Abstract
The influence of discrimination on hypothalamic pituitary adrenal (HPA) axis function is considered to be more pronounced for racial minority versus majority groups, although empirical support for this argument is not strong. This study examined whether the association of perceived discrimination was more strongly associated with long-term, retrospective cortisol output (as measured by hair cortisol concentration [HCC]) among African American compared to White adults. Participants included 141 community-dwelling adults (72 White, 69 African American; mean age 45.8 years; 67% females). The Everyday Discrimination Scale assessed perceived discrimination. The first 3 cm of proximal scalp hair were analyzed for HCC using enzyme-linked immunoassay. Associations between race, perceived discrimination and HCC were examined using hierarchical multiple regression. African Americans had higher HCC than Whites, but both groups reported perceived discrimination with similar frequency. Race moderated the association between perceived discrimination and HCC (R2 interaction = 0.03, p = 0.007) such that perceived discrimination was positively associated with HCC among African Americans (β = 0.28, p = 0.007), but not Whites (β = −0.11, p = 0.274). Perceived discrimination did not mediate the association between race and HCC (β for indirect effect = 0.025, 95% CI [−0.003, 0.087]). Although perceived discrimination did not differ between races, perceived discrimination was positively associated with retrospective levels of cortisol in scalp hair among African Americans but not Whites. This may suggest that characteristics of discrimination other than frequency are particularly salient to HPA axis function among African Americans (e.g., attribution, severity, historical context).
Keywords: hair cortisol, HPA axis, discrimination, health disparities, African American, stress
LAY SUMMARY
This study found that greater perceived discrimination frequency was associated with greater long-term cortisol secretion (i.e., hair cortisol concentration) among African American compared to White adults. Both groups reported similar discrimination frequency, so the uniqueness of African Americans’ experience with discrimination may be salient to HPA axis upregulation for this population.
INTRODUCTION
Discrimination, the unequal treatment of an individual or a group of individuals based on real or perceived differences, is a common and health-related stressor for racial and ethnic minorities in the United States (U.S.) (Kessler, Mickelson, & Williams, 1999; Sternthal, Slopen, & Williams, 2011). Discrimination among African Americans is a salient issue for the population, with approximately 92% of African American adults reporting that discrimination exists in the U.S. Among that group, 75% stated that individual discrimination is an important social problem (NPR, Robert Wood Johnson Foundation, & Harvard School of Public Health, 2017). Moreover, discrimination exposure among African Americans is linked with disproportionate representation of cardiovascular and metabolic conditions including hypertension, cardiovascular disease, and diabetes (Lewis, Cogburn, & Williams, 2015; Williams, 2012). Discrimination exposure can initiate a series of neuroendocrine responses via the hypothalamic pituitary adrenal (HPA) axis that may, if activated chronically, contribute to health disparities (Berger & Sarnyai, 2015). The extent to which the association between perceived discrimination and chronic HPA axis upregulation differs among African Americans relative to Whites has yet to be established (Busse, Yim, Campos, & Marshburn, 2017).
Biopsychosocial models of racial discrimination and minority health suggest that perceived discrimination engages various biological processes including the HPA axis (Clark, Anderson, Clark, & Williams, 1999; Myers, 2009). The HPA axis translates perceptions of threat via the hypothalamus, pituitary gland, and finally the adrenal cortex through a neuroendocrine cascade, ultimately releasing the steroid hormone cortisol into the bloodstream (Tsigos & Chrousos, 2002). Cortisol directs a host of physiological systems (e.g., circulatory, immune, digestive) to mobilize resources (e.g., glucose, free fatty acids, amino acids) to accommodate the perceived threat. Cortisol secretion typically follows a diurnal pattern, with high waking levels that steadily decline throughout the day. Transient cortisol increases are adaptive in handling environmental stressors, but long-term elevations or flattened declines throughout the day indicate chronic HPA axis upregulation. Exposure to stress modulates the circadian activity and total output of cortisol, being associated with flatter declines in cortisol across the day, lower waking cortisol, and elevated overall cortisol levels (Miller, Chen, & Zhou, 2007). Stressors defined by social evaluation and uncontrollability, both of which characterize discrimination, elicit the strongest cortisol responses (Dickerson & Kemeny, 2004).
Though race-based discrimination is more likely experienced by African Americans and other racial minority groups, an interracial stress and coping framework posits that discrimination is stressful regardless of the affiliation on which it is based (e.g., gender, race, age). This perspective suggests that discrimination influences the HPA axis similarly for racial majority and minority groups (Trawalter, Richeson, & Shelton, 2009). Accordingly, more frequently reported perceived discrimination has been associated with flattened salivary cortisol slopes among both African American and White young adults (Skinner, Shirtcliff, Haggerty, Coe, & Catalano, 2011). Others contend that past and present racial tensions in the U.S. render perceptions of discrimination particularly threatening for racial and ethnic minority individuals (particularly African Americans), increasing the degree to which such perceptions are manifested as altered cortisol secretion (Clark et al., 1999; Williams & Mohammed, 2009).
Among African American young adults, greater perceived everyday discrimination was associated with flatter salivary cortisol slopes compared to European Americans (Zeiders, Hoyt, & Adam, 2014). Similarly, perceived racial discrimination was associated with lower waking salivary cortisol among African American versus White adults (Adam et al., 2015). However, not all research finding racial differences in cortisol indices show that African Americans have markers of altered cortisol secretion, as one study showed that perceived discrimination was associated with flatter diurnal salivary cortisol slopes among White adults compared to African Americans (Fuller-Rowell, Doan, & Eccles, 2012). Although evidence links discrimination to altered cortisol patterns in African Americans, it is not conclusive.
The equivocal findings characterizing discrimination, cortisol, and race may be partly due to a temporal mismatch in comparing recalled accounts of past discrimination to cortisol obtained from saliva, which captures cortisol levels at the time of sampling (Kirschbaum et al., 1990). Fluctuations in cortisol levels due to circadian rhythm or daily variation due to situational factors (e.g., diet, recent stressor, infection) suggest that saliva is not ideal for capturing retrospective cortisol concentrations indexing the period (e.g., the past few months) ascertained by widely-used discrimination questionnaires (Hellhammer et al., 2007).
