Abstract
Purpose of review:
Direct oral anticoagulants (DOACs) are variably eliminated by the kidneys rendering their use potentially problematic in patients with chronic kidney disease (CKD) or necessitating appropriate dose adjustment.
Recent findings:
Both observational and limited randomized trial data for DOACs compared with no treatment or with warfarin for patients with atrial fibrillation on maintenance dialysis were recently published. In a randomized trial in patients on hemodialysis, there was no significant difference in vascular calcification between patients who received rivaroxaban with or without vitamin K2 or vitamin K antagonists. A randomized trial of apixaban versus warfarin was terminated owing to poor enrollment and preliminary results identified no difference in clinical outcomes between groups. However, valuable pharmacodynamic data will be forthcoming from that trial. In observational research, among patients newly diagnosed with atrial fibrillation there were opposing trends in the associations of apixaban initiation versus no oral anticoagulation with ischemic versus hemorrhagic stroke and no association was present with the overall risk of stroke or embolism. In another study comparing apixaban with warfarin initiation, apixaban was associated with less bleeding. Regular dose apixaban (5 mg twice daily) associated with reduced rates of ischemic stroke or systemic embolism, whereas no such association was present for those prescribed the reduced dose (2.5 mg twice daily).
Summary:
DOACs may be used after appropriate dose adjustment for an established clinical indication in patients with advanced CKD. Quality evidence for oral anticoagulation, with any specific agent or dose, for stroke prevention in hemodialysis continues to be lacking.
Keywords: rivaroxaban, apixaban, warfarin, chronic kidney disease, hemodialysis
1. Introduction
Direct oral anticoagulants (DOACs) were developed to simplify management of patients with venous thromboembolic disease (VTE) or atrial fibrillation (AF). These agents are particularly attractive because they offer predictable anticoagulant activity at fixed doses with less frequent drug-drug or food-drug interactions compared with the traditionally used vitamin K antagonists (VKAs), and no need for therapeutic drug monitoring. Several well-conducted randomized controlled trials (RCTs) have established their superiority or non-inferiority compared with VKAs for the prevention of thromboembolic events as well as their safety profile (major bleeding events) in the general population. However, all DOACs are eliminated by the kidneys at various degrees rendering their use potentially problematic in patients with chronic kidney disease (CKD) or necessitating appropriate dose adjustment. In this review article, we focus on the most recent data on DOACs in CKD and discuss their indications and dosing.
2. Estimation of kidney function and CKD definition
The Cockroft-Gault equation for creatinine clearance (CrCl) has been used to estimate kidney function in pharmacokinetic studies. This equation does not correct for body surface area and tends to underestimate kidney function in older individuals with low body weight. It remains unclear whether use of more precise equations for the estimated glomerular filtration rate (eGFR) would provide more accurate pharmacokinetic estimations.
While the gold standard to define kidney function is measurement of glomerular filtration rate using a variety time-dilution techniques, estimation of kidney function using a variety of equations based on standardized serum creatinine and demographic characteristics is more commonly used. While eGFR has been recommended and used to define the ‘G’-domain among stages of CKD [1], much of the pharmacologic literature uses CrCl owing to regulatory guidance. Clinically meaningful changes in drug levels may occur at a CrCl <50 ml/min for most agents. For this review, we will define CKD as a CrCl < 60 ml/min and classify it as moderate if the CrCl is between 30 and 59 ml/min and advanced if the CrCl is < 30 ml/min. Patients on maintenance dialysis will be examined separately. It is important to note, however, that CrCl does not equal eGFR and that findings using one cannot be automatically transferred to a setting where the other estimation approach is being used.
3. Pharmacokinetic data
All DOACs are eliminated by the kidneys in various degrees. For dabigatran, a direct thrombin inhibitor, kidney clearance represents 80% of the total elimination. The three direct factor Xa inhibitors have lower excretion by the kidneys: edoxaban at 50% of the absorbed dose, rivaroxaban at 35%, and apixaban at 27% [2].
Based on pharmacokinetic data, dose adjustment has been proposed for all four agents in patients with various degrees of kidney function impairment. For apixaban, age ≥80 years and body weight ≤60 kg are also to be considered for dose adjustment per package insert. However, the guidelines or statements from different societies have yielded conflicting recommendations and are presented in Table 1 [3–7].
Table 1.
