Severe acute respiratory syndrome coronavirus 2 is the virus responsible for the coronavirus disease 2019 pandemic. This review provides greater insights into the role of the renin-angiotensin- aldosterone system (RAAS) axis in acute lung injury and the effects of RAAS inhibitors on severe acute respiratory syndrome coronaviruses. Specifically, the hypothesis that RAAS inhibitors facilitate viral insertion and the alternative hypothesis of the beneficial role of these drugs are discussed. Up-to-date published data concerning the RAAS inhibitors and coronavirus disease 2019 are summarized.
Key Words: angiotensin-converting-enzyme inhibitors, angiotensin-converting enzyme 2 receptor, angiotensin receptor blockers, COVID-19
Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the virus responsible for the coronavirus disease 2019 (COVID-19) pandemic. The angiotensin-converting enzyme 2 (ACE2) has been proven to be used by SARS-CoV-2 for host cell entry. Considering that angiotensin receptor blockers and ACE inhibitors (ACEIs) upregulate the expression of ACE2 in animal studies, there may be a concern about whether these drugs may increase COVID-19 susceptibility and severity. Recently, there has been a debate among clinicians about whether to continue or to stop ACEIs and angiotensin receptor blockers in the context of COVID-19. Also, some media outlets and health systems have called for the discontinuation of these drugs in the context of suspected COVID-19. This has necessitated an urgent release of guidance on the use of such medications in COVID-19 patients. To date, multiple theories relating to the pure effects of renin-angiotensin-aldosterone system (RAAS) inhibitors on COVID-19 infections have been postulated. Favorable effects include blocking the ACE2 receptors, preventing viral entry into the heart and lungs, and protecting against lung injury in COVID-19. Adverse effects include a possible retrograde feedback mechanism that upregulates ACE2 receptors. This review provides greater insight into the role of the RAAS axis in acute lung injury and the effects of RAAS inhibitors on SARS-CoVs. The hypothesis that RAAS inhibitors facilitate viral insertion and the alternative hypothesis of the beneficial role of these drugs are discussed. Up-to-date published data concerning the RAAS inhibitors and COVID-19 are summarized.
Key Points
There is no compelling experimental or clinical evidence that angiotensin receptor blockers and angiotensin-converting enzyme inhibitors either increase vulnerability to severe acute respiratory syndrome coronavirus 2 or aggravate coronavirus disease 2019 severity and outcomes, whereas the protective role of angiotensin-converting enzyme 2 in the lung is supported by ample evidence.
Hypertensive patients using angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers should continue these medications during the coronavirus disease 2019 pandemic.
Coronavirus disease 2019 (COVID-19) is a pandemic disease caused by a novel coronavirus called severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). It is an enveloped RNA virus found in wildlife and humans. It was discovered for the first time in Wuhan City, Hubei Province, China. On December 31, 2019, the disease was initially reported to the World Health Organization.1 The disease has a clinical spectrum ranging from asymptomatic upper respiratory tract infections to severe pneumonia linked with acute respiratory distress syndrome (ARDS).2 On January 30, 2020, the World Health Organization declared the COVID-19 epidemic a global health emergency.3
The early reports from China revealed that old age, diabetes mellitus, hypertension, and cardiovascular disease were prevalent in COVID-19-infected patients, and patients with these comorbid conditions seemed to have higher case fatality rates.4,5 Patients with these comorbidities were admitted into intensive care units, required mechanical ventilation, and died more often than patients without these comorbidities. In a study that included 1099 patients with confirmed COVID-19 infection, many of the 173 individuals who developed severe disease had comorbidities, including hypertension (23.7%), diabetes mellitus (16.2%), coronary artery disease (5.8%), and cardiovascular diseases (2.3%).6 In another study, many of the 140 patients admitted to the hospital with COVID-19 infection had hypertension (30%) or diabetes mellitus (12%).7 In a third study done by Zhou et al, 191 confirmed COVID-19 cases in Wuhan, China were enrolled in a retrospective, multicenter cohort study that found that hypertension was associated with a hazard ratio of 3.05 for in-hospital mortality.8 This has raised concerns about the influence of hypertension and antihypertensive medications on the infectivity and severity of COVID-19.
