Abstract
Background
Drug-resistant gram-negative bacteria (GNB) are a global public health threat, especially in intensive care units (ICU). This study explored the prevalence of drug-resistant Enterobacteriaceae infections in an ICU in Saudi Arabia. The appropriateness of the antibiotic therapies used and their ability to improve the clinical outcomes were also assessed.
Methods
A retrospective study was conducted from 2015 to 2018 in the different ICUs of a tertiary-care hospital in Saudi Arabia. Positive cultures for multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) Enterobacteriaceae, including Klebsiella pneumoniae, Escherichia coli, and Enterobacter sp., were included. The primary outcomes involved microbiological cure and 30 days in-hospital mortality rate, while the secondary outcome included the length of hospital stay (LOS). Regression models were used to assess the relationship between appropriateness of the antibiotic therapy and clinical outcomes.
Results
Of the 227 Enterobacteriaceae cultures included in this study, 60% were either MDR (n= 130) or XDR (n= 8) infections; no PDR Enterobacteriaceae cultures were identified. Majority of the patients were female (54%), and the average age was 60.1 ± 17.7 years. MDR/XDR cultures primarily comprised E. coli (51.4%), followed by K. pneumoniae (33%) and Enterobacter sp. (16%). Most commonly used antibiotics were piperacillin/tazobactam (53%), carbapenems (47%), and cephalosporins (21.3%). Antibiotic therapy was considered appropriate in only 85 of 138 (61.59%) patients. Microbiological cure rate was achieved in 40% of the cases, and in-hospital death rate was 84%. The average LOS was 27 days. Appropriateness of the antibiotic therapy prescribed could not predict any of the study outcomes.
Conclusion
The study revealed a high prevalence of drug-resistant Enterobacteriaceae infections, which were associated with a high mortality rate. Therefore, it is essential to assess the effectiveness of antimicrobial stewardship program and infection prevention and control practices, particularly in critically ill patients.
Keywords: multidrug-resistant bacteria, extensively drug-resistant bacteria, Enterobacteriaceae
Background
Infections caused by drug-resistant gram-negative bacteria (GNB), particularly the hospital-acquired antibiotic-resistant infections pose a significant threat to global public health.1,2 Organisms expressing in vitro resistance to three or more antimicrobial classes are referred to as multidrug-resistant organisms.2 According to the Centers for Disease Control and Prevention (CDC), more than 70% of the bacteria causing hospital-acquired infections are resistant to at least one of the antimicrobial agents that are commonly used to treat them.3 EnterobacteriaceaePseudomonas aeruginosa and Acinetobacter baumannii are the most common hospital-acquired drug-resistant GNBs.4
There are three types of antimicrobial resistance exhibiting microorganisms: multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR).2 MDR microorganisms acquired non-susceptibility to at least one agent in three or more antimicrobial classes.2 XDR microorganisms are non-susceptible to at least one agent in all but two or fewer antimicrobial classes (i.e., bacterial isolates remain susceptible to only one or two categories).2 Microorganisms with non-susceptibility to all agents in all antimicrobial classes are referred to as PDR.2 In addition, few novel resistance categories have been identified while practicing, such as difficult-to-treat resistance (DTR) and modified DTR.5
Globally, there has been a significant increase in the rate of MDR-GNB infections with limited treatment options in the intensive care units (ICUs).4–7 Critically ill patients are highly vulnerable to these infections owing to multiple factors, including the use of mechanical ventilators and prolonged administration of antibiotics.8 The likelihood of the appropriateness of antimicrobial therapy decreases with increasing drug-resistant GNB.9,10 Organisms resistant to even colistin, a last-resort antibiotic used to treat many drug-resistant GNB infections have been also reported.11 Unfortunately, the pace at which new antibacterial agents are being developed cannot keep up with the rapidly increasing rate of resistant microorganisms.12–14
The impact of MDR-GNB infections can be determined by analyzing the clinical outcomes, such as hospital death rate and the length of stay (LOS) in the hospital or ICU.15 Nevertheless, the association of drug-resistant GNB with prolonged LOS and mortality remains controversial.15–18 While some studies reported a direct association between MDR-GNB and mortality, others were unable to find a relationship between drug-resistant GNB and either hospital LOS or mortality.15–18 The underlying reason for the likely association of resistant bacteria with higher mortality rates could be the delayed appropriate antibiotic therapy compared to that in infections caused by antibiotic-susceptible bacteria.19
Generally, the available data regarding the prevalence, treatments, and clinical outcomes of drug-resistant GNB infections in the ICUs of hospitals worldwide and in Saudi Arabia are limited. Moreover, drug-resistant GNB differs from one place to the other. Therefore, in this study we described the prevalence of MDR, XDR, and PDR Enterobacteriaceae infections, including Klebsiella pneumoniae, Escherichia coli, and Enterobacter sp. in ICUs. The study also listed the empirical antibiotic therapies used and assessed their appropriateness, and reported the microbiological cure rate, ICU LOS, and 30-day mortality rate.
Methods
Study Design and Setting
A retrospective cohort study was conducted in the adult medical, surgical, and cardiac ICUs of King Saud University Medical City (KSUMC), a tertiary-care teaching hospital located in Riyadh, Saudi Arabia. Medical records from June 2015 to January 2018 were accessed to collect the data.
