Abstract
BACKGROUND AND OBJECTIVES:
In 10% to 20% of cases, Kawasaki disease is refractory to intravenous immunoglobulin (IVIg), an expensive medication under a national shortage. Data suggest that infliximab is a viable alternative to a second dose of IVIg, with similar efficacy and safety. We compared the cost of a second IVIg dose to that of infliximab in the treatment of refractory Kawasaki disease (rKD).
METHODS:
A decision analysis model was used to compare rKD treatments: a second dose of IVIg at 2 g/kg versus infliximab at 10 mg/kg. Infliximab monitoring times were 24, 36, and 48 hours. Direct hospital costs beginning at rKD diagnosis were estimated by using 2016–2017 Truven MarketScan data. Redbook was used for drug costs. Calculations were applied to 3 hypothetical cohorts of 100 patients aged 2 (12.5 kg), 4 (16 kg), and 8 years (25.5 kg). Indirect costs included parental missed workdays.
RESULTS:
The total direct cost for children receiving IVIg was $1 677 801, $1 791 652, and $2 100 675 for the 2-, 4-, and 8-year-old cohorts. The direct cost of infliximab with 24 hours of monitoring was $853 042, $899 096, and $1 024 101, respectively. A 20% bidirectional sensitivity analysis revealed stability of our model, with overall cost savings with use of infliximab. With monitoring 48 hours after infliximab treatment, 20% changes in length of stay (LOS) tipped the balance for the 2- and 4-year-old cohorts. Overall, IVIg and infliximab LOS had the most influence on our model.
CONCLUSIONS:
Infliximab has potential to yield shorter LOS and significant cost savings in the treatment of rKD. Infliximab treatment, followed by 24 hours of monitoring, nearly halved hospital costs, regardless of age.
Kawasaki disease (KD) is a systemic vasculitis that is the leading cause of acquired pediatric heart disease in developed countries, with up to 25% of untreated patients developing coronary artery aneurysms.1,2 Intravenous immunoglobulin (IVIg) with aspirin is the first-line therapy recommended by American Heart Association guidelines.1,3,4
Refractory Kawasaki disease (rKD) occurs in 10% to 20% of cases and is defined by the American Heart Association as a fever for >36 hours at <7 days from completion of IVIg treatment.1,2 There is not a consensus regarding the most effective therapy or monitoring duration for rKD.
IVIg is often the first-line treatment of rKD at the same dose, infusion time, and monitoring time as the initial treatment. The use of infliximab, a chimeric monoclonal antibody that binds to tumor necrosis factor α receptors, to treat rKD appears to be on the rise in the United States, with some studies revealing a greater response rate compared to repeat IVIg treatment.5,6 Several studies reveal that infliximab results in decreased levels in inflammatory markers, shorter fever duration, decreased length of stay (LOS), and a decreased likelihood that the patient will require further treatment.3,5–11 The literature suggests that infliximab for rKD is, overall, well tolerated and safe, with minimal risk of infusion reactions or serious adverse effects.2,3,7–10,12 A multicenter phase III (Kawasaki Disease Comparative Effectiveness [KIDCARE]) clinical trial by Roberts et al13 is currently underway, in which researchers compare a 10 mg/kg dose of infliximab to a 2 g/kg dose of IVIg for rKD, looking at the rate of defervescence, the effect on inflammation, and whether there is a decrease in the baseline coronary artery z score.
