluated by patient blood type, blood type A and type O patients were much more likely to receive compatible platelets, compared to B or AB patients (data not shown). Reaction rate for females (1.20%) was not statistically different than that observed for males (1.27%).
Transfusion reaction rates for ABO compatible versus ABOi platelets
The 167 transfusion reactions included in the study resulted in an overall reaction rate of 1.24%. This includes all reactions reported to the blood bank, including those that would be classified as unrelated or “unknown” by CDC/NHSN definitions.8 This reaction rate is similar to those reported by others, and the rates of allergic reactions (0.53%) or FNHTRs (0.38%) are consistent with US hemovigilance reports.11 Hemolytic reactions (0.02%) and respiratory reactions (TACO, TRALI, TAD; 0.04%) were also observed. Suspected reactions that did not meet CDC/NHSN criteria were classified as unknown/unrelated and occurred at a rate of 0.26%.
Transfusion reaction rates were determined based on the compatibility of the platelet transfusion. Patients who received compatible platelet transfusions had the lowest rate of transfusion reactions. This was true whether all reactions were included, when only reactions that met CDC/NHSN criteria were included, or when just FNHTRs or allergic reactions were examined (Fig. 3). As allergic reactions are often mild, we graded these reactions for severity using CDC/NHSN criteria. Of note, the allergic reactions with ABOi platelets were significantly more likely to be classified as severe (26.3%) compared to ABO-compatible allergic reactions (8.8% severe; p < 0.01).
Fig. 3.

Reaction rates for ABO compatible (Comp) and ABO-incompatible (ABOi) reactions reported to the blood bank (A), for those reactions that met CDC/NHSN criteria (C), or an allergic reaction (D) are shown. For each of these analyses, the number of significantly higher (p < 0.001) than the number of reactions observed for the compatible transfusions. The reaction rate for all transfusion (B), for those that met criteria for a FNHTR observed reactions for the ABOi group was group.
Unit age and reaction rate
One potential explanation for the higher reaction rates observed in ABOi platelet transfusions may be that these transfusions are more likely to be older units that are closer to the 5-day expiration of the product. This is the point at which the blood bank must decide whether to risk outdating a 5-day platelet by issuing a younger ABO-compatible platelet to a patient. Thus, we analyzed whether unit age could account for the increased reaction rates in ABOi platelets. As expected, most units issued to the subjects studied were issued on Day 3, 4, or 5, with more than 96% of the units issued to patients in this window (Fig. 4A). The percentage of compatible units decreased slightly as age of the units increased, going from 69% on Day 3 to 58% on Day 5 (Fig. 4B). Because so few platelets were issued before or after these time points, only reaction rates for platelets issued on Days 3 to 5 were calculated. While overall reaction rates did increase with unit age, ABOi platelets consistently had a higher reaction rate than ABO compatible platelets at each time point analyzed (Fig. 4C). Chi-square analysis for the platelets transfused on Day 5 showed that reaction rates for ABOi platelets were significantly different than for compatible platelets (p < 0.0001). These results demonstrate that transfusion reaction rates are independently affected by platelet unit age and by ABO incompatibility.
Fig. 4.

Increased reaction rates with ABOi platelets is independent of platelet unit age at time of issue. (A) The number of units transfused versus unit age. (B) The percentage of ABO-compatible units transfused versus unit age. (C) Transfusion reaction rates versus unit age between 3 and 5 days is shown for all units (middle line), compatible units (bottom line), and ABOi units (top line). The reaction rates for platelets transfused on Day 1 (13), Day 2 (304), Day 6 (136) and Day 7 (28) are not shown due to the small number of transfusions on those dates. The reaction rates for all platelets on Days 3–5 were compared using the chi-square test and Day 3 versus Day 4 (P = 0.009), Day 3 versus Day 5 (p = 0.002), and Day 4 versus Day 5 (p = 0.011) were all significantly different.
Hemolytic reactions
We also determined the rates of specific types of transfusion reactions in the different compatibility categories. While only three acute hemolytic reactions were observed, two of these occurred with minor-incompatible transfusions (type O platelets into type AB and type B patients), and one occurred in a bidirectional-incompatible transfusion (type B platelet into type A patient). Thus, the overall rate of hemolytic transfusion reactions from plasma-incompatible platelet units was 3 in 2522 transfusions (or about 1 in 840 transfusions; 0.12%). No hemolytic reactions were observed in compatible or major-incompatible transfusions.