The analysis of cortisol in scalp hair is a valid indicator of retrospective cortisol secretion over several months (Stalder & Kirschbaum, 2012), but few studies have examined the association between self-reported discrimination and hair cortisol concentration (HCC). In the English Longitudinal Study of Aging, comprised of mostly White participants, perceived discrimination attributed to weight, age, and sex were all positively associated with HCC (Jackson & Steptoe, 2018). Among a racially diverse U.S. sample, lifetime discrimination positively correlated with HCC (O’Brien, Meyer, Tronick, & Moore, 2017), and this association was similar across races. However, the discrimination scale used in that study assessed primarily socioeconomic barriers, which do not capture general interpersonal occurrences of unfair treatment towards racial minority individuals surveyed by more commonly-used discrimination measures such as the Everyday Discrimination Scale (Sternthal et al., 2011). A study examining the association of perceived general discrimination with HCC among a racially diverse sample is warranted to advance understanding of discrimination and long-term cortisol secretion.
The present study examined the association between perceived everyday discrimination and HCC among a sample of African American and White adults. The primary hypothesis (H1) was: Race will moderate the relationship between perceived everyday discrimination and HCC such that discrimination will be more strongly associated with HCC among African American versus White adults. Additionally, because African Americans have exhibited higher HCC than Whites (Wosu et al., 2015) and often report more frequent discrimination compared to Whites (Kessler et al., 1999; Sternthal et al., 2011), perceived discrimination has been considered a mediator between race and cortisol levels (Lee et al., 2018). Thus, the secondary hypothesis (H2) was: Perceived discrimination will partially mediate the association of African American race and HCC, such that African American race will be associated with elevated HCC via increased perceived discrimination.
METHODS
Participants and Procedures
Participants were 141 community-dwelling adults (72 White, 69 African American, mean age 45.8 years, 67% females) recruited through flyers posted on campus, at neighborhood establishments, and on a university research website. A majority (n = 96) were University employees. Exclusion criteria were baldness/shaved head, pregnant/lactating, or use of glucocorticoid-containing medication. Participants provided written informed consent approved by the Institutional Review Board and were compensated $20 for their time. All study variables were assessed during a single study visit.
Measures
Demographics and hair hygiene
Demographic variables included age, sex (0 = male, 1 = female), and annual household income (from 1 = less than $20,000 to 5 = $100,000 or more, in $20,000 increments). Hair hygiene included number of hair washes/week and use of treatments (conditioners, bleach, permanent wave, straightening) during the three months preceding the study.
Perceived everyday discrimination
Perceived everyday discrimination was assessed using the 5-item version (Sternthal et al., 2011) of the Everyday Discrimination Scale (Williams, Yan Yu, Jackson, & Anderson, 1997). Participants were asked “In your day-to-day life, how often have any of the following things happened to you?” such as, “You are treated with less courtesy or respect than other people” and “People act as if they are afraid of you.” Responses included 1 = never, 2 = less than a few times a year, 3 = a few times a year, 4 = a few times a month, 5 = at least once a week, and 6 = almost every day. The Everyday Discrimination Scale score summed the 5 items. Reliability was acceptable (α = .79).
According to scoring instructions, discrimination attribution was asked of participants (n = 80) answering 3 (a few times a year) or higher for any Everyday Discrimination Scale item. Participants were asked, “What do you think is the main reason for these experiences? (If more than one applies, please mark “1” for the most significant reason, and “2” for the second-most significant reason).” Responses included ancestry of national origins, gender, race, age, religion, height, weight, some other aspect of your physical appearance, sexual orientation, and education or income level. Ancestry and race were combined into one category. The number of attributions were summed to create a measure of intersectionality, i.e., the number of identities that participants reported as contributing to perceived discrimination experiences.
Hair cortisol
Hair was cut using thinning shears as close to the scalp as possible near the posterior vertex of the scalp, as described previously (Hoffman, D’Anna-Hernandez, Benitez, Ross, & Laudenslager, 2017; Wright et al., 2018), stored in aluminum foil, and transferred to the Behavioral Immunology and Endocrinology Lab at the University of Colorado Anschutz Medical Campus. This laboratory participates in a quality control program provided by the Society of Hair Testing and is designated as a Center of Excellence by Salimetrics, Ltd. After hair segments were measured and the proximal 3 cm from the scalp was cut, samples were placed in a pre-weighed 2 ml cryovial (Wheaton, Millville, NJ, USA) and washed three times in 100% isopropanol and dried as previously described (Hoffman et al., 2017). After washing, drying, and re-weighing on a precision (± 0.01mg) electronic balance (Mettler, Switzerland), the hair was ground in the same tube using a ball mill (Retsch, Haan, Germany) with one 3/16-inch stainless steel ball bearing. Specially milled aluminum cassettes were designed to hold three of these cryovials. The cassettes containing the cryotubes were submerged in liquid nitrogen for approximately 3 minutes to freeze hair samples rendering them brittle for enhanced grinding. Samples were ground for 4–5 minutes. The powdered hair (5–20 mg) was extracted at room temperature in the same cryovial with HPLC grade methanol overnight on a side-to-side shaker platform. Following methanol extraction overnight, the cryovial was spun for three minutes in a centrifuge at 1700g to pellet the hair and supernatants were removed, placed into a second microcentrifuge tube, and dried under a stream of nitrogen. The extracts were reconstituted with assay diluent based on hair weight. Steroid levels were determined using a commercial high sensitivity enzyme-linked immunoassay kit (Salimetrics LLC, State College, PA, USA) per manufacturer’s protocol. Results are reported as pg/mg after precisely correcting for weight of hair extracted. A pooled control of previously ground hair was extracted as above and included on each EIA plate in duplicate for determination of inter-assay coefficients of variation. Inter-assay coefficient of variation (CV) for the control hair pool for cortisol was 9.2% and intra-assay CV was 2.8%.
Physical and psychosocial health covariates
Physical activity was operationalized as “Thinking about the last three months, how many days/week did you perform at least 30 minutes of physical activity?” Waist and hip circumferences were measured to the nearest 0.1 cm using a non-stretchable standard tape measure: 0.1 cm above the iliac crest on a horizontal plane and at the widest portion of the hip, respectively. Waist-hip ratio (WHR) was calculated as waist circumference divided by hip circumference.