Given the current IVIg shortage,14 the high cost of IVIg, and the preliminary data suggesting infliximab may have comparable efficacy and adverse event rates, we sought to compare the cost of a second IVIg dose to that of infliximab for rKD.3,6,8,10,11,15
Methods
Population
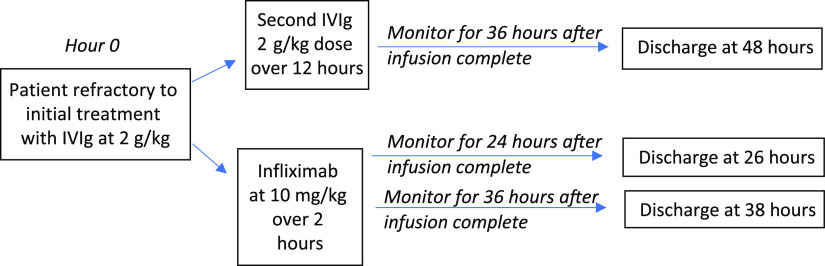
We developed a decision analysis model using a decision tree to explore 2 treatment options for rKD (Fig 1). The model starts when the patient is diagnosed with rKD. Model inputs were obtained through literature review (Table 1). Given that medications in pediatrics are often weight based, we ran the model with 3 different 100-patient cohorts of varying ages and weights (Table 2, Fig 1). Using 100-patient cohorts is standard for this study design. Because the highest incidence of KD is in those 1 to 5 years old, we introduced a cohort of 2-year-olds at the 50th percentile for weight (12.5 kg) and a cohort of 4-year-olds at the 50th percentile for weight (16 kg). A third cohort of 8-year-olds at the 50th percentile for weight (25.5 kg) was included to explore how cost would change in atypical cases outside the usual age range.
FIGURE 1.
Decision analysis for alternative treatment for refractory Kawasaki disease. Each option included 3 cohorts of children with different ages and weights.
TABLE 1.
Key Assumptions for the Decision Tree
| Assumption | Model Input | Source, Year |
|---|---|---|
| Cost of IVIg | $0.155 per mga | Redbook,16 2017 |
| Cost of infliximab | $12.54 per mga | Redbook,16 2017 |
| Hospital cost per subsequent day | $6355.96a | MarketScan,16 2016 and 2017 |
| Monitoring duration after IVIg treatment | 36 h | McCrindle et al,1 2017 |
| Monitoring duration after infliximab treatment | 12 h | Roberts et al,13 2019 |
| Monitoring duration after infliximab treatment | 48 h | Mori et al,3,8 2012 and 2018 |
| IVIg infusion time | 12 h | McCrindle et al,1 2017 |
| Infliximab infusion time | 2 h | Roberts et al,13 2019 |
| IVIg dose | 2000 mg/kg | McCrindle et al,1 2017 |
| Infliximab dose | 10 mg/kg | Roberts et al,13 2019; Vande Casteele et al,17 2018 |
| PPD placement at admission | No additional cost because it occurs before | MUSC KD algorithm |
| 50th percentile wt for 2 y old | 12.5 kg | CDC,18 2017 |
| 50th percentile wt for 4 y old | 16 kg | CDC,18 2017 |
| 50th percentile wt for 8 y old | 25.5 kg | CDC,18 2017 |
| Typical workday length for parent | 8.5 h | US Bureau of Labor Statistics,19 2020 |
| Hourly wage for adult in the US | $19.03 | US Bureau of Labor Statistics,19 2019 |
| Parental workdays lost/each day a child misses school | 0.82:1 | Flores et al,20 2009 |
| No aneurysm large enough to warrant long-term anticoagulation, no ICU admission, no death | — | — |
CDC, Centers for Disease Control and Prevention; MUSC, Medical University of South Carolina; —, not applicable.
All 2016 and 2017 costs were subsequently converted to 2019 dollars by using the US Inflation Calculator before final calculations.21
TABLE 2.
Decision Tree Analysis Results: Additional Cost Difference Between a Second Dose of IVIg and Infliximab for rKD
| Cohort Age (wt) | Medication | LOS (h) | Medication Cost, $ | Total Direct Cost per Patient, $ | Indirect Cost Per Patient, $ | Total Cost for Cohort (100 Patients), $ |
|---|---|---|---|---|---|---|
| 2 y (12.5 kg) | ||||||
| IVIg | 48 | 4066 | 16 778 | 265 | 1 704 300 | |
| Infliximab | 26 | 1645 | 8530 | 144 | 867 400 | |
| Infliximab | 38 | 1645 | 11 708 | 210 | 1 191 800 | |
| Infliximab | 50 | 1645 | 14 886 | 276 | 1 516 200 | |
| 4 y (16 kg) | ||||||
| IVIg | 48 | 5205 | 17 917 | 265 | 1 818 200 | |
| Infliximab | 26 | 2105 | 8991 | 144 | 913 500 | |
| Infliximab | 38 | 2105 | 12 169 | 210 | 1 237 900 | |
| Infliximab | 50 | 2105 | 15 347 | 276 | 1 562 300 | |
| 8 y (25.5 kg) | ||||||
| IVIg | 48 | 8295 | 21 007 | 265 | 2 127 200 | |
| Infliximab | 26 | 3355 | 10 241 | 144 | 1 038 500 | |
| Infliximab | 38 | 3355 | 13 419 | 210 | 1 362 900 | |
| Infliximab | 50 | 3355 | 16 597 | 276 | 1 687 300 |
Costs are in 2019 US dollars, and calculations were derived from the US Inflation Calculator.21 Hospital costs for LOS were derived from MarketScan 2016 and 2017 data. Medication costs were from Redbook16 and were calculated as an average per milligram across all brands of the medication. Indirect costs to account for missed parental work days were calculated with information from the US Bureau of Labor Statistics,19 Flores et al,20 and MarketScan LOS. Total direct cost includes medication cost and LOS.
