Abstract
The molecular mechanisms underlying the diverse psychiatric and neuropathological sequalae documented in subsets of athletes with concussion have not been identified. We have previously reported elevated quinolinic acid (QuinA), a neurotoxic kynurenine pathway metabolite, acutely following concussion in football players with prior concussion. Similarly, work from our group and others has shown that increased functional connectivity strength, assessed using resting state fMRI, occurs following concussion and is associated with worse concussion-related symptoms and outcome. Moreover, other work has shown that repetitive concussion may have cumulative effects on functional connectivity and is a risk factor for adverse outcomes. Understanding the molecular mechanisms underlying these cumulative effects may ultimately be important for therapeutic interventions or the development of prognostic biomarkers. Thus, in this work, we tested the hypothesis that the relationship between QuinA in serum and functional connectivity following concussion would depend on the presence of a prior concussion. Concussed football players with prior concussion (N=21) and without prior concussion (N=16) completed a MRI session and provided a blood sample at approximately 1 days, 8 days, 15 days, and 45 days post-injury. Matched, uninjured football players with (N=18) and without prior concussion (N=24) completed similar visits. The association between QuinA and global connectivity strength differed based on group (F(3, 127)=3.46, p=0.019); post-hoc analyses showed a positive association between QuinA and connectivity strength in concussed athletes with prior concussion (B=16.05, SE = 5.06, p=0.002, 95%CI[6.06, 26.03]), but no relationship in concussed athletes without prior concussion or controls. Region-specific analyses showed that this association was strongest in bilateral orbitofrontal cortices, insulae, and basal ganglia. Finally, exploratory analyses found elevated global connectivity strength in concussed athletes with prior concussion who reported depressive symptoms at the 1-day visit compared to those who did not report depressive symptoms (t(15)=2.37, mean difference=13.50, SE=5.69, p=0.032, 95%CI[1.36, 25.63], Cohen’s d =1.15.). The results highlight a potential role of kynurenine pathway (KP) metabolites in altered functional connectivity following concussion and raise the possibility that repeated concussion has a “priming” effect on KP metabolism.
Keywords: kynurenine pathway, resting state, mild traumatic brain injury
1. Introduction
Reports of increased psychiatric (e.g., major depressive disorder [MDD]) and neurodegenerative disease (e.g., chronic traumatic encephalopathy) in retired and deceased athletes have led to an increase in public concern regarding the potential adverse, long-term effects of sport-related concussion (SRC) on athletes of all ages (Guskiewicz et al., 2007; Manley et al., 2017; McKee et al., 2013). However, the molecular mechanisms underlying the diverse psychiatric and neuropathological sequalae documented in subsets of athletes with concussion have yet to be definitively determined. Here, we focus on the kynurenine pathway (KP; Supplementary Figure 1), a key energy-regulating pathway that has been hypothesized to link inflammation and glutamatergic signaling in numerous psychiatric (e.g., MDD) and neurodegenerative diseases (e.g., Alzheimer’s disease) (Savitz, 2020).
Activated immune cells require more energy than naïve cells and the KP is the major endogenous source of nicotinamide adenine dinucleotide (NAD+). Inflammatory mediators increase the catabolism of tryptophan (TRP) into kynurenine (KYN) via the enzyme indoleamine 2,3-dioxygenase (IDO). Kynurenine-3-monooxygenase (KMO), which is also activated by inflammatory cytokines, converts KYN into 3-hydroxykynurenine (3HK), ultimately leading to the formation of quinolinic acid (QuinA) and NAD+. 3HK is a potent free-radical generator while QuinA is a N-methyl-D-aspartate (NMDA) receptor agonist that potentiates the release and impairs the reuptake of glutamate (Guillemin, 2012). Alternatively, KYN is metabolized by kynurenine aminotransferase (KAT) enzymes into kynurenic acid (KynA), a competitive antagonist of ionotropic excitatory amino acid receptors, including NMDA, that is generally considered to be neuroprotective (Foster et al., 1984; Kessler et al., 1989). As an illustrative example, we have previously shown reductions in KynA relative to QuinA (KynA/QuinA; a putative neuroprotective index) in MDD patients compared to controls (Savitz et al., 2015c, 2015b), and decreases in KynA and/or elevations in QuinA have been reported in several neurodegenerative disorders (Heilman et al., 2020; Sorgdrager et al., 2019).
Evidence suggests that after concussion, and traumatic brain injury (TBI) of all severities, the KP is activated and metabolism down the QuinA/NAD+ branch of the KP is favored - although the increase in QuinA could also arise from decreased breakdown of QuinA into NAD+, as has been shown in a number of in vitro and non-TBI preclinical models (Jones et al., 2015; Minhas et al., 2019; Poyan Mehr et al., 2018; Sahm et al., 2013). Irrespective, elevated KYN relative to TRP (KYN/TRP; a proxy for IDO activity) has been reported in chronic TBI patients (Mackay et al., 2006) and acutely injured TBI patients have elevated QuinA in cerebral spinal fluid compared to controls (Bell et al., 1999; Sinz et al., 1998; Yan et al., 2015). Regarding concussion, our group has previously shown decreased KynA/QuinA and elevated QuinA in plasma in concussed football players at approximately 1 day, 1 week and 1 month post-concussion relative to uninjured football players (Singh et al., 2016). Likewise, we have reported elevated plasma QuinA in football players with a remote history of concussion (i.e., latest concussion on average 10 months prior to visit) versus football players without prior concussion (Meier et al., 2016b). Finally, we have recently shown in an independent cohort of high school and collegiate athletes that recently concussed football players with a prior concussion had reduced serum KynA/QuinA and elevated QuinA across multiple time points up to 45 days post-injury compared to controls and acutely concussed athletes without a prior concussion (Meier et al., 2020b). Taken together, these findings suggest that prior concussion may result in a predisposition towards metabolism down the QuinA pathway or its preferential accumulation.
Parallel findings demonstrate that acute concussion is associated with differences in intrinsic brain connectivity as measured by resting state functional magnetic resonance imaging (rs-fMRI). Functional connectivity abnormalities have been repeatedly demonstrated acutely and sub-acutely following SRC, though specific findings have varied across studies likely due to differences in methodology (e.g., selection of seed-regions or networks versus global metrics), differences in cohorts, and differences in study design (e.g., time since injury, comparison groups) (for review; [Mayer et al., 2015; McCrea et al., 2017]). In an overlapping sample with the aforementioned study investigating KP metabolites in football players up to 45 days post-injury, we recently found elevated global connectivity strength at the sub-acute phase in concussed football players relative to controls, with sub-analyses demonstrating that this effect was driven by symptomatic athletes after concussion, rather than those that no longer reported symptoms (Kaushal et al., 2019). Additional work further supports the hypothesis that hyperconnectivity (i.e., stronger connectivity relative to controls) may be pathological following concussion (i.e., is associated with poorer recovery or more symptoms) (N. W. Churchill et al., 2017; Meier et al., 2020a; van der Horn et al., 2017), though opposite patterns have also been reported (for review; (Puig et al., 2020)).
There is also evidence of altered functional connectivity due to chronic or repetitive SRC. Prior work has shown both positive and negative associations between connectivity and prior concussion. For example, retired football players with multiple prior concussions had increased connectivity between the anterior temporal lobe and orbitofrontal cortex relative to healthy controls (Goswami et al., 2016). Asymptomatic hockey players with prior concussion had region specific increases and decreases in connectivity of the default mode network relative to players with no prior concussion (Orr et al., 2016). Finally, the number of prior concussions was inversely or positively associated with connectivity across several seed regions in men and women collegiate athletes (N. Churchill et al., 2017).
