Behmoaras and Gil describe shared cellular features between senescent cells and macrophages and outline the interaction between the two cell types during homeostasis and disease.
Abstract
Senescence is a cellular program that prevents the replication of old, damaged, or cancerous cells. Senescent cells become growth arrested and undergo changes in their morphology, chromatin organization, and metabolism, and produce a bioactive secretome. This secretome, the senescence-associated secretory phenotype (SASP), mediates many of the pathophysiological effects associated with senescent cells, for example, recruiting and activating immune cells such as macrophages. The relation between senescent cells and macrophages is intriguing: senescent cells recruit macrophages, can induce them to undergo senescence, or can influence their polarization. Senescent cells and macrophages share multiple phenotypic characteristics; both have a high secretory status, increased lysosome numbers, or the ability to activate the inflammasome. Senescent cells accumulate during aging and disease, and killing them results in widespread benefits. Here we discuss similarities between senescent cells and macrophages and interpret the latest developments in macrophage biology to understand the molecular mechanisms of cellular senescence. We describe evidence and effects of senescence in macrophages and speculate on the ontogeny of the senescent-like state in macrophages. Finally, we examine the macrophage–senescent cell interplay and its impact on macrophage effector functions during inflammatory conditions and in the tumor microenvironment.
Introduction
Cellular senescence is a stress response that is characterized by an irreversible arrest of proliferation and the acquisition of a pro-inflammatory secretory phenotype referred to as the senescence-associated (SA) secretory phenotype (SASP; Gorgoulis et al., 2019). Cells undergoing senescence experience other profound phenotypic changes, such as alterations in their morphology, chromatin organization, and metabolism (Herranz and Gil, 2018). The aberrant accumulation of senescent cells is associated with aging and contributes to disease (Baker et al., 2016; McHugh and Gil, 2018). However, acute induction of senescence in response to tissue damage is a beneficial response that avoids the replication of preneoplastic, old, and damaged cells. This is achieved by a two-pronged approach: senescence induces the growth arrest of damaged cells, while the SASP recruits different immune cells that clears them (MacIver et al., 2008; Muñoz-Espín and Serrano, 2014). SASP can also lead to an antitumor immune response through recruitment of immune cells (Rao and Jackson, 2016; Toso et al., 2014). Among the innate immune cells recruited by senescent cells are macrophages. Macrophages are professional phagocytes, which participate in tissue homeostasis in almost every organ of the human body. One of their main characteristics is the removal of apoptotic cells in several tissues, a process also called efferocytosis. Macrophages can also sense and interact with cells that undergo a wide range of intrinsic or extrinsic insults (Gordon and Pluddemann, 2017; Gordon et al., 2014). In addition to cell clearance, recent evidence shows that macrophages can clear damaged cellular components in subcellular particles called exophers to preserve tissue homeostasis (Nicolas-Avila et al., 2020).
Macrophages interact with senescent cells, and this interaction is indispensable for major pathophysiological processes in mammals. The removal of aged and senescent RBCs by macrophages is a process whereby ∼2 million RBCs are recycled each second to ensure the iron fluxes that sustain erythropoiesis (Kay, 1975; Korolnek and Hamza, 2015). Likewise, studies within the murine liver and endometrium have reported key roles for monocytes/macrophages in the physiological clearance of senescent cells (Egashira et al., 2017; Kang et al., 2011). During the aging process, the clearance of accumulating senescent cells through phagocytosis is a well-known strategy to counteract their potential harmful effects (Oishi and Manabe, 2016). The senescent cell–macrophage interaction also occurs during pathology such as obesity-induced metabolic syndrome. For instance, the recruitment of macrophages to senescent adipocytes results in the formation of “crown-like structures” in the visceral fat, a hallmark of exacerbated inflammation in the adipose tissue (Murano et al., 2008).
Unlike cell death, senescence is a technically challenging cellular state to detect and quantify at a whole-body level. As a result, our understanding of the macrophage–senescent cell interaction has been partial and blunted by the overwhelming efforts to decipher mechanisms underlying efferocytosis. Crown-like structures, originally described as macrophages engulfing dead adipocytes, are most probably a more complex histological feature, also representing macrophages recruited to senescent adipocytes.
Recent advances to characterize senescence in vivo during aging or acute inflammation revealed that macrophages share phenotypic similarities with senescent cells such as induction of p16Ink4a expression (Grosse et al., 2020; Liu et al., 2019). Further extending these findings, a recent study showed that chemotherapy-induced senescent cells show transcriptional changes characteristic of phagocytosis and are able to engulf neighboring cells (Tonnessen-Murray et al., 2019). These recent studies confirm a long-standing observation by cell biologists pointing toward similarities at signaling, gene expression, metabolism, and organelle level between macrophages and senescent cells. These shared features translate into similar effector functions such as acquisition of a secretory phenotype. In this review, we focus on the shared cellular features between senescent cells and macrophages, namely secretion/secretory apparatus and phagocytosis. Given that detailed molecular mechanisms of most of these events are described elsewhere (Aderem and Underhill, 1999; Gordon, 2016; Murray and Stow, 2014), we focus on shared cellular pathways and hypothesize that the latest developments in macrophage biology can inform us to better understand the molecular mechanisms of cellular senescence. We describe evidence of senescence in macrophages and its effect on macrophage function and speculate on the ontogeny of senescent-like macrophages. Finally, we describe the interplay between macrophages and senescent cells and the effect of this interaction on macrophage effector functions during inflammatory conditions and in the tumor microenvironment.
Shared cellular features between senescent cells and macrophages
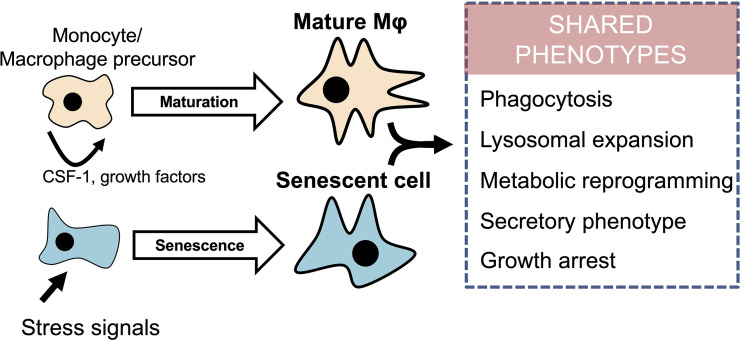
In addition to growth arrest, senescent cells undergo morphological changes, chromatin remodeling, and metabolic reprogramming, which lead to the secretion of mainly pro-inflammatory mediators, known as the SASP (Herranz and Gil, 2018). Similarly, terminally differentiated macrophages adopt a secretory phenotype that is further exacerbated upon stimulation with pro-inflammatory stimuli such as the TLR4 agonist lipopolysaccharide (LPS). The acquisition of a secretory pro-inflammatory phenotype in macrophages is accompanied by a cell cycle arrest (Vadiveloo et al., 1996; Vairo et al., 1992), which points toward a fundamental resemblance with senescent cells. One could therefore hypothesize that some cellular events between the inhibition of proliferative capacity and induction of the secretory phenotype are shared between macrophages and senescent cells (Fig. 1). These include changes in several intracellular organelles, as exemplified by changes in lysosomes, that are crucial for the function of both cell types.