Model Inputs
Drug Doses and Infusion Time
Standard IVIg dosing is 2 g/kg over 10 to 12 hours; however, in some studies, it has been administered over 20 or even 24 hours.8,22 In our baseline model, we have an assumed infusion time of 12 hours and an observation time of 36 hours.
Infliximab dosing is 5 to 10 mg/kg, with monitoring time ranging from 12 to 48 hours.1,7,8,13,23 The infliximab dose in this model is 10 mg/kg, with an infusion time of 2 hours and an observation time of 24, 36, or 48 hours. This infliximab dose and the 24 hour monitoring time were modeled on the KIDCARE clinical trial by Roberts et al.13 This dose is based on the pharmacokinetics study by Vande Casteele et al.17
Other Model Assumptions
Model assumptions included purified protein derivative (PPD) placement at admission in case of IVIg nonresponse and need for infliximab. We limited our study to the initial treatment of rKD and did not include a third treatment.
Cost Comparison
Direct Costs
Direct costs include the costs of medications and hospitalization. The 2017 Red Book: Pharmacy’s Fundamental Reference was used to estimate infliximab and IVIg costs.16 We averaged the cost of all formulations to calculate cost per milligram.16 We assumed a cost of $6355.96 per subsequent hospital day, calculated as an average per day payment from 2016 and 2017 MarketScan data for KD admissions. This value was then used to calculate a cost per hour to calculate the cost of LOS; LOS duration (in hours) is the sum of the infusion time and the observation period for the medication. MarketScan is a national commercial claims database that represents >26 million covered lives during the period studied and has been used for financial analysis in many respected journals for more than a decade24–27; the total payments reflect what is paid by insurance companies and patient families rather than what was charged.
Indirect Costs
To assess the overall impact at the societal level, indirect costs secondary to missed parental workdays were included. One missed school day was assumed for each day of hospitalization. We used a ratio of 1:0.82 for missed school days/missed parental workdays on the basis of previously published reports on children with asthma.20 Hospital admission duration (mean 3.7 days) was obtained by using 2016 and 2017 MarketScan data for KD admissions. Using data from the US Bureau of Labor Statistics, we then applied a median hourly wage of $19.03 and assumed a workday of 8.5 hours per day to calculate the indirect costs attributed to missed parental work.19 All costs were subsequently converted to 2019 US dollars for final calculations (Table 1).
Sensitivity Analysis
A sensitivity analysis was performed to determine the stability of our model. We applied a 20% bidirectional variation to all model assumptions.1 This included cost of medications, cost of hospital stay, and LOS. All analyses were conducted in Excel 2016 (Microsoft Corporation, Redmond, WA). The resulting tornado diagram enables users to visualize the relative impact of each model input on total cost.
Results
Infliximab treatment produced cost savings over IVIg treatment across all cohorts. Our model of 2-year-olds with rKD predicted that, when compared to the standard second dose of 2 g/kg of IVIg for rKD, 10 mg/kg of infliximab with a 26-hour LOS results in direct cost savings of $824 759 per 100 patients. Direct cost savings increased to $892 556 for a cohort of 100 4-year-olds and to $1 076 574 for a cohort of 8-year-olds (Table 2). Even with the 50-hour infliximab LOS, there were direct cost savings of $189 163, $256 960, and $440 978 across the 3 ages. Total indirect costs per cohort ranged from $14 400 for the infliximab 26-hour LOS group to $27 600 for the 50-hour infliximab LOS group.