ACE2 and the Renin-Angiotensin-Aldosterone System (RAAS)
Angiotensin-converting enzyme 2 (ACE2), is a type I transmembrane aminopeptidase that is mainly anchored at the apical surface of cells of the gastrointestinal system, heart, kidneys, blood vessels, and in type II alveolar cells of the lungs9 (Fig.). In addition to the membrane-bound form, there are soluble forms in the plasma and urine. ACE2 receptors are profoundly displayed in the heart and lungs. ACE2 was first discovered in 2000 as an ACE1 homolog that shares approximately 42% homology with ACE1. ACE2 is capable of producing a lung-protective factor, angiotensin 1–7, from angiotensin II. ACE2 also converts angiotensin I to angiotensin1–9. The affinity of ACE2 is higher for angiotensin II than for angiotensin I degradation10 (Fig.). The ACE2/ angiotensin 1–7 axis counterbalances theACE1/ Angiotensin II axis.11 Angiotensin II causes strong vasoconstriction, proinflammatory effects, and profibrotic effects, whereas angiotensin 1–7 shows antifibrotic, antiproliferative, vasodilatory, diuretic, and natriuretic effects. The ACE2-angiotensin 1–7 axis protects the cardiovascular system against heart failure, arrhythmia, and thrombosis. It also prevents myocardial hypertrophy and reduces vascular dysfunction associated with the metabolic syndrome.12 The equilibrium between these two opposing parts of the RAAS, at least partially, determines whether tissue injury may occur in response to stimuli, particularly in the heart and kidneys. Normally, the circulatory level of soluble ACE2 is low and its role in the lungs is minimal.13
Fig.
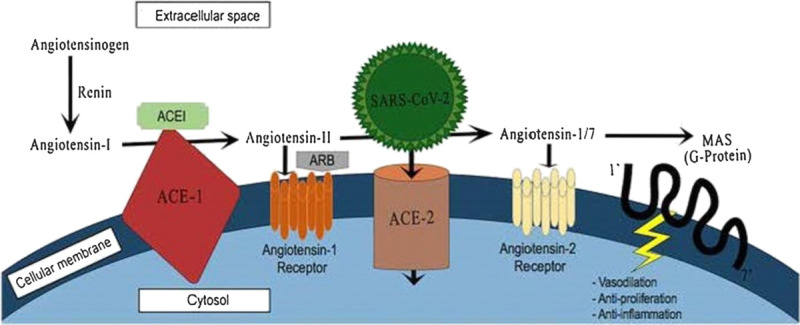
Mechanisms of action of ACEIs and ARBs and SARS-CoV-2 on the cellular RAAS receptors. Reproduced with permission from Springer Science+Business Media. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
SARS-CoV-2 and ACE2
Research has revealed that the ACE2 receptor is used by SARS-CoV-2 as a receptor to enter host cells14 (Fig.). Both types of SARS coronaviruses have four structural proteins: membrane, envelope, nucleocapsid, and spike proteins. Nucleocapsid, membrane, and envelope proteins play a role in viral replication, viral structure, and viral-induced host responses, among other roles.15 The spike protein, S-protein, is essential for fastening to and invading host cells. The S-protein merging with ACE2, along with proteolytic splitting of ACE2 by transmembrane serine protease 2, allows viral entry into host cells, viral replication, and cell-to-cell transmission.14 The expression of ACE2 receptors correlates with susceptibility to SARS-CoV infection in vitro.16 Consequently, it has been postulated that increased expression of ACE2 receptors may lead to an increased risk of infection of the lung (and possibly other tissues) by SARS-CoV-2. ACE2 receptor is also the receptor for the SARS-CoV that was responsible for the 2002–2004 SARS epidemic; however, the SARS-CoV-2 affinity for ACE2 is 10 to 20 times higher than that of SARS-CoV, which may underlie its immense transmission ability and the differences between the two epidemics.
