Abstract
Introduction
The baboon is a well-characterized model of human early stage atherosclerosis. However, histological and morphological changes involved in atherogenesis in baboons are not known. Previously we challenged baboons with a high-cholesterol, high-fat diet for two years and observed fatty streak and plaque lesions in iliac arteries (RCIA).
Methods
We evaluated histological and morphological changes of baboon arterial lesions and control arteries. In addition, we evaluated the vascular expression of CD68 and SMαA markers with progression of atherosclerosis.
Results
We observed changes that correlated with extent of atherosclerosis, including increased maximum intimal thickness. We demonstrated at molecular level the infiltration of smooth muscle cells and macrophages into the intimal layer. Further, we observed histological and morphological discordancy between the affected and adjacent areas of the same RCIA.
Conclusion
Atherogenesis in baboons is accompanied by histological, morphological and molecular changes, as in humans, providing insights to evaluate the mechanisms underlying early stage atherosclerosis in target tissues.
Keywords: Atherosclerosis, Intima-media thickness, Smooth muscle cells, Macrophages
INTRODUCTION
Atherosclerosis is a progressive, chronic inflammatory disease. The clinical end-point of atherosclerosis is cardiovascular disease (CVD), primarily caused by thickening and/or occlusion of large and medium-size arteries. CVD is the leading cause of death in the developed countries and is projected to be the major cause of death in the world by 2030.1, 2
Atherogenesis is a multifactorial process that is not well understood. Several hypotheses describe events leading to initiation and progression of atherosclerosis.3, 4 The most compelling hypotheses reviewed by Nakajima et al.5 concur that initial events during atherogenesis include deposition of modified or oxidized remnant lipoproteins cholesterol (ox-RLP-C) and LDL-C on the endothelial layer of the artery wall, expression of endothelial lectin-like oxidized LDL receptors (LOX-1), internalization of ox-RLP-C, resulting in endothelial dysfunction and expression of endothelial adhesion molecules; adhesion of circulating monocytes onto the endothelium, entry of LDL-C and monocytes into the sub-endothelial space where LDL-C is oxidized and monocytes activated to macrophages; expression of macrophage scavenger receptors, including LOX-1, internalization of oxidized LDL-C and RLP-C by macrophages to form foam cells; production of pro-inflammatory cytokines and connective matrix, conversion and proliferation of vascular smooth muscle cells (VSMC) and augmentation of inflammatory response, cell apoptosis, and intimal thickening. During these processes, atherosclerotic lesions, which are grossly and microscopically heterogeneous, develop on the intimal arterial surface. In humans, the early stage lesions are fatty streaks, which are not elevated on the intimal surface and contain predominately smooth muscle cells. These lesions may advance to plaque lesions that are elevated above the intimal surface and characterized by lipid-filled foam cells. The plaques may progress to advanced atheroma with a fibrous cap, lipid cores and accumulated connective matrices.6
Among the animal models for atherosclerosis, nonhuman primates are closest to humans and they are similar in many aspects of physiology, anatomy and genetics, and thus ideal for translational studies. Animal models such as mice, rabbits and pigs, are less expensive to maintain, engender minimal ethical concerns, and have contributed tremendously to our understanding of mechanisms of atherosclerosis.7, 8 Although small animal models are less useful in translational studies due to dissimilarities from humans, knowledge gained from the small animal models inform nonhuman primate studies for translation to humans. Baboons, in particular resemble humans in many physiological characteristics of lesions, lipid metabolism, response to dietary challenge, and spontaneous development of lesions. In addition, baboons develop atherosclerosis at many arterial sites as do humans, including coronary, iliac, aorta and carotid arteries. Furthermore, baboons, like humans, exhibit remarkable individual variation in atherosclerotic phenotypes9, allowing dissection of functional mechanisms underlying initiation and progression of atherosclerosis for early intervention and for precision medicine.
Previously we reported macro-histological characteristics of early atherosclerosis in baboons challenged with a high cholesterol, high fat (HCHF) diet for two years, including the composite correlation between lipoprotein concentrations and extent of lesions. The aim of our study is to address the micro-phenotypic variation of atherosclerotic lesions in baboons challenged with a HCHF diet for two years, and elucidate individual variation in lipoprotein and extent of atherosclerosis. Accurate assessment of individual atherosclerotic variation is fundamental for: 1) identification of tissue-specific genetic and epigenetic factors that underlie progression of atherosclerosis, which is not possible in humans; and 2) identification of molecular biomarkers indicative of early stage atherosclerosis.
To our knowledge no study has documented histological and morphometric variation of atherosclerotic lesions induced by a HCHF diet for a prolonged period of time in baboons. We report individual variation of atherosclerosis in right common iliac arteries (RCIA) by assessing histological and morphometric variation of lesion types, and between the affected and adjacent areas of RCIA. We hypothesized that maximum intimal thickness increases with the extent atherosclerosis, and that the maximum intimal thickness of an affected region is greater than that of an adjacent region. We chose to study the histology of atherosclerosis in RCIA because in our previous study9 we observed significant atherosclerotic lesion variation in this artery. In addition, previous studies have indicated that the extent of atherosclerosis in iliac arteries correlates with atherosclerosis in coronary arteries.10
MATERIALS AND METHODS
Baboon humane care
Adult baboons (Papio hamadryas) were maintained by the Southwest National Primate Research Center (SNPRC) at the Texas Biomedical Research Institute (Texas Biomed), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Practices in the housing and care of animals conformed to the regulations and standards of the US National Research Council’s Guide for the Care and Use of Laboratory Animals, Public Health and Safety Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals. The Texas Biomed Institutional Animal Care and Use Committee approved experimental protocols.
Dietary challenge
Baboons (n = 9) were challenged with a HCHF diet for two years. The HCHF diet contained saturated fat (40% of calories) and 1.7 mg cholesterol/kcal. The dietary challenge protocol has been described.9 In this study, we report microscopic evaluation of atherosclerotic lesions in the nine baboons that were challenged with the HCHF diet.