Dosing recommendations for direct oral anticoagulants in chronic kidney disease (stage 3 and 4)
| CKD stage | Guideline-statement | Year- type | Apixaban | Rivaroxaban | Edoxaban | Dabigatran |
|---|---|---|---|---|---|---|
| Stage 3 | AHA/ACC/HRS3 | 2019- guideline | 5 bid* | 15 qd | 30 qd | 150 bid |
| ACCP4 | 2018- guideline | 5 bid* | 15 qd | 30 qd | 150 or 110 bid | |
| EHRA5 | 2018- statement | 5 bid* | 15 qd¶ | 30 qd | 150 or 110 bid | |
| KDIGO6 | 2018- statement | 5 bid* | 15 qd | 30 qd | 150 or 110 bid | |
| ESC7 | 2016- guideline | 5 bid* | 15 qd | 30 (or 15 mg) qd | 150 or 110 bid | |
| Stage 4 | AHA/ACC/HRS3 | 2019- guideline | 5 bid* | 15 qd | Not recommended | 75 bid |
| ACCP4 | 2018- guideline | 2.5 bid | 15 qd | 30 qd | 75 bid (US only) | |
| EHRA5 | 2018- statement | 2.5 bid | 15 qd | 30 qd | Not recommended | |
| KDIGO6 | 2018- statement | Consider 2.5 bid | Consider 15 qd | Consider 30 qd | Consider 75 bid | |
| ESC7 | 2016- guideline | Not recommended | Not recommended | Not recommended | Not recommended |
, 2.5 mg bid if two of the three criteria are met: creatinine ≥1.5 mg/dl, body weight ≤60 kg, age ≥80
, 20 mg qd if creatinine clearance >50 ml/min
CKD, chronic kidney disease; stage 3 defined as CrCl of 30–50 ml/min (AHA/ACC/HRS, EHRA), of 30–59 ml/min (ACCP), of 31–50 ml/min (KDIGO), of 25–50 ml/min (KDIGO for apixaban), of 30–49 ml/min (ESC); stage 4 defined as a CrCl of 15–30 ml/min (AHA/ACC/HRS, ACCP, EHRA, KDIGO), of <30 ml/min (ESC) or of <25 ml/min (ESC for apixaban). AHA, American Heart Association; ACC, American College of Cardiology; HRS, Heart Rhythm Society; ACCP, American College of Chest Physicians; CrCl, creatinine clearance; EHRA, European Heart Rhythm Association; KDIGO, Kidney Disease – Improving Global Outcomes; ESC, European Society of Cardiology; qd, once daily; bid, twice daily
Adapted from Kumar et al. J Am Coll Cardiol 2019; 74:2210.2
Stanifer et al. recently published pharmacokinetic data from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial in patients with a CrCl of 25–30 ml/min [8]. There were 12 patients with advanced CKD randomized to apixaban 5 mg twice daily and 19 patients randomized to apixaban 2.5 mg twice daily who had pharmacokinetic data assessed. The median area under the curve at steady state was only moderately higher in patients with advanced CKD who received the 5 mg dose compared with patients with moderate CKD. Apixaban exposure was nearly identical for all patients who received the 2.5 mg dose across a CrCl range of 25 to >60 ml/min [8].
Specific pharmacokinetic studies have been conducted in patients on maintenance hemodialysis. De Vries et al. administered 10 mg of rivaroxaban once daily for seven days on 18 patients on hemodialysis without residual kidney function [9]. They demonstrated that rivaroxaban at this dose resulted in a similar area under the curve compared with the standard 20 mg dose in healthy volunteers. Mavrakanas et al. administered apixaban in seven patients on hemodialysis for a total of eight days [10]. They showed that apixaban at the reduced dose of 2.5 mg twice daily resulted in drug exposure near the lower end of the range achieved with the standard those of 5 mg twice daily in patients without kidney function impairment, whereas 5 mg twice daily resulted in doses within the range in 3 individuals. However, as 2 patients were well above the range seen in persons with normal kidney function, the authors suggested that the standard apixaban dose may lead to supratherapeutic levels in patients on maintenance dialysis and should be avoided. Hemodialysis per se did not affect rivaroxaban or apixaban levels [9–10].
The RENal hemodialysis patients ALlocated apixaban versus warfarin in Atrial Fibrillation trial (RENAL-AF) randomized patients on maintenance dialysis with AF to receive warfarin or apixaban 5 mg twice daily, unless a dose reduction to 2.5 mg twice daily was indicated per drug label. Detailed pharmacokinetic data were collected for ~50 patients on apixaban and detailed findins are expected to be published later in 2020. This will be the largest pool of patients with end-stage kidney disease (ESKD) on apixaban ever reported and will provide important information from detailed samples collected over a longer period of time. At least one other trial of similar design is currently underway (Compare Apixaban and Vitamin K Antagonists in Patients with Atrial Fibrillation and End-stage Kidney Disease, AXADIA, NCT09233697).
4. DOACs versus VKAs in patients with CKD
4.1. Patients with moderate CKD (CrCl 30–59 ml/min)
The large RCTs in VTE or AF enrolled patients with a CrCl as low as 30 ml/min (and a small number of patients with a CrCl of 25–30 ml/min for apixaban) [8,11–18]. These trials were summarized in two recent meta-analyses [19–20]. Since these agents have different pharmacokinetic profiles, degree of elimination by the kidneys, and pharmacodynamic properties, results are presented individually for each agent rather than lumping them together as a single DOAC group. All agents were of non-inferior or superior efficacy to VKAs with respect to an outcome of stroke or embolism. A non-inferior or lower incidence of hemorrhagic stroke was observed with DOACs compared with VKAs. Based on these data, all four DOACs can be used in moderate CKD at their respective recommended dose (Table 2).
Table 2.