We have previously reported increased connectivity between the motor cortex and supplementary cortex in football players with prior concussion relative to those without (Meier et al., 2017). Moreover, in that same work we identified associations between rs-fMRI and neuroactive KP metabolites in collegiate athletes with varying concussion history, consistent with the indirect relationship between glutamatergic neurotransmitter flux (i.e., cycling of glutamate and glutamine) and the blood-oxygen-level-dependent (BOLD) signal (Hyder et al., 2002; Smith et al., 2002). Specifically, across all participants, lower plasma KynA/QuinA was associated with greater functional connectivity between the anterior cingulate cortex, orbitofrontal cortex, hippocampus, and motor cortex to several regions including the insula, superior temporal gyrus, and visual cortex. Most relevant to the current work, prior concussion status moderated the association between connectivity and KP metabolites. That is, football players with prior concussion predominantly showed an inverse association between KynA/QuinA and connectivity of the ACC (to frontal cortex and anterior insula) as well as connectivity of the hippocampus (to visual cortex) (Meier et al., 2017).
The current study expands upon the aforementioned findings of increased functional connectivity and elevated QuinA (and reduced KynA/QuinA) following concussion in overlapping samples of high school and collegiate athletes (Kaushal et al., 2019; Meier et al., 2020b). Our goal was to determine the extent to which associations between functional connectivity and neuroactive KP metabolites differ based on acute concussion status (i.e., recent concussion versus. no recent concussion) and prior concussion history (i.e., prior concussion versus no prior concussion). Based on our prior work (Kaushal et al., 2019; Meier et al., 2020b, 2017, 2016b; Singh et al., 2016), we hypothesized that elevated QuinA and lower KynA/QuinA would be associated with increased global functional connectivity in acutely injured athletes with prior concussion.
2. Materials and Methods
2.1. Participants
High school and collegiate football players were enrolled as part of a prospective study of concussion, which has been detailed previously (Kaushal et al., 2019; Meier et al., 2020b). Exclusion criteria for the current study included: injury precluding participation in the study or other contraindications to study procedures, current narcotic use, conditions known to cause cognitive dysfunction (e.g., moderate to severe TBI, epilepsy), psychopathology (e.g., mood disorders), migraines or recurrent headaches, attention deficit/hyperactivity disorder, memory difficulties, structural MRI findings that required clinical follow-up (Klein et al., 2019), and a history of a potentially confounding illness/disease (e.g., meningitis; full list of exclusionary diseases can be found in Supplementary Table 1). The study was approved by the institutional review board at the Medical College of Wisconsin. Adult participants and parents of minors provided written informed consent; minors provided written assent.
Football players completed preseason baseline clinical assessments. Players that sustained a concussion during the study period completed up to four follow-up visits that included an MRI session and blood collection: approximately 24–48 hours (1d), 8 days (8d), 15 days (15d), and 45 days (45d) post-injury. Certified athletic trainers or team physicians trained in sports medicine initially identified and diagnosed concussions. Study investigators screened all injuries to ensure they met the study definition of concussion, which was based on the Centers for Disease Control and Prevention HEADS UP educational initiative: “An injury resulting from a forceful bump, blow, or jolt to the head that results in rapid movement of the head and causes a change in the athlete’s behavior, thinking, physical functioning, or the following symptoms: headache, nausea, vomiting, dizziness/balance problems, fatigue, difficulty sleeping, drowsiness, sensitivity to light/noise, blurred vision, memory difficulty, and difficulty concentrating”.
Uninjured football players without concussion in the last 6 months were selected from enrolled athletes to match injured athletes based on the following criteria: level of competition, institution, team, estimated intellectual functioning (word reading performance at baseline), race, handedness, concussion history, and position. Control participants completed the same study protocol as concussed participants at similar intervals.
A total of 37 football players with concussion and 42 uninjured football players met the study criteria and had MRI and blood data from at least one follow-up visit. For the purposes of the current study, concussed and uninjured athletes were further characterized based on concussion history, as in our prior work (Meier et al., 2020b). Final groups included concussed athletes without prior concussion (SRC-; n=16), concussed athletes with prior concussion (SRC+; n=21), contact controls without prior concussion (CC-; n=24), and contact controls with prior concussion (CC+; n=18). The demographic details for each group are presented in Table 1.
Table 1:
Sample Characteristics and Demographics
| SRC− | SRC+ | CC− | CC+ | Statistic | |
|---|---|---|---|---|---|
| Total No. Participants | 16 | 21 | 24 | 18 | |
| No. by Visit (1d/8d/15d/45d) | 13/12/12/12 | 17/19/15/15 | 21/21/20/19 | 16/18/17/15 | X2(9)=0.47, p=1.00 |
| Age | 17.81(1.83) | 18.05(1.75) | 18.08(1.47) | 18.39(1.91) | F(3, 75)=0.32, p=0.81 |
| Race, No. White/Non-White, NR, or Unknown | 10/6 | 15/6 | 14/10 | 15/3 | X2(3)=3.33, p=0.34 |
| Ethnicity, No. Not Hispanic/Hispanic, NR or Unknown | 16/0 | 16/5 | 19/5 | 17/1 | FET=6.06, p=0.08 |
| WTAR Standard Score | 99.94(18.88) | 99.10(11.97) | 98.17(12.40) | 105.22(14.41) | F(3, 75)=0.94, p=0.42 |
| No. Participants in College | 12 | 15 | 19 | 12 | FET=0.99, p=0.86 |
| Body Mass Index | 27.68(4.83) | 28.12(4.56) | 30.31(5.74) | 28.20(5.22) | F(3, 75)=1.14, p=0.34 |
| Years of Participation in Sport | 7.13(2.50) | 7.60(2.80) | 7.83(2.33) | 8.39(2.75) | F(3, 74)=0.71, p=0.55 |
| Median [IQR] No. of Prior Concussions | 0 [0,0] | 1 [1,2] | 0 [0,0] | 1 [1,2] | H=69.59, p<0.001 |
| Clinical Measures at Baseline | |||||
| BSI-GSI Raw Score | 1.88(2.68) | 4.76(8.83) | 2.79(3.22) | 1.94(2.59) | Welch’s F(3, 40)=0.94, p=0.43 |
| SCAT-3 Symptom Severity | 1.88(2.66) | 3.24(6.46) | 3.04(6.23) | 1.83(2.62) | F(3, 74)=0.41, p=0.74 |
| BESS Total Score | 12.87(2.80) | 11.71(4.44) | 12.04(3.11) | 9.72(3.80) | F(3, 74)=2.34, p=0.08 |
| SAC Total Score | 26.13(2.31) | 26.19(2.02) | 25.83(2.08) | 25.78(2.96) | F(3, 75)=0.15, p=0.93 |
| Clinical Measures at 1d | |||||
| BSI-GSI Raw Score | 7.31(6.93) | 4.24(5.07) | 0.81(1.33) | 1.00(1.63) | Welch’s F(3, 28)=5.73, p=0.003 |
| SCAT-3 Symptom Severity | 25.08(23.31) | 18.12(16.57) | 2.24(3.42) | 0.69(1.40) | Welch’s F(3, 28)=11.25, p<0.001 |
| BESS Total Score | 13.00(6.81) | 9.75(5.26) | 9.50(4.21) | 8.31(3.77) | F(3, 60)=2.16, p=0.10 |
| SAC Total Score | 25.62 (2.22) | 26.41(2.00) | 25.71(2.63) | 25.81(3.31) | F(3, 63)=0.31, p=0.82 |
| Median [IQR] Duration of Symptoms in Days | 6.5 [4.75, 9.5] | 7 [5, 12] | - | - | H=0.14, p=0.71 |
Note: Values are expressed as mean (standard deviation) unless otherwise noted. SRC- = concussed participants without prior concussion, SRC+ = concussed participants with prior concussion, CC- = contact controls without prior concussion, CC+ = contact controls with prior concussion, No. = Number, 1d = 1 day visit, 8d = 8 day visit, 15d = 15 day visit, 45d = 45 day visit, NR = not reported, WTAR = Wechsler Test of Adult Reading, IQR = interquartile range, BSI-GSI = Brief Symptom Inventory Global Severity Index, SCAT = The Sport Concussion Assessment Tool, BESS = Balance Error Scoring System, SAC = Standardized Assessment of Concussion, FET = Fisher’s Exact Test, H = Kruskal-Wallis chi-square.