Sample collection
After two years of HCHF dietary challenge, the animals were euthanized using pentobarbital, 100mg/kg (Vortech Pharmaceuticals LTD, Dearborn, MI). Arteries, including RCIA were collected, carefully trimmed of all serosal fibro adipose tissue, opened lengthwise, pinned on a flat surface, and fixed in 10% neutral buffered formalin.
Artery staining and digital quantification of lesions
Staining of arteries:
RCIA were stained as described.6, 11 Briefly, the opened and pinned RCIA were stained with Sudan IV (Sigma-Aldrich, St Louis, MO) in 40% isopropanol for 18 hrs at room temperature. Tissues were rinsed twice in water and stored in 10% neutral buffered formalin.
Visual grading and quantification of lesions:
RCIA atherosclerotic lesions were graded from stained specimens as described.6 The extent of lesions was quantified previously9 using BioQuant Image Analysis software (BioQuant Image Analysis Corporation, Nashville, TN).
Tissue sampling for microscopic histological characterization:
Cross-sections were excised from stained affected areas and adjacent unstained areas. The sections were processed conventionally, embedded in paraffin, cut at 5μm, stained with Verhaeff van Gieson to delineate the arterial layers, internal elastic lamina and elastin fibers. Three samples for each phenotypic gross specimen, including a normal control were examined.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on 5μm sections of the RCIA using standard protocols.12 After optimization, we interrogated the localization of VSMC using smooth muscle cell alpha actin (SMαA) antibody and CD68 for macrophages. Sections were deparaffinized in xylene, rehydrated in descending grades of alcohol (100, 70, and 45%) to water, immersed in citrate buffer [0.01 m citrate buffer (pH 6.0)], and heated to boiling for 10–15 min for antibody retrieval. After cooling for 15 min, the sections were rinsed in potassium PBS [KPBS; 0.04 m K2HPO4, 0.01 m KH2PO4, 0.154 m NaCl (pH 7.4); seven times, 6 min each)] and washed for 10 min in a solution of 1.5% H2O2/methanol and then for 5 min in KPBS. Sections were placed in diluted (10%) normal serum for 20 min and covered with primary antibody overnight at 4 C. After overnight incubation, sections were rinsed in KPBS and incubated for 1 h at room temperature with secondary antibody. After serial dehydration in 50, 70, 95, and 100% ethanol and Histoclear (three times, 2–5 min each), sections were mounted and coverslipped (Histomount; National Diagnostics, Atlanta, GA). Images were acquired with a SPOT cooled camera (Diagnostic Instruments, Inc., Sterling Heights, MI) at a magnification of ×100.
Measurement of maximum intimal thickness
The maximum intimal thickness for each section stained with Verhaeff van Gieson, presenting a particular phenotype was measured using imageJ software (http://rsb.info.nih.gov/ij/). Scaled digital images of tissue sections were imported to imageJ software and the maximum intimal thickness (nm) measured perpendicular from internal elastic lamina to the endothelial layer.
Statistical analysis
Statistical significance between groups was tested using student t-test, p < 0.05.
RESULTS
Baboon RCIA atherosclerotic lesion phenotypes
After a two-year HCHF diet challenge we observed two RCIA early stage atherosclerotic phenotypes in baboons (Figure 1). The phenotypes were fatty streaks and plaque lesions. The control tissue had no lesions. For tissues with fatty streaks or plaques, there are contiguous regions with no lesions. The fatty streak lesions represent the initial stage of the disease. Plaques are considered advanced lesions. Lesions were profound at the bifurcation of the arteries.
Figure 1:
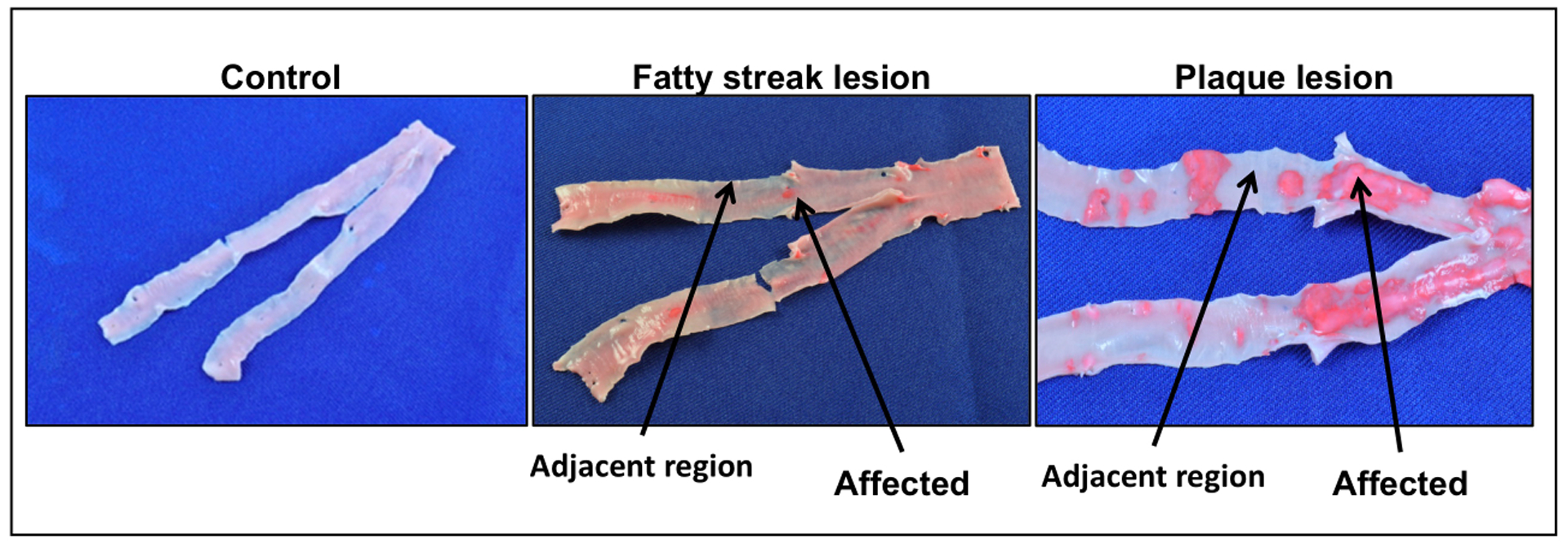
Baboon RCIA atherosclerotic phenotypes identified after 2-year HCHF diet challenge. Arrows indicate diseased and adjacent healthy regions. Lesions stained red with Sudan IV.