Outcomes with direct oral anticoagulants compared with vitamin K antagonists in patients with creatinine clearance between 30 and 50 ml/min and atrial fibrillation or with direct oral anticoagulants compared with low-molecular weight heparin/ vitamin K antagonists in patients with creatinine clearance between 30 and 50 ml/min and venous thromboembolism.
| Indication | Trial | DOAC – dose | N | Outcome | HR (95% CI) |
|---|---|---|---|---|---|
| Atrial fibrillation | ARISTOTLE10 | Apixaban 5 bid* | 3017 | Stroke/embolism | 0.79 (0.55–1.14) |
| Major bleeding | 0.50 (0.38–0.66) | ||||
| ENGAGE TIMI 4811 | Edoxaban 30 qd | 2740 | Stroke/embolism | 0.87 (0.65–1.18) | |
| Major bleeding | 0.76 (0.58–0.98) | ||||
| ROCKET AF12 | Rivaroxaban 15 qd | 2950 | Stroke/embolism | 0.84 (0.57–1.23) | |
| Major bleeding | 0.98 (0.73–1.30) | ||||
| RE-LY13 | Dabigatran 110 bid | 3374 | Stroke/embolism | 0.85 (0.59–1.24) | |
| Major bleeding | 0.99 (0.77–1.28) | ||||
| Dabigatran 150 bid | Stroke/embolism | 0.56 (0.37–0.85) | |||
| Major bleeding | 1.01 (0.79–1.30) | ||||
| Venous thromboembolism | AMPLIFY14 | Apixaban 10 bid × 7 days, then 5 bid | 338 | Recurrent VTE or VTE-related death | 0.93 (0.34–2.61)¶ |
| Major bleeding | 0.52 (0.18–1.51)¶ | ||||
| HOKUSAI-VTE15 | Edoxaban 30 qd | 363 | Recurrent VTE | 0.49 (0.19–1.27)¶ | |
| Major bleeding | 0.66 (0.37–1.18)¶ | ||||
| EINSTEIN16 | Rivaroxaban 15 bid × 3 weeks, then 20 qd | 636 | Recurrent VTE | 1.05 (0.44–2.47) | |
| Major bleeding | 0.23 (0.06–0.81) | ||||
| RE-COVER17 | Dabigatran 150 bid | 237 | Recurrent VTE | - | |
| Major bleeding | 6.71 (3.34–13.48) |
, 2.5 mg bid if two of the three criteria are met: creatinine ≥1.5 mg/dl, body weight ≤60 kg, age ≥80
, relative risk reported
N, total number of patients with chronic kidney disease; DOAC, direct oral anticoagulant; VKAs, vitamin K antagonists; bid, twice daily; HR, hazard ratio; CI, confidence interval; VTE, venous thromboembolism
4.2. Patients with advanced CKD (CrCl <30 ml/min)
Randomized data have been reported for stroke or systemic embolism in patients with AF who had advanced CKD [8]. A lower incidence of major bleeding and a similar incidence of stroke or systemic embolism was observed in patients who received apixaban compared with patients treated with warfarin. Similarly, several observational cohort studies examined the efficacy and safety of anticoagulation in CKD but they either included a very small number of patients on DOACs or very few patients had advanced CKD and the results were not separately reported for this subgroup [21–24]. We present in this section the cohort studies that have reported outcomes with DOACs in advanced CKD.
The first of these studies enrolled patients >65 years old from England with newly diagnosed AF and had an eGFR<50 ml/min/1.73m2 comparing 2424 patients on anticoagulants (641 on DOACs) with 2424 propensity score-matched patients not receiving any anticoagulation [25]. In this cohort, approximately 16% had advanced CKD and anticoagulation was associated with higher incidence of ischemic stroke and of gastrointestinal or cerebral hemorrhage but a paradoxically lower incidence of all-cause mortality, compared with no anticoagulation, that could be due to selection bias.
Coleman et al. used the United States MarketScan database to compare 1896 rivaroxaban users with 4848 warfarin users with new or prevalent AF and stage 4 or 5 CKD (including patients on renal replacement therapy) using inverse probability treatment weighting [26]. Approximately 40% of rivaroxaban users received a renally-adapted dose of <20 mg per day. There was no difference in stroke or systemic embolism between the two groups, but rivaroxaban was associated with lower incidence of bleeding-related hospitalizations, compared with warfarin. A study of similar design was conducted by Weir et al. [27]. They used the Optum Health medical record to compare 781 rivaroxaban-treated patients with 1536 propensity score-matched warfarin-treated patients with AF and stage 4 or 5 CKD. There was no significant difference in the incidence of ischemic stroke or systemic embolism (hazard ratio [HR] 0.93, 95% confidence interval [CI] 0.46–1.90) or in major bleeding (HR 0.91, 95% CI 0.65–1.28) between the two groups.
Two additional cohort studies included patients who received anticoagulation for various indications. A sizeable proportion of patients had moderate or advanced CKD. Ashley et al. used the Ontario Provincial Database to compare 27,552 new DOAC users with matched new warfarin users [28]. There was a lower incidence in cardiovascular events or mortality and in bleeding requiring hospitalization with DOACs compared with VKAs. The magnitude of the association of DOACs with cardiovascular events or mortality was greatest at an eGFR<30 ml/min/1.73m2. Edgett et al. used the OptumLabs Data Warehouse in the United States to compare 4,587 DOAC or warfarin users with stage 5 CKD or ESRD [29]. DOAC use was associated with a significantly lower incidence of ischemic stroke compared with warfarin.