Pharmacology of ACEIs/ARBs and the Role of ACE2
The main pharmacological effects of ACE inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) are to inhibit ACE1 and block angiotensin II type 1 receptor (AT1), respectively (Fig). ACEIs only partially affect the production of angiotensin II from angiotensin I, because 40% of angiotensin II production does not occur in the ACE pathway.17 As a result of producing angiotensin II, ACE1 levels are in turn reduced by a negative feedback loop because of the raised angiotensin II levels. This decrease in ACE1 allows the angiotensin II to be metabolized by ACE 2 to angiotensin 1–7.18 Angiotensin 1–7 works on angiotensin II receptors to produce antifibrotic, antiproliferative, vasodilatory, diuretic, and natriuretic effects.
Angiotensin mediates its effects through two receptors. The AT1 receptor mediates the vasoconstrictive and aldosterone-stimulating effects of angiotensin II. The action of angiotensin II at the AT2 receptor tends to antagonize the pressor effects of the AT1 receptor. ARBs specifically block the action of angiotensin II at the AT1 receptor and thereby block the arteriolar contraction and sodium-retention effects of the RAAS.
Hypothesized Harmful Effects of RAAS Inhibitors in COVID-19
As mentioned above, ACE2 is used by SARS-CoV-2 as a receptor to enter host cells. Increased ACE2 expression due to upregulation leads to enhanced binding sites for SARS-CoV-2 and consequently increased liability for COVID-19 infection (Fig.). Animal models indicate that ARBs can increase the expression of messenger RNA (mRNA) or protein levels of ACE2 in animals with heart diseases19 and chronic kidney diseases such as hypertensive nephropathy and diabetic nephropathy.20,21 Increased ACE2 mRNA or protein levels also have been documented in the aorta of hypertensive rats.22 In another animal study, the decreased expression of ACE2 receptors and circulating angiotensin 1–9 levels in animals with late ventricular dysfunction postmyocardial infarction were prevented using enalapril, an ACEI.23 Another animal study concluded that the expression of ACE2 is increased after telmisartan administration, an ARB.24 Other animal studies have suggested that the RAAS inhibitors had no impact on ACE2 expression, however. Ramipril, valsartan, and their combination in a myocardial infarction model have shown no effect on cardiac ACE2 expression.25
All of these animal models have raised concerns that patients receiving RAAS inhibitors may be more susceptible to infection with SARS-CoV-2 and become more severely ill when such an infection occurs. There is a lack of studies showing the effects of ACEIs or ARBs on the expression or activity of ACE2 receptors in the lung. In these studies, the observed effects of these drugs on ACE2 receptor expression and activity may not be comparable to humans because the doses of ACEIs and ARBs used in these animal studies were much higher than those used in humans.
In contrast to animal studies, there are few studies in humans regarding the effects of RAAS inhibitors on ACE2 expression. In a study of coronary artery disease patients who were given ACEIs intravenously, the inhibition of ACE1 with the resultant increase in angiotensin I failed to raise angiotensin 1–9 levels (Fig.). This suggests a minor function for ACE2 in the metabolism of angiotensin I, and brings into doubt whether ACEIs have any direct role in the ACE2 metabolism of angiotensin II.26
In a study performed in patients with mild to moderate essential hypertension, initial treatment with captopril produced no impact on angiotensin 1–7 plasma levels; however, continuous therapy with captopril for a period of 6 months delineated an increase in angiotensin 1–7 plasma levels.27
In a study performed in healthy humans, the mean duodenal mRNA expression level of ACE2 was found to be increased 1.9-fold in those given ACEI in comparison to the nontreated controls. No major variations in levels of expression were found in ARB-treated patients, however.28 Cross-sectional human studies reveal that plasma ACE2 levels is patients with heart failure,29 coronary artery disease,30 atrial fibrillation,31 and aortic stenosis32 were not different whether patients did or did not receive ACEIs or ARBs.