Emotional stability was measured with two items from the 10-item Personality Inventory (Gosling, Rentfrow, & Swann, 2003). Participants were asked, “I see myself as: anxious, easily upset” and “I see myself as: calm, emotionally stable.” Responses ranged from 1 = disagree strongly to 7 = agree strongly. Items were coded such that higher scores represented greater emotional stability. The reliability of the emotional stability measure was poor (α = .47).
Perceived stress was measured with four items from the 4-item Perceived Stress Scale (Cohen & Williamson, 1988). Participants were asked to rate how often stressful events occurred during the past 3 months on a scale from 0 = never to 4 = very often. Sample items include: “How often have you felt that you were unable to control the important things in your life?” and “How often have you felt difficulties were piling up so high that you could not overcome them?” Reliability of the Perceived Stress Scale was adequate (α = .68).
Statistical analyses
Two HCC values were beyond three standard deviations from the mean and were winsorized to three standard deviations (Ghosh & Vogt, 2012). HCC was positively skewed and was thus log-transformed to achieve a normal distribution. Racial differences in study variables were examined using Pearson chi-square tests and independent samples t-tests. Associations among variables were analyzed using bivariate correlations. Supplemental analysis examined whether intersectionality was related to log-HCC.
To examine associations of race and perceived discrimination with log-HCC while controlling for potential confounding variables, hierarchical multiple regression was performed. Step 1 of the model included covariates: age, sex, annual household income, physical activity, WHR, emotional stability, and perceived stress. Race was added at step 2 and perceived discrimination was added at step 3. To determine whether the association of perceived discrimination with log-HCC differed by race, a race × discrimination interaction term was added at step 4.
Mediation analysis was performed using PROCESS (Hayes, 2013) to test whether the association between African American race and log-HCC was mediated by perceived everyday discrimination. Bootstrapping (5,000 repetitions) was used to derive 95% confidence intervals for the indirect effect, which is the product of the path from race to discrimination and the path from discrimination to log-HCC. All covariates from the moderation analysis were included in the mediation analysis. Alpha was set at .05 for all hypothesis testing. Analyses were performed using Mplus version 7.4 (Muthen & Muthen, 2015).
RESULTS
Descriptive information of the study sample is provided in Table 1, and attributions for discrimination are shown in Figure 1. African Americans reported more race-based discrimination attributions than Whites (χ2 [1,78] = 53.43, p < 0.001), whereas Whites reported more age (χ2 [1,78] = 23.00, p < 0.001) and physical appearance attributions for discrimination (χ2 [1,78] = 7.48, p = 0.006). Of the 80 participants reporting discrimination attributions, 25 reported one attribution and 55 reported two attributions.
Table 1.
Descriptive information of the study sample
| Full Sample (N = 141) | White (n = 72) | African American (n = 69) | t or χ2 | |
|---|---|---|---|---|
| Age, years | 45.77 (15.17) | 42.35 (15.04) | 49.33 (14.58) | −2.80** |
| Sex, n female (%) | 95 (67) | 41 (57) | 54 (78) | 7.28** |
| Annual household income, n ≥ $40,000/year (%) | 77 (55) | 48 (67) | 29 (42) | 8.63** |
| Hair washes/week | 3.10 (2.27) | 4.60 (2.01) | 1.54 (1.24) | 10.91*** |
| Hair Treatment Use | ||||
| Conditioner, n yes (%) | 112 (79) | 51 (71) | 61 (88) | 6.66* |
| Bleach, n yes (%) | 19 (14) | 8 (11) | 11 (16) | 0.71 |
| Permanent Wave, n yes (%) | 23 (16) | 1 (1) | 22 (32) | 24.00*** |
| Straightening, n yes (%) | 34 (24) | 5 (7) | 29 (42) | 24.70*** |
| Days of ≥ 30 min physical activity/week | 3.48 (2.09) | 4.13 (1.92) | 2.81 (2.06) | 3.92*** |
| Waist to hip ratio | 0.88 (0.09) | 0.87 (0.08) | 0.89 (0.09) | −1.33 |
| Emotional stability | 5.20 (1.32) | 5.20 (1.21) | 5.19 (1.43) | 0.06 |
| Perceived stress | 1.21 (0.72) | 1.17 (0.71) | 1.26 (0.75) | −0.80 |
| Perceived everyday discrimination | 10.06 (4.38) | 9.54 (3.22) | 10.61 (5.29) | −1.44 |
| Hair cortisol concentration, pg/mg, median (IQR) | 7.7 (16.6) | 6.0 (7.2) | 8.6 (21.2) | −2.27* |
Note. All data provided as mean (standard deviation) unless indicated otherwise. Group comparisons were performed using
t-tests for continuous variables and Pearson chi-square tests for categorical variables. IQR = interquartile range.
p < 0.05;
p < 0.01;
p < 0.001.
Figure 1.