Population
Adult patients who were consecutively hospitalized and admitted to the ICU during the study period were selected for the study. All positive cultures of GNB Enterobacteriaceae, including K. pneumoniae, E. coli, and Enterobacter sp., identified by the Microbiology lab, were retrospectively classified as MDR, XDR, or PDR, regardless of the infection site and infection acquisition site. The institutional review board approved the study of King Saud University (Institutional review board number: E-18-2874).
Data Collection
Information collected from the medical records included age, gender, type of infection (MDR, XDR, PDR), source of infection, type of bacteria, acute physiology and chronic health evaluation (APACHE) II score, use of antibiotics within the previous four weeks and 90 days, presence of a urinary catheter, mechanical ventilation, dialysis, and presence of comorbid conditions (such as cardiovascular disease, lung disease, diabetes mellitus, solid tumors or hematological malignancy, liver disease, and renal failure). Information on ICU LOS, microbiological cure rate, empirical regimens, and 30-day mortality were also collected.
Definitions
Bacterial Resistance
The definitions of MDR, XDR, and PDR strains were based on the standardized international terminology proposed by CDC and the European Centre for Disease Prevention and Control (ECDC) standardized international terminology.2 Extended-spectrum beta-lactamase (ESBL)-producing organisms are defined as the ones that confer resistance to most beta-lactam antibiotics, including penicillin, cephalosporins, and aztreonam.20 They generally remain susceptible to carbapenems and may be inhibited in vitro by beta-lactam/beta-lactamase inhibitor combinations. Although CTX-M-type ESBLs belonging to the ambler class A are the most common ESBLs, TEM- and SHV-ESBLs are also detected. Carbapenemase-producing Enterobacteriaceae (CPE) are defined as organisms resistant to imipenem, meropenem, doripenem, or ertapenem, or documented to possess a carbapenemase.20 A molecular platform can be further used to detect carbapenemases of ambler class A, B, and D, including Klebsiella pneumoniae carbapenemase (KPC), New Delhi metallo-beta-lactamase (NDM), and oxacillinase-48 (OXA-48).
Treatment Regimen
Antibiotic therapy was categorized based on the number of medications used for monotherapy or combination therapy. The antibiotic therapy was defined as appropriate if the causative microorganisms were susceptible to at least one of the antimicrobial agents administered to the patient, within 24 hours of culture collection. This definition was adapted from previous studies that assessed the use of antimicrobials in drug-resistant GNB infected patients.10
Clinical Outcomes
The primary outcomes were the microbiological cure rate and all-cause mortality. The microbiological cure rate was defined as the absence of bacteria in a culture obtained from the same infection source. All-cause mortality was collected as documented in the medical records over 30 days. The secondary outcome was the LOS, calculated based on the number of days the patient was hospitalized in the ICU from the culture date.
Microbiological Procedures
Pathogen identity and antibiotic susceptibilities were determined using one of the two automated machines: VITEK 2 system (bioMerieux, Craponne, France) or MicroScan WalkAway 96 plus (Beckman Coulter, Inc., Brea, CA, USA). The machines displayed the identity of the organism with the percentage of assurance and susceptibility to 15–20 drugs (sensitive, intermediate, or resistant). For ESBL detection, an automated susceptibility platform through MicroScan WalkAway 96 plus or VITEK 2 system was used. In the case of CPE, GeneXpert (Xpert®Carba-R; Cepheid, Sunnyvale, CA, USA) was used to detect and differentiate KPC, NDM, Verona integron–encoded metallo-beta-lactamase (VIM), IMP, and OXA-48. The breakpoints and susceptibility interpretive criteria were based on the ones defined by the Clinical and Laboratory Standards Institute (CLSI).
Statistical Analysis
Frequency and percentage analysis described the categorical variables, such as gender, co-existing chronic conditions, type of bacteria, and source of infection. Mean and standard deviation (SD) or median and interquartile range (IQR) were used to describe continuous variables (age and LOS). Chi-square and Fisher’s exact tests were used to examine the factors related to bacterial mortality. Univariate and multiple regression analyses identified the variables associated with clinical outcomes (microbiological cure rate, 30-day mortality, and LOS) in a separate regression model. In the multiple regression models, we controlled for variables that were statistically significant (p-value ≤0.1) in univariate analyses. All statistical analyses were performed using R version 3.5.0.
Results
Study Population
Of the total 227 Enterobacteriaceae cultures identified during the study period, 130 (57.3%) were MDR and 8 (3.5%) were XDR cultures. No PDR cultures were isolated. Death during the hospital stay was reported in 84.1% (116/138) of the patients. Majority of the study population were female (54%) and the mean age expressed as mean ± SD was 60.1 ± 17.7 years. About half of the patients had a history of diabetes and 19.6% of the patients were previously or actively diagnosed with cancer. The most prevalent bacteria were E. coli (51.4%), followed by K. pneumonia (32.6%) and Enterobacter sp. (15.9%). Seventy-one (51.4%) of the total MDR/XDR cultures were ESBL producers and 14 (10.1%) were CPE. Urine was the most common source of infection. Most of the patients were on ventilators (61.6%) and used antibiotics for 4 weeks (71.7%), 90 days (79.7%) before enrollment. When divided by survival status, no significant differences in the clinical characteristics were evident among the patients, except for gender. Majority of the patients who survived were females. These results are summarized in Table 1.
Table 1.