Figure 1.
Macrophage maturation and senescence in non-immune cells share a multitude of cellular features. Mature macrophages (Mϕ) and senescent cells share multiple common phenotypes. Both cell types display phagocytosis, lysosomal expansion, and metabolic reprogramming; have a secretory phenotype; and are cell cycle–arrested. While macrophage maturation is a differentiation process driven by CSF-1 and other growth factors, senescence is a cellular response to stress signals such as oncogenic activation, replicative exhaustion, or drugs that cause DNA damage.
Lysosomes
Macrophages and senescent cells are characterized by an expanded lysosomal compartment. The most widely used senescence marker is SA lysosomal β-galactosidase activity, which is robustly enhanced in senescent cells as a result of increased lysosomal content (Kurz et al., 2000; Lee et al., 2006). Lysosomal expansion is also a feature of macrophages and cells derived from the monocyte/macrophage lineage such as Kupffer cells of liver and osteoclasts of bone (Bursuker et al., 1982; Kopp et al., 2007). Indeed, one of the drawbacks mentioned in using SA–β-galactosidase as a marker of senescence is that it stains macrophages (Sharpless and Sherr, 2015). More generally, monocyte-to-macrophage differentiation is associated with an increase in lysosome numbers (Daigneault et al., 2010), which suggests that tissue macrophages are lysosome-rich in general. In mononuclear phagocytes such as macrophages and dendritic cells, the endosomes and lysosomes (endo-lysosomes) are essential organelles for eliminating pathogens and process and present antigens. Interestingly, macrophages and dendritic cells expand their endo-lysosomal volume during LPS stimulation, suggesting that these organelles are remodeled upon pro-inflammatory stimulation to sustain the effector function of the cells (Hipolito et al., 2019). Importantly, during fungal infection, lysosome fusion maintains phagosome integrity in macrophages (Westman et al., 2020). Cellular senescence involves remodeling of chromatin structure through lysosomes (Ivanov et al., 2013), though direct evidence linking the lysosomal expansion to SASP in senescent cells is lacking.
Lysosomes are organelles at the interplay between cell function and metabolism. In particular, they are signaling platforms to sense the nutritional state of the cells, which impacts on the overall energy metabolism and cell growth. Activation of mTORC1 on the lysosomal surface induces anabolic pathways while repressing catabolic processes such as autophagy (see Kim and Guan [2019] for a detailed review). The lysosome is therefore a crucial organelle involved in fine-tuning the overall metabolic state of the cell. In oxidative stress–induced senescence, lysosomal dysfunction is associated with impairment of autophagy (Tai et al., 2017), though a previous report suggested that the acquisition of senescent phenotype is driven by autophagy (Young et al., 2009). While coupling mTOR activity and autophagy contributes to SASP production on senescence (Narita et al., 2011), the exact role of autophagy in senescence might depend on multiple factors. In mononuclear phagocytes, the role of autophagy in effector functions is more clearly defined. For instance, autophagy is part of the antimicrobial response to infection in macrophages (Visvikis et al., 2014). Furthermore, mTORC1 is essential for antigen presentation by dendritic cells and the subsequent regulation of T cell functions. Autophagy induction by inhibitors of the mammalian target of rapamycin enhances antigen presentation in dendritic cells (Jagannath et al., 2009).
In addition to activating mTORC1, recent reports show a link between lysosomes and cell survival through regulation of mitochondrial function. Mitochondrial number correlates with that of lysosomes during monocyte–macrophage differentiation (Daigneault et al., 2010), and it is also established that senescent cells accumulate mitochondria (Korolchuk et al., 2017). Generally, age-related mitochondrial deterioration is associated with impaired lysosome-like vacuoles (Hughes et al., 2020), so it is tempting to infer a causal relationship between lysosomal remodeling and mitochondrial function in macrophages and senescent cells. Indeed, mitochondrial function and metabolites are the focus of increasing reports studying the immune and metabolic interplay in macrophage effector functions (Martínez-Reyes and Chandel, 2020) and during senescence (Kaplon et al., 2013; López-Otín et al., 2013; Moiseeva et al., 2009). In senescent cells, a recent report has shown that defective mitochondria induce SASP through activation of the innate immune cytosolic DNA sensing the cyclic GMP-AMP synthase–stimulator of interferon genes pathway (generally known as the cGAS-STING pathway; Vizioli et al., 2020). Stimulator of interferon genes is an ER-associated sensor expressed by monocytes/macrophages that regulates nuclear factor–κB (NF-κB) and IRF3-dependent cytokine production (Ishikawa and Barber, 2008; Ishikawa et al., 2009). Thus, an attractive hypothesis could postulate the existence of a lysosome–mitochondria axis shared by senescent cells and macrophages that is essential for SASP in senescent cells and macrophage maturation and/or activation.
Intracellular iron as a messenger between lysosomes and mitochondria
Several recent reports have linked intracellular iron homeostasis as a “messenger” between lysosomal and mitochondrial function in homeostatic and inflammatory conditions (Hughes et al., 2020; Weber et al., 2020; Yambire et al., 2019). These results are worth highlighting as iron accumulation occurs in replicative senescent fibroblasts in vitro (Killilea et al., 2003), and the intracellular iron storage protein ferritin is enriched in aging tissues (DeRuisseau et al., 2013). In many cell types, including macrophages, iron transit through the lysosomal compartment is essential for oxidative phosphorylation and synthesis of tricarboxylic cycle metabolites in human macrophages (Pereira et al., 2019). These observations suggest the existence of a lysosome–iron–mitochondria pathway in senescent cells that could be connected to SASP induction. Interfering with mitochondrial membrane potential and usage of iron chelators reduces interleukin (IL)–1β synthesis in macrophages (Mills et al., 2016; Pereira et al., 2019). Similar strategies could be undertaken in senescent cells to modulate SASP.
Inflammasome activation
The identification of mechanisms underlying SASP induction and regulation is an active area of research. Compounds inducing senescence (referred to as senomorphic) could be used therapeutically to complement, or as an alternative to, those that preferentially kill senescent cells (i.e., senolytics). Recent reports have shown overlapping pathways between senescent cells and activated macrophages. Among those, inflammasome activation is a well-described feature of activated macrophages and is involved in SASP induction in senescent cells.