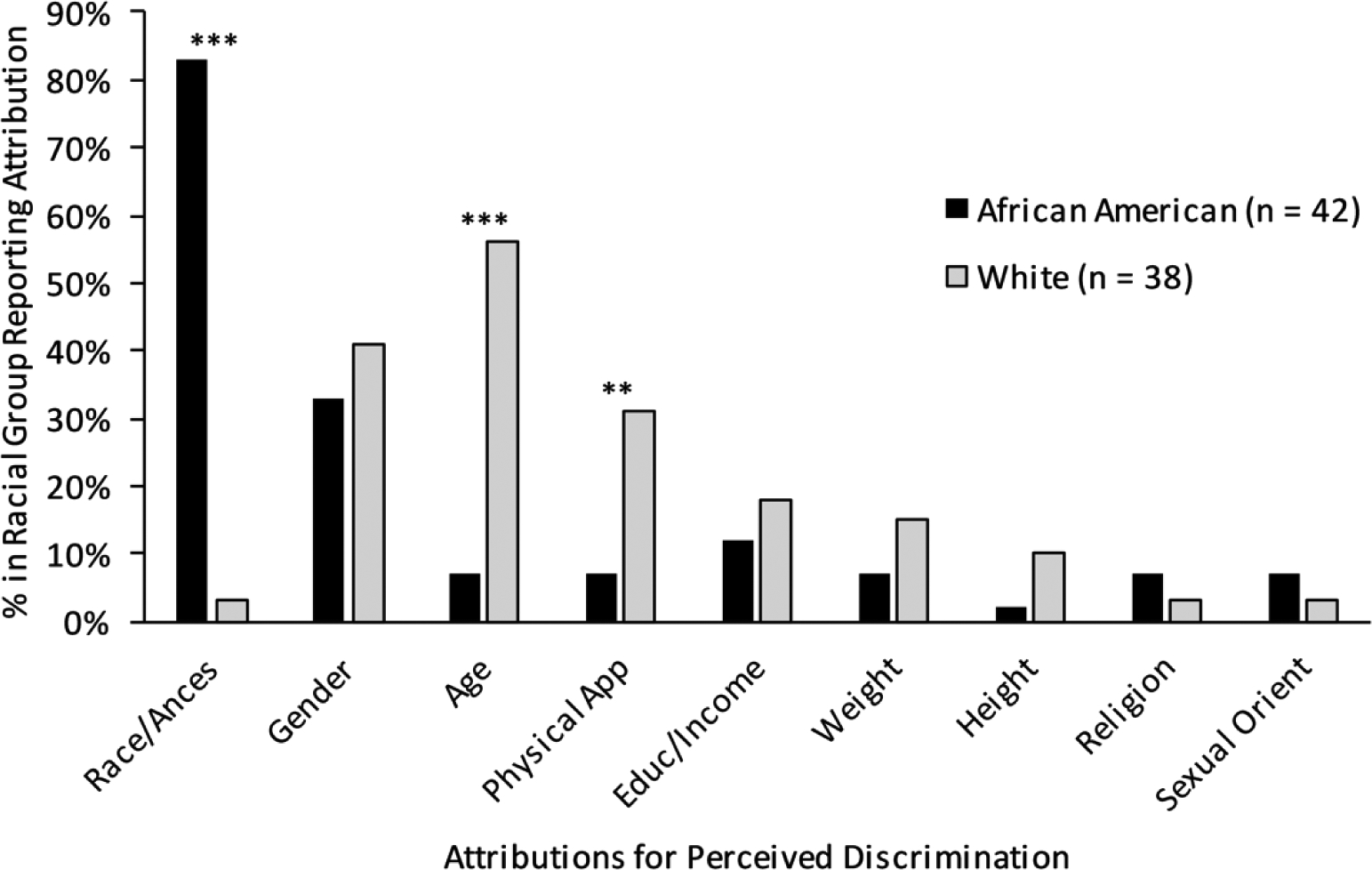
Bar graphs depicting the distribution of perceived discrimination attributions by race. Race/Ances = Race/Ancestry; Physical App = Physical Appearance; Educ/Income = Education/Income; Sexual Orient = Sexual Orientation.
** p < 0.01; *** p < 0.001.
Bivariate correlations are shown in Table 2. Perceived everyday discrimination was associated with lower annual household income, less frequent physical activity, lower emotional stability, and greater perceived stress. Additionally, log-HCC was positively associated with older age, African American race, and greater WHR. Perceived everyday discrimination was not associated with log-HCC (p = 0.084). Log-HCC was not associated with hair washing frequency (p = 0.160) or use of hair treatments: conditioner (p = 0.123), bleach (p = 0.229), permanent wave (p = 0.490), and straightening (p = 0.447). The number of discrimination-related identities was not associated with log-HCC (r = −0.06, p = 0.589).
Table 2.
Bivariate correlations among variables involved in inferential analyses (N = 141).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Age | - | ||||||||
| Sex | .17* | - | |||||||
| Race | .23** | .23* | - | ||||||
| Annual Household Income | .22* | −.05 | −.24** | - | |||||
| Physical Activity | −.13 | −.09 | −.32*** | .15 | - | ||||
| WHR | .36*** | −.24** | .11 | −.08 | −.09 | - | |||
| Emotional Stability | .03 | −.07 | .00 | .25** | .16 | .02 | - | ||
| Perceived Stress | −.13 | .04 | .07 | −.29*** | −.15 | .03 | −.38*** | - | |
| Perceived Discrimination | −.12 | −.06 | .12 | −.21* | −.20* | .01 | −.31** | .45*** | - |
| Log-HCC | .20* | −.07 | .19* | −.11 | .07 | .25** | −.04 | .05 | .15 |
Note. Sex (Male = 0, Female = 1); Race (White = 0, African American = 1). WHR = waist to hip ratio.
p < 0.05;
p < 0.01;
p < .001.
Moderation analysis
Table 3 shows the linear regression model predicting log-HCC. Covariates included in step 1 of the model explained 11.5% of the variance in log-HCC (F [7,133] = 3.03). The addition of race to the model in step 2 explained an additional 2.7% of the variance in log-HCC, and the addition of perceived discrimination in step 3 explained 2.1% of the log-HCC variance beyond step 2. The race × discrimination interaction term, added in step 4, accounted for an additional 2.6% of the variance in log-HCC beyond the step 3 model (F [1,130] = 4.35). The final model explained 19% of the variance in log-HCC (F [10,130] = 3.46). Given the significant R2 change statistic indicating that race moderated the association between perceived everyday discrimination and log-HCC, the interaction was probed to determine the association of perceived everyday discrimination with log-HCC among White and African American participants (Figure 2). Perceived everyday discrimination was positively associated with log-HCC among African American (β = 0.28, p = 0.007) but not White (β = −0.11, p = 0.274) participants, thus supporting Hypothesis 1.
Table 3.
