Abstract
Introduction:
This study examines the dose–response relationship between moderate-to-vigorous physical activity and cardiometabolic measures in adolescents.
Methods:
Cross-sectional spline analyses were performed using 2003–2016 National Health and Nutrition Examination Survey data among adolescents (aged 12–19 years, N=9,195) on objectively measured (2003–2006) and self-reported weekly mean minutes (2007–2016) of moderate-to-vigorous physical activity and cardiometabolic measures (systolic and diastolic blood pressure, total cholesterol, high-density lipoprotein, BMI, and cardiorespiratory fitness. Inflection points were determined for non-linear relationships.
Results:
For objective moderate-to-vigorous physical activity, female adolescents had significant non-linear associations with inflection points at 90 minutes/week for BMI percentile and systolic blood pressure. Male adolescents had inflection points at 150 weekly minutes of objective activity for BMI percentile and cardiorespiratory fitness. BMI percentile was about 7% lower for female and male adolescents at 150 weekly minutes of objectively measured moderate-to-vigorous physical activity versus 0 minutes. For self-reported moderate-to-vigorous physical activity, inflections points were at 375 minutes/week (diastolic blood pressure for female adolescents) and 500 minutes/week (systolic blood pressure for male adolescents).
Conclusions:
Among several significant dose–response relationships between physical activity and cardiometabolic health in adolescents, consistent and often non-linear relationships were identified for BMI, with inflection points at 90–150 minutes of objective moderate-to-vigorous physical activity. Notable differences in associations and linearity were identified by sex and physical activity measure (objective or self-report). These results support calls for any increase in physical activity among adolescents and suggest that recommendations closer to the adult guidelines of 150 weekly minutes of physical activity may be health promoting and more attainable for youth than the current recommendation of 420 weekly minutes.
INTRODUCTION
Many health benefits of physical activity (PA) for adolescents have been described,1,2 including improving cardiometabolic health in adolescence and over the lifespan.3–7 Engaging in regular moderate-to-vigorous PA (MVPA) in adolescence has been associated with lower blood pressure (BP), lower serum triglycerides, and improved cardiorespiratory fitness.5,8,9 BMI is a commonly assessed cardiometabolic metric in adolescents. MVPA has generally been associated with lower BMI and slower weight gain in adolescents, but results have been mixed.10–13
However, adolescents in the U.S. are not physically active at the recommended levels.14–16 Only 20% of a nationally representative sample of adolescents met the PA guidelines of at least 60 minutes of MVPA daily.14 PA in adolescence predicts activity levels in adulthood, heightening the importance of encouraging adolescent PA.17 The adolescent period is crucial to understand, given the emerging differences in behavioral choices (e.g., boys being more active than girls), disparities in activity (e.g., lower activity in older adolescents and some racial and ethnic minority groups), and physiologic changes (e.g., puberty) in adolescence, all of which can differentially affect the health of young men and women.14,18–23
Prior studies examining the association of PA and cardiometabolic health have predominantly defined PA as a categorical variable (e.g., top quartile of minutes of MPVA achieved), rather than as a continuous variable. Analyzing PA categorically precludes a more nuanced understanding of the dose–response relationship between PA and health.3–5,8,9,24 Prior studies have also typically used PA measured either objectively with an activity-tracking device or by self-report responses to a survey. Each type of activity measure has limitations: Objective data are often collected over short time periods, capturing variable or incomplete (i.e., detects only uniaxial movement) activity, whereas self-reported data may be subject to response bias, which can overestimate true activity levels.25–27 This study adds to the existing literature by using contemporary nationally representative data to assess the dose–response relationship between adolescent PA—measured both objectively and by self-report as a continuous variable—and cardiometabolic health, stratified by sex. The authors hypothesize that significant dose–response relationships exist between PA and cardiometabolic health measures for adolescents and that these relationships differ by sex.
METHODS
Study Population
Investigators examined data from adolescents (aged 12–19 years) in the 2003–2016 National Health and Nutrition Examination Survey (NHANES). The NHANES is a stratified, multistage probability sample, providing a nationally representative sample of the U.S. population. The data include physical examination, in-home interviews, and laboratory testing in Mobile Examination Centers.28
Measures
Primary exposures were objective and self-reported weekly mean minutes of MVPA. Accelerometers (ActiGraph AM-7164) were used to measure minutes of objective PA via uniaxial movement in NHANES for 2003–2006.29 The intensity of movements summed over 1-minute time intervals were measured and recorded as counts.30,31 Participants were instructed to wear the accelerometers for 7 consecutive days while awake and remove them nightly at bedtime.4 A minimum of 3 wear days for ≥10 hours/day were required for inclusion in these analyses.32 Sample weights were adjusted for this subgroup (n=761 female and 1,168 male adolescents with any ActiGraph data) to generate nationally representative estimates.
The NHANES self-reported PA data from 2007–2016 were derived from the WHO Global Physical Activity Questionnaire.33–36 Adolescents responded to the questionnaire without parental assistance and were asked the average number of days and duration spent on recreational MVPA daily for ≥10 minutes at a time. Sports, fitness, or recreational activities of moderate or vigorous intensity were included, such as small increase in breathing or heart rate like brisk walking, bicycling, swimming, or golf for moderate and large increase in breathing or heart rate like running or basketball for vigorous activity.28 The primary self-reported MVPA measure was defined as the sum of weekly minutes of recreational activity. Additional questions capturing PA at work and transportation were used in a sensitivity analysis where recreational, occupational, and transportation activity were summed for total recreational activity.