Inflammasome activation is pivotal in innate immune defense against microbial insults. Assembly of the inflammasome complex initiates the release of IL-1β and preferentially occurs in myeloid cells with phagocytic activity such as monocytes/macrophages (Erlich et al., 2019). Inflammasome activation is a two-step process that includes priming and activation steps, under transcriptional and posttranscriptional regulation, respectively (Schroder and Tschopp, 2010). In macrophages, the priming step triggers NF-κB and transcription of pro-inflammatory genes such as IL-1β after a pro-inflammatory stimulus such as LPS. In senescent cells, the induction of NF-κB has been reported, even though the priming signal was either oxidative stress (Han et al., 2020) or oncogenic activation (Chien et al., 2011). A recent study has found de novo cohesin peaks enriched in SA genes during oncogene-induced senescence. An example is de novo cohesin binding involved in new loop formation in the IL1B locus. Interestingly, this organization is commonly observed in cells undergoing oncogene-induced senescence and terminally differentiated macrophages (Olan et al., 2020). When it comes to the activation step, stimuli such as nigericin toxin, extracellular ATP, silica, or cholesterol crystals (i.e., lysosome destabilizers) cause the activation of inflammasome in primed macrophages. This implicates NLRP3 polymerization with adaptor protein ASC, recruitment of pro–caspase-1, and subsequent cleavage of pro–IL-1β and pro–IL-18, together with gasdermin-D, which facilitates the release of mature cytokines through lytic pores (Shi et al., 2015). This is a well-established innate immune response in macrophages. The activation of Nlrp3 inflammasome in senescent cells is most probably context and environment dependent.
We have shown that cells undergoing oncogene-induced senescence secreted and processed mature forms of IL-1α and IL-1β, suggesting inflammasome activation preceding SASP (Acosta et al., 2013). More recently, caspase-5 was shown to be essential for IL-1α–dependent SASP through noncanonical inflammasome activation (Wiggins et al., 2019). Interestingly, the authors investigated the same pathway in macrophages and further demonstrated that caspase-5 can directly process IL-1α in human macrophages, while release of cleaved IL-1α requires caspase-11 in murine macrophages (Wiggins et al., 2019). These findings show that noncanonical inflammasome activation can operate in both senescent cells and macrophages, though the exact role of Nlrp3 inflammasome in SASP requires clarification. Nlrp3 inflammasome activation has been linked to immune senescence (Spadaro et al., 2016), described as a plausible driver of SASP (Acosta et al., 2013; Latz and Duewell, 2018).
The cumulative data on the implication of inflammasome in SASP point toward the noncanonical activation of IL-1α. Indeed, this atypical member of the IL-1 family is a cytokine with dual functions. The nuclear localization sequence in the precursor region of the cytokine allows IL-1α to enter the nucleus and act as a transcription factor to increase gene expression of IL-8 (Werman et al., 2004). Given that SASP is primarily associated with pro-inflammatory cytokines such as IL-8 (Rodier et al., 2009), these findings make IL-1α a major driver of SASP. Interestingly, in macrophages, IL-1α confers host resistance to fungal infection via expression of caspase-11 in an IFN-β–dependent manner (Ketelut-Carneiro et al., 2019). This study is a typical example where one can learn from macrophage biology to speculate on the mechanisms of cellular senescence. Indeed, the fungal agent Paracoccidioides brasiliensis induces an IL-1α –mediated production of IL-6 in macrophages (Ketelut-Carneiro et al., 2019). IL-6 is a crucial factor in T helper type 17 lymphocyte differentiation and is also associated with SASP (Rodier et al., 2009). Based on these observations, it is tempting to speculate on the existence of a caspase-11/IL-1α/IL-6 pathway during cellular senescence. Furthermore, a recent report showed that surface pro–IL-1α on macrophages can be cleaved and activated by thrombin (Burzynski et al., 2019), a finding that could be extrapolated to cellular senescence, as mice treated with senescent-inducing doxorubicin show increased blood clotting (Wiley et al., 2019).
In summary, the secretion of IL-1 family members, and in particular IL-1α, is associated with senescence and context-dependent macrophage activation through the activation of caspase family members. The secretion of IL-1 cytokines is broadly associated with cell death, though IL-1α release is seen during necrosis and pyroptosis, an inflammatory form of cell death induced by caspase-1, activation of Nlrp3 inflammasome, and active IL-1β secretion. Since apoptotic cell death is mediated by caspases different from those involved in necrosis and pyroptosis (Man and Kanneganti, 2016), one could hypothesize that senescence, necrosis, pyroptosis, and apoptosis have unique caspases that drive context-dependent secretion of pro-inflammatory mediators such as IL-1 cytokines. However, recent evidence shows a significant crosstalk and compensation between apoptotic and pyroptotic cell death mechanisms, and caspases such as caspase-6 having a master-regulatory role in both events (Zheng et al., 2020). It is therefore plausible that senescence shares common caspases that have been reported to induce pyroptosis or apoptosis.
Phagocytosis
Phagocytosis could be described as a fundamental characteristic of myeloid cells, a process whereby microorganisms, damaged or dead cells, and foreign substances such as nanoparticles are ingested and eliminated. Most tissue-resident macrophages are highly phagocytic and participate in tissue homeostasis. For instance, thymic macrophages clear apoptotic thymocytes, and splenic red pulp macrophages phagocytose old erythrocytes (Gordon, 2016). In a thought-provoking study, Tonnessen-Murray et al. (2019) found that chemotherapy-induced senescent breast cancer cells could engulf neighboring cells through phagocytosis and process them in the lysosome compartment. Notably, when compared with the RAW264 macrophage cell line, senescent cell phagocytosis was more pronounced (Tonnessen-Murray et al., 2019). The authors also argued that this acquired phagocytic capacity of senescent cells could be an upstream feature of SASP, but additional studies will be required to confirm this hypothesis.
Recent advances made in phenotyping tissue macrophages could help us to contextualize senescent cell phagocytosis and its potential link with SASP. Transcriptomics studies have indeed reported organ-specific gene and enhancer patterns for a range of tissue macrophage populations (Lavin et al., 2014). More recently, phagocytosis was shown to imprint a distinct anti-inflammatory profile in tissue-resident macrophages (A-Gonzalez, 2017). Phagocytic macrophages showed blunted expression of Il1b and supported tissue homeostasis (A-Gonzalez, 2017). These results are interesting and suggest that a tissue-resident, nonphagocytic macrophage has a phenotype distinct from those that participate in the clearance of senescent or apoptotic cells. When considered in the context of senescence, one can thus wonder whether senescent cells display different states of activation and whether phagocytosis confer on them an additional phenotype with anti-inflammatory and antagonizing effects on SASP.