2.2. Clinical battery
The clinical battery has been described in detail previously (Kaushal et al., 2019; Meier et al., 2020b). Data collected at baseline included demographic and health information and the Wechsler Test of Adult Reading to estimate intellectual functioning, (WTAR). In addition, athletes were asked about their concussion history at baseline after being provided with a standard definition based on the United States Department of Defense (Carney et al., 2014). The clinical battery included measures of psychological distress (Brief Symptom Inventory–18; BSI-18), concussion symptom severity (The Sport Concussion Assessment Tool–3rd Edition symptom; SCAT), balance deficits (Balance Error Scoring System; BESS), and neurocognitive performance (Standardized Assessment of Concussion; SAC). Information regarding acute injury characteristics and length of recovery was collected at follow-up visits.
2.3. Blood biomarker data
Venous blood was collected using Red Top BD Vacutainer tubes, left to clot at room temperature for 30 min, centrifuged at 1,500 RCF for 15 min and stored at -80 °C. Quinolinic acid (QuinA), kynurenic acid (KynA), 3-hydroxykynurenine (3HK), tryptophan (TRP), and kynurenine (KYN) concentrations were determined from serum blind to diagnosis using high-performance liquid chromatography with tandem mass spectrometry detection by Charles River Laboratories, Inc. according to their standard protocol.
2.4. Imaging parameters and processing
Imaging data were obtained on a 3 Tesla General Electric MR750 whole‐body MR scanner using 32‐channel receiver coil array. Rs-fMRI data were collected using a gradient‐echo echo‐planar image (EPI) with the following parameters: 501 volumes, FOV=210 mm, acquisition matrix=104 × 104, slice thickness=2 mm, 72 sagittal slices, TR/TE=720/30 ms, flip angle=50°, hyperband acceleration factor=8. During the rs-fMRI scan, participants were instructed to keep their eyes open and think of nothing in particular. A reverse phase‐encoded scan was collected to allow susceptibility‐induced distortion correction. High‐resolution T1‐weighted structural images were obtained for anatomical reference using a magnetization‐prepared rapid gradient‐echo sequence with the following parameters: FOV=256 mm, acquisition matrix=256, slice thickness=1 mm, 160 slices, TR/TE/TI=7.592/3.008/900 ms, flip angle=8°.
Unless otherwise noted, preprocessing was performed using Analysis of Functional NeuroImages programs (AFNI) (Cox, 1996) as previously described (Kaushal et al., 2019). Anatomical images were skull‐stripped in native space using a union mask of segmented gray matter and white matter from SPM 12. The skull‐stripped brain was registered to the MNI‐152 template using an affine registration with correlation ratio cost function and trilinear interpolation followed by a nonlinear warp, implemented in FSL (Jenkinson et al., 2002). The first 29 volumes of the resting‐state scan were removed to account for auto‐calibration data and allow for stabilization of longitudinal magnetization, and the AFNI program 3dDespike was used to remove signal spike artifacts. Susceptibility‐induced distortion correction was performed using FSL’s topup (Andersson et al., 2003). Volumes were registered to the first volume to account for head motion. A single transformation matrix was created for spatial normalization by concatenating the anatomy-to-MNI‐152 matrix and the matrix resulting from a 6°‐of‐freedom registration of the rs-fMRI volume to the anatomical scan calculated using FSL’s FLIRT with the boundary‐based registration cost‐function (Greve and Fischl, 2009; Jenkinson et al., 2002). The resulting matrix and the nonlinear warp from the anatomy-to-MNI‐152 brain were applied to the motion corrected image to bring the image in standard space with 2 mm isotropic resolution.
Signals of no‐interest, including the average CSF signal, average white matter signal, the six motion parameters and their derivatives, and the zero‐ through third‐order polynomial trends were then regressed from the rs-fMRI data. Volumes with excessive head motion (i.e., Euclidian norm of the six motion parameters >0.30) were removed along with the preceding volume and replaced using interpolation. Denoised images were then bandpass filtered (0.01 to 0.10 Hz). Resting‐state scans with visually-identified artifacts and scans in which the average Euclidian norm of motion parameters was greater than 0.2 were excluded from analyses to minimize potential effects of motion on group analyses. Table 1 shows the sample size with usable data at each time point.
The AFNI program 3dNetCorr was used to calculate a connectivity matrix for each participant at each time point using regions-of-interest (ROI; or nodes) derived from the automated anatomical labeling atlas (AAL2) (Rolls et al., 2015; Taylor and Saad, 2013). The connectivity strength (i.e., nodal strength) of each individual ROI was calculated from the weighted connectivity matrices as the sum of weights of all connections to that ROI, implemented in the BRain analysis using GraPH theory (BRAPH) software (Mijalkov et al., 2017; Rubinov and Sporns, 2010). The average strength across all ROIs was calculated as a measure of global connectivity strength (i.e., average nodal strength). Negative correlations were not included in calculations of connectivity strength. To ensure that the observed results were not sensitive to the selected brain atlas, identical procedures were performed using the Craddock whole-brain functional atlas (200 ROI atlas based on rt 2-level parcellation) (Craddock et al., 2012).
2.5. Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics version 24 (Armonk, NY) unless otherwise indicated. Analyses of variance, Kruskal-Wallis tests, chi-square tests, or Fisher’s exact tests compared demographic variables, clinical measures, and head motion during scanning across groups. For descriptive purposes, clinical variables (i.e., BSI-18 global severity index, SCAT, BESS, SAC) were compared between groups at baseline and at the 1d visit. Individual KP markers (e.g., QuinA) and the relevant ratios (e.g., KynA/QuinA) were natural log-transformed to normalize their distribution. Linear mixed-effects (LME) models were fit to determine the interaction between group (SRC+, SRC-, CC+, CC-) and log-transformed KP metabolite (each metabolite and ratio run separately) on the global connectivity strength measure; the main effects of group and KP metabolite were included. To account for repeat scans and KP measurements, visit was modeled and participant was included as a random factor. Primary analyses focused on the global connectivity strength measure derived using the AAL2 parcellation. To confirm that the results were not biased by the parcellation scheme, identical LME were fit using the Craddock parcellation as sensitivity analyses. An alpha of 0.05 was used for primary KP metabolites (QuinA, KynA/Quin); while an alpha of 0.0083 (Bonferroni corrected 0.05/6) was considered significant for secondary KP outcomes (KynA/3HK, KynA, 3HK, KYN/TRP, KYN, TRP).
Based on significant global connectivity results of the above analyses, LME were fit in the SRC+ group to characterize the association between QuinA and connectivity strength of each individual node from the AAL2 parcellation. Visit was modeled and participant treated as a random factor as above. For these analyses, Benjamini-Hochberg False-Discovery Rate (FDR) of q<0.05 determined significance to account for multiple testing across 120 nodes (i.e., regions).
Given the known associations between QuinA and depression (Savitz, 2017), additional exploratory analyses were conducted in SRC+ participants to determine if either global connectivity strength or connectivity strength of individual ROI (limited to those showing associations with QuinA, see Results) were associated with BSI-18 depression sub-scale scores. SRC+ were differentiated into two subgroups based on the presence (n=8) or absence (n=9) of depressive symptoms from the BSI depression sub-scale at the 1d visit (i.e., the visit with the most severe post-concussion depressive symptoms). Exploratory independent samples t-tests compared connectivity strength at the 1d visit in SRC+ athletes with or without depressive symptoms. For individual ROI, an FDR of q<0.05 determined significance to account for multiple testing.
3. Results
3.1. Demographic and clinical data
Groups did not differ in demographic information or clinical data at baseline (Table 1), with the exception of number of prior concussions (p<0.001). By design, SRC+ and CC+ had more prior concussions than SRC- and CC- (all p<0.001). There were no group differences in head motion during the rs-fMRI scan at any visit (all p>0.10). Acutely following injury (i.e., 1d visit), SRC+ and SRC- reported more severe SCAT and BSI-GSI symptoms than CC+ and CC- (all p<0.05); SRC- reported less severe BSI-GSI symptoms than SRC+ (p<0.05). One SRC+ reported post-traumatic amnesia and one SRC- reported retrograde amnesia following their current injury; no injured athletes reported loss of consciousness due to their current injury.