In a Japanese cohort study, 617 hypertensive patients on ACEIs, ARBs, and calcium-channel blockers were enrolled in addition to nonhypertensive patients (n = 101).33 The urinary ACE2 level was found to be higher in the olmesartan-treated group (an ARB), but not in those who took the other drugs.
Combining all of these studies, the upregulation of ACE2 receptors was noted only in animal studies using various ARBs or ACEIs at doses higher than typically used in humans. Such results also suggest that results from animal models may not be applicable in humans. Also, it must be noted that the biologic relevance of ACE2 differs according to tissue and the clinical state. As mentioned before, there is a lack of studies showing the effects of these RAAS inhibitors on the lung-specific expression of ACE2. The levels of the soluble forms of ACE2 in plasma and urine do not constitute a strong indicator of the membrane-bound form, as the latter is normally shed out of the membrane.34 As a result, there is no convincing evidence that the ARBs/ACE-enhanced ACE2 increment can enhance human susceptibility to SARS-CoV-2 (Table 1).
Table 1.
Hypothesized harmful effects of RAAS inhibitors in COVID-19

Potential Benefits of RAAS Inhibitors in COVID-19
Following the initial binding of the S-protein of SARS-CoV-2, there is downregulation of the membrane-bound ACE2.35 This downregulation of ACE2 receptor activity in the lungs leads to unopposed accumulation of angiotensin II, the substrate for ACE2 (Fig.). Accumulated angiotensin II leads to increased neutrophil accumulation, increased vascular permeability, and exacerbated pulmonary edema, and eventually leads to ARDS.36 Consequently, by reducing angiotensin II levels, ACEIs and ARBs may protect against lung injury in individuals with COVID-19.
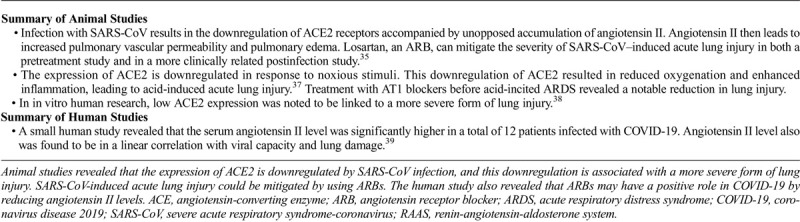
In mice studies, Kuba et al noted that after infection with SARS-CoV occurs and ARDS ensues, the downregulation of ACE2 occurs and is accompanied by unopposed accumulation of angiotensin II.35 Angiotensin II then leads to increased pulmonary vascular permeability and pulmonary edema. Injection of the SARS-CoV spike protein in mice substantially reduces lung ACE2 protein levels (downregulated the ACE2 expression in the lung) with consequent acute lung injury. The study delineated that losartan, an ARB, can mitigate the severity of SARS-CoV–induced acute lung injury in both a pretreatment study and a more clinically related postinfection study.35 Noting that the structure of the S-protein of both types of SARS viruses is almost identical, SARS-CoV-2 infection also may downregulate the ACE2 expression in the lung.11 In this situation, ARBs may prove useful to mitigate the severity of acute lung injury caused by SARS-CoV-2.