Future studies to understand senescent cell phagocytosis should also consider the surface receptors involved in the regulation of phagocytosis. Phagocytic cells present nonopsonic receptors, apoptotic cell receptors, and opsonic phagocytic receptors, and the investigation of these in senescent cells could facilitate our understanding of the mechanisms underlying phagocytosis in senescent cells. One nonopsonic scavenger receptor is CD36, which mediates phagocytosis of infected RBCs in rodent macrophages (Patel et al., 2004) and initiates the secretory phenotype during the establishment of cellular senescence (Chong et al., 2018). CD36 plays an essential role in the clearance of apoptotic cells in vivo by macrophages (Greenberg et al., 2006), so the investigation of its role in senescent cell phagocytosis could be of potential interest.
Senescent-like macrophages: Just another polarization state?
Growth arrest is a major characteristic of terminally differentiated macrophages (Klappacher et al., 2002). Macrophage colony-stimulating factor (CSF-1) controls the proliferation, differentiation, and survival of cells of the macrophage lineage and has been widely used for in vitro culture of these cells. After prolonged stimulation with CSF-1 (usually days), growth arrest is accompanied by acquisition of macrophage-specific markers, including those related to a secretory phenotype. Stimuli other than CSF-1 can promote monocyte-to-macrophage differentiation, and the phorbol ester–stimulated myeloid leukemia cell line THP-1 is a good example, which led to the identification of transcriptional networks that control growth arrest and differentiation in vitro (Gažová et al., 2020). The silencing of cell cycle genes also occurs in human macrophages stimulated with LPS (Baillie et al., 2017), suggesting that macrophage differentiation/maturation and pro-inflammatory activation are inversely correlated with a transcriptional program that controls growth.
A mature macrophage can therefore be defined as a tissue-resident myeloid cell that can acquire different phenotypic states, including one that is characterized by growth arrest and a secretory and/or phagocytic phenotype. Whether this can be called “senescent” is open to debate, and the definition of senescence has not reached a consensus among scientists. One conceivable scenario is that macrophage senescence could reflect one of the numerous activation states that a tissue-resident macrophage adopts in response to environmental stimuli. We thus designate the senescent state in macrophages as “senescent-like macrophages.” Recent reports on senescent-like macrophages support this interpretation (Grosse et al., 2020; Hall et al., 2017; Liu et al., 2019).
A hallmark of senescent cells is high expression of Cdkn2a and its protein product p16Ink4a. Evidence for phagocytes contributing to p16Ink4a promoter-driven reporter signal was first reported using clodronate liposomes that selectively kill professional phagocytes in chronologically aged mice harboring a hemizygous p16(Ink4a) knock-in of luciferase (p16 LUC; Hall et al., 2016). More recently, two groups have generated knock-in mouse models targeting p16Ink4a (Grosse et al., 2020; Liu et al., 2019). Despite the limitations of these models (i.e., ability of the reporter mice to reflect true Cdkn2a expression), both studies identified p16Ink4a-high macrophages in vivo, which suggests that these cells show phenotypic similarities with non-immune senescent cells. Interestingly, the most significant accumulation of p16Ink4a-high cells was observed in the liver with ≈70% vascular endothelial cells and ≈30% F4/80-positive macrophages (Grosse et al., 2020). Whether these cells are resident myeloid Kupffer cells remains to be identified, and if they are, it will confirm a previous study reporting that Kupffer cells show signs of senescence such as IL-1α–driven production of IL-6, promoting hepatocarcinogenesis (Sakurai et al., 2008).
The evidence that local liver macrophages have a “senescent-like” phenotype raises the question of whether resident macrophages could acquire senescence upon tissue damage or inflammation. In a rat model of gliosis and motor neuron loss, p16INK4a-positive nuclei were localized in a subset of microglia, the resident macrophages of the central nervous system (Trias et al., 2019). In keeping with this, cultured brain microglia have been shown to present morphological features and markers of senescence, including positive labeling for p16Ink4a and SASP (Trias et al., 2019). Similarly, in a model of peritonitis induced in the murine reporter strain p16Ink4a, macrophages displayed features of senescence (Liu et al., 2019). Last, LPS could induce the formation of senescent-like macrophages, characterized by lipid accumulation, SASP, and a persistent DNA damage response (Wang et al., 2020). Once again, a parallel with senescence is evident as senescent glial cells exhibit excessive fat deposits, a phenotype that was termed ‘‘accumulation of lipids in senescence’’ (Ogrodnik et al., 2019).
Senescent cells influencing macrophages and vice versa
A possible explanation for the presence of p16INK4a macrophages in tumors or aged tissues, is the induction of paracrine senescence (Acosta et al., 2013) in these macrophages. Nevertheless, it seems that tissue macrophages, in certain conditions, can have high p16Ink4a expression, but this should not necessarily be interpreted as senescence. As elegantly demonstrated by Hall et al. (2017), expression of SAβG and p16INK4a in macrophages is reversible and p53-independent, suggesting that p16Ink4a is another checkpoint in the tissue-specific polarization of these macrophages. Using a method for in vivo elicitation of SAβG and p16INK4a-positive macrophages with intraperitoneal injection of alginate beads encapsulated with senescent cells, the authors also showed that these “senescent-associated macrophages” were more M2-like (Hall et al., 2017). Using the same experimental setup, the authors had previously reported that senescent cells preferentially attract macrophages characterized by p16Ink4a gene expression and β-galactosidase activity (Hall et al., 2016), raising the possibility of an interplay between senescent cells and macrophages that contributes to the shared high p16Ink4a expression in each cell type.
Deciphering the senescent cell–macrophage interaction without the process of elicitation will be crucial, as elicitation itself could influence macrophage function. In fact, the exact nature of macrophage polarization following recruitment to senescent cells in vivo is likely to be context-dependent. p53-expressing senescent stellate cells release factors that skew macrophage polarization toward a cytotoxic M1 state (Lujambio et al., 2013), whereas senescent thyrocytes induce an M2-like polarization in human monocytes (Mazzoni et al., 2019). To account for the effect of microenvironment in cell–cell interactions, single-cell transcriptomic approaches will therefore be helpful in clarifying the exact activation phenotype of macrophage interactions with senescent cells (e.g., computational methods for receptor/ligand pairs in single-cell RNA sequencing). Recent efforts to generate an atlas of aging tissues in the mouse could achieve this aim (Tabula Muris Consortium, 2020).
In certain conditions, a context-dependent macrophage phenotype can also cause senescence of surrounding cells. During chronic venous leg ulcers, iron overloading of macrophages induced a p16INK4a-dependent senescence program in resident fibroblasts, eventually leading to impaired wound healing (Sindrilaru et al., 2011). Similarly, elevated local senescence in diabetic wound healing is linked to pathological repair via macrophage CXCR2-mediated SASP (Wilkinson et al., 2019). Interestingly, reprogramming of tumor-associated macrophages by CXCR2 inhibition drives senescence and tumor inhibition in advanced prostate cancer (Di Mitri et al., 2019).