3.2. Association of KP markers and global connectivity strength
There was a significant interaction between group and QuinA on connectivity strength, F(3, 127)=3.46, p=0.019. Follow-up analyses showed that this effect was driven by a significant association between QuinA and connectivity strength in SRC+, B=16.05, SE = 5.06, p=0.002, 95%CI[6.06, 26.03] (Figure 1). The association between QuinA and connectivity was not significant in SRC-, B=-2.76, SE=4.11, p=0.50, 95%CI[-10.91, 5.38], CC-, B=4.22, SE=4.41, p=0.34, 95%CI[-4.54, 12.97], or CC+, B=-4.28, SE=5.64, p=0.45, 95%CI[-15.42, 6.86]. There were no significant main effects or interactions for KynA/QuinA or any secondary KP marker (all p>0.10; Table 2).
Figure 1:
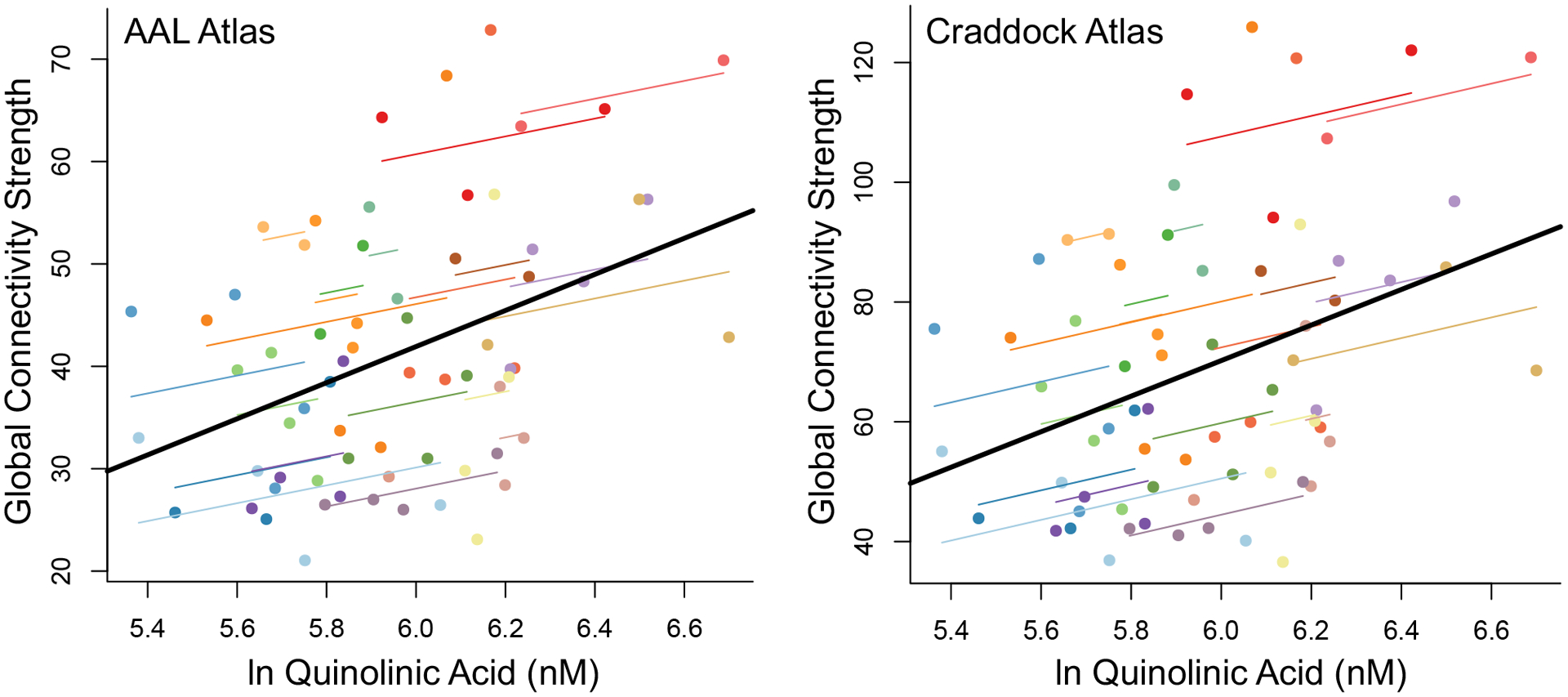
Shown is the association between natural log transformed (ln) quinolinic acid and global connectivity strength for both the Automated Anatomical Labeling atlas, version 2 (AAL2) and the Craddock atlas for concussed participants with prior concussion. For illustrative purposes, individual participants are indicated using a single color, with smaller lines demonstrating the relationship between connectivity and quinolinic acid in each participant over the repeated sessions. The solid black line represents the association between connectivity and quinolinic acid across all participants and time points. nM = nanomolar
Table 2:
Associated statistics for kynurenine pathway marker effects on global connectivity strength
| AAL2 | AAL2 | Craddock | Craddock | |
|---|---|---|---|---|
| Measure | Main Effect, KP marker | KP marker × Group Interaction | Main Effect, KP marker | KP marker × Group Interaction |
| Primary | ||||
| QuinA | F(1, 134)=1.86, p=0.18 | F(3, 127)=3.46, p=0.019 | F(1, 130)=1.53, p=0.22 | F(3, 123)=3.27, p=0.024 |
| KynA/QuinA | F(1, 149)=0.00, p=0.99 | F(3, 147)=0.32, p=0.81 | F(1, 143)=0.00, p=0.96 | F(3, 141)=0.26, p=0.86 |
| Secondary | ||||
| KynA/3HK | F(1, 185)=0.01, p=0.94 | F(3, 190)=1.00, p=0.39 | F(1, 177)=0.00, p=0.98 | F(3, 181)=0.92, p=0.43 |
| KynA | F(1, 243)=1.11, p=0.29 | F(3, 241)=0.77, p=0.51 | F(1, 240)=1.28, p=0.26 | F(3, 238)=1.00, p=0.39 |
| 3HK | F(1, 245)=1.60, p=0.21 | F(3, 239)=0.44, p=0.73 | F(1, 243)=2.16, p=0.14 | F(3, 237)=0.63, p=0.60 |
| KYN/TRP | F(1, 212)=0.03, p=0.86 | F(3, 179)=0.49, p=0.69 | F(1, 207)=0.04, p=0.84 | F(3, 173)=0.38, p=0.76 |
| KYN | F(1, 170)=0.86, p=0.35 | F(3, 158)=0.15, p=0.93 | F(1, 164)=0.79, p=0.38 | F(3, 153)=0.24, p=0.87 |
| TRP | F(1, 228)=0.91, p=0.34 | F(3, 223)=0.92, p=0.43 | F(1, 221)=0.84, p=0.36 | F(3, 216)=1.10, p=0.35 |
Note: AAL2 = Automated Anatomical Labeling atlas, Craddock = Craddock atlas, KP = kynurenine pathway, QuinA = quinolinic acid, KynA = kynurenic acid, 3HK = 3-hydroxykynurenine, KYN = kynurenine, TRP = tryptophan.
Sensitivity analyses were performed as above using an alternative atlas to define ROI. As in the primary analysis, there was a significant interaction between group and QuinA on connectivity strength, F(3, 123.34)=3.27, p=0.024. As above, SRC+ had a significant association between QuinA and connectivity strength, B=27.92, SE=9.22, p=0.003, 95%CI[9.71, 46.12] (Figure 1). There were no significant associations for SRC-, B=-3.39, SE=7.44, p=0.65, 95%CI[-18.15, 11.38], CC-, B=7.72, SE=7.99, p=0.34, 95%CI[-8.14, 23.57], or CC+, B=-10.44, SE=10.29, p=0.31, 95%CI[-30.76, 9.88]. As in the primary analysis, there were no significant main effects or interactions for KynA/QuinA or any secondary KP marker (all p>0.10; Table 2).