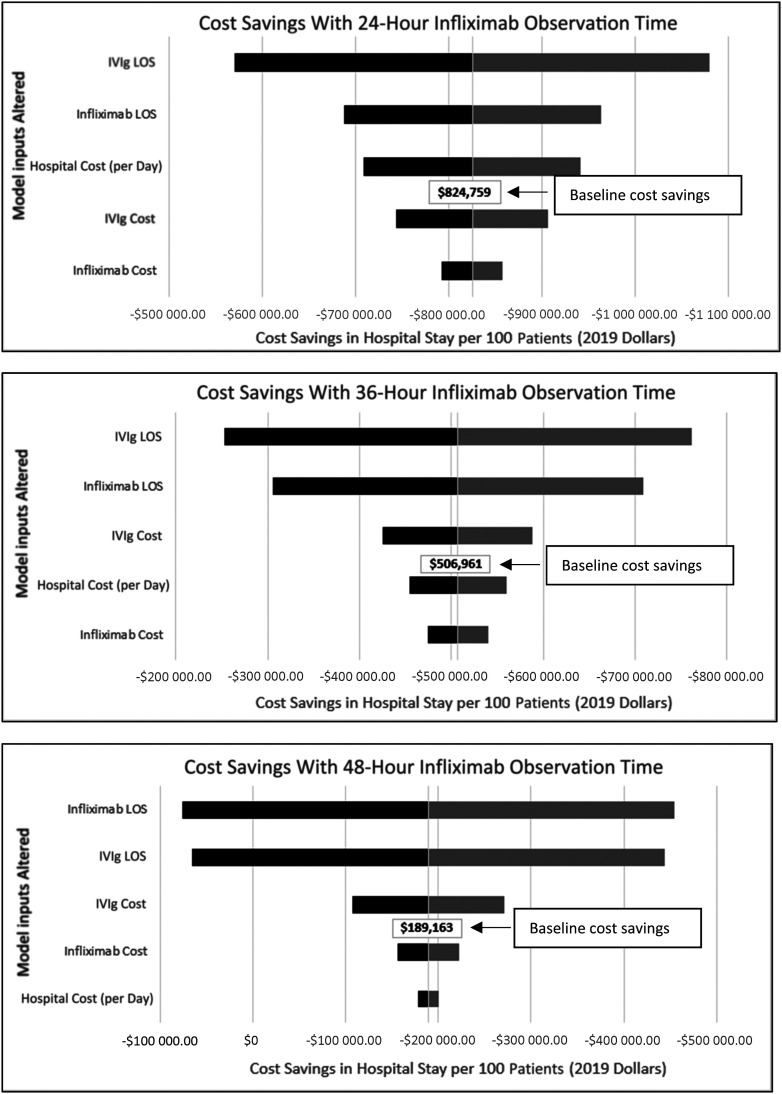
The results of our sensitivity analysis with 20% bidirectional adjustment on model inputs reveal that our model was stable and that the prediction of cost savings with use of infliximab persists when infliximab monitoring time is ≤36 hours. However, once infliximab monitoring time increases to 48 hours, there is a possibility of no cost savings or of increased overall cost for the 2 and 4 year old cohorts if there is significant variation in the infliximab LOS model input assumption. In a sample tornado diagram for the 2-year-old cohort (Fig 2), a second IVIg dose is compared to infliximab. Medication LOS had the greatest influence on degree of cost savings.
FIGURE 2.
Tornado diagrams for sensitivity analysis of predicted cost savings per 100 patients in the 2-year old cohort with a second IVIg dose versus infliximab with varying monitoring times. With 20% bidirectional variability of model inputs (black = 20% less, gray = 20% more), the length of the bars reveal the relative importance of each variable in the overall cost of the hospital stay. A midline value of baseline cost savings is noted. The margins of the bars represent the potential impact on hospital stay and cost savings between cohorts.
Discussion
This decision analysis model compares 2 common medications for the treatment of rKD and predicts that infliximab can result in substantial cost savings. This is primarily driven by a lower cost per dose, a shorter infusion time, and a potentially shorter monitoring time, resulting in shorter overall LOS.