RCIA histology and morphology
Histological and morphological variation of RCIA lesions are shown in Figures 2.
Figure 2:
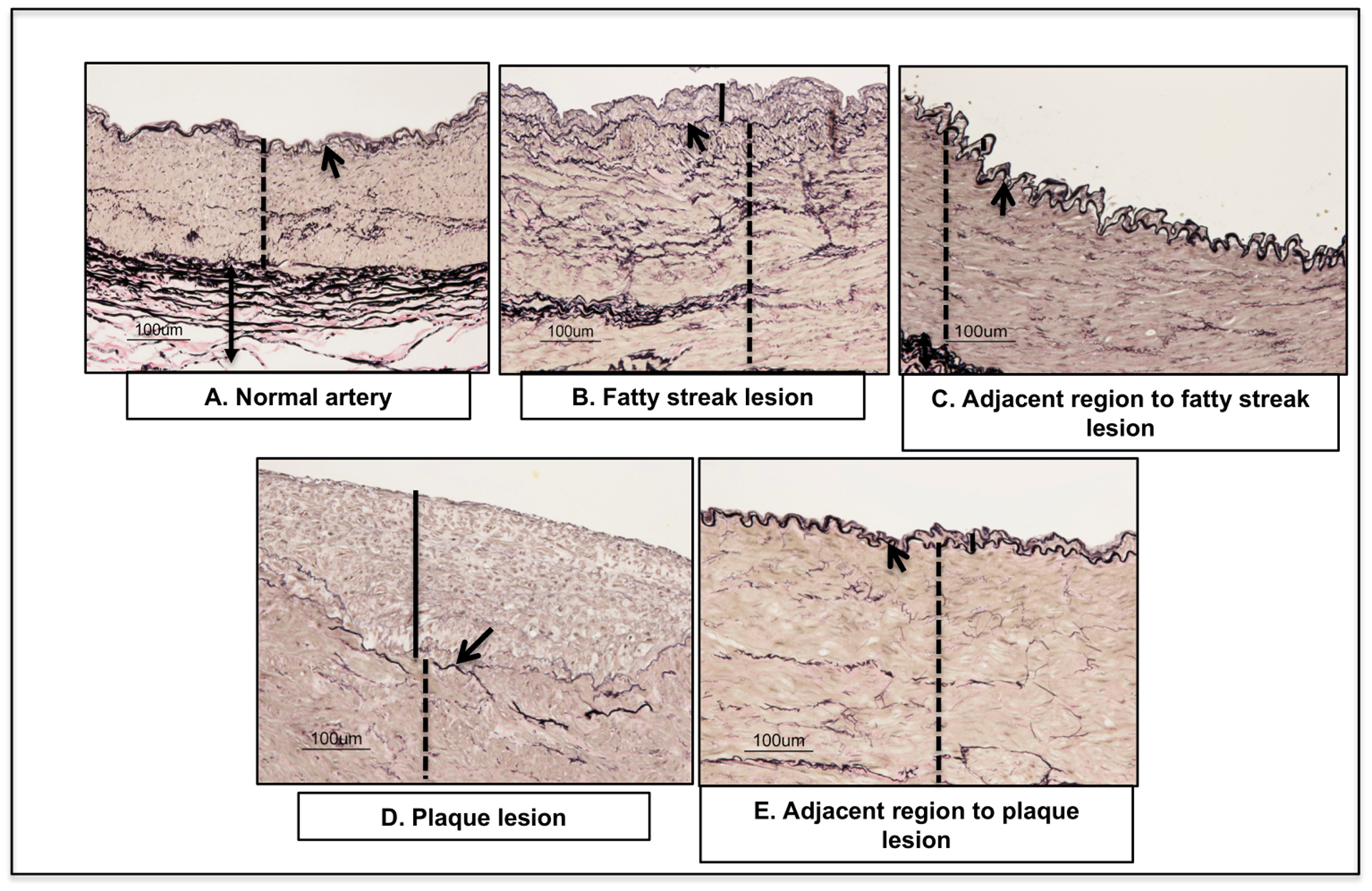
Validation of RCIA phenotypes: Cross-sections of the control, affected and adjacent regions stained with Verhaeff van Gieson displaying arterial layers: intima, and media layers). Arrow indicates fiber-like internal and external elastin lamina; arrow heads denote intimal. Solid line indicates the intimal layer; Dotted line indicates media layer.
Histologic changes in the fatty streak lesions were mild in nature and characterized by a combination of degenerative and hyperplastic changes. In all three samples, the intima was focally or multi-focally expanded by pale, loosely-arranged eosinophilic material, scattered VSMC and fibroblasts, and fine, wispy elastin fibers. The internal elastic lamina was variably duplicated or occasionally absent.
Histologic changes in regions adjacent to the fatty streak lesions varied. In two of the three samples, the artery was histologically unremarkable. In the third, much of the internal elastic lamina was duplicated and the intima expanded by pale, loosely-arranged eosinophilic material, scattered VMSC, and fibroblasts. This is likely an extension of the lesion into the adjacent, grossly normal-appearing tissue.
Histologic changes in the plaque lesions were minimal to mild in nature and characterized by extensive degenerative changes. In two of the three samples, the intima was expanded by a mixture of dense, eosinophilic, homogenous material and pale, loose areas that contained cells consistent with foamy macrophages, mononuclear cells, smooth muscle cells, and fibroblasts, with rare fine, wispy, disorganized elastin fragments. Changes in the third sample were similar, but extended into the media and were associated with scattered basophilic material.
Histologic changes in regions adjacent to the plaque lesions varied. As in the adjacent areas bordering fatty streak lesions, samples varied from being histologically unremarkable to exhibiting minimal expansion of the intima by VSMC, fibroblasts, and increased elastic fibers. This observation was consistent with an extension and reduction in severity of the lesion into the adjacent grossly normal appearing tissue.