3.3. Association of QuinA and connectivity strength in individual regions
Analyses were performed to determine the association between QuinA and connectivity strength in individual regions-of-interest in SRC+ given the observed association between global QuinA and global connectivity strength. There was a significant association in 31 of the 120 regions following FDR correction (Table 3). As seen in Figure 2, several of the strongest associations were observed bilaterally in the orbitofrontal cortices, insulae, and basal ganglia.
Table 3:
Association of quinolinic acid and functional connectivity strength in SRC+
| Region-of-Interest | Beta | SE | t | df | p-value | FDR-corrected p |
|---|---|---|---|---|---|---|
| Pallidum_R | 27.66 | 7.18 | 3.85 | 43.12 | < 0.001 | < 0.001 |
| Caudate_L | 25.80 | 6.77 | 3.81 | 31.45 | 0.001 | 0.017 |
| Olfactory_L | 25.55 | 7.37 | 3.47 | 45.39 | 0.001 | 0.017 |
| Caudate_R | 25.30 | 7.22 | 3.51 | 46.09 | 0.001 | 0.017 |
| Olfactory_R | 25.04 | 7.38 | 3.39 | 39.94 | 0.002 | 0.018 |
| Heschl_L | 24.69 | 7.64 | 3.23 | 42.42 | 0.002 | 0.018 |
| Frontal_Med_Orb_L | 24.67 | 6.76 | 3.65 | 55.46 | 0.001 | 0.017 |
| Frontal_Sup_2_R | 24.16 | 7.08 | 3.41 | 56.49 | 0.001 | 0.017 |
| Frontal_Med_Orb_R | 23.62 | 7.01 | 3.37 | 47.39 | 0.001 | 0.017 |
| Insula_L | 22.00 | 7.22 | 3.05 | 40.95 | 0.004 | 0.030 |
| Putamen_L | 21.98 | 6.79 | 3.24 | 44.81 | 0.002 | 0.018 |
| Frontal_Sup_Medial_L | 21.68 | 7.33 | 2.96 | 51.57 | 0.005 | 0.034 |
| Putamen_R | 20.95 | 6.38 | 3.28 | 44.35 | 0.002 | 0.018 |
| Cingulate_Ant_R | 20.53 | 7.16 | 2.87 | 47.68 | 0.006 | 0.034 |
| Rectus_R | 20.46 | 6.74 | 3.04 | 47.94 | 0.004 | 0.030 |
| Insula_R | 19.68 | 7.14 | 2.76 | 33.43 | 0.009 | 0.043 |
| Paracentral_Lobule_L | 19.54 | 7.47 | 2.62 | 48.54 | 0.012 | 0.046 |
| OFCmed_R | 19.50 | 5.87 | 3.32 | 34.07 | 0.002 | 0.018 |
| Cerebellum_9_R | 19.42 | 6.06 | 3.21 | 60.94 | 0.002 | 0.018 |
| Rolandic_Oper_L | 19.33 | 6.85 | 2.82 | 32.92 | 0.008 | 0.042 |
| Precuneus_L | 19.10 | 7.06 | 2.70 | 48.82 | 0.009 | 0.043 |
| Frontal_Sup_2_L | 19.05 | 6.66 | 2.86 | 47.02 | 0.006 | 0.034 |
| ParaHippocampal_L | 18.99 | 6.55 | 2.90 | 44.99 | 0.006 | 0.034 |
| OFCpost_R | 18.93 | 6.98 | 2.71 | 36.45 | 0.010 | 0.044 |
| Occipital_Mid_L | 18.90 | 6.67 | 2.83 | 52.81 | 0.006 | 0.034 |
| Calcarine_R | 18.88 | 6.75 | 2.80 | 45.16 | 0.008 | 0.042 |
| Angular_R | 18.72 | 7.22 | 2.59 | 59.77 | 0.012 | 0.046 |
| Lingual_L | 18.64 | 7.12 | 2.62 | 44.09 | 0.012 | 0.046 |
| Lingual_R | 18.63 | 7.00 | 2.66 | 51.31 | 0.010 | 0.044 |
| Cerebellum_Crus2_L | 17.52 | 5.81 | 3.02 | 44.48 | 0.004 | 0.030 |
| Cerebellum_Crus1_L | 17.10 | 6.58 | 2.60 | 52.82 | 0.012 | 0.046 |
Note: Shown are the parameter estimates for the significant associations between natural log transformed quinolinic acid and connectivity strength for specific regions-of-interest from the Automated Anatomical Labeling (version 2) atlas following False Discovery Rate (FDR) correction. SE = standard error, df = degrees of freedom, t = t statistic, _R = right hemisphere, _L = left hemisphere, Med = medial, Orb = orbital, Sup = superior, Ant = anterior, Oper = operculum, Post = posterior, Mid = middle, OFC = orbitofrontal cortex.
Figure 2:
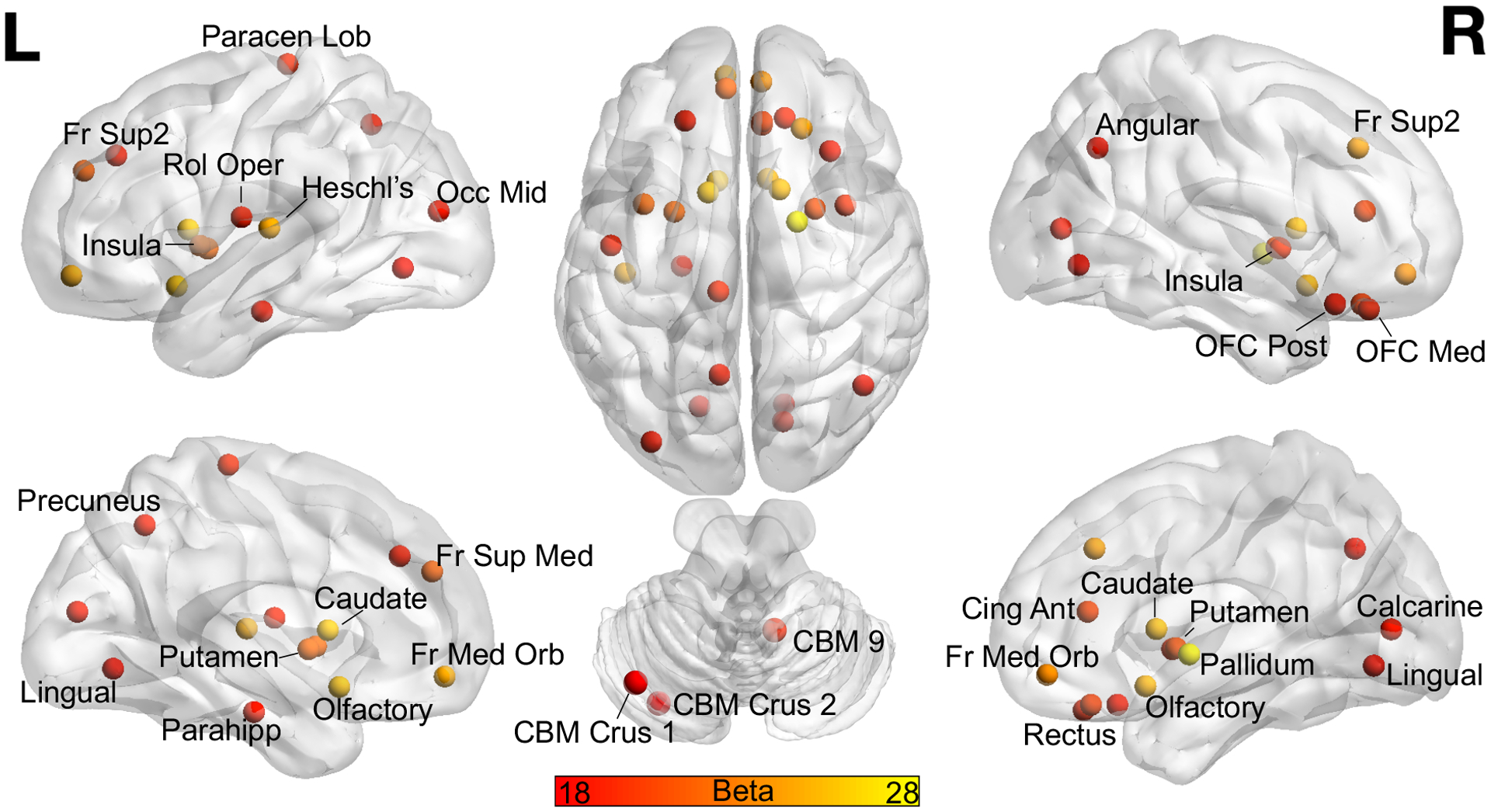
Displayed are the regions of interest from the Automatic Anatomical Labeling atlas, version 2 that showed a significant association between natural log transformed quinolinic acid and connectivity strength in concussed athletes with prior concussion following False Discovery Rate correction. Regions are labeled once. Color bar corresponds to the unstandardized Beta. L = left, R = Right, Paracen Lob = paracentral lobule, Fr = frontal, Sup = superior, Rol Oper = rolandic operculum, Occ = occipital, Mid = middle, Med = medial, Orb = orbital, OFC = orbitofrontal cortex, Cing = cingulate, Ant = anterior, Parahipp = parahippocampal, CBM = cerebellum, Post = posterior.