Transfusion reaction rates based on type of incompatibility
To complete our analysis, the transfusion reaction rates were determined based on the type of incompatibility of the platelet transfusion. We found that all three categories of ABOi platelet transfusions had higher reaction rates than seen with compatible platelet transfusions. This was true when either all reactions or only CDC/NHSN classifiable reactions were considered (Fig. 5A,B). These results suggest that plasma-incompatible as well as antigen-incompatible transfusions may contribute to the increased rates seen with ABOi platelets. When specific reaction types were considered, we found that both FNHTRs and allergic reactions were more commonly seen with all incompatible subgroups as well (Fig. 5C,D). To determine if any of these differences were statistically significant, univariate logistic regression analysis was conducted to determine the odds ratio (and 95% confidence interval) for transfusion reaction rates with major-, minor-, and bidirectional-incompatible platelet transfusions compared to the rates with compatible platelet transfusions (Table 1). These data show that ABOi platelets as a group, as well as the major-incompatible platelet transfusion subgroup, had statistically significant increased chances of being associated with transfusion reactions compared to ABO-compatible platelets. These significant differences with major-incompatible transfusions (N = 2651) held true when CDC/NHSN qualifying reaction, FNHTR, and allergic reaction rates were examined (Table 1). While bidirectional (N = 607) and minor-incompatible (N = 1890) transfusions had higher rates of reactions, none of these subgroups reached statistical significance when compared to ABO-compatible transfusions. The lack of statistical significance could be due to the smaller number of transfusions in these groups, though it is of interest that major-incompatible transfusions had the highest reaction rates of any of the groups in this series. Univariate analysis was also performed for patient age (<18 vs. adult), patient ABO type (type O as reference), unit ABO type (type AB as reference) and sex, but none of these analyses showed significant differences between the groups (data not shown).
Fig. 5.
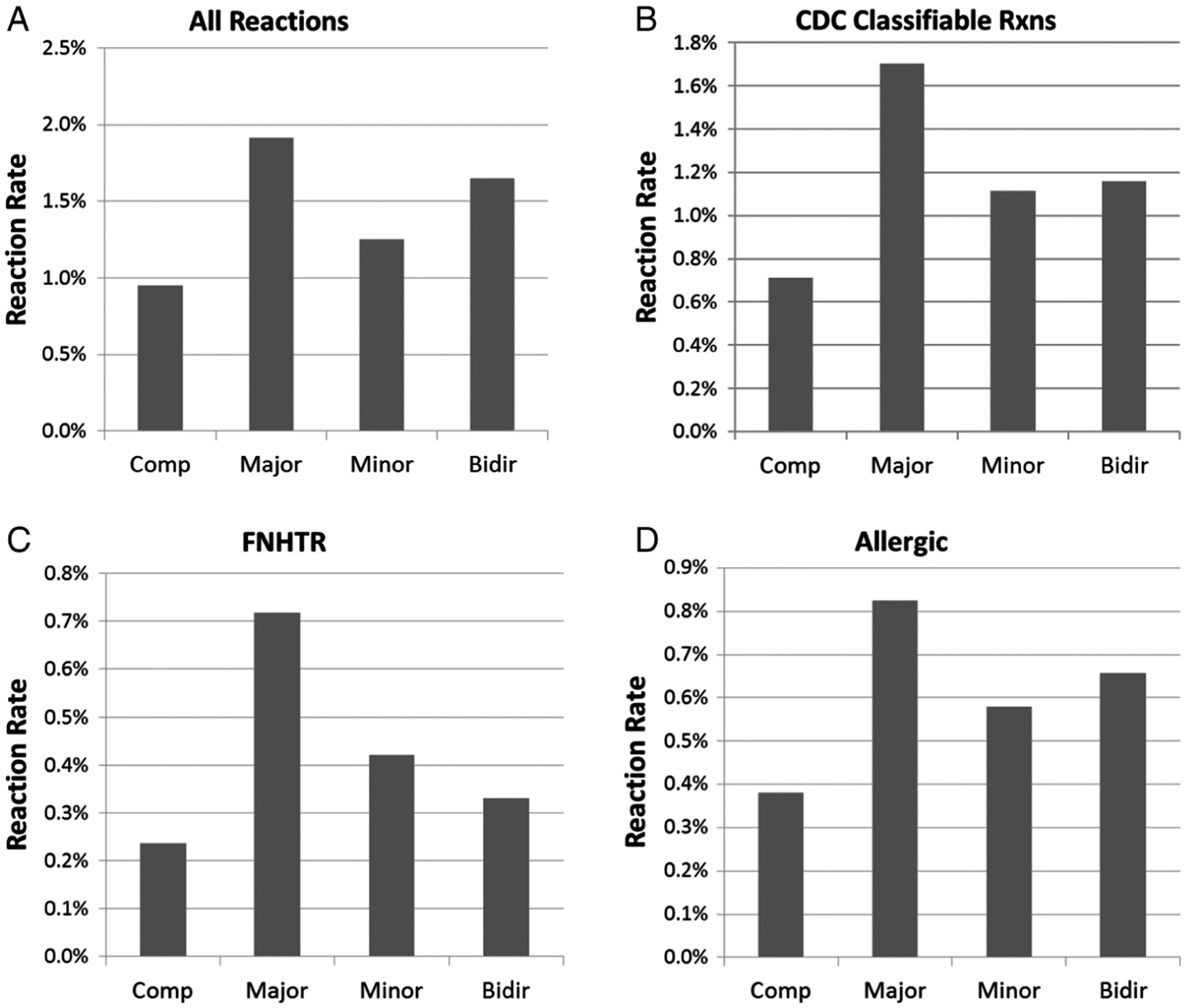