Standardized coefficients from regression models predicting log-hair cortisol concentration (N = 141).
| Step 1 | Step 2 | Step 3 | Step 4 | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Age | 0.21* | 0.024 | 0.17† | 0.073 | 0.18† | 0.051 | 0.15† | 0.097 |
| Sex | −0.07 | 0.448 | −0.10 | 0.256 | −0.08 | 0.360 | −0.03 | 0.781 |
| Annual Household Income | −0.15 | 0.069 | −0.10 | 0.246 | −0.09 | 0.254 | −0.09 | 0.275 |
| Physical Activity | 0.14 | 0.113 | 0.19* | 0.040 | 0.20* | 0.020 | 0.19* | 0.029 |
| WHR | 0.16† | 0.052 | 0.16† | 0.059 | 0.16† | 0.053 | 0.21** | 0.009 |
| Emotional Stability | −0.03 | 0.774 | −0.05 | 0.604 | −0.02 | 0.823 | 0.01 | 0.907 |
| Perceived Stress | 0.04 | 0.693 | 0.04 | 0.704 | −0.02 | 0.817 | −0.01 | 0.898 |
| Race | 0.19* | 0.030 | 0.17† | 0.054 | −0.29† | 0.172 | ||
| Perceived Discrimination | 0.17* | 0.045 | −0.11 | 0.283 | ||||
| Race × Discrimination | 0.59** | 0.007 | ||||||
| Model R2 | 0.115 | 0.142 | 0.163 | 0.189 | ||||
| F for R2 Change | 3.03* | 0.022 | 4.22* | 0.030 | 3.20* | 0.045 | 4.35** | 0.007 |
Note. Sex (Male = 0, Female = 1); Race (White = 0, African American = 1). WHR = waist to hip ratio.
p < 0.10;
p < 0.05;
p < 0.01;
p < .001.
Figure 2.
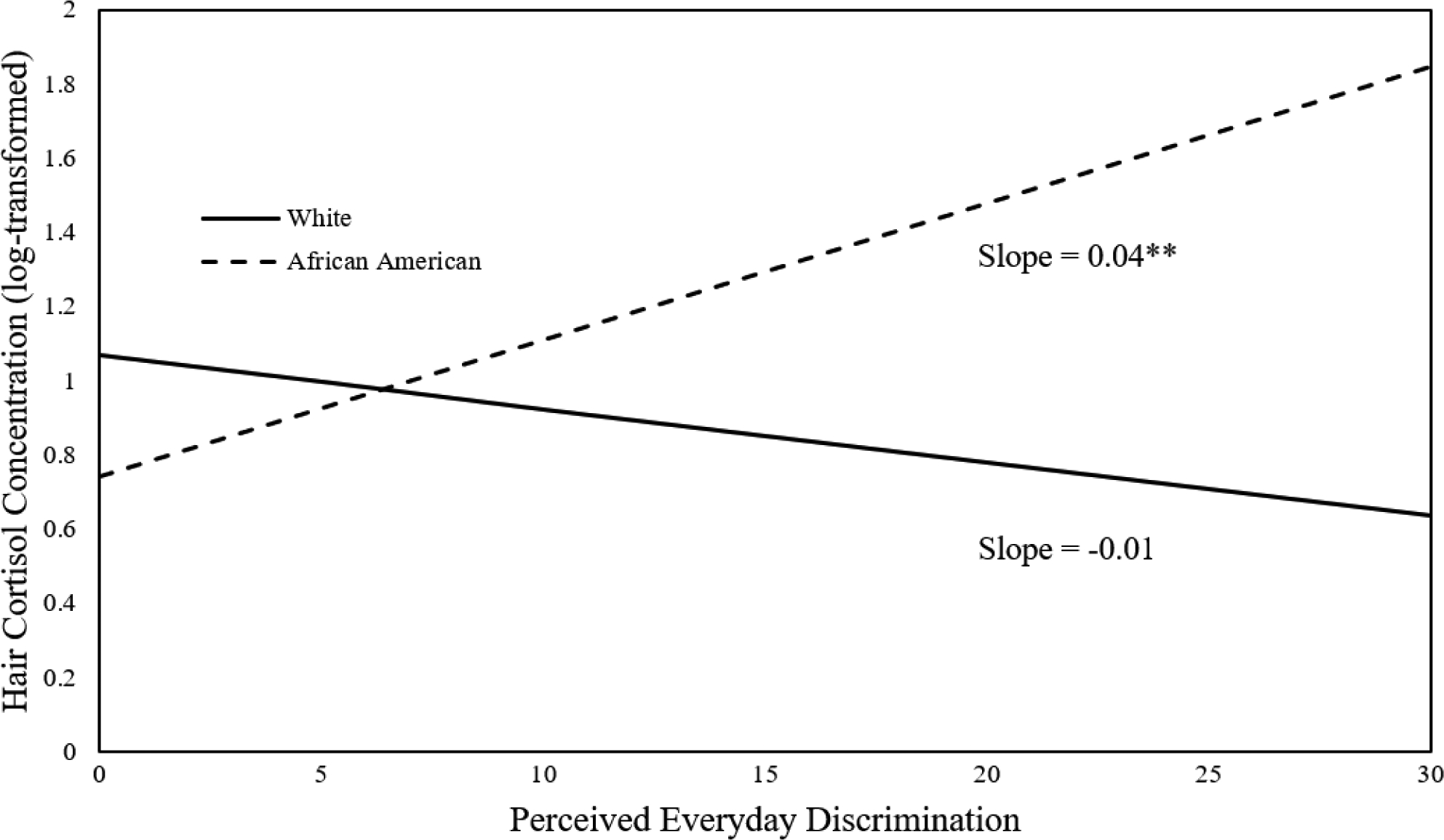
Simple slopes depicting the association between perceived everyday discrimination and hair cortisol concentration for White and African American participants. N = 141.
** p < 0.01.
Mediation analysis
When entered in a model predicting log-HCC (controlling for covariates), African American race was associated with elevated log-HCC. Upon addition to the model, perceived everyday discrimination was also positively associated with log-HCC, and the association of race with log-HCC was attenuated (Table 3, Step 3). However, African American race was not associated with perceived discrimination (β = .13, p = 0.087). The indirect effect, linking African American race to increased log-HCC via increased perceived discrimination, was not significantly different from zero (β = 0.025, 95% CI [−0.003, 0.087]), indicating that perceived discrimination did not mediate the association of African American race and log-HCC. Hypothesis 2 was thus not supported.
DISCUSSION
This study provides evidence that perceived discrimination is associated with long-term cortisol upregulation in African Americans relative to Whites. Although researchers have posited the relationship between discrimination and elevated cortisol output among groups disproportionately exposed, empirical support demonstrating an association is lacking. This study examined the association between perceived everyday discrimination and long-term retrospective cortisol levels in scalp hair among African American and White adults. Race moderated the association, such that perceived discrimination was positively associated with HCC among African American but not White adults. Additionally, perceived discrimination frequency did not mediate the association of African American race and elevated HCC. These results offer critical evidence supporting an association between perceived discrimination and elevated HCC in African Americans and suggest that some aspect of discrimination other than reported frequency of occurrence (e.g., uniqueness of African American experience with discrimination) may account for observed racial differences in HCC.