Yao et al. used an administrative database to examine the effectiveness and safety of three DOACs, rivaroxaban, apixaban, and dabigatran, compared with warfarin for stroke prevention in patients with AF across an eGFR range of 15 to ≥90 ml/min/1.73m2 [30]. All three DOACs were of at least similar effectiveness and safety compared with warfarin. No significant interaction was detected between treatment group and CKD stage for any outcome. In the subgroup of patients with advanced CKD, the incidence of stroke or major bleeding was not different between rivaroxaban or apixaban-treated patients, compared with patients who received warfarin [30].
From the available observational studies, the following conclusions can be drawn: i) there are no high-quality data for DOACs in patients with advanced CKD; ii) no major safety signal has been detected with DOACs compared with warfarin; iii) appropriate dose reduction is not universally applied in patients with advanced CKD; iv) data on whether DOACs (or warfarin) reduce the incidence of stroke compared with no treatment in patients with advanced CKD is inconclusive.
4.3. Risk of acute kidney injury or progression of CKD with DOACs versus VKAs
Cases of unexplained acute kidney injury (AKI) with macroscopic hematuria in patients on warfarin with supratherapeutic international normalized ratio levels led to the description of warfarin-related nephropathy. Biopsy findings of these cases identified the red cell casts in numerous renal tubules but unremarkable glomeruli [31]. It was hypothesized that supratherapeutic anticoagulation was unmasking an underlying glomerular disorder or was leading to hematuria and tubular damage in patients with substantially reduced number of nephrons rendering them vulnerable to hemorrhage. The term evolved into anticoagulant-related nephropathy (ARN) to include cases of AKI in patients on DOACs. Prognosis of ARN is not uniform with some of the patients developing CKD of variable severity after one or multiple ARN episodes.
Five retrospective cohort studies tried to estimate incidence of AKI and/or CKD progression in patients with or without underlying CKD who were receiving DOACs or warfarin for AF [32–36]. Rivaroxaban or dabigatran, but not apixaban, were superior to warfarin for both clinical outcomes in the studied populations. It has been hypothesized that warfarin use is more frequently associated with time periods of supratherapeutic anticoagulation, essential to induce ARN, compared with the DOACs that have a more predictable pharmacodynamic profile. Similarly, progression to CKD may be due to multiple unresolving or partially resolving ARN episodes in the same patient that more commonly occur with warfarin than DOACs. If these hypotheses are correct, failure of apixaban to show better renal outcomes than warfarin may be caused by supratherapeutic anticoagulation with this agent due to lack of dose adjustment: indeed, according to current dosing recommendations, apixaban is prescribed at the standard dose regardless of kidney function unless the patient also has a low body weight (<60 kg) or is older than 80 years. This is not the case of rivaroxaban or edoxaban: for these two agents, dose reduction is recommended when the CrCl drops below 50 ml/min.
In summary, the magnitude of protection seen in the available analyses was small and additional prospective data, in particular from randomized trials, are needed before any recommendations for the selection of DOACs versus VKAs for the point of protective benefit for kidney function can be made.
5. VKAs and DOACs in patients with ESKD
Although VTE is more common in patients with ESRD than the general population, there are no data with VKAs or DOACs for this indication [37]. Therefore, for patients on maintenance dialysis, we will focus on available data for stroke prevention in AF [38].
5.1. VKAs or DOACs versus no anticoagulation in ESKD
5.1.1. Warfarin versus no anticoagulation
Anticoagulation in AF is a preventive strategy against stroke or embolism in patients who are considered to be at high risk for this complication. Benefit from anticoagulation compared with antiplatelet therapy or no treatment has been established in the general population. Multiple retrospective analyses and systematic reviews of those analyses have analyzed outcomes of warfarin compared to no anticoagulation among ESKD with AF. These studies were recently reviewed [38–39]. There was no significant benefit from VKAs versus no anticoagulation with respect to ischemic stroke. Moreover, a significantly higher incidence of major bleeding was seen with VKAs compared with no treatment. Although these studies have engendered concerns about whether there is a net benefit to anticoagulation for AF in the setting of ESKD, inconsistency in study design and quality preclude making firm conclusions. A randomized trial is underway and will compare the traditionally used VKAs with no anticoagulation in ESKD (NCT02886962).
5.1.2. DOACs versus no anticoagulation
Almost no studies have been conducted examining the question of DOAC effectiveness compared to a strategy not using oral anticoagulation. While a single observational study of apixaban has recently been published, there are no studies comparing rivaroxaban, dabigatran, or edoxaban to no anticoagulation in ESKD patients.
In a cohort study of patients with newly diagnosed AF and ESKD in the USRDS [40], Mavrakanas et al. compared 521 patients initiating apixaban with 1561 propensity-matched patients who did not initiate anticoagulation. Apixaban was not associated with the incidence of any stroke (ischemic or hemorrhagic) in this population compared with no treatment, although precision of the estimated association was low. Similar to findings with oral anticoagulation using VKA, apixaban was associated with higher incidence of fatal or intracranial bleeding compared with no anticoagulation [40].