Points for discussion
In recent years, senescent cells have been recognized to play key roles in aging and contribute to a wide range of diseases including cancer and fibrosis. Strategies to target senescent cells have been proposed as a new therapeutic paradigm. Often those strategies take advantage of characteristics of senescent cells, such as targeting the increased β-galactosidase activity present on senescent cells (e.g., galacto-oligosaccharide–encapsulated nanoparticles containing cytotoxic or senolytic drugs (Munoz-Espin et al., 2018) and galactose-derived pro-drugs (González-Gualda et al., 2020; Guerrero et al., 2019). Since macrophages and senescent cells share many phenotypic characteristics, a double question arises. Are the benefits of certain senolytic medicines related to eliminating sub-populations of macrophages? This might be the case, at least in atherosclerosis (Childs et al., 2016). Or, on the contrary, are there unintended consequences of drugs aiming to kill senescent cells also target macrophages? Cardiac glycosides such as digoxin and digitoxin show in situ senolytic activity at a nanomolar range concentration that is close to the one observed in cardiac patients treated with this drug (Guerrero et al., 2019; López-Lázaro, 2007). We have observed that cardiac glycosides cause cytotoxicity in human macrophages and ameliorate homeostasis in white adipose tissue from obese patients (Olona et al., 2020), raising the possibility that senolytics also cause selective cytotoxicity in mononuclear phagocytes. Tailoring these drugs for specifically one type of cell (macrophage or senescent cell) will require an in-depth knowledge of phenotypic similarities and interplay between macrophages and senescent cells.
Acknowledgments
Core support was provided by the Medical Research Council (MC_U120085810), and grants from Worldwide Cancer Research (18-0215) and CRUK (C15075/A28647) funded research in J. Gil’s laboratory. This work was also supported by a grant from the Medical Research Council (MR/N01121X/1) to J. Behmoaras.
J. Gil has acted as a consultant for Unity Biotechnology, Geras Bio, and Merck KGaA; owns equity in Unity Biotechnology and Geras Bio; and is a named inventor in Imperial College and Medical Research Council patents related to senolytic therapies.
Author contributions: J. Behmoaras and J. Gil conceptualized an co-wrote the manuscript.
References
- Acosta, J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang T.W., Lasitschka F., Andrulis M., et al. 2013. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15:978–990. 10.1038/ncb2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem, A., and Underhill D.M.. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593–623. 10.1146/annurev.immunol.17.1.593 [DOI] [PubMed] [Google Scholar]
- Baillie, J.K., Arner E., Daub C., De Hoon M., Itoh M., Kawaji H., Lassmann T., Carninci P., Forrest A.R., Hayashizaki Y., et al. 2017. Analysis of the human monocyte-derived macrophage transcriptome and response to lipopolysaccharide provides new insights into genetic aetiology of inflammatory bowel disease. PLoS Genet. 13:e1006641 10.1371/journal.pgen.1006641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., Saltness R.A., Jeganathan K.B., Verzosa G.C., Pezeshki A., et al. 2016. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 530:184–189. 10.1038/nature16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursuker, I., Rhodes J.M., and Goldman R.. 1982. Beta-galactosidase–an indicator of the maturational stage of mouse and human mononuclear phagocytes. J. Cell. Physiol. 112:385–390. 10.1002/jcp.1041120312 [DOI] [PubMed] [Google Scholar]
- Burzynski, L.C., Humphry M., Pyrillou K., Wiggins K.A., Chan J.N.E., Figg N., Kitt L.L., Summers C., Tatham K.C., Martin P.B., et al. 2019. The Coagulation and Immune Systems Are Directly Linked through the Activation of Interleukin-1alpha by Thrombin. Immunity. 50:1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien, Y., Scuoppo C., Wang X., Fang X., Balgley B., Bolden J.E., Premsrirut P., Luo W., Chicas A., Lee C.S., et al. 2011. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 25:2125–2136. 10.1101/gad.17276711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs, B.G., Baker D.J., Wijshake T., Conover C.A., Campisi J., and van Deursen J.M.. 2016. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 354:472–477. 10.1126/science.aaf6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, M., Yin T., Chen R., Xiang H., Yuan L., Ding Y., Pan C.C., Tang Z., Alexander P.B., Li Q.J., and Wang X.F.. 2018. CD36 initiates the secretory phenotype during the establishment of cellular senescence. EMBO Rep. 19:e45274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigneault, M., Preston J.A., Marriott H.M., Whyte M.K., and Dockrell D.H.. 2010. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 5:e8668 10.1371/journal.pone.0008668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuisseau, K.C., Park Y.M., DeRuisseau L.R., Cowley P.M., Fazen C.H., and Doyle R.P.. 2013. Aging-related changes in the iron status of skeletal muscle. Exp. Gerontol. 48:1294–1302. 10.1016/j.exger.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mitri, D., Mirenda M., Vasilevska J., Calcinotto A., Delaleu N., Revandkar A., Gil V., Boysen G., Losa M., Mosole S., et al. 2019. Re-education of Tumor-Associated Macrophages by CXCR2 Blockade Drives Senescence and Tumor Inhibition in Advanced Prostate Cancer. Cell Rep. 28:2156–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira, M., Hirota Y., Shimizu-Hirota R., Saito-Fujita T., Haraguchi H., Matsumoto L., Matsuo M., Hiraoka T., Tanaka T., Akaeda S., et al. 2017. F4/80+ Macrophages Contribute to Clearance of Senescent Cells in the Mouse Postpartum Uterus. Endocrinology. 158:2344–2353. 10.1210/en.2016-1886 [DOI] [PubMed] [Google Scholar]
- Erlich, Z., Shlomovitz I., Edry-Botzer L., Cohen H., Frank D., Wang H., Lew A.M., Lawlor K.E., Zhan Y., Vince J.E., and Gerlic M.. 2019. Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat. Immunol. 20:397–406. 10.1038/s41590-019-0313-5 [DOI] [PubMed] [Google Scholar]
- Gažová, I., Lefevre L., Bush S.J., Clohisey S., Arner E., de Hoon M., Severin J., van Duin L., Andersson R., Lengeling A., et al. 2020. The Transcriptional Network That Controls Growth Arrest and Macrophage Differentiation in the Human Myeloid Leukemia Cell Line THP-1. Front. Cell Dev. Biol. 8:498 10.3389/fcell.2020.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gualda, E., Paez-Ribes M., Lozano-Torres B., Macias D., Wilson J.R. III, Gonzalez-Lopez C., Ou H.L., Miron-Barroso S., Zhang Z., Lerida-Viso A., et al. 2020. Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell. 19:e13142 10.1111/acel.13142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, S. 2016. Phagocytosis: An Immunobiologic Process. Immunity. 44:463–475. 10.1016/j.immuni.2016.02.026 [DOI] [PubMed] [Google Scholar]