Demographics and Baseline Characteristics Based on Resistance Type
| Characteristics | Overall (n=138) | Alive (n=22/138) | Dead (n=116/138) | p-value* |
|---|---|---|---|---|
| Gender | 0.004 | |||
| Male | 64 (46.4%) | 4 (18.2%) | 60 (51.7%) | |
| Female | 74 (53.6%) | 18 (81.8%) | 56 (48.3%) | |
| Age | 0.93 | |||
| Mean ± SD | 60.1 ± 17.7 | 59.7 ± 21.4 | 60.1 ± 17.1 | |
| Median [min, max] | 63.5 [14.0, 94.0] | 69.5 [15.0, 87.0] | 63.0 [14.0, 94.0] | |
| History of DM | 75 (54.3%) | 12 (54.5%) | 63 (54.3%) | 0.98 |
| History of cancer | 27 (19.6%) | 5 (22.7%) | 22 (19.0%) | 0.68 |
| Bacteria | 0.26 | |||
| E. coli | 71 (51.4%) | 12 (54.5%) | 59 (50.9%) | |
| K. pneumoniae | 45 (32.6%) | 9 (40.9%) | 36 (31.0%) | |
| Enterobacter species | 22 (15.9%) | 1 (4.5%) | 21 (18.1%) | |
| MDR/XDR strains | 0.78 | |||
| MDR | 130 (94.2%) | 21 (95.5%) | 109 (94.0%) | |
| XDR | 8 (5.8%) | 1 (4.5%) | 7 (6.0%) | |
| Resistance type | 0.48 | |||
| ESBL | 71 (51.4%) | 13 (59.1%) | 58 (50.0%) | |
| CPE | 14 (10.1%) | 3 (13.6%) | 11 (9.5%) | |
| Other | 53 (38.4%) | 6 (27.3%) | 47 (40.5%) | |
| Source | 0.56 | |||
| Urine | 34 (24.6%) | 7 (31.8%) | 27 (23.3%) | |
| Wound | 16 (11.6%) | 4 (18.2%) | 12 (10.3%) | |
| Blood | 15 (10.9%) | 0 (0%) | 15 (12.9%) | |
| Sputum | 14 (10.1%) | 4 (18.2%) | 10 (8.6%) | |
| Tracheal aspirate | 12 (8.7%) | 2 (9.1%) | 10 (8.6%) | |
| Central line | 12 (8.7%) | 3 (13.6%) | 9 (7.8%) | |
| Urinary catheter | 8 (5.8%) | 1 (4.5%) | 7 (6.0%) | |
| Peritoneal fluid | 7 (5.1%) | 1 (4.5%) | 6 (5.2%) | |
| Tissue | 7 (5.1%) | 0 (0%) | 7 (6.0%) | |
| Others | 13 (9.3) | 0 (0%) | 13 (11.2%) | |
| Ventilation | 85 (61.6%) | 11 (50.0%) | 74 (63.8%) | 0.22 |
| ESRD | 26 (18.8%) | 4 (18.2%) | 22 (19.0%) | 0.93 |
| Prior 4 weeks antibiotic use | 99 (71.7%) | 12 (54.5%) | 87 (75.0%) | 0.05 |
| Prior 90 days antibiotic use | 110 (79.7%) | 18 (81.8%) | 92 (79.3%) | 0.79 |
| Prior 4 weeks antibiotic use | 99 (71.7%) | 12 (54.5%) | 87 (75.0%) | 0.05 |
| Inotropes | 75 (54.3%) | 8 (36.4%) | 67 (57.8%) | 0.06 |
| ICU length of stay ≥ 72 hours before culture | 63 (45.7%) | 11 (50.0%) | 52 (44.8%) | 0.66 |
Notes: *Chi-square test was used to compare categorical data and independent sample t-test to compare continuous data. Data presented as mean ± standard deviation for continuous variables, or number and (percentages) for categorical variables.
Abbreviations: CPE, carbapenem-producing Enterobacteriaceae; DM, diabetes mellitus; ESBL, extended-spectrum β-lactamase; ESRD, end-stage renal disease; ICU, intensive care unit; MDR, multidrug-resistant; SD, standard deviation; XDR, extensively drug-resistant.