Although there are a growing number of studies exploring the efficacy and safety of alternative treatment options with promising results, including randomized controlled trials, indirect meta-analysis, and systematic reviews, it remains difficult to determine comparative efficacy. Many studies are underpowered because of small or unequal groups sizes, their single-center nature, homogenous international populations that may not apply to the United States, use of adjunctive or combination therapies, and varying definitions of rKD.3,6,8–11,13,15
Large randomized controlled trial results are not currently available for clinicians to weigh the efficacy or safety of IVIg versus infliximab. Rather than pursue a cost-effectiveness analysis under the assumption of equal efficacy, we decided to create a decision analysis model, which uses the best current evidence to make reasonable assumptions, explore outcomes, and enable clinicians to make informed decisions. Clinicians are tasked with being judicious with resources while delivering quality care to patients, and emerging diagnoses, such as multisystem inflammatory syndrome in children, may require IVIg with no known alternative options. At a time when IVIg is under a national shortage and there is no clear evidence that infliximab is inferior in outcomes or safety for treatment of rKD, cost should be considered. The results of studies underway, such as the KIDCARE trial, will allow for an effectiveness outcome, which can be used to update this effort to a cost-effectiveness analysis in the future.
There are several limitations to this study. It was built on several assumptions, such as placing a PPD on admission for all KD diagnoses; failure to do so could delay infliximab administration another 48 hours or require obtaining a QuantiFERON gold test. Averaging the cost of medications per milligram does not account for discounts hospitals may receive or rounding doses to avoid waste. The hospital costs used in this analysis are from MarketScan, a database that may not fully reflect geographic or plan-type variations in the United States. Societal costs, as measured with indirect costs from parents missing work, may be overestimated or underestimated; the ratio used is not from studies on KD specifically. Although we apply cost per day to cost per hour, a hospital day actually starts at any time point at which the patient remains admitted after midnight. We applied hard cutoff points for monitoring in calculating LOS for each medication. Furthermore, this model does not account for those with more severe clinical courses. Despite all of this, our sensitivity analysis reveals that the relative difference in cost remains great between use of infliximab and IVIg.
Conclusions
This decision analysis suggests that the use of infliximab over a second dose of IVIg for children with rKD can result in cost savings.
Footnotes
Deidentified individual participant data will not be made available.
Dr Brinton participated in study design, acquisition of data and model inputs, data analysis, and critical revision of the article; Mr Chew participated in MarketScan data analysis, acquisition of data, and revising the article for important intellectual content; Dr Andrews participated in study design and concept, sensitivity analyses, and critical review and revision of the manuscript; Dr Johnson participated in study design and concept, drafting the initial manuscript, data acquisition, sensitivity analyses, and revision of the manuscript; Dr Williams participated in study design and concept and critical revision of the manuscript for important intellectual content; Dr Simpson participated in study design and concept, data analysis and interpretation, and critical revision of the manuscript for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: A South Carolina Clinical & Translational Research Institute voucher supported use of the MarketScan data for this article. Supported by a grant from the National Institutes of Health and the National Center for Advancing Translational Sciences (UL1TR001450). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Epidemiology and Prevention. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927–e999 [DOI] [PubMed] [Google Scholar]
- 2.Tremoulet AH, Jain S, Jaggi P, et al. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9930):1731–1738 [DOI] [PubMed] [Google Scholar]
- 3.Mori M, Imagawa T, Hara R, et al. Efficacy and limitation of infliximab treatment for children with Kawasaki disease intractable to intravenous immunoglobulin therapy: report of an open-label case series. J Rheumatol. 2012;39(4):864–867 [DOI] [PubMed] [Google Scholar]