- Gordon, S., and Pluddemann A.. 2017. Tissue macrophages: heterogeneity and functions. BMC Biol. 15:53 10.1186/s12915-017-0392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, S., Pluddemann A., and Martinez Estrada F.. 2014. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol. Rev. 262:36–55. 10.1111/imr.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis, V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C., Campisi J., Collado M., Evangelou K., Ferbeyre G., et al. 2019. Cellular Senescence: Defining a Path Forward. Cell. 179:813–827. 10.1016/j.cell.2019.10.005 [DOI] [PubMed] [Google Scholar]
- Greenberg, M.E., Sun M., Zhang R., Febbraio M., Silverstein R., and Hazen S.L.. 2006. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 203:2613–2625. 10.1084/jem.20060370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse, L., Wagner N., Emelyanov A., Molina C., Lacas-Gervais S., Wagner K.D., and Bulavin D.V.. 2020. Defined p16(High) Senescent Cell Types Are Indispensable for Mouse Healthspan. Cell Metab. 32:87–99. [DOI] [PubMed] [Google Scholar]
- Guerrero, A., Herranz N., Sun B., Wagner V., Gallage S., Guiho R., Wolter K., Pombo J., Irvine E.E., Innes A.J., et al. 2019. Cardiac glycosides are broad-spectrum senolytics. Nat. Metab. 1:1074–1088. 10.1038/s42255-019-0122-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, B.M., Balan V., Gleiberman A.S., Strom E., Krasnov P., Virtuoso L.P., Rydkina E., Vujcic S., Balan K., Gitlin I., et al. 2016. Aging of mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY). 8:1294–1315. 10.18632/aging.100991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, B.M., Balan V., Gleiberman A.S., Strom E., Krasnov P., Virtuoso L.P., Rydkina E., Vujcic S., Balan K., Gitlin I.I., et al. 2017. p16(Ink4a) and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (Albany NY). 9:1867–1884. 10.18632/aging.101268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X., Chen H., Gong H., Tang X., Huang N., Xu W., Tai H., Zhang G., Zhao T., Gong C., et al. 2020. Autolysosomal degradation of cytosolic chromatin fragments antagonizes oxidative stress-induced senescence. J. Biol. Chem. 295:4451–4463. 10.1074/jbc.RA119.010734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz, N., and Gil J.. 2018. Mechanisms and functions of cellular senescence. J. Clin. Invest. 128:1238–1246. 10.1172/JCI95148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipolito, V.E.B., Diaz J.A., Tandoc K.V., Oertlin C., Ristau J., Chauhan N., Saric A., McLaughlan S., Larsson O., Topisirovic I., and Botelho R.J.. 2019. Enhanced translation expands the endo-lysosome size and promotes antigen presentation during phagocyte activation. PLoS Biol. 17:e3000535 10.1371/journal.pbio.3000535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, C.E., Coody T.K., Jeong M.Y., Berg J.A., Winge D.R., and Hughes A.L.. 2020. Cysteine Toxicity Drives Age-Related Mitochondrial Decline by Altering Iron Homeostasis. Cell. 180:296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, H., and Barber G.N.. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 455:674–678. 10.1038/nature07317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, H., Ma Z., and Barber G.N.. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 461:788–792. 10.1038/nature08476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, A., Pawlikowski J., Manoharan I., van Tuyn J., Nelson D.M., Rai T.S., Shah P.P., Hewitt G., Korolchuk V.I., Passos J.F., et al. 2013. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 202:129–143. 10.1083/jcb.201212110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath, C., Lindsey D.R., Dhandayuthapani S., Xu Y., Hunter R.L. Jr., and Eissa N.T.. 2009. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat. Med. 15:267–276. 10.1038/nm.1928 [DOI] [PubMed] [Google Scholar]
- Kang, T.W., Yevsa T., Woller N., Hoenicke L., Wuestefeld T., Dauch D., Hohmeyer A., Gereke M., Rudalska R., Potapova A., et al. 2011. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 479:547–551. 10.1038/nature10599 [DOI] [PubMed] [Google Scholar]
- Kaplon, J., Zheng L., Meissl K., Chaneton B., Selivanov V.A., Mackay G., van der Burg S.H., Verdegaal E.M., Cascante M., Shlomi T., et al. 2013. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 498:109–112. 10.1038/nature12154 [DOI] [PubMed] [Google Scholar]
- Kay, M.M. 1975. Mechanism of removal of senescent cells by human macrophages in situ. Proc. Natl. Acad. Sci. USA. 72:3521–3525. 10.1073/pnas.72.9.3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelut-Carneiro, N., Souza C.O.S., Benevides L., Gardinassi L.G., Silva M.C., Tavares L.A., Zamboni D.S., and Silva J.S.. 2019. Caspase-11-dependent IL-1alpha release boosts Th17 immunity against Paracoccidioides brasiliensis. PLoS Pathog. 15:e1007990 10.1371/journal.ppat.1007990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea, D.W., Atamna H., Liao C., and Ames B.N.. 2003. Iron accumulation during cellular senescence in human fibroblasts in vitro. Antioxid. Redox Signal. 5:507–516. 10.1089/152308603770310158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., and Guan K.L.. 2019. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 21:63–71. 10.1038/s41556-018-0205-1 [DOI] [PubMed] [Google Scholar]
- Klappacher, G.W., Lunyak V.V., Sykes D.B., Sawka-Verhelle D., Sage J., Brard G., Ngo S.D., Gangadharan D., Jacks T., Kamps M.P., et al. 2002. An induced Ets repressor complex regulates growth arrest during terminal macrophage differentiation. Cell. 109:169–180. 10.1016/S0092-8674(02)00714-6 [DOI] [PubMed] [Google Scholar]
- Kopp, H.G., Hooper A.T., Shmelkov S.V., and Rafii S.. 2007. Beta-galactosidase staining on bone marrow. The osteoclast pitfall. Histol. Histopathol. 22:971–976. [DOI] [PubMed] [Google Scholar]
- Korolchuk, V.I., Miwa S., Carroll B., and von Zglinicki T.. 2017. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine. 21:7–13. 10.1016/j.ebiom.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolnek, T., and Hamza I.. 2015. Macrophages and iron trafficking at the birth and death of red cells. Blood. 125:2893–2897. 10.1182/blood-2014-12-567776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz, D.J., Decary S., Hong Y., and Erusalimsky J.D.. 2000. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 113:3613–3622. [DOI] [PubMed] [Google Scholar]