Immunohistochemistry
Figure 3 shows the detection of (A) SMαA marker and (B) CD68 marker in different lesion types in RCIA. SMαA was profoundly detected in the media layer of all lesion types and adjacent regions. SMαA signal in the intimal layer decreased in parallel with progression of atherosclerosis. SMαA was detected minimally on the plaque lesions. CD68 was only detected on the neointima of the plaque lesions.
Figure 3:
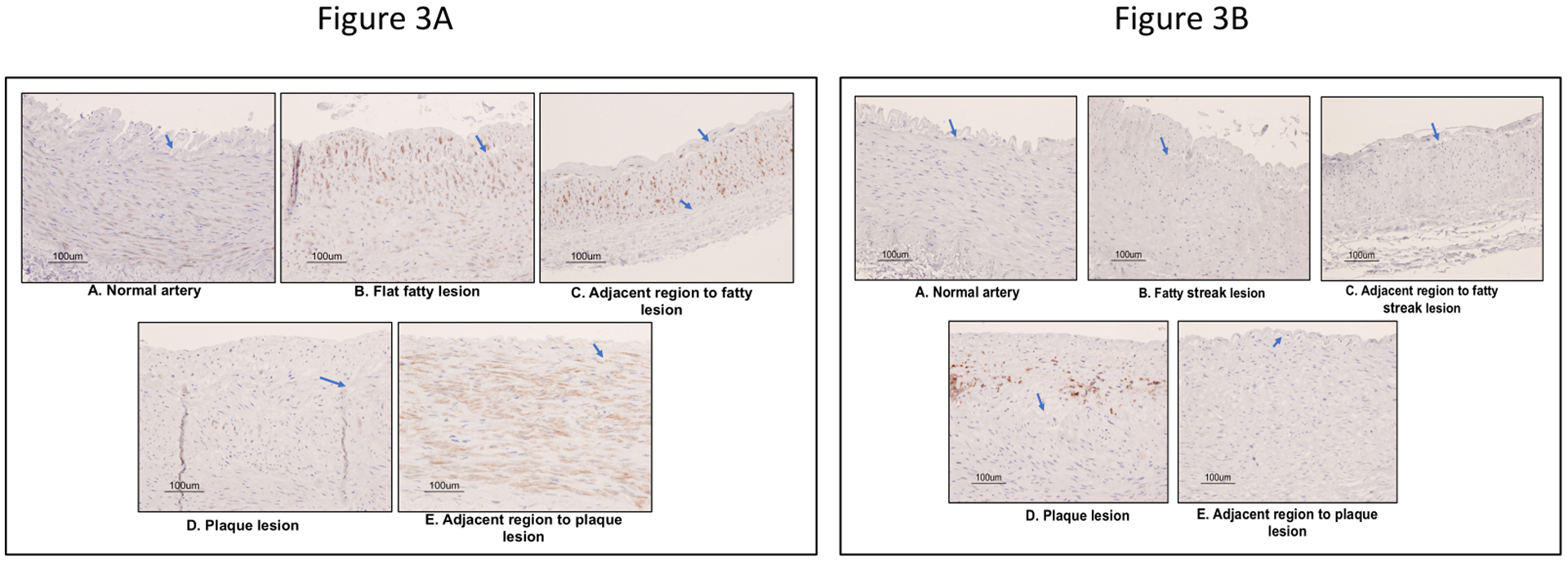
(A) Detection of vascular smooth muscle cell contractile phenotype marker (SMαA) and (B) macrophage marker (CD68) in early stage atherosclerotic lesions in baboons by immunohistochemistry. Brown stain indicates the detection of SMαA or CD68. Blue stain deficit the nucleus of vascular cells. The arrow indicates the elastic lamina.
RCIA maximum intimal thickness
Table 1 and Figure 4 depict maximal intimal thickness for the RCIA phenotypes. We observed that the maximal intimal thickness increased with extent of atherosclerosis and that the difference between the mean intimal thickness of varying lesions was significant. In addition, we found a significant difference in intimal thickness between the affected and the unaffected adjacent regions of the same artery.
Table 1:
Maximum intimal thickness of the lesions and the adjacent regions in baboon RCIA measured after two-year HCHF dietary challenge.
| Baboon # | Lesion (μm) | Adjacent Region (AR) (μm) |
|---|---|---|
| Fatty streak | AR of a fatty streak | |
| 1 | 100.62 | 26.09 |
| 2 | 104.97 | 23.9 |
| 3 | 65.64 | 33.95 |
| Mean ± SD | 90.41±21.56 | 27.98±5.29 |
| Plaque | AR of a plaque | |
| 4 | 238.75 | 35.8 |
| 5 | 203.75 | 26.54 |
| 6 | 329.56 | 24.85 |
| Mean ± SD | 257.35±64.94 | 29.06±5.90 |
| Control | ||
| 7 | 58.29 | |