Imai et al used a mouse model of acute lung injury/ARDS triggered by acid aspiration to demonstrate that expression of ACE2 is downregulated in response to toxic stimuli.37 This downregulation of ACE2 resulted in reduced oxygenation and enhanced inflammation, leading to acid-induced acute lung injury.37 Treatment with AT1 blockers before acid-incited ARDS revealed a notable reduction in lung injury, which confirmed the function of the AT1 receptors in the physiological response. Exogenous ACE2 administration also has been shown to rescue acid aspiration-induced acute lung injury.37 In in vitro human research, low ACE2 expression was noted to be linked to a more severe form of lung injury.38
In concordance with the results of these studies, the result of a small human study performed in Wuhan, China revealed that the serum angiotensin II level was significantly high in a total of 12 patients infected with COVID-19. Also, it showed that the angiotensin II level was in a linear correlation with viral capacity and lung damage.39 These findings lead us to propose that ARBs may have a positive role in COVID-19 by reducing angiotensin II levels (Table 2).
Table 2.
Potential benefits of RAAS inhibitors in COVID-19
New Data
A large case-control study has been performed in Italy. The study compared 6272 people with established COVID-19 infection between February 21 and March 11, 2020, with a control group of 30,759 people who were comparable in sex, age, and the city of residency who did not have COVID-19 infection. The use of ACEIs and ARBs was more common among case-patients than among controls. Using multivariate conditional logistic regression analysis, the likelihood of SARS-CoV-2 infection was not associated with either ACEIs or ARBs. Further analysis also showed no relation between these drugs and the severity of COVID-19, when matching subjects with severe or fatal infections with paired controls.40
A case-population study of 1139 adult cases was conducted in Madrid, Spain, in patients who were 18 years or older diagnosed as having COVID-19 using polymerase chain reaction and required hospitalization between March 1 and March 24, 2020, in any of seven hospitals in Madrid. To give an aggregate of 11,390 matched controls, the patients were each thoughtfully paired with 10 population controls using data from 2018. The study measured the hospitalization rates of polymerase chain reaction–confirmed COVID-19 patients using RAAS inhibitors versus other antihypertensive drugs. It delineated that compared with other antihypertensive medications, the use of RAAS inhibitors was not shown to be correlated with an augmented risk of hospital admission because of COVID-19 or an expanded risk of serious complications from COVID-19 requiring intensive care unit admission or leading to fatal consequences.41
A large database study has been conducted that enrolled 8910 hospitalized patients in 11 countries of three continents. The covered patients were those who had been diagnosed as having COVID-19, who were admitted to the hospital between December 20, 2019 and March 15, 2020, and who had either died in the hospital or had lived until discharge. In concordance with the results of the previously mentioned Italian study, this study delineated that neither ACEIs nor ARBs were correlated with an expanded risk of in-hospital mortality. An additional interpretation limited to hypertensive patients (those for whom RAAS inhibitors would be indicated) also did not reveal an adverse correlation.42
Another study was conducted at the New York University Langone Health system using data from electronic health records. A total of 12,594 patients were enrolled who were tested for COVID-19 between March 2020 and April 15, 2020. The study aimed to assess whether previous treatment with either ARBs, ACEIs, calcium-channel blockers, β-blockers, or thiazide diuretics has an impact on the likelihood that a COVID-19 test will become positive or negative. In addition, it measured the relation between previous treatment with these medications and the likelihood that positively tested patients will develop severe illness, which is defined as the admission to an intensive care unit, the need for mechanical ventilation, or even death. A total of 5894 (46.8%) patients tested positive for COVID-19, 1002 of whom (17.0%) had severe illness. The study revealed that 4357 (34.6%) patients had a history of hypertension, 2573 of whom (59.1%) tested positive, and 634 (24.6%) patients had severe illness. The study concluded that none of these drugs was found to increase the likelihood of a positive COVID-19 test. Also, none of these medications was associated with a substantial increase in the risk of severe illness among patients with positive tests.43
Similar conclusions were achieved by Mehta and colleagues in a cohort study of 18,472 patients who had been tested for COVID-19 infection in the Cleveland Clinic Health System in Ohio and Florida. Overlap propensity score weighting revealed no correlation between the use of ACEIs or ARBs and the likelihood of a positive test (odds ratio 0.97, 95% confidence interval 0.81–1.15).44
A retrospective, multicenter study was conducted in Hubei Province, China, including 1128 patients with a history of hypertension, 188 patients (17%) of whom received an ACEI/ARB drug during hospitalization.45 The study concluded that inpatient use of ACEI/ARB was found to be associated with a lower risk of all-cause mortality in comparison with those who do not use these drugs. Although analysis of the study needs to consider the potential for residual confounders, in-hospital use of ACEI /ARB is unlikely to be associated with an increased mortality rate.