- Latz, E., and Duewell P.. 2018. NLRP3 inflammasome activation in inflammaging. Semin. Immunol. 40:61–73. 10.1016/j.smim.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Lavin, Y., Winter D., Blecher-Gonen R., David E., Keren-Shaul H., Merad M., Jung S., and Amit I.. 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 159:1312–1326. 10.1016/j.cell.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B.Y., Han J.A., Im J.S., Morrone A., Johung K., Goodwin E.C., Kleijer W.J., DiMaio D., and Hwang E.S.. 2006. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 5:187–195. 10.1111/j.1474-9726.2006.00199.x [DOI] [PubMed] [Google Scholar]
- Liu, J.Y., Souroullas G.P., Diekman B.O., Krishnamurthy J., Hall B.M., Sorrentino J.A., Parker J.S., Sessions G.A., Gudkov A.V., and Sharpless N.E.. 2019. Cells exhibiting strong p16 (INK4a) promoter activation in vivo display features of senescence. Proc. Natl. Acad. Sci. USA. 116:2603–2611. 10.1073/pnas.1818313116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Lázaro, M. 2007. Digitoxin as an anticancer agent with selectivity for cancer cells: possible mechanisms involved. Expert Opin. Ther. Targets. 11:1043–1053. 10.1517/14728222.11.8.1043 [DOI] [PubMed] [Google Scholar]
- López-Otín, C., Blasco M.A., Partridge L., Serrano M., and Kroemer G.. 2013. The hallmarks of aging. Cell. 153:1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujambio, A., Akkari L., Simon J., Grace D., Tschaharganeh D.F., Bolden J.E., Zhao Z., Thapar V., Joyce J.A., Krizhanovsky V., and Lowe S.W.. 2013. Non-cell-autonomous tumor suppression by p53. Cell. 153:449–460. 10.1016/j.cell.2013.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver, N.J., Jacobs S.R., Wieman H.L., Wofford J.A., Coloff J.L., and Rathmell J.C.. 2008. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J. Leukoc. Biol. 84:949–957. 10.1189/jlb.0108024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man, S.M., and Kanneganti T.D.. 2016. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 16:7–21. 10.1038/nri.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Reyes, I., and Chandel N.S.. 2020. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11:102 10.1038/s41467-019-13668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni, M., Mauro G., Erreni M., Romeo P., Minna E., Vizioli M.G., Belgiovine C., Rizzetti M.G., Pagliardini S., Avigni R., et al. 2019. Senescent thyrocytes and thyroid tumor cells induce M2-like macrophage polarization of human monocytes via a PGE2-dependent mechanism. J. Exp. Clin. Cancer Res. 38:208 10.1186/s13046-019-1198-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh, D., and Gil J.. 2018. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 217:65–77. 10.1083/jcb.201708092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, E.L., Kelly B., Logan A., Costa A.S.H., Varma M., Bryant C.E., Tourlomousis P., Dabritz J.H.M., Gottlieb E., Latorre I., et al. 2016. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 167:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva, O., Bourdeau V., Roux A., Deschenes-Simard X., and Ferbeyre G.. 2009. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol. Cell. Biol. 29:4495–4507. 10.1128/MCB.01868-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Espín, D., and Serrano M.. 2014. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15:482–496. 10.1038/nrm3823 [DOI] [PubMed] [Google Scholar]
- Munoz-Espin, D., Rovira M., Galiana I., Gimenez C., Lozano-Torres B., Paez-Ribes M., Llanos S., Chaib S., Munoz-Martin M., Ucero A.C., et al. 2018. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 10:e9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano, I., Barbatelli G., Parisani V., Latini C., Muzzonigro G., Castellucci M., and Cinti S.. 2008. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 49:1562–1568. 10.1194/jlr.M800019-JLR200 [DOI] [PubMed] [Google Scholar]
- Murray, R.Z., and Stow J.L.. 2014. Cytokine Secretion in Macrophages: SNAREs, Rabs, and Membrane Trafficking. Front. Immunol. 5:538 10.3389/fimmu.2014.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A-Gonzalez , N., J.A. Quintana, S. Garcia-Silva, M. Mazariegos, A. Gonzalez de la Aleja, J.A. Nicolas-Avila, W. Walter, J.M. Adrover, G. Crainiciuc, V.K. Kuchroo, et al. 2017. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J. Exp. Med. 214:1281–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita, M., Young A.R., Arakawa S., Samarajiwa S.A., Nakashima T., Yoshida S., Hong S., Berry L.S., Reichelt S., Ferreira M., et al. 2011. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 332:966–970. 10.1126/science.1205407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas-Avila, J.A., Lechuga-Vieco A.V., Esteban-Martinez L., Sanchez-Diaz M., Diaz-Garcia E., Santiago D.J., Rubio-Ponce A., Li J.L., Balachander A., Quintana J.A., et al. 2020. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell. 183:94–109. [DOI] [PubMed] [Google Scholar]
- Ogrodnik, M., Zhu Y., Langhi L.G.P., Tchkonia T., Kruger P., Fielder E., Victorelli S., Ruswhandi R.A., Giorgadze N., Pirtskhalava T., et al. 2019. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab. 29:1061–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi, Y., and Manabe I.. 2016. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech. Dis. 2:16018 10.1038/npjamd.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olan, I., Parry A.J., Schoenfelder S., Narita M., Ito Y., Chan A.S.L., Slater G.S.C., Bihary D., Bando M., Shirahige K., et al. 2020. Transcription-dependent cohesin repositioning rewires chromatin loops in cellular senescence. Nat. Commun. 11:6049 10.1038/s41467-020-19878-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olona, A., Hateley C., Guerrero A., Ho J.K., Johnson M.R., Thomas D., Gil J., and Behmoaras J.. 2020. Cardiac glycosides cause selective cytotoxicity in human macrophages and ameliorate white adipose tissue homeostasis. bioRxiv. doi: 10.1101/2020.09.18.293415 (Preprint posted September 18, 2020) [DOI] [PubMed]
- Patel, S.N., Serghides L., Smith T.G., Febbraio M., Silverstein R.L., Kurtz T.W., Pravenec M., and Kain K.C.. 2004. CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J. Infect. Dis. 189:204–213. 10.1086/380764 [DOI] [PubMed] [Google Scholar]
- Pereira, M., Chen T.D., Buang N., Olona A., Ko J.H., Prendecki M., Costa A.S.H., Nikitopoulou E., Tronci L., Pusey C.D., et al. 2019. Acute Iron Deprivation Reprograms Human Macrophage Metabolism and Reduces Inflammation In Vivo. Cell Rep. 28:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, S.G., and Jackson J.G.. 2016. SASP: Tumor Suppressor or Promoter? Yes! Trends Cancer. 2:676–687. 10.1016/j.trecan.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Rodier, F., Coppe J.P., Patil C.K., Hoeijmakers W.A., Munoz D.P., Raza S.R., Freund A., Campeau E., Davalos A.R., and Campisi J.. 2009. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11:973–979. 10.1038/ncb1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai, T., He G., Matsuzawa A., Yu G.Y., Maeda S., Hardiman G., and Karin M.. 2008. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 14:156–165. 10.1016/j.ccr.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder, K., and Tschopp J.. 2010. The inflammasomes. Cell. 140:821–832. 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- Sharpless, N.E., and Sherr C.J.. 2015. Forging a signature of in vivo senescence. Nat. Rev. Cancer. 15:397–408. 10.1038/nrc3960 [DOI] [PubMed] [Google Scholar]