Primary cardiometabolic outcomes were continuous measures of systolic BP (SBP), diastolic blood pressure (DBP), total cholesterol, high-density lipoprotein (HDL), BMI 95th percentile, and cardiorespiratory fitness (VO2 max). Repeated SBP and DBP measurements were averaged per NHANES guidelines.28 BMI was calculated using height and weight data as percentage of the 95th percentile (hereafter referred to as BMI percentile) per Centers for Disease Control and Prevention guidelines and to account for BMI percentile distribution.37 Serum total cholesterol and HDL were sampled non-fasting at the Mobile Examination Center. VO2 max estimates were available in the NHANES for years 2003–2004 and derived from an equation incorporating age, submaximal VO2 max, and heart rate during a treadmill physical exercise test.38
Covariates selected a priori based on prior literature included sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other Hispanic, and other Multiracial), federal poverty level ratio, health insurance (no insurance, Medicaid, private insurance), survey year, and 6-month calendar period (November to April, May to October) to account for seasonal variation in PA.21,39 For the objective activity models, number of wear days was also a covariate.
Statistical Analysis
Demographic characteristics are presented overall, by sex, and by NHANES cohort (2003–2006, 2007–2016). Continuous PA and cardiometabolic measure variables are presented as medians with first and third quartiles; categorical variables are presented as counts and percentages with 95% CIs. PA and cardiometabolic measures’ descriptive statistics are presented among participants who completed any amount of PA and had a measure recorded, respectively. Objective weekly PA minutes >500 were truncated to 501 minutes/week, and self-report minutes >1,260/week were truncated to 1,261 minutes/week to reduce the effects of likely implausible values.
The dose–response relationships between MVPA and cardiometabolic measures were assessed using unadjusted sex-stratified linear regression models. The functional form of each relationship was evaluated for linearity using natural cubic splines, with 3 knots placed at the 5th, 50th, and 95th percentiles of MVPA. When non-linearity was observed, natural cubic splines were used and inflection points, defined as the minutes of PA at which the relationship between a cardiometabolic measure and PA changes, were determined.
The unadjusted and adjusted (for covariates) estimated difference with SE, comparing 150 with 0 minutes of objective PA and 420 with 0 minutes of self-reported PA, are presented. These thresholds were selected based on the following criteria: existing PA guidelines (i.e., 150 minutes/week for adults and 420 minutes/week for youth),16 and the distribution of PA among study participants (e.g., few NHANES participants reached 420 minutes/week for objective MVPA).
Adjusted effect sizes estimates with 95% CIs are presented to quantify the amount by which the cardiometabolic outcome changed given a 1-minute change in the PA predictor; for non-linear associations, two different points were selected based on where the effect sizes best represented the average slopes.
Two sensitivity analyses were conducted: one broadening self-reported PA to include the sum of MVPA in occupational, transportation, and recreational settings,40 and the other defining adolescents as aged 12–17 years, per the child/adolescent age limit used in some guidelines.41
All analyses accounted for the complex NHANES survey design through stratification, clustering, and post-stratification weighting to maintain a nationally representative sample. Sampling weights were adjusted to better represent the U.S. population for the subpopulation of participants who provided objective MVPA and estimated VO2 max data—which had missing values >10%. Otherwise, no adjustments were needed for variable missingness.29 Two-tailed p-values <0.05 were considered statistically significant. All statistical analyses were performed by the Duke Clinical Research Institute using SAS, version 9.4. The present study was deemed exempt from further review by the Duke IRB.
RESULTS
Table 1 shows the demographic characteristics of the sample with a median age of 14.8 years. The study sample (N=9,915) was 49.0% female, 58.5% non-Hispanic White, 14.7% non-Hispanic Black, and 13.1% Mexican American. The 2003–2006 and 2007–2016 cohort characteristics can be found in Appendix Tables 2 and 3.
Table 1.
Adolescent Sample Characteristics (Age 12‒19 Years), NHANES 2003‒2016
| Characteristics | Overall (N=9,195) % (95% CI) |
Females (N=4,456) % (95% CI) |
Males (N=4,739) % (95% CI) |
|---|---|---|---|
| Age, median (Q1, Q3) | 14.8 (12.9, 16.7) | 14.8 (13.0, 16.7) | 14.7 (12.9, 16.7) |
| Race/Ethnicity | |||
| Non-Hispanic White | 58.5 (55.1, 61.8) | 58.3 (54.9, 61.6) | 58.7 (54.9, 62.4) |
| Non-Hispanic Black | 14.7 (12.8, 16.7) | 15.0 (12.9, 17.1) | 14.5 (12.5, 16.6) |
| Mexican American | 13.1 (11.2, 15.0) | 12.9 (10.9, 14.9) | 13.3 (11.3, 15.2) |
| Other Hispanic | 6.3 (5.3, 7.4) | 6.5 (5.3, 7.7) | 6.2 (5.0, 7.3) |
| Other | 7.4 (6.4, 8.4) | 7.4 (6.3, 8.5) | 7.4 (6.2, 8.6) |
| Federal poverty level ratio distribution | |||
| <1 | 21.6 (19.7, 23.5) | 22.3 (20.2, 24.5) | 20.9 (18.7, 23.1) |
| 1‒1.9 | 22.2 (20.6, 23.8) | 22.8 (20.7, 24.9) | 21.7 (19.8, 23.6) |
| 2‒2.9 | 16.5 (15.2, 17.9) | 17.1 (15.1, 19.2) | 16.0 (14.3, 17.6) |
| 3‒3.9 | 13.8 (12.3, 15.3) | 13.3 (11.5, 15.0) | 14.3 (12.4, 16.1) |
| ≥4 | 25.9 (23.6, 28.2) | 24.5 (21.6, 27.3) | 27.2 (24.6, 29.9) |
| Missing | 6.2 (5.3, 7.1) | 6.4 (5.3, 7.5) | 6.0 (5, 7.1) |
| Insurance coverage | |||
| No insurance | 11.9 (10.7, 13.1) | 11.7 (10.2, 13.1) | 12.1 (10.9, 13.3) |
| Private insurance | 58.8 (56.3, 61.3) | 59.4 (56.5, 62.2) | 58.3 (55.5, 61.1) |
| Medicaid | 20.0 (18.4, 21.7) | 20.6 (18.7, 22.6) | 19.4 (17.6, 21.3) |
NHANES, National Health and Nutrition Examination Survey.