5.2. VKAs versus DOACs in ESKD
5.2.1. Rivaroxaban and dabigatran
The first study to assess the safety of rivaroxaban and dabigatran in patients on hemodialysis was a cohort study of 281 dabigatran-treated and 244 rivaroxaban-treated patients who were compared with 8064 patients started on warfarin. Both rivaroxaban and dabigatran were independently associated with significantly higher risks of hospitalization and death from bleeding compared with warfarin [41]. Both doses of dabigatran and the standard rivaroxaban dose were associated with more bleeding events compared with warfarin. On the contrary, similar rates of bleeding events were observed with the reduced rivaroxaban dose (15 mg daily) or with warfarin. Furthermore, none of the patients in this cohort received the reduced dose of 10 mg of rivaroxaban that has been proposed for patients on maintenance hemodialysis [9]. Therefore, the increased incidence of bleeding with DOACs in this cohort could possibly be due to supratherapeutic anticoagulation in the absence of appropriate dose adjustment or a warfarin comparison group that spent most of their time in the subtherapeutic range [42].
The Valkyrie study was the first RCT using rivaroxaban 10 mg once daily with or without menaquinone-7, a form of vitamin K2, against VKAs in 132 patients on chronic hemodialysis with AF [43]. The trial was designed to examine the effect of vitamin K deficiency on vascular calcification as assessed by calcium scores on heart computed tomography and pulse wave velocity. There was no significant difference in calcification at 18 months between the three groups. Although the study was not powered for clinical outcomes, the total number of life-threatening or major bleeding events was lower with rivaroxaban compared with VKAs. The authors suggested that rivaroxaban at 10 mg daily might be safely used in patients on maintenance hemodialysis. They also hypothesized that vascular calcifications may have already progressed beyond a point of no return in this prevalent dialysis cohort with most patients already on VKAs upon inclusion. In this case, use of DOACs at earlier stages of CKD instead of warfarin could potentially have a beneficial effect on vascular calcification. This hypothesis, that has never been studied, merits further investigation.
Miao et al. used the MarketScan database to retrospectively compare 787 rivaroxaban with 1836 apixaban users with AF and ESRD, using inverse probability weighting to adjust for differences at baseline [44]. Only 29% of patients received a reduced DOAC dose. There was no significant difference between the two studied agents for all clinical outcomes (stroke or embolism, ischemic stroke, or major bleeding).
5.2.2. Apixaban versus warfarin
Apixaban, the DOAC least excreted by the kidneys, became increasingly popular in the United States since 2014 and has been prescribed at either the full or the reduced dose. Siontis et al. used the United States Renal Data System (USRDS) to compare effectiveness and safety of apixaban to warfarin in this population [45]. There was no difference in the incidence of ischemic stroke or embolism between the two groups. However, when examining this association by dosing group (5 mg vs. 2.5 mg) the standard dose was associated with significantly lower rates of stroke compared with warfarin, whereas the reduced dose was not significantly different. In addition, apixaban was superior to warfarin with respect to major bleeding regardless of dose.
The RENAL-AF trial randomized patients on maintenance dialysis with AF to receive warfarin or standard dose apixaban (5 mg twice daily), unless a criterion for dose reduction was present, and was designed as a non-inferiority safety trial for clinically significant bleeding [46]. The study was initially planned to recruit 760 patients but had to terminate enrollment at 154 patients due to slower than anticipated enrollment. A total of 71% of patients received the standard apixaban dose. In an exploratory analysis, there were no significant differences in the incidences of stroke or major bleeding between the apixaban and warfarin groups.
6. Conclusions
Based on the data discussed above, the following suggestions could be made. All four DOACs can be used in moderate CKD at the recommended dose. DOACs may be used after appropriate dose adjustment for an established clinical indication in patients with advanced CKD. Suggested doses based on current evidence are proposed in Figure 1. Prescription of a DOAC (or VKA) for stroke prevention in ESKD is currently not an evidence based practice and hampered by near absence of quality data regarding the net harms and benefits. If oral anticoagulation is being considered, choice and dosing should be individualized taking into account the patient’s risk profile and/or preferences. While rivaroxaban at 10 mg daily and apixaban at 2.5 mg twice daily seem to present acceptable safety profiles compared with warfarin, they may not achieve the level of anticoagulation needed for the objective of such treatment, prevention of stroke and other thromboembolic events and whether higher doses (e.g. 5 mg twice daily of apixaban) are likely to be optimal remains uncertain. Appropriate dose reduction for DOACs is commonly overlooked in CKD-ESRD and should deliberately be considered at the time of DOAC initiation in these patients.
Figure 1.