- Shi, J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., and Shao F.. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 526:660–665. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- Sindrilaru, A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., et al. 2011. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 121:985–997. 10.1172/JCI44490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro, O., Goldberg E.L., Camell C.D., Youm Y.H., Kopchick J.J., Nguyen K.Y., Bartke A., Sun L.Y., and Dixit V.D.. 2016. Growth Hormone Receptor Deficiency Protects against Age-Related NLRP3 Inflammasome Activation and Immune Senescence. Cell Rep. 14:1571–1580. 10.1016/j.celrep.2016.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabula Muris Consortium 2020. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. 583:590–595. 10.1038/s41586-020-2496-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, H., Wang Z., Gong H., Han X., Zhou J., Wang X., Wei X., Ding Y., Huang N., Qin J., et al. 2017. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy. 13:99–113. 10.1080/15548627.2016.1247143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnessen-Murray, C.A., Frey W.D., Rao S.G., Shahbandi A., Ungerleider N.A., Olayiwola J.O., Murray L.B., Vinson B.T., Chrisey D.B., Lord C.J., and Jackson J.G.. 2019. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J. Cell Biol. 218:3827–3844. 10.1083/jcb.201904051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso, A., Revandkar A., Di Mitri D., Guccini I., Proietti M., Sarti M., Pinton S., Zhang J., Kalathur M., Civenni G., et al. 2014. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9:75–89. 10.1016/j.celrep.2014.08.044 [DOI] [PubMed] [Google Scholar]
- Trias, E., Beilby P.R., Kovacs M., Ibarburu S., Varela V., Barreto-Nunez R., Bradford S.C., Beckman J.S., and Barbeito L.. 2019. Emergence of Microglia Bearing Senescence Markers During Paralysis Progression in a Rat Model of Inherited ALS. Front. Aging Neurosci. 11:42 10.3389/fnagi.2019.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadiveloo, P.K., Vairo G., Novak U., Royston A.K., Whitty G., Filonzi E.L., Cragoe E.J. Jr., and Hamilton J.A.. 1996. Differential regulation of cell cycle machinery by various antiproliferative agents is linked to macrophage arrest at distinct G1 checkpoints. Oncogene. 13:599–608. [PubMed] [Google Scholar]
- Vairo, G., Royston A.K., and Hamilton J.A.. 1992. Biochemical events accompanying macrophage activation and the inhibition of colony-stimulating factor-1-induced macrophage proliferation by tumor necrosis factor-alpha, interferon-gamma, and lipopolysaccharide. J. Cell. Physiol. 151:630–641. 10.1002/jcp.1041510324 [DOI] [PubMed] [Google Scholar]
- Visvikis, O., Ihuegbu N., Labed S.A., Luhachack L.G., Alves A.F., Wollenberg A.C., Stuart L.M., Stormo G.D., and Irazoqui J.E.. 2014. Innate host defense requires TFEB-mediated transcription of cytoprotective and antimicrobial genes. Immunity. 40:896–909. 10.1016/j.immuni.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizioli, M.G., Liu T., Miller K.N., Robertson N.A., Gilroy K., Lagnado A.B., Perez-Garcia A., Kiourtis C., Dasgupta N., Lei X., et al. 2020. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. 34:428–445. 10.1101/gad.331272.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Fu H., Zhu R., Wu X., Ji X., Li X., Jiang H., Lin Z., Tang X., Sun S., et al. 2020. BRD4 contributes to LPS-induced macrophage senescence and promotes progression of atherosclerosis-associated lipid uptake. Aging (Albany NY). 12:9240–9259. 10.18632/aging.103200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, R.A., Yen F.S., Nicholson S.P.V., Alwaseem H., Bayraktar E.C., Alam M., Timson R.C., La K., Abu-Remaileh M., Molina H., et al. 2020. Maintaining Iron Homeostasis Is the Key Role of Lysosomal Acidity for Cell Proliferation. Mol. Cell. 77:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman, A., Werman-Venkert R., White R., Lee J.K., Werman B., Krelin Y., Voronov E., Dinarello C.A., and Apte R.N.. 2004. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc. Natl. Acad. Sci. USA. 101:2434–2439. 10.1073/pnas.0308705101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman, J., Walpole G.F.W., Kasper L., Xue B.Y., Elshafee O., Hube B., and Grinstein S.. 2020. Lysosome Fusion Maintains Phagosome Integrity during Fungal Infection. Cell Host Microbe. 28:798–812.e6. 10.1016/j.chom.2020.09.004 [DOI] [PubMed] [Google Scholar]
- Wiggins, K.A., Parry A.J., Cassidy L.D., Humphry M., Webster S.J., Goodall J.C., Narita M., and Clarke M.C.H.. 2019. IL-1alpha cleavage by inflammatory caspases of the noncanonical inflammasome controls the senescence-associated secretory phenotype. Aging Cell. 18:e12946 10.1111/acel.12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley, C.D., Liu S., Limbad C., Zawadzka A.M., Beck J., Demaria M., Artwood R., Alimirah F., Lopez-Dominguez J.A., Kuehnemann C., et al. 2019. SILAC Analysis Reveals Increased Secretion of Hemostasis-Related Factors by Senescent Cells. Cell Rep. 28:3329–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, H.N., Clowes C., Banyard K.L., Matteuci P., Mace K.A., and Hardman M.J.. 2019. Elevated Local Senescence in Diabetic Wound Healing Is Linked to Pathological Repair via CXCR2. J. Invest. Dermatol. 139:1171–1181. [DOI] [PubMed] [Google Scholar]
- Yambire, K.F., Rostosky C., Watanabe T., Pacheu-Grau D., Torres-Odio S., Sanchez-Guerrero A., Senderovich O., Meyron-Holtz E.G., Milosevic I., Frahm J., et al. 2019. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. eLife. 8:e51031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, A.R., Narita M., Ferreira M., Kirschner K., Sadaie M., Darot J.F., Tavare S., Arakawa S., Shimizu S., Watt F.M., and Narita M.. 2009. Autophagy mediates the mitotic senescence transition. Genes Dev. 23:798–803. 10.1101/gad.519709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, M., Karki R., Vogel P., and Kanneganti T-D.. 2020. Caspase-6 Is a Key Regulator of Innate Immunity, Inflammasome Activation, and Host Defense. Cell. 181:674–687.e13. 10.1016/j.cell.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]