Official Statements
Before the results of the previous large COVID-19 studies, the Council on Hypertension of the European Society of Cardiology, the American Heart Association, the Heart Failure Society of America, and the American College of Cardiology navigated the uncertainty about whether to continue or discontinue the use of RAAS inhibitors in COVID-19, and have spoken with one voice in advising that ACEI or ARB therapy should be continued during the COVID-19 pandemic, as there is no convincing evidence that they can increase susceptibility to infection, severe illness, or death.46–48
Conclusions
The controversy regarding the use of ACEIs and ARBs in COVID-19 came after finding that the COVID-19 virus binds to the ACE2 receptor to gain host cell entry. Many studies, mainly animal studies, proposed that ACEIs and ARBs may increase the expression of ACE2, which has raised concerns that the use of these drugs may increase the susceptibility for contracting SARS-CoV-2. Other studies showed that these drugs may play a protective role against lung injury in COVID-19 patients, however. To date, there is no compelling experimental or clinical evidence that ARBs and ACEIs either increase vulnerability to SARS-CoV-2 or aggravate COVID-19 severity and outcomes, whereas the protective role of ACE2 in the lung is supported by ample evidence. In this regard, several professional societies have released statements and agreed upon the continuation of ACEIs/ARBs during the COVID-19 outbreak. Results from newly published studies have contributed to diminishing the speculation about the safety of RAAS inhibitors during the COVID-19 pandemic. Large-scale clinical studies with convincing evidence are needed to navigate the uncertainty regarding whether ACEIs and ARBs have favorable, harmful, or neutral effects regarding the susceptibility to SARS-CoV-2 and the severity and outcomes of COVID-19.
Acknowledgment
There are no proper words to convey my deep gratitude and respect to The University of Gezira Faculty of Medicine.
Footnotes
The author did not report any financial relationships or conflicts of interest.
References
- 1.Centers for Disease Control and Prevention 2019 novel coronavirus, Wuhan, China. https://www.cdc.gov/coronavirus/2019-ncov/about/index.html. Published January 26, 2020. Accessed January 27, 2020.
- 2.Guan WJ Ni ZY Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramzy A, McNeil DG. W.H.O. declares global emergency as Wuhan coronavirus spreads. https://nyti.ms/2RER70M. Published January 30, 2020. Accessed January 30, 2020.