Empirical Therapy
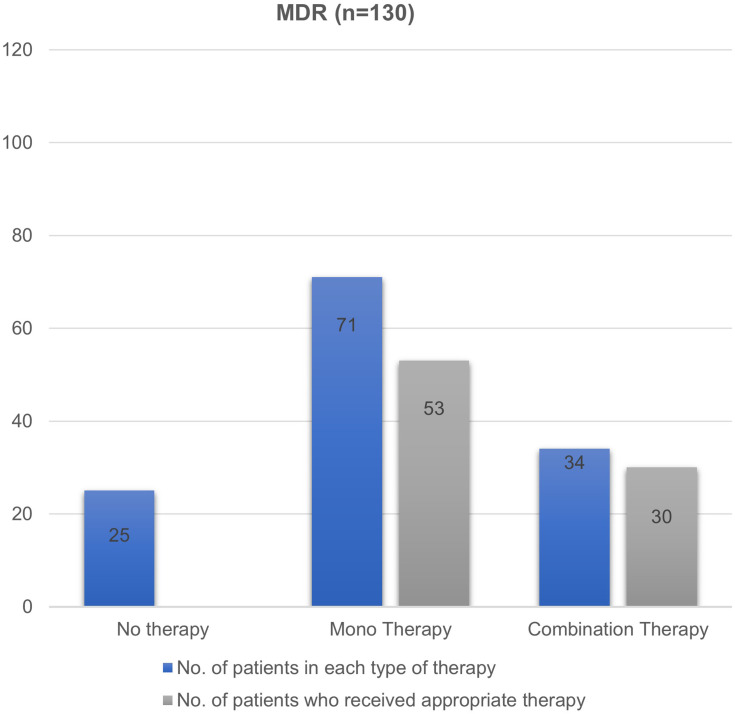
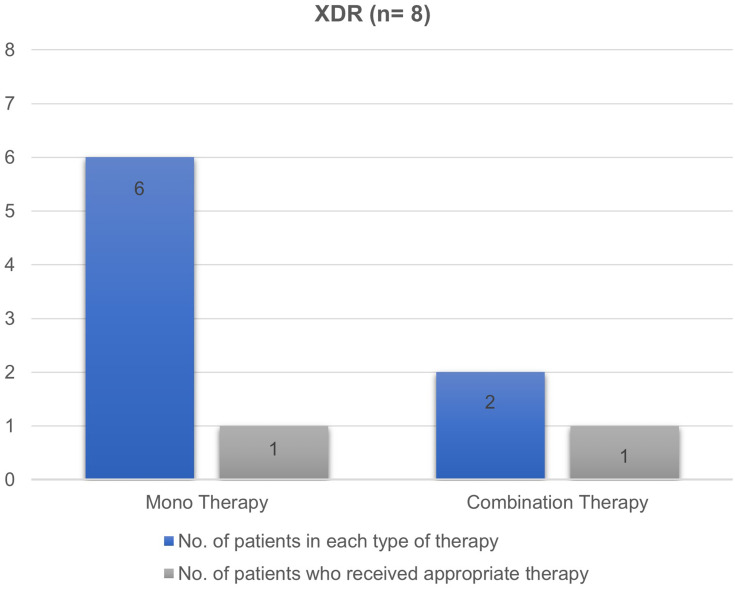
Majority of patients with MDR infections were subjected to treatment with monotherapy (52%), followed by combination therapy (30%) and no therapy (18%) (Figure 1). In 10 patients who received no antibiotics (25/138), the site of infection was urine. Among those who received monotherapy, the most commonly used antibiotic was piperacillin/tazobactam (n= 31, 46%). In contrast, carbapenems along with aminoglycoside was the most frequent combination therapy for MDR infections. XDR infections were primarily treated with monotherapy (n= 6, 75%) involving carbapenems as the most commonly used drug in half of the cases (Figure 2). Two patients received combination therapy involving carbapenems with aminoglycoside and the other with fluoroquinolone.
Figure 1.
Number of antibiotic therapy and their appropriateness in MDR Enterobacteriaceae isolates.
Abbreviation: MDR, multidrug-resistant.
Figure 2.
Number of antibiotic therapy and their appropriateness in XDR Enterobacteriaceae isolates.
Abbreviation: XDR, extensively drug-resistant.
Antibiotic therapy was found to be appropriate in 61.59% (85/138) of the patients only. In the MDR and XDR groups, 64% (83/130) and 25% (2/8) of the patients received appropriate antibiotic therapy, respectively (Figures 1 and 2). A detailed description of the antibiotics received by the study participants and their appropriateness are listed in Supplementary Tables S1 and S2.
Clinical Outcomes
Of the 130 patients with MDR infections, 108 (83%) died at the end of follow-up, 52 (40%) reached microbiological cure, and the mean LOS was 25.8 days. On the contrary, in the XDR group (n= 8), 7 (79%) patients died, microbiological cure was achieved in 4 (50%) patients, and the mean LOS was 48.0 days (Table 2). Univariate regression analysis revealed mortality to be significantly associated with the male patients (Table 3). Antibiotic use in the previous 90 days was related to lower odds of microbiological cure rate (Table 4). Since no other variables were associated with clinical outcomes at a p-value <0.1, multivariate regression models were not performed (Tables 3 and 4). Neither the type of resistance and/or bacterial strains nor the appropriateness of antibiotics prescribed, were associated with LOS using multivariate linear models (Table 5). However, the use of ventilators (coefficient= 11.49, 95% confidence interval [CI]= 1.02–21.97) and an ICU stay of >72 hours before culture results were associated with an increased LOS (coefficient= 28.65, 95% CI= 17.85–39.46). The use of inotropes resulted in shorter stays (Table 5).
Table 2.
Clinical Outcomes of Study Subjects Divided by MDR/XDR Status
| Outcome | Overall (n=138) | MDR Group (n=130) | XDR Group (n=8) | p-value* |
|---|---|---|---|---|
| ICU LOS in days mean ± SD | 27.1 ± 32.7 | 25.8 ± 31.9 | 48.0 ± 39.4 | 0.06 |
| Culture negative, n (%) | 56 (40.6%) | 52 (40.0%) | 4 (50.0%) | 0.58 |
| Hospital mortality, n (%) | 116 (84.1%) | 109 (83.8%) | 7 (87.5%) | 0.78 |
Notes: *Chi-square test was used to compare categorical data and independent sample t-test to compare continuous data. Data presented as mean ± standard deviation for continuous variables, or number and (percentages) for categorical variables.
Abbreviations: ICU, intensive care unit; LOS, length of stay; MDR, multidrug-resistant; SD, standard deviation; XDR, extensively drug-resistant.
Table 3.