Measures of PA revealed that 56.4% (median=37 minutes/week) of female and 81.3% (median=68 minutes/week) of male adolescents had any objectively measured activity, while 65.2% (median=266 minutes/week) of female and 80.7% (median=372 minutes/week) of male adolescents had any self-reported activity (Appendix Table 1). The distribution of the cardiometabolic measures is presented in Appendix Table 1.
In female adolescents, unadjusted non-linear and significant dose–response relationships were identified for objective MVPA with SBP and BMI; inflection points were at 90 weekly minutes of MVPA for both measures (Table 2 and Figure 1). For male adolescents, unadjusted non-linear and significant associations were identified for objective MVPA with SBP, BMI percentile, and VO2 max, all at inflection points of 150 weekly minutes (Table 2 and Figure 1).
Table 2.
Estimated Mean Change in Cardiometabolic Measures for Physical Activity in Adolescents (Age 12‒19 Years), NHANES
| Measure | Objective activity (150 vs 0 weekly minutes)a |
Self-report activity (420 vs 0 weekly minutes)b |
||
|---|---|---|---|---|
| Unadjusted (SE) |
Adjusted (SE) |
Unadjusted (SE) |
Adjusted (SE) |
|
| Systolic blood pressure | ||||
| Female | −2.95 (0.99)c | −2.39 (1.01) | 0.06 (0.37) | 0.22 (0.36) |
| Male | −4.27 (1.26)c | −2.92 (1.14) | −1.08 (0.56)c | 0.41 (0.51) |
| Diastolic blood pressure | ||||
| Female | −1.45 (0.84) | −1.54 (0.83) | −1.31 (0.70)c | −1.59 (0.66) |
| Male | −2.19 (0.72) | −1.22 (0.67) | −0.19 (0.31) | −0.39 (0.30) |
| Total cholesterol | ||||
| Female | −2.06 (3.20) | −0.94 (3.46) | −1.49 (1.02) | −1.22 (1.05) |
| Male | 0.18 (1.64) | −0.59 (1.85) | −1.46 (0.85) | −1.41 (0.87) |
| High density lipoprotein | ||||
| Female | −0.47 (1.37) | −0.08 (1.70) | 0.96 (0.39) | 1.01 (0.41) |
| Male | 3.27 (0.95) | 2.06 (0.97) | 1.13 (0.35) | 1.16 (0.35) |
| BMI | ||||
| Female | −6.55 (1.98)c | −7.10 (2.15) | −2.14 (0.55) | −1.45 (0.59) |
| Male | −7.26 (1.92)c | −7.65 (2.13) | −0.30 (0.57) | 0.01 (0.60) |
| VO2 max | ||||
| Female | 0.99 (1.03) | −0.11 (1.02) | N/Ad | N/Ad |
| Male | 5.74 (1.80)c | 7.05 (1.90) | N/Ad | N/Ad |
Notes: Boldface indicates statistical significance (p<0.05). BMI represented as percent of the 95th percentile.
Data from NHANES 2003‒2006. Change in cardiometabolic measure at 150 weekly minutes (per adult physical activity guideline recommendations) compared to 0 minutes of activity.
Data from NHANES 2007‒2016. Change in cardiometabolic measure at 420 weekly minutes (per adolescent physical activity guideline recommendations) compared to 0 minutes of activity.
Indicates non-linear relationship.
Measure not available for survey years used for self-report physical activity data.
NHANES, National Health and Nutrition Examination Survey.
Figure 1.
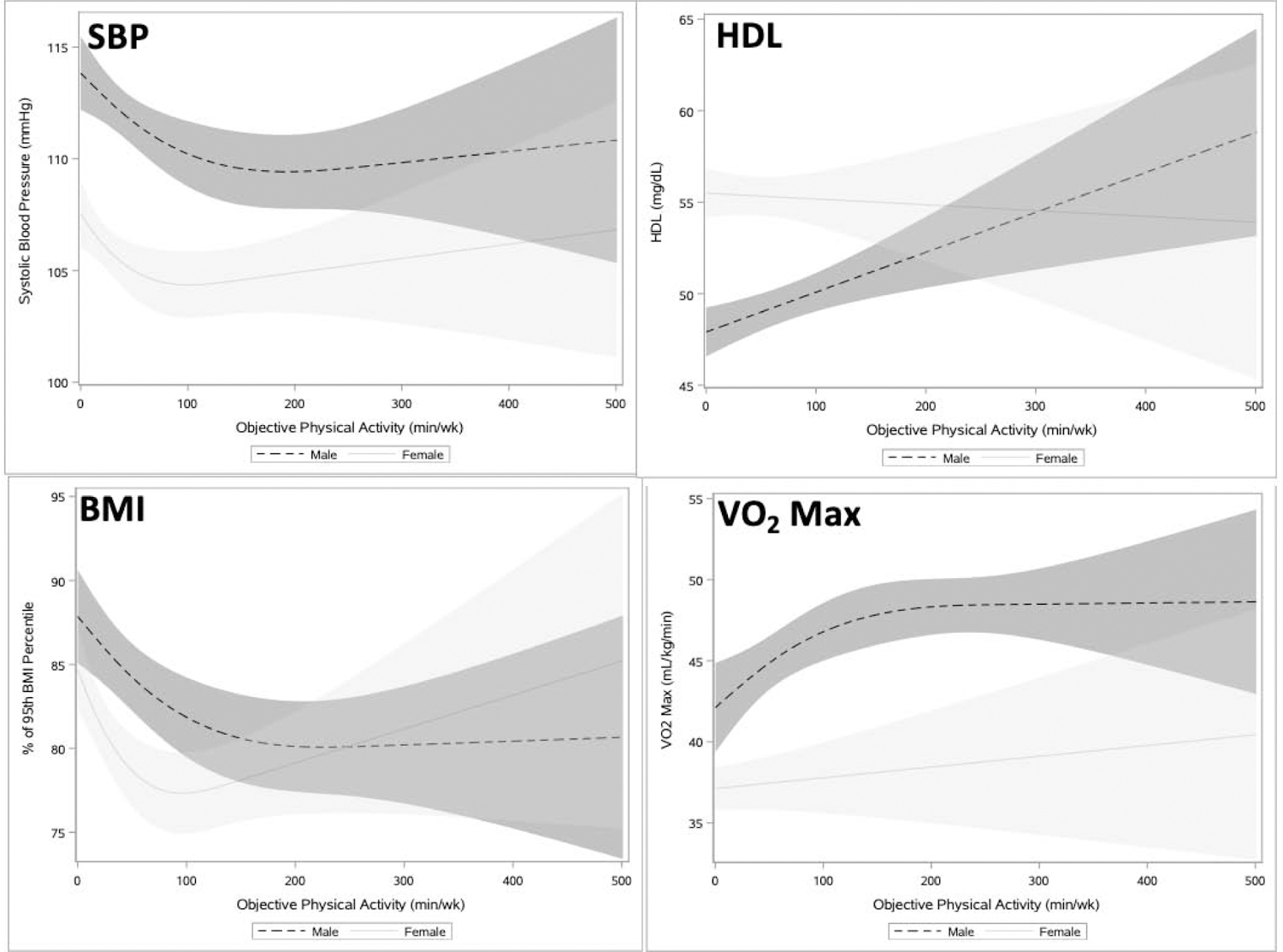
Association between objective physical activity and cardiometabolic outcomes among adolescents (age 12‒19 years), NHANES 2003‒2006.
Notes: Unadjusted Models. Shading represents 95% CI.
SBP, systolic blood pressure; HDL, high density lipoprotein; NHANES, National Health and Nutrition Examination Survey.