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 5.Huang C Wang Y Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu L Wang B Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect 2020;80:656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JJ Dong X Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–1741. [DOI] [PubMed] [Google Scholar]
- 8.Zhou F Yu T Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P Yang XL Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice GI Thomas DA Grant PJ, et al. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 2004;383(Pt 1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos RAS Sampaio WO Alzamora AC, et al. The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7). Physiol Rev 2018;98:505–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo J Huang Z Lin L, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc 2020;9:e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serfozo P Wysocki J Gulua G, et al. Ang II (angiotensin II) conversion to angiotensin-(1–7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension 2020;75:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M Kleine-Weber H Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J 2019;16:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann H Geier M Marzi A, et al. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem Biophys Res Commun 2004;319:1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollenberg NK, Fisher ND, Price DA. Pathways for angiotensin II generation in intact human tissue: evidence from comparative pharmacological interruption of the renin system. Hypertension 1998;32:387–392. [DOI] [PubMed] [Google Scholar]
- 18.Campbell DJ. Endogenous angiotensin II levels and the mechanism of action of angiotensin-converting enzyme inhibitors and angiotensin receptor type 1 antagonists. Clin Exp Pharmacol Physiol 1996;23(suppl 3):S125–S131. [DOI] [PubMed] [Google Scholar]
- 19.Ferrario CM Jessup J Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 20.Iwanami J Mogi M Tsukuda K, et al. Role of angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis in the hypotensive effect of azilsartan. Hypertens Res 2014;37:616–620. [DOI] [PubMed] [Google Scholar]
- 21.Lakshmanan AP Thandavarayan RA Palaniyandi SS, et al. Modulation of AT-1R/CHOP-JNK-caspase12 pathway by olmesartan treatment attenuates ER stress-induced renal apoptosis in streptozotocin-induced diabetic mice. Eur J Pharm Sci 2011;44:627–634. [DOI] [PubMed] [Google Scholar]
- 22.Zhong JC Ye JY Jin HY, et al. Telmisartan attenuates aortic hypertrophy in hypertensive rats by the modulation of ACE2 and profilin-1 expression. Regul Pept 2011;166(1–3):90–97. [DOI] [PubMed] [Google Scholar]
- 23.Ocaranza MP Godoy I Jalil JE, et al. Enalapril attenuates downregulation of angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension 2006;48:572–578. [DOI] [PubMed] [Google Scholar]
- 24.Soler MJ Ye M Wysocki J, et al. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol 2009;296:F398–F405. [DOI] [PubMed] [Google Scholar]
- 25.Burchill LJ Velkoska E Dean RG, et al. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond) 2012;123:649–658. [DOI] [PubMed] [Google Scholar]
- 26.Campbell DJ Zeitz CJ Esler MD, et al. Evidence against a major role for angiotensin converting enzyme-related carboxypeptidase (ACE2) in angiotensin peptide metabolism in the human coronary circulation. J Hypertens 2004;22:1971–1976. [DOI] [PubMed] [Google Scholar]
- 27.Luque M Martin P Martell N, et al. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1–7) in essential hypertension. J Hypertens 1996;14:799–805. [DOI] [PubMed] [Google Scholar]
- 28.Vuille-dit-Bille RN Camargo SM Emmenegger L, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 2015;47:693–705. [DOI] [PubMed] [Google Scholar]
- 29.Epelman S Shrestha K Troughton RW, et al. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail 2009;15:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramchand J Patel SK Srivastava PM, et al. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLOS One 2018;13:e0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters TE Kalman JM Patel SK, et al. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace 2017;19:1280–1287. [DOI] [PubMed] [Google Scholar]
- 32.Ramchand J Patel SK Kearney LG, et al. Plasma ACE2 activity predicts mortality in aortic stenosis and is associated with severe myocardial fibrosis. JACC Cardiovasc Imaging 2020;13:655–664. [DOI] [PubMed] [Google Scholar]
- 33.Furuhashi M Moniwa N Mita T, et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens 2015;28:15–21. [DOI] [PubMed] [Google Scholar]
- 34.Lambert DW Yarski M Warner FJ, et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 2005;280:30113–30119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuba K Imai Y Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care 2017;21:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai Y Kuba K Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia HP Look DC Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 2005;79:14614–14621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y Yang Y Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mancia G Rea F Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Abajo FJ Rodríguez-Martín S Lerma V, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 2020;395:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehra MR Desai SS Kuy S, et al. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020;382:e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Reynolds HR Adhikari S Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández-Ruiz I. RAAS inhibitors do not increase the risk of COVID-19. Nat Rev Cardiol 2020;17:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang P Zhu L Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.European Society of Cardiology Position statement of the ESC Council on Hypertension on ACE-inhibitors and angiotensin receptor blockers. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang. Published March 13, 2020. Accessed March 20, 2020.
- 47.American College of Cardiology HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. Published March 17, 2020. Accessed March 20, 2020.
- 48.Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA 2020;323:1769–1770. [DOI] [PubMed] [Google Scholar]