Characteristics Associated with Hospital Mortality in Study Subjects (N=138)
| Unadjusted Analysis OR (95% CI) | |
|---|---|
| Male gender | 4.82 (1.68–17.47) |
| Age (years) | 1.00 (0.97–1.03) |
| History of DM | 0.99 (0.39–2.48) |
| History of cancer | 0.80 (0.28–2.62) |
| XDR strain | 1.35 (0.22–25.90) |
| Bacteria | |
| K. pneumoniae | Reference |
| E. coli | 1.22 (0.46–3.20) |
| Enterobacter species | 5.23 (0.90–100.28) |
| Resistance type | |
| ESBL | 0.57 (0.19–1.56) |
| CPE | 0.46 (0.11–2.48) |
| Other | Reference |
| Ventilation | 1.76 (0.70–4.46) |
| ESRD | 1.05 (0.35–3.92) |
| Prior 90 days antibiotic use | 0.85 (0.23–2.54) |
| Prior 4 weeks antibiotic use | 2.5 (0.96–6.41) |
| Inotropes | 2.39 (0.95–6.41) |
| ICU length stay ≥ 72hrs until culture | 0.81 (0.32–2.04) |
| Appropriateness of therapy | 1.58 (0.63–3.98) |
Abbreviations: CI, confidence interval; CPE, carbapenem-producing Enterobacteriaceae; DM, diabetes mellitus; ESBL, extended-spectrum β-lactamase; ESRD, end-stage renal disease; ICU, intensive care unit; OR, odds ratio, SD, standard deviation; XDR, extensively drug-resistant.
Table 4.
Characteristics Associated with Negative Cultures in Study Subjects (N=138)
| Unadjusted Analysis OR (95% CI) | |
|---|---|
| Male gender | 0.62 (0.31–1.22) |
| Age (years) | 1.00 (0.98–1.02) |
| History of DM | 0.74 (0.37–1.47) |
| History of cancer | 1.47 (0.62–3.44) |
| XDR Strain | 1.5 (0.34–6.60) |
| Bacteria | |
| K. pneumoniae | Reference |
| E. coli | 0.98 (0.46–2.11) |
| Enterobacter species | 1.25 (0.44–3.52) |
| Resistance type | |
| ESBL | 0.67 (0.32–1.39) |
| CPE | 2.35 (0.71–8.54) |
| Other | Reference |
| Ventilation | 0.30 (0.14–0.60) |
| ESRD | 1.05 (0.35–3.92) |
| Prior 90 days antibiotics use | 0.43 (0.18–0.99) |
| Prior 4 weeks antibiotics use | 0.54 (0.25–1.15) |
| Inotropes | 0.60 (0.29–1.16) |
| ICU length stay ≥ 72hrs until culture | 1.19 (0.60–2.36) |
| Appropriateness of therapy | 0.67 (0.33–1.33) |
Abbreviations: CI, confidence interval; CPE, carbapenem-producing Enterobacteriaceae; DM, diabetes mellitus; ESBL, extended-spectrum β-lactamase; ESRD, end-stage renal disease; ICU, intensive care unit; OR, odds ratio, SD, standard deviation; XDR, extensive drug-resistant.
Table 5.
Characteristics Associated with LOS in Study Subjects (N=138)
| Unadjusted Analysis Coefficient (95% CI) | Adjusted Analysis Coefficient (95% CI) | |
|---|---|---|
| Male gender | −3.33 (−14.42–7.76) | – |
| Age (years) | −0.16 (−0.47–0.18) | – |
| History of DM | 7.83 (−3.21–18.87) | – |
| History of cancer | −10.40 (−24.21–3.42) | – |
| XDR Strain | 22.21 (−1.12–45.54)* | 2.03 (−19.28–23.33) |
| Bacteria | ||
| K. pneumoniae | Reference | Reference |
| E. coli | −14.69 (−26.86- [−2.51])* | −4.64 (−15.80–6.51) |
| Enterobacter species | −0.18 (−16.75–16.39) | − 3.62 (−18.36–11.12) |
| Resistance type | ||
| ESBL | −2.44 (−14.24–9.36) | – |
| CPE | 3.69 (−16.43–23.81) | – |
| Other | Reference | – |
| Ventilation | 17.34 (6.31–28.38)* | 11.49 (1.02–21.97)** |
| ESRD | 6.45 (−7.86–20.76) | – |
| Prior 90 days antibiotics use | 11.51 (−2.09–25.11)* | −1.33 (−13.67–11.01) |
| Prior 4 weeks antibiotics use | 3.53 (−8.74–15.79) | – |
| Inotropes | −12.24 (−23.16- [−1.32]) | −11.72 (−21.54- [−1.89])** |
| ICU length stay ≥ 72hrs until culture | 33.10 (23.50–42.71)* | 28.65 (17.85–39.46)** |
| Appropriateness of therapy | −7.71 (−18.90–3.49) | – |
Notes: *Variables with p-values below 0.1 were included in the adjusted model. **p-value <0.05.
Abbreviations: CI, confidence interval; CPE, carbapenem-producing Enterobacteriaceae; DM, diabetes mellitus; ESBL, extended-spectrum β-lactamase; ESRD, end-stage renal disease; ICU, intensive care unit; OR, odds ratio, SD, standard deviation; XDR, extensively drug-resistant.