The main finding of race moderating the association between perceived discrimination and HCC supports theories suggesting that discrimination may be more detrimental to HPA axis regulation for racial minority groups compared to majority groups (Clark et al., 1999; Myers, 2009; Williams & Mohammed, 2009). This finding is in line with prior studies showing that perceived discrimination is related to indicators of HPA axis dysregulation in the form of flattened diurnal salivary cortisol slopes (Zeiders et al., 2014) and lower waking salivary cortisol levels (Adam et al., 2015) among African American compared to White adults. The current study provides evidence that perceived discrimination is disproportionately related to another indicator of altered cortisol secretion—elevated long-term cortisol levels—among African Americans. HCC has been associated with adverse health indicators in this population, including elevated hemoglobin A1C, a marker of poor glycemic control (Lehrer, Dubois, Maslowsky, Laudenslager, & Steinhardt, 2016).
Although discrimination is suggested to be stressful for all individuals according to interracial stress models (Trawalter et al. 2009), there was no association of perceived discrimination with HCC among White participants in this study. In a population study of perceived discrimination and hair cortisol among primarily White adults, reported discrimination due to age, sex, and weight were all positively associated with HCC (Jackson & Steptoe, 2018). In that study, participants were classified as yes if they regularly reported discrimination in any of the above three domains and no if they did not, allowing for comparison of high vs. low perceived discrimination. Age-, gender-, and weight-based discrimination were common forms of discrimination reported by White participants in the current study, but the sample was not sufficiently powered to examine associations of HCC with these specific types of discrimination or dichotomize participants into high vs low discrimination on specific attribution domains.
It is important to note that due to the legacy of slavery and systemic racism in the U.S., African Americans are disproportionately exposed to ongoing experiences of discrimination and unfair treatment across the life course (Reskin, 2012). Consequently, exposure to discrimination is chronic, shaping health risks across generations and starting as early as in-utero (Goosby, Cheadle, & Mitchell, 2018). For example, Kuzawa and Sweet (2009) argue that exposure of African American women to discrimination can not only shape their own HPA axis function but can also prepare their offspring in anticipation of similar discriminatory environments through epigenetic processes linked to in-utero exposure to elevated cortisol levels. Race differences in HPA function between African American and White infants can be seen as early as one year with differences partially explained by maternal exposure to discrimination (Dismukes et al., 2018). This suggests a unique social experience for African Americans that distinguishes their risk for HPA axis upregulation in a way that does not impact Whites.
The present study’s finding of racial differences characterizing the association between everyday discrimination and HCC add to the emerging discrimination and hair cortisol literature. In previous work, lifetime discrimination was positively associated with HCC in a racially diverse sample of young adults (O’Brien et al., 2017). No moderation by race was found, possibly due to the lifetime discrimination scale assessing major past discrimination events involving socioeconomic restriction (e.g., being denied a loan or college admission), while the Everyday Discrimination Scale used in the present study measures day-to-day occurrences of unfair treatment. Restricting socioeconomic advancement may be detrimental regardless of race, while perceptions of unfair treatment may be more salient to racial minority individuals. This distinction may have contributed to the positive association between everyday discrimination and HCC only for African Americans in the present study.
This study’s secondary hypothesis was that perceived discrimination would partially mediate the association of African American race and HCC, such that African American race would be positively associated with HCC via increased perceived discrimination. Although African American race and perceived discrimination were both positively associated with HCC (steps 2 and 3 of regression model in Table 3), this hypothesis was not supported, largely because African American and White participants reported similar levels of perceived everyday discrimination. It is not clear why both groups reported similar discrimination scores, given that African Americans generally report more frequent discrimination than Whites (Kessler et al., 1999; Sternthal et al., 2011). Considerably more Whites reported age- and physical appearance-based discrimination compared to African Americans, which likely contributed to the higher than expected perceived discrimination scores among Whites. Regardless of why perceived discrimination scores were similar, it remains that racial differences in HCC were not explained by differences in perceived discrimination frequency in this study.
African American participants reported considerably more race-based discrimination than did Whites, so the attribution for discrimination (i.e., race-based vs other discrimination types) may be particularly important for HPA axis function. In past research, racial discrimination was associated with greater psychological distress than non-racial discrimination among African Americans (Chae, Lincoln, & Jackson, 2011). Further, African American women attributing mistreatment to race had higher blood pressure reactivity than those attributing mistreatment to other factors (Guyll, Matthews, & Bromberger, 2001). Because most participants in the present study reporting race-based discrimination were African American, it is reasonable to assume that racial discrimination is a highly salient form of discrimination for African Americans that may account for differences in cortisol output.
African Americans display elevated HCC relative to other racial groups, which has been attributed in part to slower hair growth rate allowing greater cortisol accumulation in hair (Wosu et al., 2015). We similarly found higher HCC in African Americans than Whites, although we did not measure hair growth rate, which is a limitation of the present study. This difference in HCC did not likely affect the finding of race moderating the association between everyday discrimination and HCC, because African Americans had comparable HCC to Whites at lower discrimination levels (Figure 2).
In addition to not assessing hair growth rate, there were several study limitations. Given the cross-sectional design, temporal associations between discrimination and HCC were not determined. Because participants possibly experienced discrimination beyond what they reported, prior discrimination exposure could influence HCC. Prospective studies could investigate how chronic discrimination longitudinally influences HCC. In addition, our study did not account for anticipatory stress and vigilance which are possible pathways through which discrimination impacts HPA axis function. To maximize efficiency, the HPA axis adjusts its activity based on immediate, continuing, and predicted forthcoming adversity. In the presence of a chronic stressor, the HPA axis upregulates in response (Sterling, 2004). For African Americans, discrimination experiences are chronic stressors experienced and anticipated both emotionally and physiologically (Goosby, Straley, & Cheadle, 2017). Anticipation of racial discrimination is associated with metabolic and cardiovascular risk factors (Clark, Benkert, & Flack, 2006; Hicken, Lee, Ailshire, Burgard, & Williams, 2013; Powell, Jesdale, & Lemon, 2016). Thus, future research should assess anticipatory stress and vigilance related to discrimination in addition to discrimination experiences. It should also be noted that HCC does not capture HPA axis dynamics (e.g., amplitude/duration of cortisol response to stress, diurnal cortisol patterns). Thus, it is unknown whether elevated HCC for African Americans reporting high discrimination reflects increased responsiveness to daily stressors (e.g., discrimination), higher baseline cortisol, or both. Further research should characterize this upregulation.