For female adolescents in adjusted analyses, 150 minutes versus 0 minutes of objective MVPA was significantly associated with lower BMI percentile (7.10%) (Table 2). Effect sizes, representing the average slope/1 minute of activity at selected points, for these associations were the following: SBP= −0.023 (95% CI= −0.044, −0.003) from 49 to 50 minutes and 0.005 (95% CI= −0.010, 0.021) from 199 to 200 minutes; and BMI percentile= −0.076 (95% CI= −0.114, − 0.038) from 49 to 50 minutes and 0.034 (95%=0.004, 0.065) from 199 to 200 minutes.
Among male adolescents in adjusted analyses, 150 minutes versus 0 minutes of objective MVPA was significantly associated with higher HDL (2.06 mg/dL), lower BMI percentile (7.65%), and higher VO2 max (7.05 mL/kg[minute]) (Table 2). Effect sizes were the following: HDL=0.014 (95% CI=0.001, 0.027) in a linear relationship; BMI percentile= −0.063 (95% CI= −0.102, − 0.024) from 49 to 50 minutes and −0.010 (95% CI= −0.028, 0.009) from 199 to 200 minutes; and VO2 max=0.059 (95% CI=0.022, 0.095) from 49 to 50 minutes and 0.010 (95% CI= −0.012, 0.032) from 199 to 200 minutes.
For female adolescents, self-reported MVPA had a non-linear and significant dose–response relationship for DBP with an inflection point at 375 minutes (Table 2 and Figure 2). Male adolescents had a non-linear and significant association between self-reported MVPA and SBP with an inflection point at 500 minutes of MVPA (Table 2 and Figure 2).
Figure 2.
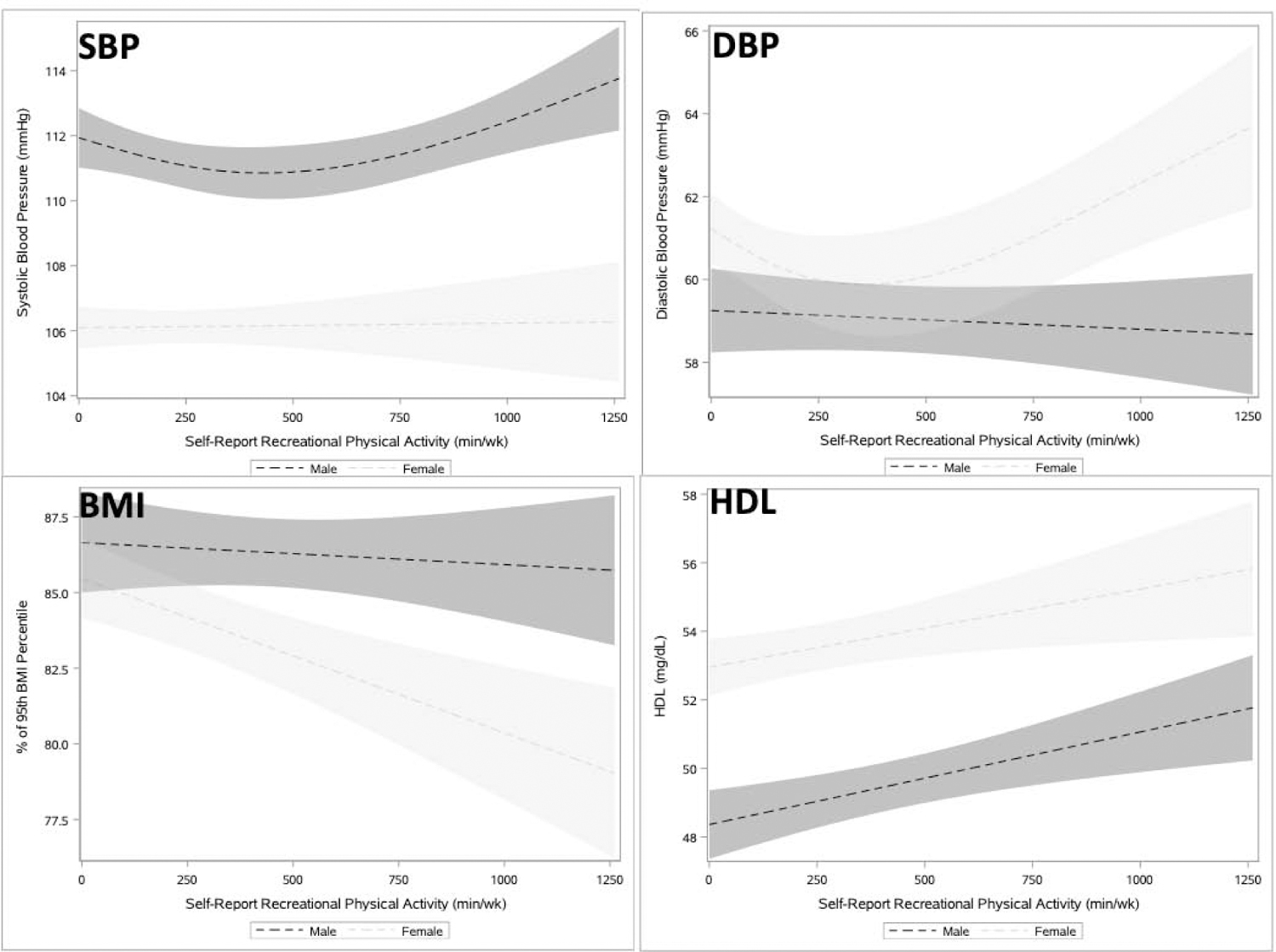
Association between self-reported recreational physical activity and cardiometabolic measures among adolescents (age 12‒19 years), NHANES 2007‒2016.
Notes: Unadjusted Models. Shading represents 95% CI.
SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; NHANES, National Health and Nutrition Examination Survey.
For female adolescents in adjusted analyses, 420 minutes versus 0 minutes of MVPA was associated with lower DBP (1.59 mmHg) and BMI percentile (1.45%), and higher HDL (1.01 mg/dL) (Table 2). For DBP, the effect size, representing the average slope/1 minute of activity, was −0.006 (95% CI= −0.011, −0.002) from 99 to 100 minutes and 0.004 (95% CI=0.002, 0.007) from 749 to 750 minutes. Effect sizes for the significant linear associations were BMI percentile −0.003 (95% CI= −0.006, −0.001) and HDL 0.002 (95% CI=0.000, 0.004).
For male adolescents, 420 minutes versus 0 minutes of MVPA was associated with higher HDL (1.16 mg/dL) (Table 2). The effect size for the linear relationship with HDL was 0.003 (95% CI=0.001, 0.004).
Total self-reported recreational, transport, and occupational activity showed nearly all linear associations, except with HDL for male adolescents, which had an inflection point at 900 weekly activity minutes (Appendix Figure 1). Total self-reported MVPA was not significantly associated with any cardiometabolic measures for female adolescents after adjustment, whereas HDL was significantly associated for male adolescents (Appendix Table 4).
When adolescents were defined as those aged 12–17 years, adjusted results were qualitatively similar for objectively measured MVPA, with activity significantly associated with BMI percentile for female adolescents, and HDL, BMI percentile, and VO2 max significantly associated for male adolescents (Appendix Table 5). Consistent with the primary analysis, the inflection points for female adolescents occurred at 90 weekly minutes of objective activity for SBP and BMI percentile, and at 375 weekly minutes of self-reported activity for DBP. Male adolescents also had an inflection point at 150 minutes of objective activity for BMI percentile, like the primary analysis (Appendix Figures 2 and 3).