3.4. Association between connectivity strength and depressive symptoms
Exploratory analyses were conducted to determine if connectivity strength was association with post-concussion depressive symptom status in SRC+ participants, limited to the global connectivity measure and individual ROIs that showed associations with QuinA (see above). Global connectivity strength was significantly higher in SRC+ athletes with depressive symptoms compared to those without, t(15)=2.37, mean difference=13.50, SE=5.69, p=0.032, 95%CI[1.36, 25.63], Cohen’s d =1.15. Several individual ROI showed similar associations with depressive symptom status at 1d post-concussion (Table 4), though they did not survive FDR correction at q<0.05 despite several having large effect sizes (i.e., Cohen’s d > 1.0).
Table 4:
Differences in individual ROI connectivity strength based on depression symptom status in SRC+
| Region-of-Interest | MD* | SE | t | df | p-value | FDR-corrected p | Cohen’s d |
|---|---|---|---|---|---|---|---|
| Lingual_L | −19.205 | 6.580 | −2.919 | 15 | 0.011 | 0.075 | −1.418 |
| Cingulate_Ant_R | −21.310 | 7.573 | −2.814 | 15 | 0.013 | 0.075 | −1.367 |
| Heschl_L | −20.173 | 7.575 | −2.663 | 15 | 0.018 | 0.075 | −1.294 |
| Frontal_Sup_2_L | −17.606 | 6.869 | −2.563 | 15 | 0.022 | 0.075 | −1.245 |
| Occipital_Mid_L | −15.896 | 6.243 | −2.546 | 15 | 0.022 | 0.075 | −1.237 |
| Rolandic_Oper_L | −17.910 | 7.033 | −2.547 | 15 | 0.022 | 0.075 | −1.237 |
| Frontal_Med_Orb_R | −18.290 | 7.194 | −2.542 | 15 | 0.023 | 0.075 | −1.235 |
| Lingual_R | −17.840 | 7.068 | −2.524 | 15 | 0.023 | 0.075 | −1.227 |
| Pallidum_R | −14.385 | 5.403 | −2.662 | 9.540* | 0.025 | 0.075 | −1.348 |
| Putamen_R | −14.375 | 5.836 | −2.463 | 15 | 0.026 | 0.075 | −1.197 |
| Putamen_L | −13.683 | 5.659 | −2.418 | 15 | 0.029 | 0.075 | −1.175 |
| Calcarine_R | −15.678 | 6.629 | −2.365 | 15 | 0.032 | 0.075 | −1.149 |
| ParaHippocampal_L | −17.036 | 7.271 | −2.343 | 15 | 0.033 | 0.075 | −1.138 |
| Frontal_Sup_Medial_L | −17.932 | 7.665 | −2.339 | 15 | 0.034 | 0.075 | −1.137 |
| Insula_L | −14.768 | 6.474 | −2.281 | 15 | 0.038 | 0.076 | −1.108 |
| Caudate_R | −18.285 | 7.761 | −2.356 | 9.827* | 0.041 | 0.076 | −1.191 |
| Insula_R | −15.070 | 6.760 | −2.229 | 15 | 0.042 | 0.076 | −1.083 |
| Frontal_Med_Orb_L | −14.993 | 6.830 | −2.195 | 15 | 0.044 | 0.076 | −1.067 |
| Frontal_Sup_2_R | −17.028 | 8.077 | −2.108 | 15 | 0.052 | 0.085 | −1.024 |
| Paracentral_Lobule_L | −16.507 | 8.003 | −2.063 | 15 | 0.057 | 0.087 | −1.002 |
| Precuneus_L | −16.238 | 7.941 | −2.045 | 15 | 0.059 | 0.087 | −0.994 |
| Angular_R | −15.685 | 8.577 | −1.829 | 15 | 0.087 | 0.122 | −0.889 |
| Cerebellum_9_R | −11.894 | 6.946 | −1.712 | 15 | 0.107 | 0.142 | −0.832 |
| OFCpost_R | −13.794 | 8.112 | −1.700 | 15 | 0.110 | 0.142 | −0.826 |
| Olfactory_L | −14.420 | 8.879 | −1.624 | 15 | 0.125 | 0.150 | −0.789 |
| Cerebellum_Crus1_L | −13.025 | 8.033 | −1.621 | 15 | 0.126 | 0.150 | −0.788 |
| Olfactory_R | −14.340 | 8.904 | −1.610 | 9.495* | 0.140 | 0.161 | −0.816 |
| Rectus_R | −9.302 | 6.977 | −1.333 | 15 | 0.202 | 0.223 | −0.648 |
| Caudate_L | −11.613 | 8.566 | −1.356 | 8.708* | 0.209 | 0.223 | −0.691 |
| OFCmed_R | −8.561 | 7.329 | −1.168 | 15 | 0.261 | 0.270 | −0.568 |
| Cerebellum_Crus2_L | −1.434 | 6.034 | −0.238 | 15 | 0.815 | 0.815 | −0.115 |
Note: Shown are the associated statistics for comparison of connectivity strength for specific regions-of-interest in concussed athletes with prior concussion based on depression symptom status at 1day post-injury. Asterisk indicates t-test with unequal variance, MD = mean difference, SE = standard error, DF = degrees of freedom, T = T-statistic, FDR = False Discovery Rate, _R = right hemisphere, _L = left hemisphere, Med = medial, Orb = orbital, Sup = superior, Ant = anterior, Oper = operculum, Post = posterior, Mid = middle, OFC = orbitofrontal cortex.
Comparison is athletes without versus those with depression symptoms.
4. Discussion
The current study in high school and collegiate football players tested the hypothesis that the association between neuroactive KP metabolites and a global metric of functional connectivity would depend on both acute injury status and prior concussion. Consistent with our hypothesis, QuinA was significantly associated with functional connectivity strength in acutely injured athletes with prior concussion across the acute to sub-acute phase post-concussion, but not in injured athletes without prior concussion or in uninjured controls. Follow-up analyses identified several regions where this association was the strongest, including multiple regions in medial prefrontal cortex, insulae, and basal ganglia. Finally, exploratory analyses found that greater global connectivity strength was associated with the presence of acute depressive symptoms in concussed athletes with prior concussion. These results are discussed in detail in the following sections.