Discussion
The study estimated the prevalence rate of drug-resistant Enterobacteriaceae infections in the adult medical, surgical, and cardiac ICUs of a tertiary-care hospital in Saudi Arabia. The antibiotic regimens used to treat these infections and the factors associated with 30-day mortality and microbiological cure rate were also investigated in this study. While 60% of the Enterobacteriaceae cultures isolated from the ICU during the study period were either MDR or XDR, no patients with PDR infections were identified. The prevalence rates in this study were higher compared to those reported in the previous studies conducted worldwide. A study based in India involving bacterial isolates from a tertiary-care hospital reported lower rates of drug-resistant GNB; 33.5% were MDR strains, 12.1% were XDR strains, and no PDR strains were identified.21 In 2019, a nationwide report from the United States reported that the proportion of MDR Enterobacteriaceae isolates in inpatients to be 6.6%.22 A large study on the resistance patterns of the bacterial isolates obtained from an emergency department in Hungary revealed 23.8% of the bacterial isolates to be MDR.20 Only one local study on the E. coli isolates reported 67% prevalence rate of MDR infections.23 The factor that attributed to the high prevalence rate of MDR infections observed in this study was the critically ill health of the patients, compared to the previous report on patients that involved both ICU and non-ICU patients.21–23 Other studies involving ICU patients revealed either lower or similar rates of drug resistance among the GNB compared to the current study.24,25 A study on 137 patients in Nepal reported 46% of the GNB isolates obtained from an ICU to cause MDR infections.24 Another study in India, which looked at the bacterial isolates in critically ill patients with ventilator-associated pneumonia, found that 88% of the isolates were GNB, of which 72% were responsible for MDR infections.25
There are a few significant differences between the current study and previously published reports on the prevalence of drug-resistant Enterobacteriaceae. First, the current study specifically focused on the Enterobacteriaceae species rather than GNB in general. According to the World Health Organization (WHO), microorganisms belonging to the Enterobacteriaceae family have been identified as critical and are ranked first in the WHO priority pathogen list for research, discovery, and development of new antibiotics.26 Second, both MDR and XDR cases are described here, compared to the previous studies that focused on MDR infections alone. Lastly, owing to the limited availability of data on the prevalence of infections caused by drug-resistant GNB in critically ill patients, the current study on ICU patients would indeed enrich the existing literature.
The prevalence rate (51.4%) of ESBL-producing bacteria was much higher in the current study region compared to that reported in other regions. For instance, in East Europe, the prevalence of ESBL-producing bacteria was >10%, while in Canada, it was 3.5%.27,28 In the Arabian Gulf region, the prevalence ranged from 7.5% in Kuwait to 41% in the United Arab Emirates.29,30 Furthermore, other studies in Saudi Arabia reported prevalence of ESBL-producing bacteria to be between 22% and 36% in Enterobacteriaceae.31 In this study, the prevalence of CPE (6.2%) was also higher than that reported in the previous studies.32–36 This could be due to the high number of MDR-GNB infections observed in the current study sample.
More than half of the subjects in the current study received initial monotherapy for the treatment of their infections, with piperacillin/tazobactam and carbapenems being the most used agents. However, only about 62% of the prescribed antibiotics were deemed appropriate. Furthermore, around 18% of the subjects did not receive any empirical antimicrobial therapy within the first 24 hours of culture results. It is essential to highlight that, in less than half of these subjects, the primary site of infection was the urine, which might have been bacterial colonization; therefore, no antibiotics were given. Limited studies investigated the appropriateness of antibiotic therapy in ICU patients with MDR and XDR infections. A study based in Italy reported that 61% of the therapies prescribed for MDR infections did not meet the proposed criteria for appropriateness.37 A review article that explicitly focused on CPE infections by compiling data from 889 patients revealed that combination therapy, monotherapy, and inappropriate therapy was received by 48.6%, 38.1%, and 11.3% of the patients, respectively.38 However, due to the lack of a universally accepted definition of appropriate definitive antibiotic therapy against MDR and XDR pathogens, comparison of the current study results with the existing literature was challenging.
The case fatality rate (84%) due to MDR and XDR Enterobacteriaceae in the current study was significantly higher than that reported in previous studies.17,39 A case fatality rate of 34% was observed in a study involving hospitalized patients with MDR gram-negative isolates.39 On the other hand, Blot et al after studying 328 patients with GNB infections in an ICU setting found that the case fatality rate for MDR infections was 45%.17 The high mortality rate reported in this study could be justified by the involvement of both MDR and XDR Enterobacteriaceae infections and the focus only on critically ill patients. The significant risk factors associated with mortality in the current study patients with MDR and XDR Enterobacteriaceae included previous use of antibiotics and the type of resistance (i.e., ESBL and CPE). However, significantly higher mortality rate was reported only in the male population. It has been suggested that while the classical resistance classifications, MDR, XDR, and PDR, do not correlate well with clinical outcomes, novel classification criteria, such as DTR may add value to the significance of bacterial resistance.5,40
This study suffers from several limitations. Firstly, being a single-center study, the results might have been affected by the practices exclusive to this center, thereby limiting the generalizability of the study findings. However, the ICU is a part of the large tertiary-care hospital that accepts patients from different parts of Saudi Arabia. Second, information on antibiotics administered beyond the first 24 hours of positive culture results was not collected. Whether the positive cultures were a result of bacterial colonization rather than active infections, specifically in the case of urine infection was not clear. This uncertainty might have affected the definition of appropriate antibiotic therapy. Third, since the current study is a retrospective analysis of clinical data, some data could be missing. Nevertheless, we attempted to collect all study-related information for all participants. Lastly, the small size limited the ability to perform adjusted regression analyses.
Despite the limitations, the study has several strengths. The study focused on both MDR and XDR infections and reported on important clinical outcomes, specifically the mortality rate. In addition, a detailed description on the use of antibiotics within the first 24 h of culture results was also presented.