DISCUSSION
These findings demonstrate that continuous measures of both objectively and self-reported MVPA had significant dose–response relationships with cardiometabolic measures in adolescents. Consistent and often non-linear relationships were identified between PA and BMI percentile in adolescents. Among the non-linear relationships, inflection points were determined at 90–150 weekly minutes of objectively measured activity. Notable differences in association and linearity were identified by sex and type of MVPA measure. The results support calls for any increase in PA and suggest that recommendations closer to adult guidelines of 150 weekly minutes may be health promoting and more attainable for many youth than the current adolescent recommendation of 420 minutes/week.
Though prior studies found mixed associations between PA and BMI in adolescents,11–13,42,43 this study identified that BMI percentile was on average lower by 7.25% for adolescents who were physically active for 150 versus 0 weekly minutes of objective MVPA. A systematic review of 20 PA intervention studies in overweight/obese children and adolescents demonstrated a 26.9% reduction in BMI percentile with an average of 138 weekly minutes of PA.10 The consistency of the significant inverse dose–response relationship between PA and BMI across sex and types of activity could indicate that BMI is one of the earliest cardiometabolic biomarkers modifiable by PA. Prior evidence has linked adolescent BMI with short- and long-term cardiometabolic measures, such as young adult cardiovascular health and chronic disability later in adulthood.44–.46 Although the absolute difference in cardiometabolic risk factors for each adolescent may be small with different levels of PA, these modest differences in cardiometabolic measures can have significant population-wide effects.47
Inflections points—where the relationship between cardiometabolic measure and MVPA changes—were identified at 90–150 minutes/week of objectively measured MVPA and several cardiometabolic outcomes. Prior studies identified various amounts of activity needed to impact cardiometabolic measures for adolescents including: 210–420 weekly minutes (30–60 minutes daily) of objective MVPA in a single study, 120–200 weekly minutes (40 minutes 3–5 days/week) of intervention-based activity in another study for measurable effects on blood pressure, and 70–105 weekly minutes (10 to 15–minute bouts/day) of objective activity for effects on BMI.10,48,49–51 The current inflection points often suggested decreasing marginal benefit and occurred at MVPA levels closer to the recommended activity for adults (150 minutes/week) than for adolescents (420 minutes/week).16 Notably, the shape of the splines do not causally indicate PA levels above the inflection point will worsen adolescent cardiometabolic health (i.e., increasing BMI percentile at higher objectively measured MVPA in Figure 1), though may suggest lower marginal impacts. Additionally, few adolescents were active at levels above the inflection points (90 minutes for female and 150 minutes for male adolescents) reflected in the large CIs in the tails of the spline analyses.