The study sample was primarily low-socioeconomic status (SES), which may limit generalizability to higher-SES individuals. A growing literature suggests that at higher SES, African Americans still have poorer health profiles relative to Whites. This difference is partially explained by higher rates of interpersonal discrimination (Colen, Ramey, Cooksey, & Williams, 2018; Howard & Sparks, 2015) for which higher SES African Americans report more of relative to their lower SES counterparts (NPR et al., 2017). Research assessing cortisol output demonstrates that SES can moderate the relationship between discrimination and cortisol output (Fuller-Rowell et al., 2012) and minority individuals reporting low and high SES had higher HCC than those reporting moderate SES (O’Brien, Tronick, & Moore, 2013). However, this evidence is limited, and more diverse and larger study samples are required to more clearly assess these relationships. Despite these limitations, this study suggests that perceived discrimination is associated with elevated retrospective cortisol secretion among African American but not White adults.
ACKNOWLEDGEMENTS
The authors thank the members of the data collection team, including Dina Carter, RN, Didra Risher, Wonil Park, MS, and Ken Ripperger-Suhler, PhD, as well as expert processing of the hair samples by Danielle Glenn and Robert Shelton in the Behavioral Immunology and Endocrinology Laboratory. This study was funded by a grant from St. David’s Center for Health Promotion and Disease Prevention in Underserved Populations, The University of Texas at Austin School of Nursing, Austin, TX. H. Matthew Lehrer received partial salary support during the analysis and interpretation of data and manuscript preparation by a University Continuing Fellowship and by NIH grant T32HL082610. Bridget J. Goosby received partial salary support during the preparation of this manuscript from Grant P2CHD042849 awarded to the Population Research Center at the University of Texas at Austin by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Mark L. Laudenslager received partial salary support during the preparation of this manuscript from the American Heart Association (15SFDRN24180024).
Footnotes
DECLARATION OF INTEREST
The authors declare no competing interests.
REFERENCES
- Adam EK, Heissel JA, Zeiders KH, Richeson JA, Ross EC, Ehrlich KB, … Eccles JS (2015). Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: A 20-year prospective study. Psychoneuroendocrinology, 62, 279–291. 10.1016/j.psyneuen.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, & Sarnyai Z (2015). “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. Stress, 18(1), 1–10. 10.3109/10253890.2014.989204 [DOI] [PubMed] [Google Scholar]
- Busse D, Yim IS, Campos B, & Marshburn CK (2017). Discrimination and the HPA axis: current evidence and future directions. Journal of Behavioral Medicine, 40(4), 539–552. 10.1007/s10865-017-9830-6 [DOI] [PubMed] [Google Scholar]
- Chae DH, Lincoln KD, & Jackson JS (2011). Discrimination, attribution, and racial group identification: Implications for psychological distress among Black Americans in the National Survey of American Life (2001–2003). American Journal of Orthopsychiatry, 81(4), 498–506. 10.1111/j.1939-0025.2011.01122.x [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, & Williams DR (1999). Racism as a stressor for African Americans. American Psychologist, 54(10), 805–816. [DOI] [PubMed] [Google Scholar]
- Clark R, Benkert RA, & Flack JM (2006). Large arterial elasticity varies as a function of gender and racism-related vigilance in Black youth. Journal of Adolescent Health, 39(4), 562–569. 10.1016/j.jadohealth.2006.02.012 [DOI] [PubMed] [Google Scholar]
- Cohen S, & Williamson G (1988). Perceived stress in a probability sample of the United States In Spacapan S & Oskamp S (Eds.), The Claremont Symposium on Applied Social Psychology. The social psychology of health. (Vol. 13, pp. 31–67). Thousand Oaks, CA, US: Sage Publications, Inc. [Google Scholar]
- Colen CG, Ramey DM, Cooksey EC, & Williams DR (2018). Racial disparities in health among nonpoor African Americans and Hispanics: The role of acute and chronic discrimination. Social Science & Medicine, 199, 167–180. 10.1016/j.socscimed.2017.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dismukes A, Shirtcliff E, Jones CW, Zeanah C, Theall K, & Drury S (2018). The development of the cortisol response to dyadic stressors in Black and White infants. Development and Psychopathology, 30(5), 1995–2008. 10.1017/S0954579418001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Doan SN, & Eccles JS (2012). Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology, 37(1), 107–118. 10.1016/j.psyneuen.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, & Vogt A (2012). Outliers: An evaluation of methodologies. Joint Statistical Meetings. Presented at the American Statistical Association, San Diego, CA. [Google Scholar]
- Goosby BJ, Cheadle JE, & Mitchell C (2018). Stress-related biosocial mechanisms of discrimination and African American health inequities. Annual Review of Sociology, 44(1), 319–340. 10.1146/annurev-soc-060116-053403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Straley E, & Cheadle JE (2017). Discrimination, sleep, and stress reactivity: pathways to African American-White cardiometabolic risk inequities. Population Research and Policy Review, 36(5), 699–716. 10.1007/s11113-017-9439-z [DOI] [Google Scholar]
- Gosling SD, Rentfrow PJ, & Swann WB (2003). A very brief measure of the Big-Five personality domains. Journal of Research in Personality, 37(6), 504–528. 10.1016/S0092-6566(03)00046-1 [DOI] [Google Scholar]
- Guyll M, Matthews KA, & Bromberger JT (2001). Discrimination and unfair treatment: Relationship to cardiovascular reactivity among African American and European American women. Health Psychology, 20(5), 315–325. 10.1037//0278-6133.20.5.315 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: The Guilford Press. [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, & Hagemann D (2007). Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology, 32(1), 80–86. 10.1016/j.psyneuen.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Hicken MT, Lee H, Ailshire J, Burgard SA, & Williams DR (2013). “Every shut eye, ain’t sleep”: The role of racism-related vigilance in racial/ethnic disparities in sleep difficulty. Race and Social Problems, 5(2), 100–112. 10.1007/s12552-013-9095-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MC, Karban LV, Benitez P, Goodteacher A, & Laudenslager ML (2014). Chemical processing and shampooing impact cortisol measured in human hair. Clinical & Investigative Medicine, 37(4), E252, E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JT, & Sparks PJ (2015). The role of education in explaining racial/ethnic allostatic load differentials in the United States. Biodemography and Social Biology, 61(1), 18–39. 10.1080/19485565.2014.937000 [DOI] [PubMed] [Google Scholar]