Conclusion
The rate of antimicrobial resistance among the GNBs differs significantly worldwide and rapidly changes over time. Limited data are available on the prevalence of MDR, XDR, and PDR Enterobacteriaceae in Saudi Arabia. Most local studies reported the prevalence of ESBL and carbapenemase producers, which may owe to the lack of standard definitions. This study showed that more than half of Enterobacteriaceae infections in critically ill patients were due to drug-resistant bacteria, with E. coli being the most common pathogen. The high mortality rate observed in the current study cohort has been alarming, indicating the need for further research involving larger sample of patients from different centers to identify the possible predictors of mortality. Finally, global efforts are also warranted to develop new antimicrobial agents that can minimize the negative clinical and economic consequences of antibiotic-resistant organisms.
Acknowledgments
We thank Undergraduate Research Support Program for their support and KSUMC microbiology lab for their massive support and help. This research project was supported by a grant from the “Research Center of the Center for Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Funding Statement
This research project was supported by a grant from the “Research Center of the Center for Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Abbreviations
CDC, Centers for Disease Control and Prevention; CLSI, Clinical and Laboratory Standards Institute; CPE, carbapenemase-producing Enterobacteriaceae; DTR, difficult-to-treat resistance; ECDC, European Centre for Disease Prevention and Control; ESBLs, extended-spectrum beta-lactamases; GNB, Gram-negative bacteria; ICU, intensive care unit; IQR, interquartile range; KPC, Klebsiella pneumoniae carbapenemase; KSUMC, King Saud University Medical City; LOS, length of stay; MDR, multidrug-resistant; NDM, New Delhi metallo-beta-lactamase; OXA-48, oxacillinase-48; PDR, pandrug resistant; SD, standard deviation; VIM, Verona integron–encoded metallo-beta-lactamase; WHO, World Health Organization; XDR, extensively drug-resistant.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The study was approved by the institutional review board of King Saud University (Institutional review board number E-18-2874). All data were anonymized to maintain participant’s privacy, and the study was conducted in accordance with the Declaration of Helsinki. In light of the retrospective and anonymous nature of the study, the Ethics Committee did not require written informed consent provided by participants.
Author Contributions
HA and AMA both made equal and substantial contributions to the study. In addition, all authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interests regarding this work.
References
- 1.Exner M, Bhattacharya S, Christiansen B, et al. Antibiotic resistance: what is so special about multidrug-resistant gram-negative bacteria? GMS Hyg Infect Control. 2017;12:Doc05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 3.Kang CI, Kim SH, Park WB, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760–766. doi: 10.1128/AAC.49.2.760-766.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lockhart SR, Abramson MA, Beekmann SE, et al. Antimicrobial resistance among gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45(10):3352–3359. doi: 10.1128/JCM.01284-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajdács M, Bátori Z, Ábrók M, Lázár A, Burián K. Characterization of resistance in gram-negative urinary isolates using existing and novel indicators of clinical relevance: a 10-year data analysis. Life. 2020;10(2):16. doi: 10.3390/life10020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parajuli NP, Acharya SP, Mishra SK, Parajuli K, Rijal BP, Pokhrel BM. High burden of antimicrobial resistance among gram negative bacteria causing healthcare associated infections in a critical care unit of Nepal. Antimicrob Resist Infect Control. 2017;6(1):67. doi: 10.1186/s13756-017-0222-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falagas ME, Bliziotis IA, Kasiakou SK, Samonis G, Athanassopoulou P, Michalopoulos A. Outcome of infections due to pandrug-resistant (PDR) gram-negative bacteria. BMC Infect Dis. 2005;5(1):24. doi: 10.1186/1471-2334-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druge S, Ruiz S, Vardon-Bounes F, et al. Risk factors and the resistance mechanisms involved in Pseudomonas aeruginosa mutation in critically ill patients. J Intensive Care. 2019;7(1):36. doi: 10.1186/s40560-019-0390-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollef MH. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin Infect Dis. 2008;47(Suppl S1):S3–S13. doi: 10.1086/590061 [DOI] [PubMed] [Google Scholar]
- 10.Micek ST, Welch EC, Khan J, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother. 2010;54(5):1742–1748. doi: 10.1128/AAC.01365-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zowawi HM. Antimicrobial resistance in Saudi Arabia. An urgent call for an immediate action. Saudi Med J. 2016;37(9):935–940. doi: 10.15537/smj.2016.9.16139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the infectious diseases society of America. Clin Infect Dis. 2009;48(1):1–12. doi: 10.1086/595011 [DOI] [PubMed] [Google Scholar]
- 13.Gajdács M, Albericio F. Antibiotic resistance: from the bench to patients. Antibiotics. 2019;8(3):129. doi: 10.3390/antibiotics8030129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892. doi: 10.3390/molecules24050892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Suppl 2):S82–S89. doi: 10.1086/499406 [DOI] [PubMed] [Google Scholar]
- 16.Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18(1):54–60. doi: 10.1111/j.1469-0691.2011.03478.x [DOI] [PubMed] [Google Scholar]