| 8 | 56.14 | |
| 9 | 51.00 | |
| Mean ± SD | 55.13±3.73 | |
Figure 4:
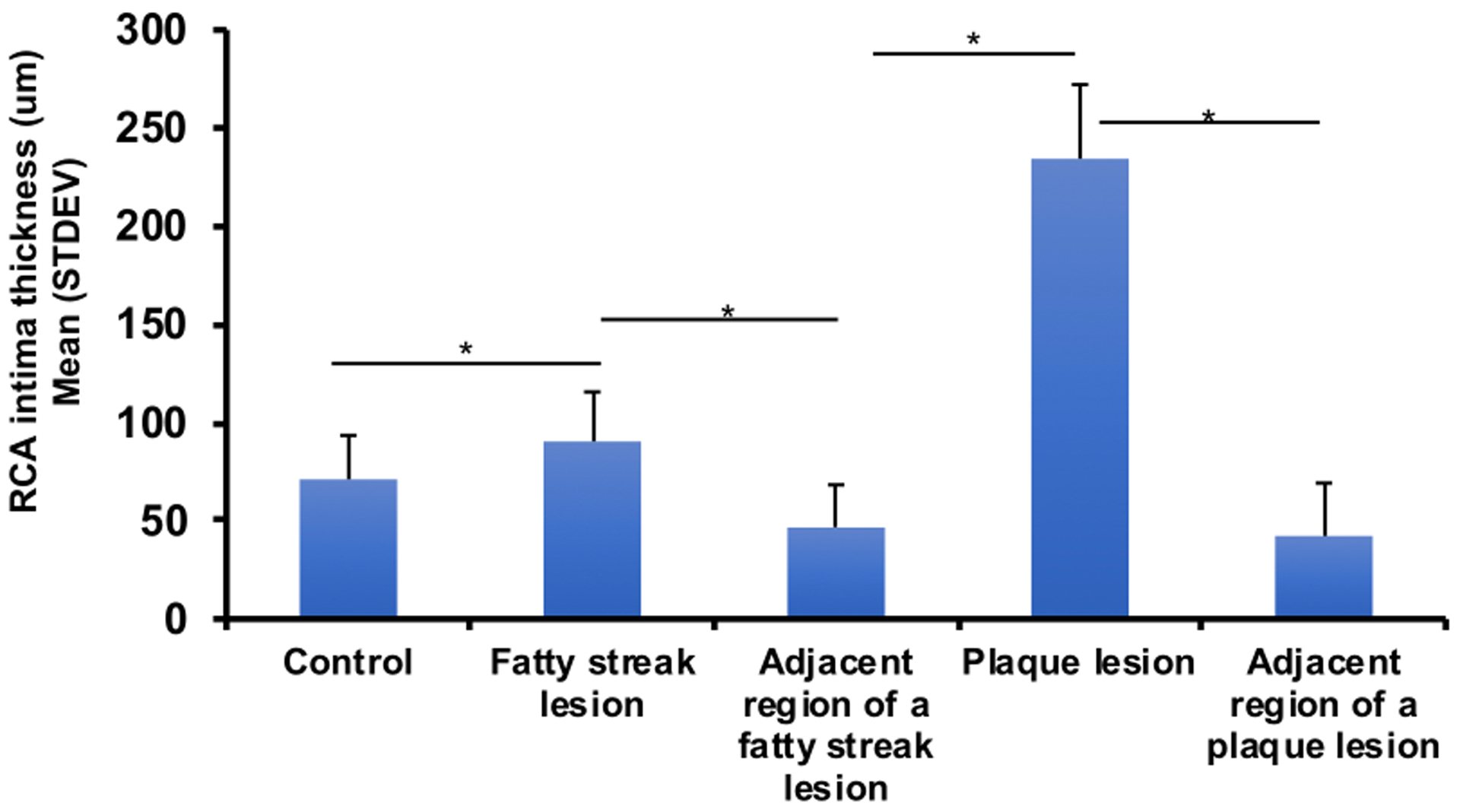
Baboon RCIA intima-media thickness after 2-year diet challenge with HCHF diet. X-axis denotes RCIA phenotypes; y-axis indicates mean ± SD of intimal thickness (um). Values embedded on bars depict sample sizes.
The maximal intimal thickness was lowest in adjacent regions to fatty streak lesions (27.98±5.29 μm) and greatest for the plaque lesions (257.35±64.94 μm). The maximal intimal thickness for adjacent regions was significantly less than that of the affected regions. In addition, the maximal intimal thickness for the control was significantly less than that of fatty streak lesion, which was less than that for the plaque lesion.
The extent of atherosclerosis was associated with intimal media thickness and concentration of plasma lipids. Figure 5 shows a scatter plot depicting the extent of atherosclerosis (measured by proportion of RCIA covered with lesions) and maximum intimal thickness. We observed that maximum intimal thickness is strongly correlated with the extent of atherosclerosis in baboons (Pearson correlation coefficient; r=0.83). Figure 6 shows the correlation between lipid profiles concentrations and extent of atherosclerosis. The concentrations of LDL-C, triglycerides (TG) and total cholesterol (TSC) were positively correlated with extent of atherosclerosis while the concentration of HDL-C was negatively correlated.
Figure 5:
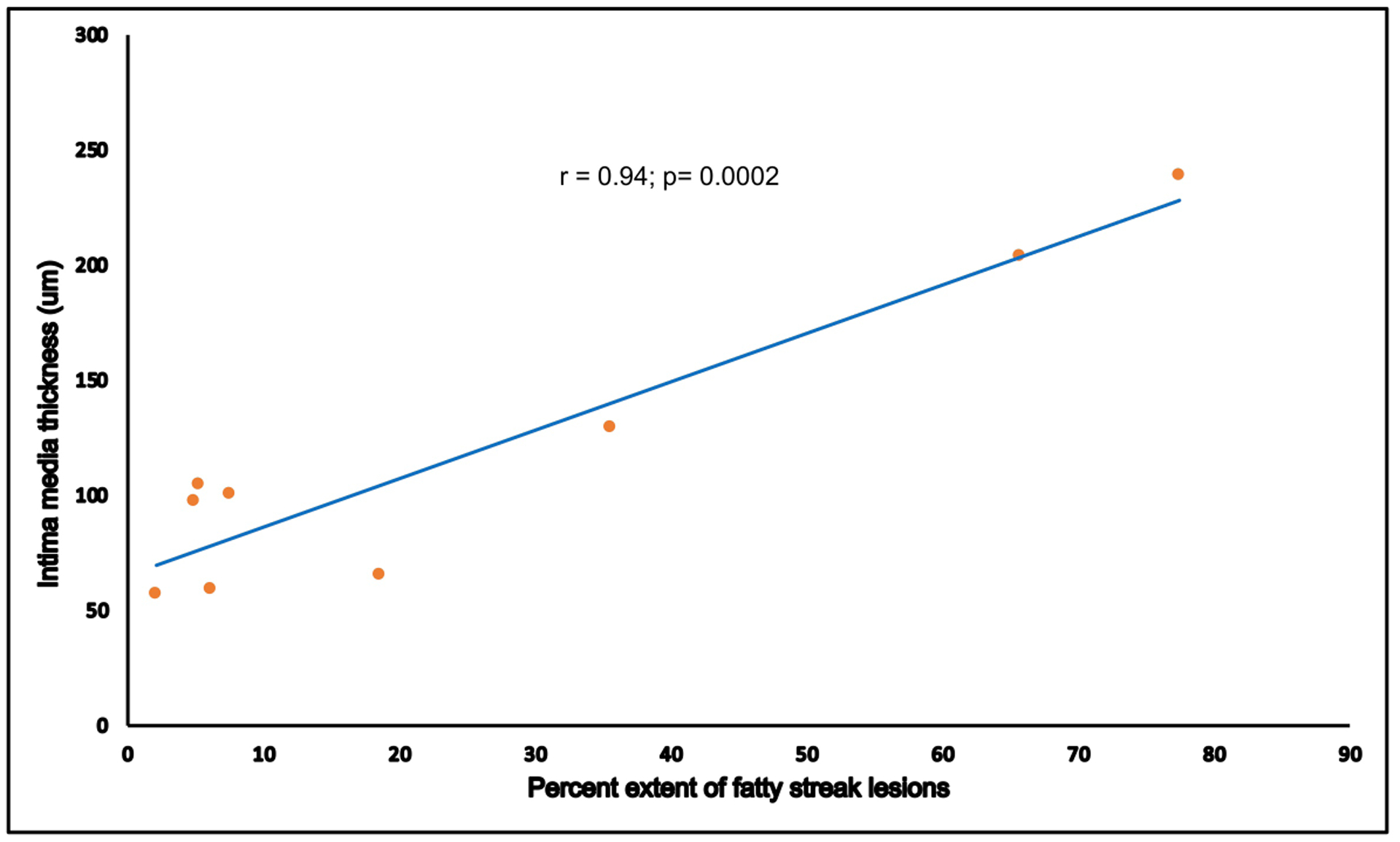
RCIA intimal thickness correlates with extent of atherosclerosis. X-axis denotes proportion of area covered with fatty streak lesions measured by digital computation using Bioquant Image Analyzer. Intimal thickness measurements are shown on the y-axis.