- Jackson SE, & Steptoe A (2018). Obesity, perceived weight discrimination, and hair cortisol: a population-based study. Psychoneuroendocrinology, 98, 67–73. 10.1016/j.psyneuen.2018.08.018 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, & Williams DR (1999). The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. Journal of Health and Social Behavior, 40(3), 208–230. 10.2307/2676349 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Steyer R, Eid M, Patalla U, Schwenkmezger P, & Hellhammer DH (1990). Cortisol and behavior: 2. Application of a latent state-trait model to salivary cortisol. Psychoneuroendocrinology, 15(4), 297–307. 10.1016/0306-4530(90)90080-S [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, & Sweet E (2009). Epigenetics and the embodiment of race: Developmental origins of US racial disparities in cardiovascular health. American Journal of Human Biology, 21(1), 2–15. 10.1002/ajhb.20822 [DOI] [PubMed] [Google Scholar]
- Lee DB, Peckins MK, Heinze JE, Miller AL, Assari S, & Zimmerman MA (2018). Psychological pathways from racial discrimination to cortisol in African American males and females. Journal of Behavioral Medicine, 41(2), 208–220. 10.1007/s10865-017-9887-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer HM, Dubois SK, Maslowsky J, Laudenslager ML, & Steinhardt MA (2016). Hair cortisol concentration and glycated hemoglobin in African American adults. Psychoneuroendocrinology, 72, 212–218. 10.1016/j.psyneuen.2016.06.018 [DOI] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, & Williams DR (2015). Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annual Review of Clinical Psychology, 11, 407–440. 10.1146/annurev-clinpsy-032814-112728, 10.1146/annurev-clinpsy-032814-112728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133(1), 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Myers HF (2009). Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. Journal of Behavioral Medicine, 32(1), 9–19. 10.1007/s10865-008-9181-4 [DOI] [PubMed] [Google Scholar]
- NPR, Robert Wood Johnson Foundation, & Harvard School of Public Health. (2017). Discrimination in America: experiences and views of African Americans. Retrieved from https://www.npr.org/assets/img/2017/10/23/discriminationpoll-african-americans.pdf
- O’Brien KM, Meyer J, Tronick E, & Moore CL (2017). Hair cortisol and lifetime discrimination: Moderation by subjective social status. Health Psychology Open, 4(1), 2055102917695176 10.1177/2055102917695176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM, Tronick EZ, & Moore CL (2013). Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress and Health, 29(4), 337–344. 10.1002/smi.2475 [DOI] [PubMed] [Google Scholar]
- Powell LR, Jesdale WM, & Lemon SC (2016). On edge: the impact of race-related vigilance on obesity status in African-Americans: Obesity and race-related vigilance. Obesity Science & Practice, 2(2), 136–143. 10.1002/osp4.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reskin B (2012). The race discrimination system. Annual Review of Sociology, 38(1), 17–35. 10.1146/annurev-soc-071811-145508 [DOI] [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, & Catalano RF (2011). Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology, 23(4), 1167–1186. 10.1017/S095457941100054X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, & Kirschbaum C (2012). Analysis of cortisol in hair – State of the art and future directions. Brain, Behavior, and Immunity, 26(7), 1019–1029. 10.1016/j.bbi.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Sterling P (2004). Principles of allostasis: Optimal design, predictive regulation, pathophysiology, and rational therapeutics In Schulkin J (Ed.), Allostasis, Homeostasis, and the Costs of Physiological Adaptation (pp. 17–64). 10.1017/CBO9781316257081.004 [DOI] [Google Scholar]
- Sternthal MJ, Slopen N, & Williams DR (2011). Racial disparities in health. Du Bois Review: Social Science Research on Race, 8(01), 95–113. 10.1017/S1742058X11000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawalter S, Richeson JA, & Shelton JN (2009). Predicting behavior during interracial interactions: A stress and coping approach. Personality and Social Psychology Review, 13(4), 243–268. 10.1177/1088868309345850 [DOI] [PubMed] [Google Scholar]
- Tsigos C, & Chrousos GP (2002). Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865–871. 10.1016/S0022-3999(02)00429-4 [DOI] [PubMed] [Google Scholar]
- Williams DR (2012). Miles to go before we sleep: Racial inequities in health. Journal of Health and Social Behavior, 53(3), 279–295. 10.1177/0022146512455804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, & Mohammed SA (2009). Discrimination and racial disparities in health: evidence and needed research. Journal of Behavioral Medicine, 32(1), 20–47. 10.1007/s10865-008-9185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Yan Yu, Jackson JS, & Anderson NB (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology, 2(3), 335–351. 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- Wright KD, Ford JL, Perazzo J, Jones LM, Mahari S, Sullenbarger BA, & Laudenslager ML (2018). Collecting hair samples for hair cortisol analysis in African Americans. Journal of Visualized Experiments, 136(06), e57288 10.3791/57288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosu AC, Gelaye B, Valdimarsdóttir U, Kirschbaum C, Stalder T, Shields AE, & Williams MA (2015). Hair cortisol in relation to socio-demographic and lifestyle characteristics in a multi-ethnic US sample. Annals of Epidemiology, 25(2), 90–95.e2. 10.1016/j.annepidem.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiders KH, Hoyt LT, & Adam EK (2014). Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: The moderating role of racial–ethnic minority status. Psychoneuroendocrinology, 50, 280–288. 10.1016/j.psyneuen.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]