- 17.Blot S, Vandewoude K, De Bacquer D, Colardyn F. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis. 2002;34(12):1600–1606. doi: 10.1086/340616 [DOI] [PubMed] [Google Scholar]
- 18.Menashe G, Borer A, Yagupsky P, et al. Clinical significance and impact on mortality of ESBL-producing gram-negative isolates in nosocomial bacteremia. Scand J Infect Dis. 2001;33(3):188–193. doi: 10.1080/00365540151060806 [DOI] [PubMed] [Google Scholar]
- 19.Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;Suppl 31(Supplement_4):S131–S138. doi: 10.1086/314079 [DOI] [PubMed] [Google Scholar]
- 20.Benkő R, Gajdács M, Matuz M, et al. Prevalence and antibiotic resistance of ESKAPE pathogens isolated in the emergency department of a tertiary care teaching hospital in hungary: a 5-year retrospective survey. Antibiotics. 2020;9(9):624. doi: 10.3390/antibiotics9090624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basak S, Singh P, Rajurkar M. Multidrug resistant and extensively drug resistant bacteria: a Study. J Pathog. 2016;2016:1–5. doi: 10.1155/2016/4065603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta V, Ye G, Olesky M, Lawrence K, Murray J, Yu K. National prevalence estimates for resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States. Int J Infect Dis. 2019;85:203–211. doi: 10.1016/j.ijid.2019.06.017 [DOI] [PubMed] [Google Scholar]
- 23.Alqasim A, Abu Jaffal A, Alyousef A. Prevalence of multidrug resistance and extended-spectrum β-lactamase carriage of clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Int J Microbiol. 2018;3026851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siwakoti S, Subedi A, Sharma A, Baral R, Bhattarai NR, Khanal B. Incidence and outcomes of multidrug-resistant gram-negative bacteria infections in intensive care unit from Nepal- a prospective cohort study. Antimicrob Resist Infect Control. 2018;7:114. doi: 10.1186/s13756-018-0404-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta R, Malik A, Rizvi M, et al. Epidemiology of multidrug-resistant gram-negative pathogens isolated from ventilator-associated pneumonia in ICU patients. J Glob Antimicrob Resist. 2017;9:47–50. doi: 10.1016/j.jgar.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization (WHO): list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Available from: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed December3, 2020.
- 27.Coque TM, Baquero F, Canton R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 2008;13(47):19044. [PubMed] [Google Scholar]
- 28.Zhanel GG, DeCorby M, Laing N, et al. Antimicrobial-resistant pathogens in intensive care units in Canada: results of the Canadian National Intensive Care Unit (CAN-ICU) study, 2005–2006. Antimicrob Agents Chemother. 2008;52(4):1430–1437. doi: 10.1128/AAC.01538-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamal W, Rotimi VO, Khodakhast F, et al. Prevalence of extended-spectrum β-lactamases in Enterobacteriaceae, Pseudomonas and Stenotrophomonas as determined by the VITEK 2 and E test systems in a Kuwait teaching hospital. Med Prin Pract. 2005;14:325–331. doi: 10.1159/000086930 [DOI] [PubMed] [Google Scholar]
- 30.Al-Zarouni M, Senok A, Rashid F, Al-Jesmi SM, Panigrahi D. Prevalence and antimicrobial susceptibility pattern of extended-spectrum β-lactamase-producing Enterobacteriaceae in the United Arab Emirates. Med Prin Pract. 2008;17:32–36. doi: 10.1159/000109587 [DOI] [PubMed] [Google Scholar]
- 31.Kandeel A. Prevalence and risk factors of extended-spectrum β-lactamases producing Enterobacteriaceae in a general hospital in Saudi Arabia. J Microbiol Infect Dis. 2014;4(2):50–54. doi: 10.5799/ahinjs.02.2014.02.0126 [DOI] [Google Scholar]
- 32.Lai CC, Wu UI, Wang JT, Chang SC. Prevalence of carbapenemase-producing Enterobacteriaceae and its impact on clinical outcomes at a teaching hospital in Taiwan. JFMA. 2013;112(8):492–496. [DOI] [PubMed] [Google Scholar]
- 33.El Wartiti MA, Bahmani FZ, Elouennass M, Benouda A. Prevalence of carbapenemase- producing Enterobacteriaceae in a University Hospital in Rabat, Morocco: a 19-Months Prospective Study. Int Arab J Antimicrob Agents. 2012;2(3:4):1–6. [Google Scholar]
- 34.Kandeel A. Epidemiology of carbapenemase producing Enterobacteriaceae in a general hospital. JMID. 2015;5(2):57–62. doi: 10.5799/ahinjs.02.2015.02.0177 [DOI] [Google Scholar]
- 35.Garbati M, Sakkijha H, Abushaheen A. Infections due to carbapenem resistant Enterobacteriaceae among Saudi Arabian hospitalized patients: a matched Case-Control Study. Biomed Res Int. 2016;2016:3961684. doi: 10.1155/2016/3961684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alotaibi F, Bukhari E, Al-Mohizea M, Hafiz T, Essa EB, AlTokhais YI. Emergence of carbapenem-resistant Enterobacteriaceae isolated from patients in a university hospital in Saudi Arabia. Epidemiology, clinical profiles and outcomes. J Infect Public Health. 2017;10(5):667–673. doi: 10.1016/j.jiph.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viceconte G, Maraolo AE, Iula VD, Catania MR, Tosone G, Orlando R. Appropriateness of antibiotic prescription for targeted therapy of infections caused by multidrug-resistant bacteria: assessment of the most common improper uses in a tertiary hospital in southern Italy. Infez Med. 2017;25(3):224–233. [PubMed] [Google Scholar]
- 38.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2014;20(9):862–872. doi: 10.1111/1469-0691.12697 [DOI] [PubMed] [Google Scholar]
- 39.Patolia S, Abate G, Patel N, Patolia S, Frey S. Risk factors and outcomes for multidrug- resistant gram-negative bacilli bacteremia. Ther Adv Infect Dis. 2018;5(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadri SS, Adjemian J, Lai YL, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67:1803–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]