Figure 6:
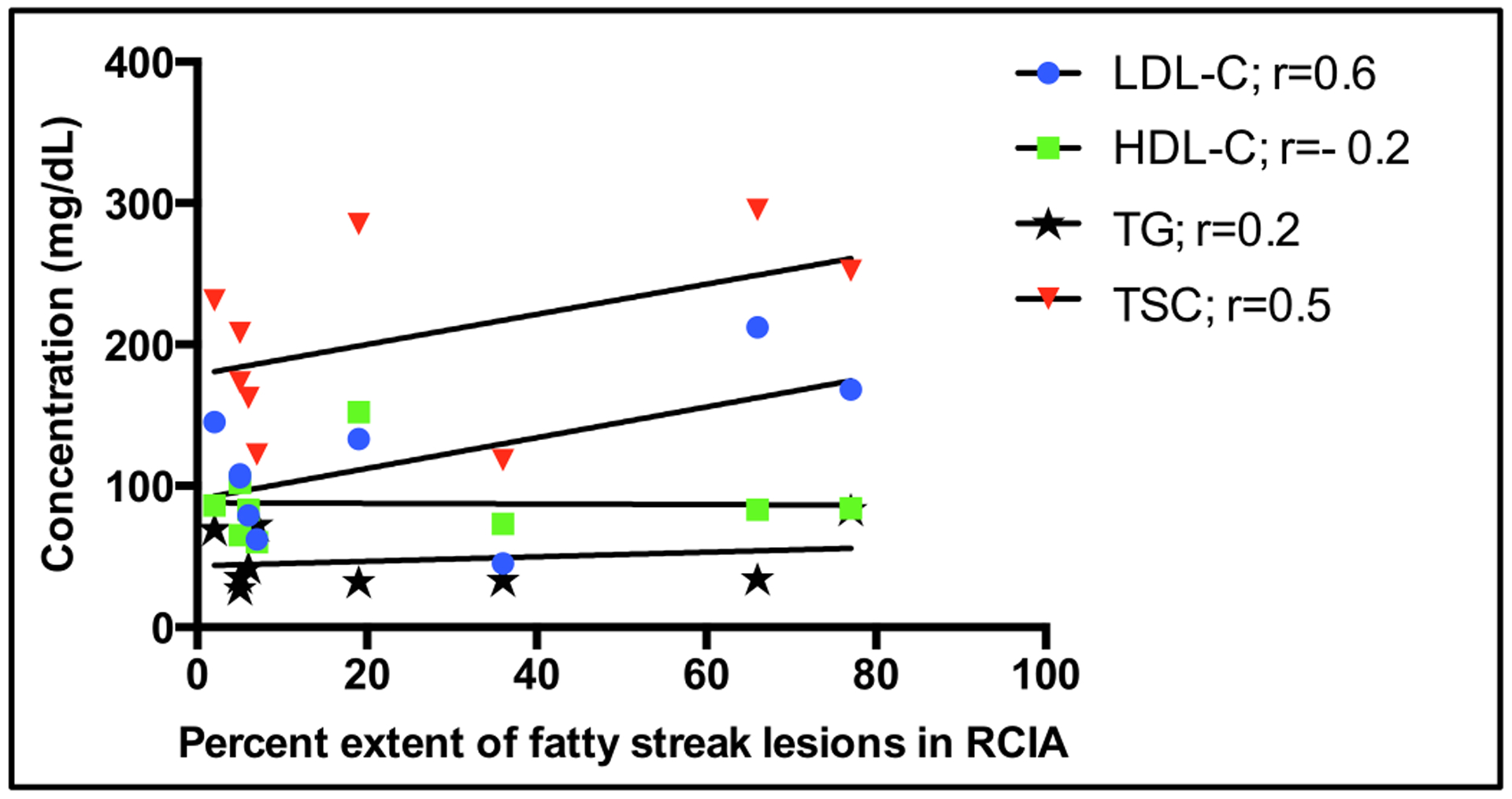
Correlation between percent extent of lesions in RCIA and serum lipid concentration in baboons (n=9) challenged with HCHF diet for two years. r = Pearson correlation coefficient.