The dose–response relationships’ significance and shape for MVPA and cardiometabolic health were substantially different for female and male adolescents, supporting some prior literature describing differential health effects of activity by sex.4,14,25 For non-linear relationships, female adolescents had inflection points at lower activity levels (e.g., 90 weekly minutes vs 150 minutes for male adolescents for objective MVPA with BMI percentile and SBP). These differences contrasted with a prior study that used self-reported data and indicated males may require less MVPA than females to achieve similar health benefits.52 The distinct dose–response relationships by sex in adolescents may be due to differential pubertal processes and timing between sexes (e.g., average female puberty beginning 2 years before male puberty), though these data were unavailable in NHANES.53,54 Male and female adolescents may also have distinct sedentary and dietary patterns.7,8,55 Understanding how pubertal onset, sedentary time, and dietary intake influence the dose–response relationship for adolescents are future areas for research. These differential responses by sex suggest that different levels of PA could be recommended for female versus male adolescents, though developing sex-specific guidelines needs to be balanced with challenges of effectively disseminating more nuanced recommendations.
Similar to prior analyses, the authors identified different associations with cardiometabolic health depending on the type of PA measurement.14,18,25 Levels of self-reported activity were substantially higher than objectively measured activity, resulting in differently shaped and scaled dose–response relationships (e.g., a non-linear association for objective but linear association for self-reported activity and female BMI) and inflection point ranges (e.g., 90–150 minutes for objective activity and 375–500 minutes for self-reported activity). More non-linear relationships were identified for objectively measured than self-reported MVPA. These differences may originate in the inherent limitations of each measure, such as overestimation of self-reported MVPA levels because of social desirability over a 30-day period, whereas objective MVPA is collected over a shorter period of time and relies on achieving bouts of activity. The findings suggest that the type of PA measurement can affect clinical or public health activity recommendations (i.e. step counts or activity duration for PA goals).
Limitations
These findings should be considered in the context of some limitations. By using cross-sectional NHANES data, causal inference is limited. For example, the authors were unable to assess the benefits of a previously inactive adolescent becoming active, which may have larger effects on cardiometabolic health. VO2 max values were estimated and therefore may not represent true cardiorespiratory fitness.28 In some associations, the directionality of the relationship may be reversed (e.g., high BMI influencing an adolescent’s PA level). Objective MVPA was collected using accelerometers worn between 3–7 days per prior validation studies, though measures may not be representative of adolescents’ typical activity level, including a potential Hawthorne effect.32,56
CONCLUSIONS
With overall low rates of adolescent PA, these new insights into dose–response relationships suggest that even incremental increases in PA have the potential to improve adolescent cardiometabolic health. This study highlights the urgency to develop strategies to increase adolescent PA and supports the “call to action” in PA guidelines that emphasize that any PA is better than none.16 The results also suggest that recommendations closer to adult guidelines of 150 weekly minutes of PA may be health promoting and also more attainable for youth than the current 1 hour/day recommendation for adolescents—a goal so high that it may be demotivating for some.57 Further investigation and causal studies are indicated before such a substantial change in guidelines for adolescents are enacted.
Supplementary Material
ACKNOWLEDGMENTS
All authors—KS, HM, HF, TSS, ACS, EMP, SCA, EDP, MJP, and CAW—are responsible for the reported research and have participated in the concept and design, analysis and interpretation of data, and drafting or revising of the manuscript. All coauthors have approved the manuscript for submission to American Journal of Preventive Medicine and support its validity. All phases of this study were supported by Dr. Charlene Wong’s grant, from the National Heart, Lung, and Blood Institute (Grant No 1K23HL141689) at the NIH.
KS, HM, and HF participated in the design, methodology, investigation, data curation, and formal analysis. ACS, EMP, SCA, EDP, MJP, and CAW participated in methodology, investigation, supervision, and funding. All authors were involved in writing and editing the manuscript.