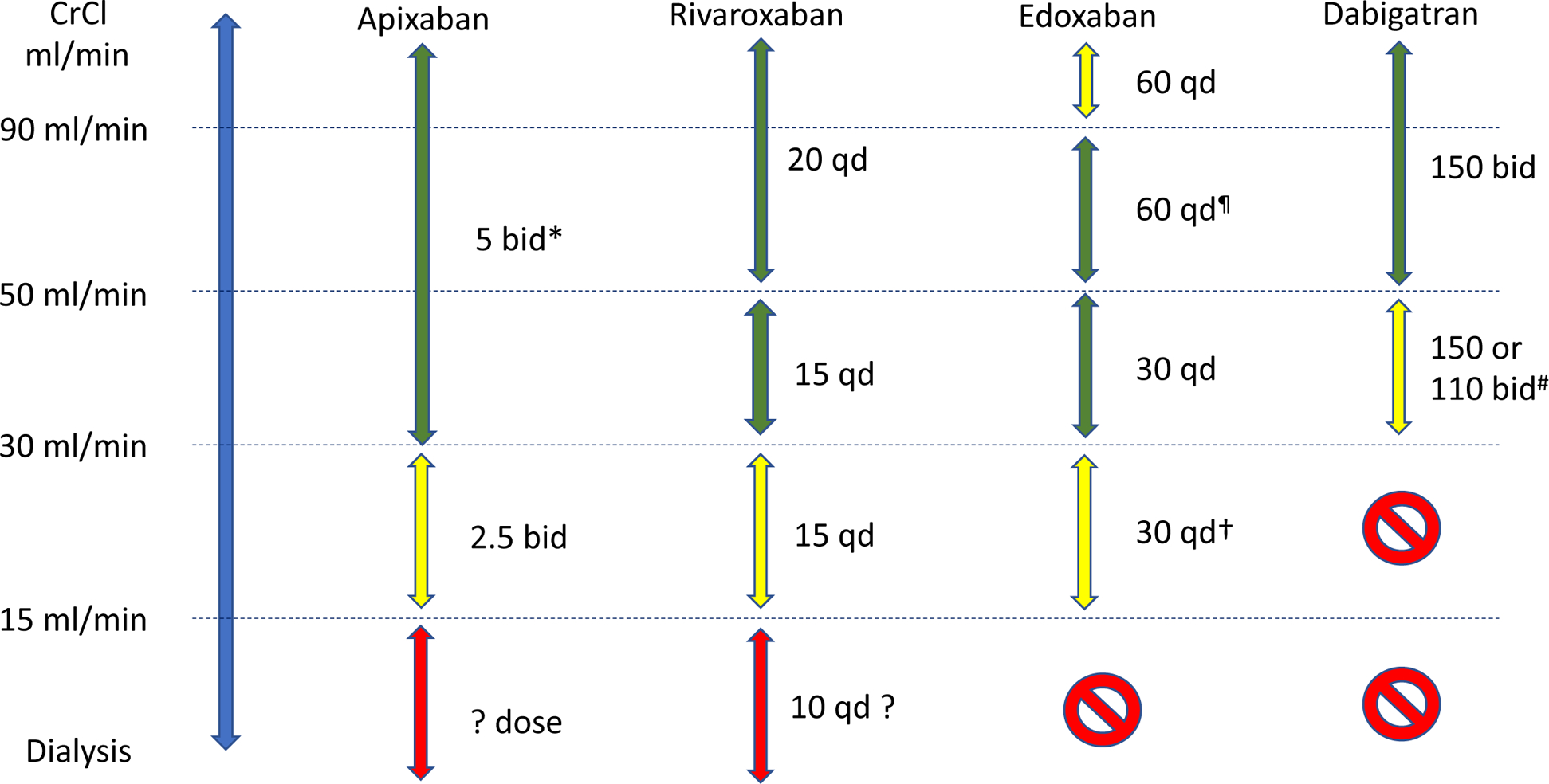
Suggested doses for direct oral anticoagulants in patients with chronic kidney disease
Green arrow: the agent can be used; yellow arrow: use with caution; red arrow: use in selected cases only after careful assessment of risk profile and potential benefits.
Suggested doses are for atrial fibrillation or venous thromboembolism after the high intensity time period (21 days for rivaroxaban, 7 days for apixaban, 5 days for edoxaban and dabigatran).
Qd, once daily; bid, twice daily; CrCl, creatinine clearance
*, 2.5 mg bid if two of the three criteria are met: creatinine ≥1.5 mg/dl, body weight ≤60 kg, age ≥80
¶, 30 mg qd if body weight ≤60 kg or use of potent P-gp inhibitor
#, especially in patients at high risk of bleeding
†, consider 15 mg qd if body weight ≤60 kg and/or use of potent P-gp inhibitor
Adapted from Steffel et al, Eur Heart J 2018; 39:1351.4
7. Key points.
All four DOACs can be used in moderate CKD at the recommended dose.
DOACs may be used after appropriate dose adjustment for an established clinical indication in patients with advanced CKD.
Prescription of a DOAC (or VKA) for stroke prevention in ESRD is not based on solid evidence and, if considered, should be individualized taking into account the patient’s risk profile and/or preferences and the limited evidence.
8. Acknowledgments
Conflicts of interest: Dr. Mavrakanas has no disclosures; Dr. Charytan discloses consulting fees or research support from AstraZeneca, Merck, Jannssen, NovoNordisk, Gilead, and Fresenius Medical Care; Dr. Winkelmayer discloses receipt of consultancy fees from from Akebia, Amgen, AstraZeneca, Bayer, Daichii-Sankyo, Janssen, Merck, Relypsa, Vifor Fresenius Medical Care Renal Pharma and speaker honoraria from Fibrogen.