Reaction rates for ABO-compatible (Comp), major incompatible (Major), minor-incompatible (Minor), and bidirectional-incompatible (Bidir) platelet transfusions. (A) Reaction rates for all reactions reported to the blood bank are shown for each type of transfusion. (B) Reaction rates for reactions that met CDC/NHSN criteria are shown for each type of transfusion. (C) Reaction rates that met criteria for FNHTRs are shown for each type of transfusion. (D) Reaction rates that met criteria for allergic transfusion reactions are shown for each type of transfusion. Statistical analysis for these data is summarized in Table 1.
TABLE 1.
Odds ratios and 95% confidence intervals for different transfusion reaction types relative to reaction rates for ABO compatible platelet transfusions
| Odds ratio | 95% confidence interval | p value | |
|---|---|---|---|
| All reported reactions | |||
| All ABOi | 1.81 | 1.33–2.46 | <0.001 |
| Bidirectional | 1.74 | 0.90–3.38 | 0.101 |
| Major | 2.16 | 1.53–3.07 | <0.001 |
| Minor | 1.34 | 0.85–2.12 | 0.214 |
| CDC/NHSN qualifying reactions | |||
| All ABOi | 2.01 | 1.42–2.84 | <0.001 |
| Bidir | 1.63 | 0.79–3.58 | 0.225 |
| Major | 2.41 | 1.63–3.56 | <0.001 |
| Minor | 1.57 | 0.95–2.59 | 0.078 |
| FNHTR | |||
| All ABOi | 2.31 | 1.32–4.04 | 0.003 |
| Bidirectional | 1.30 | 0.30–5.57 | 0.721 |
| Major | 3.00 | 1.62–5.54 | <0.001 |
| Minor | 1.68 | 0.74–3.79 | 0.215 |
| Allergic reactions | |||
| All ABOi | 1.81 | 1.14–2.88 | 0.012 |
| Bidirectional | 1.61 | 0.57–4.56 | 0.368 |
| Major | 2.13 | 1.25–3.62 | 0.005 |
| Minor | 1.42 | 0.72–2.81 | 0.310 |
ABOi = ABO-incompatible; CDC = Centers for Disease Control and Prevention; FNHTR = febrile nonhemolytic transfusion reaction; NHSN = National Healthcare Safety Network.
DISCUSSION
The relationship between transfusion reaction rates and ABOi platelet transfusions has not been well established. A comprehensive systematic review from 2009 looked at 77 studies that included more than 1500 patients and concluded that ABOi platelets could not be associated with increased transfusion reaction rates.3 However, none of the studies reviewed were powered to detect even modest increases in transfusion reaction rates. The current study, evaluating over 13,000 platelet transfusions in 2547 unique patients and the resultant 167 transfusion reactions, is the largest single study to date that evaluates the impact of ABO incompatibility on transfusion reaction rate.