Dr. Eric Peterson reports support from Amarin, Amgen, AstraZeneca, Baseline Study LLC, Bayer AG, Eli Lilly & Company, Genentech, Janssen Pharm, Merck & Co, Inc., Novartis, Reflexion Health, Regeneron, Sanofi-Aventis, and Society of Thoracic Surgeons and does consulting for Abiomed, Amgen, Inc., AstraZeneca, Baseline Study LLC, Genentech, Janssen Pharm, and Sanofi-Aventis. Dr. Michael Pencina reports past advisory consulting fee from Boehringer Ingelheim; speaking fee from Merck; and contracts to Duke University from Sanofi/Regeneron, Amgen and BMS, not related to the submitted work. Dr. Charlene Wong reports research funded by Verily Life Science. No other financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Strong WB, Malina RM, Blimkie CJ, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146(6):732–737. 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Barnett TA, Gauvin L, Craig CL, Katzmarzyk PT. Modifying effects of sex, age, and education on 22-year trajectory of leisure-time physical activity in a Canadian cohort. J Phys Act Health. 2007;4(2):153–166. 10.1123/jpah.4.2.153. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7:40 10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung AE, Skinner AC, Steiner MJ, Perrin EM. Physical activity and BMI in a nationally representative sample of children and adolescents. Clin Pediatr (Phila). 2012;51(2):122–129. 10.1177/0009922811417291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutin B, Yin Z, Humphries MC, Barbeau P. Relations of moderate and vigorous physical activity to fitness and fatness in adolescents. Am J Clin Nutr. 2005;81(4):746–750. 10.1093/ajcn/81.4.746. [DOI] [PubMed] [Google Scholar]
- 6.Levin S, Lowry R, Brown DR, Dietz WH. Physical activity and body mass index among US adolescents: Youth Risk Behavior Survey, 1999. Arch Pediatr Adolesc Med. 2003;157(8):816–820. 10.1001/archpedi.157.8.816. [DOI] [PubMed] [Google Scholar]
- 7.Elmesmari R, Martin A, Reilly JJ, Paton JY. Comparison of accelerometer measured levels of physical activity and sedentary time between obese and non-obese children and adolescents: a systematic review. BMC Pediatr. 2018;18:106 10.1186/s12887-018-1031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekelund U, Luan J, Sherar LB, et al. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. 2012;307(7):704–712. 10.1001/jama.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay J, Maximova K, Durksen A, et al. Physical activity intensity and cardiometabolic risk in youth. Arch Pediatr Adolesc Med. 2012;166(11):1022–1029. 10.1001/archpediatrics.2012.1028. [DOI] [PubMed] [Google Scholar]
- 10.Kelley GA, Kelley KS, Pate RR. Exercise and BMI in overweight and obese children and adolescents: a systematic review and trial sequential meta-analysis. Biomed Res Int. 2015;2015:704539 10.1155/2015/704539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavelle HV, Mackay DF, Pell JP. Systematic review and meta-analysis of school-based interventions to reduce body mass index. J Public Health (Oxf). 2012;34(3):360–369. 10.1093/pubmed/fdr116. [DOI] [PubMed] [Google Scholar]
- 12.Guerra PH, Nobre MR, Silveira JA, Taddei JA. The effect of school-based physical activity interventions on body mass index: a meta-analysis of randomized trials. Clinics (Sao Paulo). 2013;68(9):1263–1273. 10.6061/clinics/2013(09)14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atlantis E, Barnes EH, Singh MA. Efficacy of exercise for treating overweight in children and adolescents: a systematic review. Int J Obes (Lond). 2006;30(7):1027–1040. 10.1038/sj.ijo.0803286. [DOI] [PubMed] [Google Scholar]
- 14.Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–2028. 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katzmarzyk PT, Denstel KD, Beals K, et al. Results From the United States of America’s 2016 Report Card on Physical Activity for Children and Youth. J Phys Act Health. 2016;13(11 suppl 2):S307–S313. 10.1123/jpah.2016-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Physical Activity Guidelines for Americans 2nd Edition. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Published 2018. Accessed August 20, 2019. [DOI] [PMC free article] [PubMed]
- 17.Telama R, Yang X, Viikari J, Valimaki I, Wanne O, Raitakari O. Physical activity from childhood to adulthood: a 21-year tracking study. Am J Prev Med. 2005;28(3):267–273. 10.1016/j.amepre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 19.Butt J, Weinberg RS, Breckon JD, Claytor RP. Adolescent physical activity participation and motivational determinants across gender, age, and race. J Phys Act Health. 2011;8(8):1074–1083. 10.1123/jpah.8.8.1074. [DOI] [PubMed] [Google Scholar]
- 20.Committee on Physical Activity and Physical Education in the School Environment; Food and Nutrition Board; Institute of Medicine; Kohl HWCH III, editors. Educating the Student Body: Taking Physical Activity and Physical Education to School. Washington, DC: The National Academies Press, 2013. 10.17226/18314. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong S, Wong CA, Perrin E, Page S, Sibley L, Skinner A. Association of physical activity with income, race/ethnicity, and sex among adolescents and young adults in the United States: findings from the National Health and Nutrition Examination Survey, 2007‒2016. JAMA Pediatr. 2018;172(8):732–740. 10.1001/jamapediatrics.2018.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govindan M, Gurm R, Mohan S, et al. Gender differences in physiologic markers and health behaviors associated with childhood obesity. Pediatrics. 2013;132(3):468–474. 10.1542/peds.2012-2994. [DOI] [PubMed] [Google Scholar]
- 23.Barstad LH, Juliusson PB, Johnson LK, Hertel JK, Lekhal S, Hjelmesaeth J. Gender-related differences in cardiometabolic risk factors and lifestyle behaviors in treatment-seeking adolescents with severe obesity. BMC Pediatr. 2018;18:61 10.1186/s12887-018-1057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celis-Morales CA, Perez-Bravo F, Ibanez L, Salas C, Bailey ME, Gill JM. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7(5):e36345 10.1371/journal.pone.0036345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atienza AA, Moser RP, Perna F, et al. Self-reported and objectively measured activity related to biomarkers using NHANES. Med Sci Sports Exerc. 2011;43(5):815–821. 10.1249/mss.0b013e3181fdfc32. [DOI] [PubMed] [Google Scholar]
- 26.Kavanaugh K, Moore JB, Hibbett LJ, Kaczynski AT. Correlates of subjectively and objectively measured physical activity in young adolescents. J Sport Health Sci. 2015;4(3):222–227. 10.1016/j.jshs.2014.03.015. [DOI] [Google Scholar]
- 27.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011;40(4):454–461. 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 28.CDC. National Health and Nutrition Examination Survey, Physician Examination Procedures Manual. https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/PE.pdf. Published 2003. Accessed July 10, 2020.
- 29.National Cancer Institute, Division of Cancer Control & Population Sciences. SAS Programs for Analyzing NHANES 2003–2004 Accelerometer Data. https://epi.grants.cancer.gov/nhanes_pam/. Accessed September 10, 2018.