DISCUSSION
Atherosclerosis is the primary cause of human CVD. Although the baboon is a well-characterized model for human atherosclerosis, very little is known about cellular changes during initiation of atherogenesis in baboons. To our knowledge, the microscopic histologic and morphologic changes characterizing the different types of early stage atherosclerotic lesions in baboons challenged with a HCHF diet, have not been documented. Detailed characterization of this phenotypic variation is fundamentally important to understand the biological mechanisms that underlie early stage atherosclerotic lesions, and to develop interventions to slow lesion progression to advanced fibrous plaques. The baboon naturally develops atherosclerosis13, and, as in humans, exhibits modest serum lipid response to HCHF diet, and susceptibility to atherosclerosis.14 Moreover, baboons show a great deal of individual variation in response to a HCHF diet.9 In addition, unlike the murine model, the baboon has the advantage of having lipoprotein metabolism similar to humans with a predominance of non-HDL lipoproteins, human-like HDL subclasses, expression of cholesteryl ester transfer protein15, as well as an immune system resembling that of humans.16 We report, for the first time, the histological and morphological characterization of early stage lesions in baboons. We note that there is a general increase in maximum intimal thickness with extent atherosclerosis as well as an increase in disruption of the intimal endothelial layer, degeneration of internal elastic lamina, and infiltration of cells, including macrophages and VSMC.
We previously observed that baboons challenged with a HCHF diet for two years developed two lesion types, including fatty streaks and plaques, suggesting variation in response to a stressor.9 These lesions were equivalent to Type II and III lesions that develop in young human adults.4, 17 In addition, we reported the composite correlation between extent of atherosclerosis and some lipidomic profiles.9 In the current study, we observed individual variation in lipidemia that is correlated with extent of atherosclerosis as reported previously in baboons and in humans.18, 19 This conclusion, together with similarities between baboons and humans, reinforces the baboon as a suitable model for translational research on molecular and cellular mechanisms that underlie human atherosclerosis.
We analyzed the correlation between maximum intimal thickness and extent of atherosclerosis measured, respectively, using ImageJ and Bioquant software packages. In our assessment of maximum intimal thickness, we maintained perpendicular measurements to avoid bias. Notably, measurement of maximum intimal thickness at different angles may result in differences, which biases the data. Accurate and consistence assessment of artery phenotypes is essential for subsequent experiments to discover biomarkers indicative of early stage atherosclerosis and genetic mechanisms that influence initiation of atherosclerosis. We observed for the first time, that maximum intimal thickness is strongly correlated with the extent of atherosclerosis. This observation concurs with a human study that indicated intimal thickness, measured by ultrasound is correlated with histology sections for the thickness of intimal plaque.20
Macrophages and VSMC play a significant role in the progression of atherosclerosis.21 VSMC display most plasticity among other vascular cells. In normal physiological conditions, differentiated VSMC are quiescent in the media layer, where they provide contractile properties to the tissue and express contractile proteins, including SMαA and smooth muscle myosin heavy chain (SMMHC). Both of these proteins are markers of mature differentiated VSMC. In response to endothelial injury, these markers are suppressed and cells switch phenotypes from a contractile differentiated cell to a synthetic proliferative cell, a phenomenon also known as dedifferentiation, whereinafter the cells migrate to the intimal layer and proliferate.22–24 In the case of macrophages, there is extensive evidence that monocyte-derived macrophages localize in the neointima where they are activated to play a pivotal role in progression of atherosclerosis.25
In our study we evaluated the expression of SMαA and CD68 in relation to the progression of early stage atherosclerotic lesions. We posited that the SMαA marker will be less evident in plaque lesions compared to control, fatty streak lesions and adjacent regions, and that the macrophage marker will be detected in the lesions. CD68 was only detected in plaques. We observed that SMαA was significantly reduced in the intimal layer of the plaques compared to the control, fatty streak lesions and adjusted regions. This observation concurs with our hypothesis and the central dogma of VSMC phenotype switching. SMαA marker is expected to be sparsely detected in the lesions due to switching of the VSMC phenotype from contractile to a proliferative phenotype and migrating to intima layer in response to endothelial dysfunction. In addition, we are aware of recent studies that have indicated that VSMC in the intima are the result of differentiation of progenitor cells resident in the tissue or from bone marrow26, and/or transdifferentiation from other vascular tissue cells.27 The later observation is controversial, potentially due to the different cell tracing techniques used for these studies.28–30
The lack of detection of the CD68 and presence of SMαA in the intima layer of fatty streak lesions shows the characteristics of an intermediary phenotype between control and plaque lesions. We demonstrate for first time in vivo in nonhuman primates the suppression of SMαA parallel to progression of atherosclerosis and our results provide potential insight on the role of VSMC in early stage atherosclerosis.
In conclusion, we observed development of early stage atherosclerosis in baboons in response to a two-year HCHF diet challenge, manifested by progressive disruption of the endothelial layer; increase in the number of macrophages, lipid-filled or without, in the intimal layer; and migration of VSMC from media to intimal layer of the plaque lesions. We observed morphological differences between the affected and adjacent regions of the same RCIA, including decreased number of contractile smooth muscle cells in the intimal layer and increased maximum intimal thickness in the affected regions compared to adjacent regions. In addition, we observed a positive correlation between maximum intimal thickness and extent of atherosclerosis. The results from this study document histological characteristics of, and variation in, early stage atherosclerotic lesions in baboons. This is a first step toward understanding the molecular factors underlying initiation and progression of atherosclerosis for the purpose of discovery of novel targets to prevent and treat atherosclerosis.