A single-institution cohort study described a reduction in transfusion reactions following implementation of a policy that provided either ABO-identical platelets or minor-incompatible platelets that had been washed to remove ABO antibodies from the platelet unit.12 However, the transfusion reaction rates at this institution were quite low even before implementation of the policy (<1 in 1000). This policy also resulted in a slight increase in product wastage of about 5%. An overall reaction rate of 1.24% was observed in the present study, which is closer to usual reported reaction rates.11
Multiple authors have suggested that large randomized controlled studies are necessary to determine if ABOi platelets increase transfusion reaction rates.3,7 This study was not randomized or blinded, so it is possible that reporting bias of providers could partially explain the results. Transfusing staff (typically nurses) could be aware that the products are not ABO identical, which could lead to increased reporting for these patients. However, this seems unlikely to completely explain the results as most providers/nurses are unlikely to be aware that the compatibility of platelets may influence reaction rates. In our experience, no transfusing providers have been concerned about the ABO compatibility of a platelet transfusion as a possible cause of a reaction. Furthermore, we found that the differences were maintained even when reactions not meeting CDC/NHSN criteria were excluded. The data in this and other studies suggest that a randomized controlled trial with patients randomized to receive ABOi platelets may not be necessary and could in fact be contraindicated or unethical.
Most policies related to incompatible platelets have focused on plasma (minor) incompatibility. This likely is due to the rare but lethal consequences of giving plasma-incompatible platelets.5,6 Less attention has been given to A/B antigen (major)-incompatible platelet transfusions. This study found that major-incompatible platelet transfusions had statistically significantly higher rates of total reported reactions, reactions meeting NHSN definitions, allergic reactions, and FNHTRs. These robust findings support the conclusion that A/B antigens in the platelet product contribute to patient signs or symptoms that result in the reporting of a transfusion reaction. The mechanism of these reactions is not addressed in this study, but others have shown that circulating immune complexes have been shown to occur following transfusion of ABOi platelets.13 These complexes may be the cause of the increased allergic reaction and/or FNHTR rates observed with major-incompatible transfusions.
Allergic reactions are commonly observed with platelet transfusions, and these were the most frequent reaction types observed in this study. Importantly, allergic reaction rates also appeared to differ depending on the compatibility of the transfusion, with ABO major-incompatible platelets leading to the highest proportion of allergic transfusion reactions. Furthermore, 26% of the allergic reactions associated with ABOi platelet transfusions in this study were classified as severe. Of note, one recent study examined allergic reactions and found that 61% of products are not completed following an allergic reaction, and for platelets this results in an average of 138.4 mL of the product not being transfused.14 FNHTRs are also commonly reported during platelet transfusions, and this was the second most frequent type of transfusion reaction reported during our study period. The odds ratios for ABO major-incompatible platelet transfusions were approximately double that for compatible transfusions for both FNHTRs and allergic transfusion reactions.
This study suggests that efforts to maximize the number of ABO-compatible/identical platelet transfusions that are provided to patients could significantly reduce the rate of transfusion reactions. One approach to maximizing the percentage of compatible units could be to perform rapid bacterial testing to increase the product shelf life up to 7 days.15 This would allow additional time to provide ABO-identical platelets to patients. However, this approach would likely result in more platelets being transfused beyond the typical 5-day expiration date. Since we found that reaction rates went up with unit age (Fig. 4C), the benefits of increasing the proportion of ABO-compatible transfusions could be offset by the increased risk associated with transfusion of older platelet units. With the recent FDA guidance documents providing for longer platelet shelf life, it will be important to monitor transfusion reaction rates with these older products.
The data in this study may be useful in developing policies that reduce transfusion reactions while preserving platelet inventories. Finding a standarized approach for all situations and patients is unlikely to be easy and likely to solicit a wide range of opinions on how to mitigate these increased risks of ABOi platelet reactions. Regardless of how one views these findings, this quantitative analysis should be useful in developing processes that minimize platelet wastage while maximizing patient safety for individual institutions. At our institution, we are considering prioritizing ABO-identical platelets for those most likely to have reactions. This might include patients requiring chronic transfusions such as leukemia or stem cell transplant patients. These patients could be identified by the blood bank based on need for irradiated blood products, which are generally recommended for these patients.
In summary, this single-institution study looking at reaction rates for more than 13,000 platelet transfusions found that ABOi platelets have a 1.5- to 2.0-fold increase in the likelihood of being associated with a transfusion reaction. This increased risk appeared to be independent of unit age and was observed for all reactions reported to the blood bank, only those meeting CDC/NHSN criteria, FNHTRs, and allergic reactions. The finding that major-incompatible units had the highest reaction rates among a variety of types suggests that circulating immune complexes involving patient anti-A or anti-B antibodies combined with A or B antigens from the transfused units may be an underappreciated cause of a variety of platelet transfusion reactions.