The positive association between QuinA and functional connectivity strength is mostly consistent with our work in an independent sample of collegiate athletes. In the prior work, the association between KynA/QuinA and connectivity of seed-regions in the anterior cingulate cortex and hippocampus to multiple brain regions differed based on concussion history as well as football exposure, with football players with prior concussion typically showing inverse associations between KynA/QuinA and connectivity (Meier et al., 2017). Although we did not observe any statistically significant effects for KynA/QuinA in the current study, the patterns of lower KynA/QuinA and higher QuinA are consistent (KynA/QuinA is negatively correlated with QuinA). Moreover, the current results extend our prior work by focusing on a global metric of functional connectivity strength that did not require the a priori selection of a ROI, the inclusion of high school athletes in addition to collegiate athletes, and the availability of MRI and blood draws collected across multiple, longitudinal visits.
The current study critically extends upon our prior work in that, here, the association between connectivity and QuinA was only observed in recently concussed athletes with prior concussion, whereas prior work focused on prior concussion in isolation in an independent cohort (Meier et al., 2017). It is important to note that the same association was not observed in acutely injured athletes without prior concussion, suggesting that this effect was not solely due to acute injury. Similarly, the same association was also not observed in uninjured football players with prior concussion, highlighting the fact that the effect is not solely due to prior injury. We hypothesize that this finding reflects a priming effect of prior concussion and acute/sub-acute concussion on the relationship between connectivity and QuinA. In our previous work in an overlapping sample, QuinA was elevated (and KynA/QuinA reduced) at all visits in the recently concussed group with prior concussion (Meier et al., 2020b). Based on that finding, and the fact that QuinA is produced by microglia and macrophages (Espey et al., 1997; Guillemin et al., 2005), we hypothesized that the observed elevation in QuinA reflects the long-term priming of monocyte lineage cells by prior concussion. That is, chronic inflammation (e.g., due to prior concussion) may sensitize the immune system to subsequent triggers (e.g., recent concussion), resulting in a greater inflammatory response (Dilger and Johnson, 2008; Perry and Holmes, 2014; Witcher et al., 2015). Nevertheless, we cannot rule out the possibility that association between QuinA and hyperconnectivity reflects the residual effect of multiple concussions.
Given the proposed association between glutamatergic neurotransmission and the BOLD signal (Hyder et al., 2002; Smith et al., 2002), one possible explanation for the observed association between QuinA and functional connectivity is that the effects of QuinA on the NMDA receptor (e.g., promotion of glutamate release and inhibition of reuptake) alters functional connectivity. In addition to its role in altering glutamatergic activity, QuinA has other deleterious effects that could impact intrinsic connectivity, including blood brain barrier disruption, the generation of reactive oxygen species, and the destabilization of cellular cytoskeletons (Guillemin, 2012). Regardless of the potential mechanisms, we cannot prove a causal relationship. Nevertheless, current results are consistent with abnormalities in QuinA (elevated QuinA, reduced KynA/QuinA) reported in a variety of psychiatric and neurodegenerative diseases that are associated with brain injury (Amaral et al., 2013; Guskiewicz et al., 2007; Savitz, 2020).
Hyperconnectivity has been posited to be a common response of the brain to neurological injuries with a known inflammatory component (i.e., multiple sclerosis and TBI) (Hillary et al., 2015). Although there are reports of hypoconnectivity following concussion (for review, see (Puig et al., 2020)), hyperconnectivity has been documented in several studies at the acute, sub-acute, and chronic phase following concussion and is associated with worse symptoms or prolonged recovery time (N. W. Churchill et al., 2017; Goswami et al., 2016; Kaushal et al., 2019; Meier et al., 2020a, 2017; van der Horn et al., 2017). The current work provides further support that hyperconnectivity is pathological based on our finding in concussed athletes with prior concussion that greater connectivity strength was associated with greater global functional connectivity strength. Therefore, in the context of the primed immune system hypothesis outlined above, the increased release of QuinA in concussed athletes with prior concussion leads to greater glutamatergic dysregulation that is ultimately reflected in the rs-fMRI BOLD signal connectivity and potentially mood dysregulation. Additional work is needed to directly test this proposed pathway. However, it should be noted that we cannot rule out that the observed hyperconnectivity is not pathological, per se, but rather reflects a compensatory increase in connectivity strength in response to elevated symptoms.
Finally, although we focused on a global connectivity metric, follow-up analyses demonstrated that the strongest associations with QuinA were observed in the medial and orbital frontal cortex, insula, as well as the basal ganglia. We have previously shown that various KP measures are associated with striatal and medial PFC structure in patients with major depressive disorder (MDD) (Meier et al., 2016a; Savitz et al., 2015a). Specifically, neuroactive KP metabolites (KynA/3HK, KynA/QuinA) mediated group differences (controls versus MDD) in mPFC thickness, while KYN and KYN/TRP were inversely associated with striatal volume in MDD patients. Similarly, multiple papers have shown that inflammatory challenges (e.g., endotoxin, IFN-alpha) impact glucose metabolism, BOLD signal response, and glutamate levels in the medial frontal cortex, insula, and/or striatum (Capuron et al., 2007, 2005; Eisenberger et al., 2010; Hannestad et al., 2012; Haroon et al., 2014; Harrison et al., 2009). Therefore, the current results add to the literature documenting associations between KP activity, as well as inflammation in general, with these brain regions. This is further supported by the finding that increased functional connectivity in several of these regions was also associated with the presence of depressive symptoms with large effect sizes, though none survived multiple comparison correction.
Current results were limited to male football players; therefore, we are unable to test for potential sex-differences in the associations between concussion, KP metabolites, and functional connectivity. Sex differences have been documented in previous concussion research, with indications that women report more symptoms following concussion, take longer to recover, and have higher risk of sustaining a concussion (Bretzin et al., 2018; Covassin et al., 2003; Iverson et al., 2017; Merritt et al., 2019; Zemek et al., 2016). Moreover, we have previously documented sex differences in serum KP metabolites, with women having lower KynA/QuinA and KynA/3HK, driven by lower KynA, compared to men (Meier et al., 2018). Given these differences, as well as the well-established sex differences in immune function, in general, future work is required to determine the extent to which sex moderates the associations observed in the current study (Klein et al., 2010; Klein and Flanagan, 2016; vom Steeg and Klein, 2016).
Finally, the current study investigated associations of KP metabolites and concussion with intrinsic brain connectivity as measured by rs-fMRI. Rs-fMRI, compared to task-based fMRI, allows for the mapping of intrinsic functional connectivity without confounds associated with tasks, such as performance or effort issues (for a review of fMRI use in TBI research, see (Mayer et al., 2015). There are alternative methods to assess intrinsic brain connectivity, such as electroencephalogram (EEG), magnetoencephalography (MEG), or functional near-infrared spectroscopy (fNIRS). While some of these methods provide more direct assessment of neural activity (i.e., EEG, MEG), the spatial resolution throughout the entirety of brain (including critical subcortical regions) is typically superior for fMRI. Finally, there are neuroimaging measures of brain microstructure that are sensitive to the effects of concussion, such as diffusion MRI (Gardner et al., 2012). The possible associations of concussion and KP metabolites with these alternative neuroimaging metrics merits future research.
4.1. Limitations
Current results were limited to high school and collegiate American football players and may not generalize to other sports, women, or athletes of different ages. The presence and number of prior concussions was based on participant self-report, which may be biased. Serum samples were non-fasting and collection time of blood samples was non-standardized across the study due to confines of the parent study. Although the repeated sampling over multiple visits could help mitigate these factors, they could impact KP measurements in blood. The extent to which serum KP metabolites reflect levels in the brain is uncertain, though blood and brain/CSF levels of QuinA have been shown to be significantly correlated (Haroon et al., 2020; Heyes et al., 1992; Heyes and Morrison, 1997; Raison et al., 2010). Finally, the sample size of participants with both blood and available scan data is relatively small in terms of individual participants. Because of the longitudinal design, however, KP metabolites and connectivity strength were measured in up to four visits per participants (e.g., 262 visits with blood and imaging). Nevertheless, it is likely that certain analyses (e.g., exploratory analyses in SRC+ group) were underpowered.