Funding information:
This study received support from the following: National Institutes of Health (NIH; Grant number: K01HL130697) received by GMK; American Heart Association, Post-Doctoral Fellowship (Grant number: 14POST18150019) received by GMK; NIH grant number: P01 HL028972 received by JLV; NIH Office of the Director (Grant number: P51 OD011133) received by JLV; NIH Office of the Director (Grant number: P51 RR013986) received by JLV; NIH (Grant numbers: C06 RR14578; C06 RR15456; C06 RR013556; C06 RR017515) supported the infrastructure that was used during the investigation of the work reported in the manuscript.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P: Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D: Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006, 3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushwaha RS, McGill HC Jr.: Diet, plasma lipoproteins and experimental atherosclerosis in baboons (Papio sp.). Hum Reprod Update 1998, 4:420–9. [DOI] [PubMed] [Google Scholar]
- 4.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W Jr., Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW: A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994, 89:2462–78. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima K, Kawano M, Hasegawa M, Taki J, Fujimoto M, Takehara K, Tonami N: Myocardial damages in systemic sclerosis detected by gated myocardial perfusion SPECT and sympathetic imaging. Circ J 2006, 70:1481–7. [DOI] [PubMed] [Google Scholar]
- 6.McGill HC, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP, Ather PD: Origin of atherosclerosis in childhood and adolescence. American Journal of Clinical Nutrition 2000, 72:1307s–15s. [DOI] [PubMed] [Google Scholar]
- 7.Emini Veseli B, Perrotta P, De Meyer GRA, Roth L, Van der Donckt C, Martinet W, De Meyer GRY: Animal models of atherosclerosis. Eur J Pharmacol 2017, 816:3–13. [DOI] [PubMed] [Google Scholar]
- 8.Shim J, Al-Mashhadi RH, Sorensen CB, Bentzon JF: Large animal models of atherosclerosis - new tools for persistent problems in cardiovascular medicine. J Pathol 2016, 238:257–66. [DOI] [PubMed] [Google Scholar]
- 9.Mahaney MC, Karere GM, Rainwater DL, Voruganti VS, Dick EJ Jr., Owston MA, Rice KS, Cox LA, Comuzzie AG, VandeBerg JL: Diet-induced early-stage atherosclerosis in baboons: Lipoproteins, atherogenesis, and arterial compliance. J Med Primatol 2018, 47:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB: Dominant Social-Status and Contraceptive Hormone-Treatment Inhibit Atherogenesis in Premenopausal Monkeys. Arterioscl Throm Vas 1995, 15:2094–100. [DOI] [PubMed] [Google Scholar]
- 11.Guzman MA, Mcmahan CA, Mcgill HC, Strong JP, Tejada C, Restrepo C, Teggen DA, Robertson WB, Solberg LA: Selected Methodologic Aspects of International Atherosclerosis Project. Laboratory Investigation 1968, 18:479-+. [PubMed] [Google Scholar]
- 12.Li C, Schlabritz-Loutsevitch NE, Hubbard GB, Han V, Nygard K, Cox LA, McDonald TJ, Nathanielsz PW: Effects of Maternal Global Nutrient Restriction on Fetal Baboon Hepatic Insulin-Like Growth Factor System Genes and Gene Products. Endocrinology 2009, 150:4634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mcgill HC, Strong JP, Holman RL, Werthessen NT: Arterial Lesions in the Kenya Baboon. Circ Res 1960, 8:670–9. [Google Scholar]
- 14.Kushwaha RS, McGill HC: Diet, plasma lipoproteins and experimental atherosclerosis in baboons (Papio sp.). Human Reproduction Update 1998, 4:420–9. [DOI] [PubMed] [Google Scholar]
- 15.Chan JKG, Cox LA, VandeBerg JL: Animal Models of Diet-induced Hypercholesterolemia Hypercholesterolemia: Edited by Kumar SA. Rijeka, IntechOpen, 2015. [Google Scholar]
- 16.Mestas J, Hughes CC: Of mice and not men: differences between mouse and human immunology. Journal of immunology (Baltimore, Md : 1950) 2004, 172:2731–8. [DOI] [PubMed] [Google Scholar]
- 17.McGill HC, McMahan CA, Herderick EE, Tracy RE, Malcom GT, Zieske AW, Strong JP, Grp PR: Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. Arterioscl Throm Vas 2000, 20:836–45. [DOI] [PubMed] [Google Scholar]
- 18.Mcgill HC, Mcmahan CA, Kruski AW, Mott GE: Relationship of Lipoprotein Cholesterol Concentrations to Experimental Atherosclerosis in Baboons. Arteriosclerosis 1981, 1:3–12. [DOI] [PubMed] [Google Scholar]
- 19.McGill HC, McMahan CA, Zieske AW, Tracy RE, Malcom GT, Herderick EE, Strong JP, Ather PD: Association of coronary heart disease risk factors with microscopic qualities of coronary atherosclerosis in youth. Circulation 2000, 102:374–9. [DOI] [PubMed] [Google Scholar]
- 20.Mallery JA, Tobis JM, Griffith J, Gessert J, Mcrae M, Moussabeck O, Bessen M, Moriuchi M, Henry WL: Assessment of Normal and Atherosclerotic Arterial-Wall Thickness with an Intravascular Ultrasound Imaging Catheter. American Heart Journal 1990, 119:1392–400. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Wang Z, Zhang L, Wang Y: Roles of Cells from the Arterial Vessel Wall in Atherosclerosis. Mediators Inflamm 2017, 2017:8135934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamley JH, Campbell GR, Burnstock G: Dedifferentiation, redifferentiation and bundle formation of smooth muscle cells in tissue culture: the influence of cell number and nerve fibres. J Embryol Exp Morphol 1974, 32:297–323. [PubMed] [Google Scholar]
- 23.Owens GK: Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 1995, 75:487–517. [DOI] [PubMed] [Google Scholar]
- 24.Ross R: The pathogenesis of atherosclerosis. Mechanisms of ageing and development 1979, 9:435–40. [DOI] [PubMed] [Google Scholar]
- 25.Tabas I, Lichtman AH: Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017, 47:621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett MR, Sinha S, Owens GK: Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res 2016, 118:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji H, Atchison L, Chen Z, Chakraborty S, Jung Y, Truskey GA, Christoforou N, Leong KW: Transdifferentiation of human endothelial progenitors into smooth muscle cells. Biomaterials 2016, 85:180–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bentzon JF, Falk E: Circulating smooth muscle progenitor cells in atherosclerosis and plaque rupture: current perspective and methods of analysis. Vascul Pharmacol 2010, 52:11–20. [DOI] [PubMed] [Google Scholar]
- 29.Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, Furuya A, Kuro-o M, Sata M, Nagai R: Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation 2010, 122:2048–57. [DOI] [PubMed] [Google Scholar]
- 30.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R: Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 2002, 8:403–9. [DOI] [PubMed] [Google Scholar]