- 30.National Health and Nutrition Examination Survey: 2003‒2004 Data Documentation, Codebook, and Frequencies. National Center for Health Statistics. https://wwwn.cdc.gov/nchs/nhanes/2003-2004/PAXRAW_C.htm. Published 2006. Accessed September 10, 2019.
- 31.Edwardson CL, Gorely T. Epoch length and its effect on physical activity intensity. Med Sci Sports Exerc. 2010;42(5):928–934. 10.1249/mss.0b013e3181c301f5. [DOI] [PubMed] [Google Scholar]
- 32.Toftager M, Kristensen PL, Oliver M, et al. Accelerometer data reduction in adolescents: effects on sample retention and bias. Int J Behav Nutr Phys Act. 2013;10:140 10.1186/1479-5868-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanner M, Hartmann C, Pestoni G, Martin BW, Siegrist M, Martin-Diener E. Validation of the Global Physical Activity Questionnaire for self-administration in a European context. BMJ Open Sport Exerc Med. 2017;3(1):e000206 10.1136/bmjsem-2016-000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. 2009;6(6):790–804. 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 35.WHO. Global Physical Activity Surveillance. 2017; http://www.who.int/ncds/surveillance/steps/GPAQ/en/. Published 2017. Accessed 2019.
- 36.Cleland CL, Hunter RF, Kee F, Cupples ME, Sallis JF, Tully MA. Validity of the global physical activity questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health. 2014;14:1255 10.1186/1471-2458-14-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC. About Child and Teen BMI. https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html. Accessed February 2, 2019.
- 38.National Health and Nutrition Examination Survey. Cardiovascular Fitness Procedures Manual. https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/cv_99-04.pdf. Revised January 2004. Accessed July 10, 2020.
- 39.Haas JS, Lee LB, Kaplan CP, Sonneborn D, Phillips KA, Liang SY. The association of race, socioeconomic status, and health insurance status with the prevalence of overweight among children and adolescents. Am J Public Health. 2003;93(12):2105–2110. 10.2105/ajph.93.12.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moseley CA, Skinner AC, Perrin EM, Armstrong SC, Peterson ED, Wong CA. Adolescent and young adult recreational, occupational, and transportation activity: activity recommendation and weight status relationships. J Adolesc Health. 2019;65(1):147–154. 10.1016/j.jadohealth.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y, Granja MR, Koball H. Basic Facts about Low-Income Children. National Center for Children in Poverty. http://www.nccp.org/publications/pdf/text_1174.pdf. Published January 2017. Accessed July 10, 2020.
- 42.McGovern L, Johnson JN, Paulo R, et al. Treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clin Endocrinol Metab. 2008;93(12):4600–4605. 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- 43.Harris KC, Kuramoto LK, Schulzer M, Retallack JE. Effect of school-based physical activity interventions on body mass index in children: a meta-analysis. CMAJ. 2009;180(7):719–726. 10.1503/cmaj.080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gooding HC, Milliren C, Shay CM, Richmond TK, Field AE, Gillman MW. Achieving cardiovascular health in young adulthood: which adolescent factors matter? J Adolesc Health. 2016;58(1):119–121. 10.1016/j.jadohealth.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002;51(1):204–209. 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 46.Henriksson P, Henriksson H, Tynelius P, et al. Fitness and body mass index during adolescence and disability later in life: a cohort study. Ann Intern Med. 2019;170(4):230–239. 10.7326/m18-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155(7):701–709. 10.1001/archinte.1995.00430070053006. [DOI] [PubMed] [Google Scholar]
- 48.Schwarzfischer P, Weber M, Gruszfeld D, et al. BMI and recommended levels of physical activity in school children. BMC Public Health. 2017;17:595 10.1186/s12889-017-4492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mark AE, Janssen I. Dose-response relation between physical activity and blood pressure in youth. Med Sci Sports Exerc. 2008;40(6):1007–1012. 10.1249/mss.0b013e318169032d. [DOI] [PubMed] [Google Scholar]
- 50.Torrance B, McGuire KA, Lewanczuk R, McGavock J. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3(1):139–149. [PMC free article] [PubMed] [Google Scholar]
- 51.Tarp J, Child A, White T, et al. Physical activity intensity, bout-duration, and cardiometabolic risk markers in children and adolescents. Int J Obes (Lond). 2018;42(9):1639–1650. 10.1038/s41366-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galan I, Boix R, Medrano MJ, et al. Physical activity and self-reported health status among adolescents: a cross-sectional population-based study. BMJ Open. 2013;3(5):e002644 10.1136/bmjopen-2013-002644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JM, Kaciroti N, Appugliese D, Corwyn RF, Bradley RH, Lumeng JC. Body mass index and timing of pubertal initiation in boys. Arch Pediatr Adolesc Med. 2010;164(2):139–144. 10.1001/archpediatrics.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wohlfahrt-Veje C, Mouritsen A, Hagen CP, et al. Pubertal onset in boys and girls is influenced by pubertal timing of both parents. J Clin Endocrinol Metab. 2016;101(7):2667–2674. 10.1210/jc.2016-1073. [DOI] [PubMed] [Google Scholar]
- 55.Caine-Bish NL, Scheule B. Gender differences in food preferences of school-aged children and adolescents. J Sch Health. 2009;79(11):532–540. 10.1111/j.1746-1561.2009.00445.x. [DOI] [PubMed] [Google Scholar]
- 56.Dossegger A, Ruch N, Jimmy G, et al. Reactivity to accelerometer measurement of children and adolescents. Med Sci Sports Exerc. 2014;46(6):1140–1146. 10.1249/mss.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frank HR, Ubel PA, Wong CA. Behavioral economic insights for pediatric obesity: suggestions for translating the guidelines for our patients. JAMA Pediatr. 2020;174(4):319–320. 10.1001/jamapediatrics.2019.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