ACKNOWLEDGMENTS
The authors thank Hannah Born for secretarial assistance with the manuscript. The statistical analysis for this study was supported in part by the University of Iowa Clinical and Translational Science Award, granted with funds from the National Institutes of Health (UL1TR002537).
Source of support: Department of Pathology, University of Iowa.
ABBREVIATIONS:
- ABOi
ABO-incompatible
- AHTR
acute hemolytic transfusion reaction
- CDC
Centers for Disease Control and Prevention
- EMRs
electronic medical records
- FNHTR
febrile nonhemolytic transfusion reaction
- LIS
laboratory information system
- MRN
medical record number
- NHSN
National Healthcare Safety Network
- TACO
transfusion-associated circulatory overload
- TAD
transfusion-associated dyspnea
- TRALI
transfusion-related acute lung injury
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Dunbar NM, Katus MC, Freeman CM, et al. Easier said than done: ABO compatibility and D matching in apheresis platelet transfusions. Transfusion 2015;55:1882–8. [DOI] [PubMed] [Google Scholar]
- 2.Cooling LL, Downs TA, Butch SH, et al. Anti-A and anti-B titers in pooled group O platelets are comparable to apheresis platelets. Transfusion 2008;48:2106–13. [DOI] [PubMed] [Google Scholar]
- 3.Shehata N, Tinmouth A, Naglie G, et al. ABO-identical versus nonidentical platelet transfusion: a systematic review. Transfusion 2009;49:2442–53. [DOI] [PubMed] [Google Scholar]
- 4.Carr R, Hutton JL, Jenkins JA, et al. Transfusion of ABO-mismatched platelets leads to early platelet refractoriness. Br J Haematol 1990;75:408–13. [DOI] [PubMed] [Google Scholar]
- 5.Pierce RN, Reich LM, Mayer K. Hemolysis following platelet transfusions from ABO-incompatible donors. Transfusion 1985; 25:60–2. [DOI] [PubMed] [Google Scholar]
- 6.Sadani DT, Urbaniak SJ, Bruce M, et al. Repeat ABO-incompatible platelet transfusions leading to haemolytic transfusion reaction. Transfus Med 2006;16:375–9. [DOI] [PubMed] [Google Scholar]
- 7.Dunbar NM, Ornstein DL, Dumont LJ. ABO incompatible platelets: risks versus benefit. Curr Opin Hematol 2012;19:475–9. [DOI] [PubMed] [Google Scholar]
- 8.AuBuchon JP, Fung M, Whitaker B, et al. AABB validation study of the CDC’s National Healthcare Safety Network Hemovigilance Module adverse events definitions protocol. Transfusion 2014;54:2077–83. [DOI] [PubMed] [Google Scholar]
- 9.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med 2007;26: 3661–75. [DOI] [PubMed] [Google Scholar]
- 10.Hess AS, Hess JR. Understanding tests of the association of categorical variables: the Pearson chi-square test and Fisher’s exact test. Transfusion 2017;57:877–9. [DOI] [PubMed] [Google Scholar]
- 11.Harvey AR, Basavaraju SV, Chung KW, et al. Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012. Transfusion 2015;55:709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrichs KF, Howk N, Masel DS, et al. Providing ABO-identical platelets and cryoprecipitate to (almost) all patients: approach, logistics, and associated decreases in transfusion reaction and red blood cell alloimmunization incidence. Transfusion 2012; 52:635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heal JM, Masel D, Rowe JM, et al. Circulating immune complexes involving the ABO system after platelet transfusion. Br J Haematol 1993;85:566–72. [DOI] [PubMed] [Google Scholar]
- 14.Kasim J, Aldarweesh F, Connor JP. Blood product and laboratory resource wastage in non-severe allergic transfusion reactions: an opportunity for improvement. Transfus Med 2019;29:338–43. [DOI] [PubMed] [Google Scholar]
- 15.Harm SK, Szczepiorkowski ZM, Dunbar NM. Routine use of Day 6 and Day 7 platelets with rapid testing: two hospitals assess impact 1 year after implementation. Transfusion 2018; 58:938–42. [DOI] [PubMed] [Google Scholar]