Funding: National Institutes of Health grant R01 DK095024
Contributor Information
Thomas A. Mavrakanas, Department of Medicine, McGill University, Montreal, QC
David M. Charytan, Division of Nephrology, NYU Grossman School of Medicine, New York, NY
Wolfgang C. Winkelmayer, Selzman Institute for Kidney Health, Section of Nephrology, Baylor College of Medicine, Houston, TX
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Woek Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3:1–150. [Google Scholar]
- 2.Kumar S, Lim E, Covic A, et al. Anticoagulation in Concomitant Chronic Kidney Disease and Atrial Fibrillation: JACC Review Topic of the Week. J Am Coll Cardiol 2019; 74:2204–2215. [DOI] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019; 74:104–132. [DOI] [PubMed] [Google Scholar]
- 4.Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and expert panel report. Chest 2018; 154:1121–1201. [DOI] [PubMed] [Google Scholar]
- 5.Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018; 39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 6.Turakhia MP, Blankestijn PJ, Carrero JJ, et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 2018; 39:2314–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 8.Stanifer JW, Pokorney SD, Chertow GM, et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation 2020; 141:1384–1392. [DOI] [PubMed] [Google Scholar]; * The only pharmacokinetic study from randomized data with apixaban in patients with creatinine clearance 25–30 ml/min.
- 9.De Vriese AS, Caluwé R, Bailleul E, et al. Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis 2015; 66:91–98. [DOI] [PubMed] [Google Scholar]; * The only pharmacokinetic study on rivaroxaban in hemodialysis.
- 10.Mavrakanas TA, Samer CF, Nessim SJ, et al. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol 2017; 28:2241–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The only pharmacokinetic study on apixaban at steady state in hemodialysis.
- 11.Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2012; 33:2821–2830. [DOI] [PubMed] [Google Scholar]
- 12.Bohula EA, Giugliano RP, Ruff CT, et al. Impact of Renal Function on Outcomes With Edoxaban in the ENGAGE AF-TIMI 48 Trial. Circulation 2016; 134:24–36. [DOI] [PubMed] [Google Scholar]
- 13.Lindner SM, Fordyce CB, Hellkamp AS, et al. Treatment Consistency Across Levels of Baseline Renal Function With Rivaroxaban or Warfarin: A ROCKET AF (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) Analysis. Circulation. 2017; 135:1001–1003. [DOI] [PubMed] [Google Scholar]
- 14.Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 2014; 129:961–970. [DOI] [PubMed] [Google Scholar]
- 15.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369:799–808. [DOI] [PubMed] [Google Scholar]
- 16.Verhamme P, Wells PS, Segers A, et al. Dose reduction of edoxaban preserves efficacy and safety for the treatment of venous thromboembolism. An analysis of the randomised, double-blind HOKUSAI VTE trial. Thromb Haemost 2016; 116:747–753. [DOI] [PubMed] [Google Scholar]
- 17.Bauersachs RM, Lensing AW, Prins MH, et al. Rivaroxaban versus enoxaparin/vitamin K antagonist therapy in patients with venous thromboembolism and renal impairment. Thromb J 2014; 12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldhaber SZ, Schulman S, Eriksson H, et al. Dabigatran versus Warfarin for Acute Venous Thromboembolism in Elderly or Impaired Renal Function Patients: Pooled Analysis of RE-COVER and RE-COVER II. Thromb Haemost 2017; 117:2045–2052. [DOI] [PubMed] [Google Scholar]
- 19.Malhotra K, Ishfaq MF, Goyal N, et al. Oral anticoagulation in patients with chronic kidney disease: A systematic review and meta-analysis. Neurology 2019; 92:e2421–e2431. [DOI] [PubMed] [Google Scholar]
- 20.Ha JT, Neuen BL, Cheng LP, et al. Benefits and Harms of Oral Anticoagulant Therapy in Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann Int Med 2019; 171:181–189. [DOI] [PubMed] [Google Scholar]; ** A meta-analysis of RCTs on DOACs in patients with CKD
- 21.Shin JI, Secora A, Alexander GC, et al. Risks and Benefits of Direct Oral Anticoagulants across the Spectrum of GFR among Incident and Prevalent Patients with Atrial Fibrillation. Clin J Am Soc Nephrol 2018; 13: 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loo SY, Coulombe J, Dell’Aniello S, et al. Comparative effectiveness of novel oral anticoagulants in UK patients with non-valvular atrial fibrillation and chronic kidney disease: a matched cohort study. BMJ Open 2018; 8:e019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harel Z, Mamdani M, Juurlink DN, et al. Novel Oral Anticoagulants and the Risk of Major Hemorrhage in Elderly Patients With Chronic Kidney Disease: A Nested Case-Control Study. Can J Cardiol 2016; 32:986.e17–986.e22. [DOI] [PubMed] [Google Scholar]
- 24.Keskar V, McArthur E, Wald R, et al. The association of anticoagulation, ischemic stroke, and hemorrhage in elderly adults with chronic kidney disease and atrial fibrillation. Kidney Int 2017; 91:928–936. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, de Lusignan S, McGovern A, et al. Ischaemic stroke, haemorrhage, and mortality in older patients with chronic kidney disease newly started on anticoagulation for atrial fibrillation: a population based study from UK primary care. BMJ 2018; 360:k342. [DOI] [PubMed] [Google Scholar]
- 26.Coleman CI, Kreutz R, Sood NA, et al. Rivaroxaban Versus Warfarin in Patients With Nonvalvular Atrial Fibrillation and Severe Kidney Disease or Undergoing Hemodialysis. Am J Med 2019; 132:1078–1083. [DOI] [PubMed] [Google Scholar]; * The first cohort study with rivaroxaban in patients with stage 4–5 CKD.