- 4.Saneeymehri S, Baker K, So TY. Overview of pharmacological treatment options for pediatric patients with refractory Kawasaki disease. J Pediatr Pharmacol Ther. 2015;20(3):163–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez SR, Birkholz M, Anderson MS, et al. Diagnostic and treatment trends in children with Kawasaki disease in the United States, 2006-2015. Pediatr Infect Dis J. 2019;38(10):1010–1014 [DOI] [PubMed] [Google Scholar]
- 6.Son MB, Gauvreau K, Burns JC, et al. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: a retrospective study. J Pediatr. 2011;158(4):644–649.e1 [DOI] [PubMed] [Google Scholar]
- 7.Burns JC, Mason WH, Hauger SB, et al. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005;146(5):662–667 [DOI] [PubMed] [Google Scholar]
- 8.Mori M, Hara T, Kikuchi M, et al. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci Rep. 2018;8(1):1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youn Y, Kim J, Hong YM, Sohn S. Infliximab as the first retreatment in patients with Kawasaki disease resistant to initial intravenous immunoglobulin. Pediatr Infect Dis J. 2016;35(4):457–459 [DOI] [PubMed] [Google Scholar]
- 10.Burns JC, Best BM, Mejias A, et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153(6):833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan H, Chi H, You H, et al. Indirect-comparison meta-analysis of treatment options for patients with refractory Kawasaki disease. BMC Pediatr. 2019;19(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremoulet AH. Adjunctive therapies in Kawasaki disease. Int J Rheum Dis. 2018;21(1):76–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts SC, Jain S, Tremoulet AH, et al. ; KIDCARE Multicenter Study Group. The Kawasaki Disease Comparative Effectiveness (KIDCARE) trial: a phase III, randomized trial of second intravenous immunoglobulin versus infliximab for resistant Kawasaki disease. Contemp Clin Trials. 2019;79:98–103 [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Information about immune globulin (human) product shortage. Available at: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/information-about-immune-globulin-human-product-shortage. Accessed January 1, 2020
- 15.Crayne CB, Mitchell C, Beukelman T. Comparison of second-line therapy in IVIg-refractory Kawasaki disease: a systematic review. Pediatr Rheumatol Online J. 2019;17(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truven Health. (2017). Truven Health MarketScan Research Databases
- 17.Vande Casteele N, Oyamada J, Shimizu C, et al. Infliximab pharmacokinetics are influenced by intravenous immunoglobulin administration in patients with Kawasaki disease. Clin Pharmacokinet. 2018;57(12):1593–1601 [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention National Center for Health Statistics. Clinical Growth Charts. Available at: https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed November 11, 2020.
- 19.US Department of Labor Bureau of Labor Statistics. May 2018 national occupational employment and wage estimates United States. Available at: https://www.bls.gov/oes/2018/may/oes_nat.htm. Accessed January 17, 2020
- 20.Flores G, Snowden-Bridon C, Torres S, et al. Urban minority children with asthma: substantial morbidity, compromised quality and access to specialists, and the importance of poverty and specialty care. J Asthma. 2009;46(4):392–398 [DOI] [PubMed] [Google Scholar]
- 21.US Inflation Calculator. Xxx. 2019. Available at: https://www.usinflationcalculator.com/. Accessed February 3, 2020
- 22.Ogata S, Ogihara Y, Honda T, Kon S, Akiyama K, Ishii M. Corticosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics. 2012;129(1). Available at: https://pediatrics.aappublications.org/content/129/1/e17/e17 [DOI] [PubMed] [Google Scholar]
- 23.Song MS, Lee SB, Sohn S, et al. Infliximab treatment for refractory Kawasaki disease in Korean children. Korean Circ J. 2010;40(7):334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyamfi-Bannerman C, Zupancic JAF, Sandoval G, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Cost-effectiveness of antenatal corticosteroid therapy vs no therapy in women at risk of late preterm delivery: a secondary analysis of a randomized clinical trial. JAMA Pediatr. 2019;173(5):462–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah D, Lu X, Paly VF, Tsintzos SI, May DM. Cost-effectiveness analysis of implantable cardiac devices in patients with systolic heart failure: a US perspective using real world data. J Med Econ. 2020;23(7):690–697 [DOI] [PubMed] [Google Scholar]
- 26.Watkins KE, Burnam MA, Orlando M, Escarce JJ, Huskamp HA, Goldman HH. The health value and cost of care for major depression. Value Health. 2009;12(1):65–72 [DOI] [PubMed] [Google Scholar]
- 27.Whittington MD, McQueen RB, Ollendorf DA, et al. Assessing the value of mepolizumab for severe eosinophilic asthma: a cost-effectiveness analysis. Ann Allergy Asthma Immunol. 2017;118(2):220–225 [DOI] [PubMed] [Google Scholar]