- 27.Weir MR, Ashton V, Moore KT, et al. Rivaroxaban versus warfarin in patients with non-valvular atrial fibrillation and stage IV-V chronic kidney disease. Am Heart J 2020; 223:3–11. [DOI] [PubMed] [Google Scholar]
- 28.Ashley JM, McArthur E, Bota S, et al. Risk of Cardiovascular Events and Mortality Among Elderly Patients With Reduced GFR Receiving Direct Oral Anticoagulants. Am J Kidney Dis 2020. April 22 pii: S0272–6386(20)30639–9. [DOI] [PubMed] [Google Scholar]
- 29.Edgett DA, Dratch A, Streja E, et al. Direct oral anticoagulants vs. warfarin: stroke outcomes in CKD patients. J Am Soc Nephrol 2018; 29: FR-PO374 [abstract]. [Google Scholar]
- 30.Yao X, Inselman JW, Shah ND, et al. Comparative effectiveness and safety of oral anticoagulants across baseline kidney function. Circ Cardiovasc Qual Outcomes 2019; 12(Suppl 1):A11. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A cohort study examining the efficacy and safety of apixaban, rivaroxaban, and dabigatran versus warfarin across an eGFR range of 15 to ≥90 ml/min/1.73m2.
- 31.Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol 2018; 29:2787–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** A landmark article on anticoagulant-related nephropathy.
- 32.Chan YH, Yeh YH, See LC, et al. Acute Kidney Injury in Asians With Atrial Fibrillation Treated With Dabigatran or Warfarin. J Am Coll Cardiol 2016; 68:2272–83.27884245 [Google Scholar]
- 33.Yao X, Tangri N, Gersh BJ, et al. Renal Outcomes in Anticoagulated Patients With Atrial Fibrillation. J Am Coll Cardiol 2017; 70:2621–2632. [DOI] [PubMed] [Google Scholar]; * The first cohort study on renal outcomes in patients with AF on anticoagulation.
- 34.Shin JI, Luo S, Alexander GC, et al. Direct Oral Anticoagulants and Risk of Acute Kidney Injury in Patients With Atrial Fibrillation. J Am Coll Cardiol 2018; 71:251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez AV, Bradley G, Khan M, et al. Rivaroxaban Versus Warfarin and Renal Outcomes in Non-valvular Atrial Fibrillation Patients with Diabetes. Eur Heart J Qual Care Clin Outcomes 2019. pii: qcz047 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36.Coleman CI, Kreutz R, Sood N, et al. Rivaroxaban’s Impact on Renal Decline in Patients With Nonvalvular Atrial Fibrillation: A US MarketScan Claims Database Analysis. Clin Appl Thromb Hemost. 2019; 25:1076029619868535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molnar AO, Bota SE, McArthur E, et al. Risk and complications of venous thromboembolism in dialysis patients. NDT 2018; 33:874–880. [DOI] [PubMed] [Google Scholar]; * The first cohort study on VTE in dialysis patients.
- 38.Kuno T, Takagi H, Ando T, et al. Oral Anticoagulation for Patients With Atrial Fibrillation on Long-Term Hemodialysis. J Am Coll Cardiol 2020; 75:273–285. [DOI] [PubMed] [Google Scholar]; ** The most recent meta-analysis on oral anticoagulation in hemodialysis.
- 39.Van Der Meersch H, De Bacquer D, De Vriese AS. Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: A systematic review and meta-analysis. Am Heart J 2017; 184:37–46. [DOI] [PubMed] [Google Scholar]
- 40.Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. A retrospective cohort study. Clin J Am Soc Nephrol 2020. (accepted, in press). doi: 10.2215/CJN.11650919 [DOI] [PMC free article] [PubMed] [Google Scholar]; * The first cohort study with apixaban vs. no anticoagulation in hemodialysis.
- 41.Chan KE, Edelman ER, Wenger JB, et al. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation 2015; 131:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The first cohort study with dabigatran and rivaroxaban in hemodialysis.
- 42.Yang F, Hellyer JA, Than C, et al. Warfarin utilization and anticoagulation control in patients with atrial fibrillation and chronic kidney disease. Heart 2017; 103:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Vriese AS, Caluwé R, Pyfferoen L, et al. Multicenter Randomized Controlled Trial of Vitamin K Antagonist Replacement by Rivaroxaban with or without Vitamin K2 in Hemodialysis Patients with Atrial Fibrillation: the Valkyrie Study. J Am Soc Nephrol 2020; 31:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The first RCT with rivaroxaban in hemodialysis.
- 44.Miao B, Sood N, Bunz TJ, Coleman CI. Rivaroxaban versus apixaban in non-valvular atrial fibrillation patients with end-stage renal disease or receiving dialysis. Eur J Haematol 2020; 104:328–335. [DOI] [PubMed] [Google Scholar]
- 45.Siontis KC, Zhang X, Eckard A, et al. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation 2018; 138:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The first cohort study with apixaban vs. warfarin in hemodialysis.
- 46.Pokorney SD, RENAL AF Investigators. RENal hemodialysis patients Allocated apixaban versus warfarin in Atrial Fibrillation. Presented at the AHA 2019 meeting on November 16, 2019. Accessed online at: https://www.acc.org/education-and-meetings/image-and-slide-gallery/media-detail?id=469e5bd88fff4d2bb8ec183e71521637 [Google Scholar]; * The first randomized trial with apixaban vs. warfarin in hemodialysis.