5. Conclusions
Elevated serum concentrations of QuinA are associated with increased functional brain connectivity in recently concussed athletes with a history of prior concussion. Future studies are needed to directly test the hypothesis that negative sequalae of multiple concussions may be, at least in part, mediated by a priming of the KP that triggers greater production of QuinA upon subsequent injury.
Supplementary Material
Acknowledgements
The authors thank Ashlee Taylor and Brenda Davis from University of Oklahoma Integrative Immunology Center for handling of blood biospecimens; Ashley LaRoche and Alexa Wild from the Department of Neurosurgery at the Medical College of Wisconsin for study coordination; Dr. Andrew Nencka, Brad Swearingen, and the MRI technicians at the Center for Imaging Research at the Medical College of Wisconsin for assistance in MRI data collection; Dr. Aniko Szabo from the Department of Biostatistics at the Medical College of Wisconsin for statistical consultation; and Dr. Julien Roeser from Charles River Laboratories for quantification of kynurenine metabolites.
Funding
This work was supported by the Defense Health Program under the Department of Defense Broad Agency Announcement for Extramural Medical Research through Award No. W81XWH-14-1-0561. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. Support for this work was also provided by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R21NS099789. TM acknowledges additional support from the National Institute of Neurological Disorders And Stroke (R01NS102225) and through a project funded through the Research and Education Program, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin. JS acknowledges support from the National Institute of General Medical Sciences (P20GM121312) and the National Institute of Mental Health (R21MH113871). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The REDCap electronic database and the Adult Translational Research Unit used for this project were supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436.
References
- Amaral M, Outeiro TF, Scrutton NS, Giorgini F, 2013. The causative role and therapeutic potential of the kynurenine pathway in neurodegenerative disease. J. Mol. Med 10.1007/s00109-013-1046-9 [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Skare S, Ashburner J, 2003. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 20, 870–888. 10.1016/S1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Heyes MP, Wisniewski SR, Sinz EH, Clark RSB, Blight AR, Marion DW, Adelson PD, 1999. Quinolinic acid in the cerebrospinal fluid of children after traumatic brain injury. Crit. Care Med 10.1097/00003246-199903000-00023 [DOI] [PubMed] [Google Scholar]
- Bretzin AC, Covassin T, Fox ME, Petit KM, Savage JL, Walker LF, Gould D, 2018. Sex Differences in the Clinical Incidence of Concussions, Missed School Days, and Time Loss in High School Student-Athletes: Part 1. Am. J. Sports Med 10.1177/0363546518778251 [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH, 2005. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol. Psychiatry 10.1016/j.biopsych.2005.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH, 2007. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-α therapy. Neuropsychopharmacology. 10.1038/sj.npp.1301362 [DOI] [PubMed] [Google Scholar]
- Carney N, Ghajar J, Jagoda A, Bedrick S, Davis-O’Reilly C, Du Coudray H, Hack D, Helfand N, Huddleston A, Nettleton T, 2014. Concussion guidelines step 1: systematic review of prevalent indicators. Neurosurgery 75, S3--S15. [DOI] [PubMed] [Google Scholar]
- Churchill N, Hutchison MG, Leung G, Graham S, Schweizer TA, 2017. Changes in functional connectivity of the brain associated with a history of sport concussion: A preliminary investigation. Brain Inj 10.1080/02699052.2016.1221135 [DOI] [PubMed] [Google Scholar]
- Churchill NW, Hutchison MG, Richards D, Leung G, Graham SJ, Schweizer TA, 2017. Neuroimaging of sport concussion: Persistent alterations in brain structure and function at medical clearance. Sci. Rep 7 10.1038/s41598-017-07742-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin T, Swanikt CB, Sachs ML, 2003. Sex Differences and the Incidence of Concussions among Collegiate Athletes. J. Athl. Train [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE, Hu XP, Mayberg HS, 2012. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp 33, 1914–1928. 10.1002/hbm.21333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW, 2008. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J. Leukoc. Biol 10.1189/jlb.0208108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey MG, Chernyshev ON, Reinhard JF, Namboodiri MAA, Colton CA, 1997. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport 10.1097/00001756-199701200-00011 [DOI] [PubMed] [Google Scholar]
- Foster AC, Vezzani A, French ED, Schwarcz R, 1984. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett 10.1016/0304-3940(84)90050-8 [DOI] [PubMed] [Google Scholar]
- Gardner A, Kay-Lambkin F, Stanwell P, Donnelly J, Williams WH, Hiles A, Schofield P, Levi C, Jones DK, 2012. A systematic review of diffusion tensor imaging findings in sports-related concussion. J. Neurotrauma 29, 2521–2538. 10.1089/neu.2012.2628 [DOI] [PubMed] [Google Scholar]
- Goswami R, Dufort P, Tartaglia MC, Green RE, Crawley A, Tator CH, Wennberg R, Mikulis DJ, Keightley M, Davis KD, 2016. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Struct. Funct 221, 1911–1925. 10.1007/s00429-015-1012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B, 2009. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72. 10.1016/j.neuroimage.2009.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, 2012. Quinolinic acid, the inescapable neurotoxin. FEBS J. 10.1111/j.1742-4658.2012.08485.x [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ, 2005. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 10.1002/glia.20090 [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, Mccrea M, Harding HP, Matthews A, Mihalik JR, Cantu RC, 2007. Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc 39, 903–909. 10.1249/mss.0b013e3180383da5 [DOI] [PubMed] [Google Scholar]
- Hannestad J, Subramanyam K, DellaGioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, Carson RE, 2012. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J. Nucl. Med 10.2967/jnumed.111.097014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Welle JR, Woolwine BJ, Goldsmith DR, Baer W, Patel T, Felger JC, Miller AH, 2020. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology 10.1038/s41386-020-0607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR, Hu XP, Miller AH, 2014. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology 10.1038/npp.2014.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD, 2009. Inflammation Causes Mood Changes Through Alterations in Subgenual Cingulate Activity and Mesolimbic Connectivity. Biol. Psychiatry 10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman PL, Wang EW, Lewis MM, Krzyzanowski S, Capan CD, Burmeister AR, Du G, Escobar Galvis ML, Brundin P, Huang X, Brundin L, 2020. Tryptophan Metabolites Are Associated With Symptoms and Nigral Pathology in Parkinson’s Disease. Mov. Disord 10.1002/mds.28202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Saito K, Quearry BJ, Price RW, Lee K, Bhalla RB, Der M, Markey SP, 1992. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and β2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J. Neuroimmunol 10.1016/0165-5728(92)90214-6 [DOI] [PubMed] [Google Scholar]
- Heyes MP, Morrison PF, 1997. Quantification of local de novo synthesis versus blood contributions to quinolinic acid concentrations in brain and systemic tissues. J. Neurochem 10.1046/j.1471-4159.1997.68010280.x [DOI] [PubMed] [Google Scholar]
- Hillary FG, Roman CA, Venkatesan U, Rajtmajer SM, Bajo R, Castellanos ND, 2015. Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology. 10.1037/neu0000110 [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG, 2002. Total neuroenergetics support localized brain activity: Implications for the interpretation of fMRI. Proc. Natl. Acad. Sci. U. S. A 99, 10771–10776. 10.1073/pnas.132272299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL, Gardner AJ, Terry DP, Ponsford JL, Sills AK, Broshek DK, Solomon GS, 2017. Predictors of clinical recovery from concussion: A systematic review. Br. J. Sports Med 10.1136/bjsports-2017-097729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jones SP, Franco NF, Varney B, Sundaram G, Brown DA, De Bie J, Lim CK, Guillemin GJ, Brew BJ, 2015. Expression of the kynurenine pathway in human peripheral blood mononuclear cells: Implications for inflammatory and neurodegenerative disease. PLoS One. 10.1371/journal.pone.0131389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal M, España LY, Nencka AS, Wang Y, Nelson LD, McCrea MA, Meier TB, 2019. Resting-state functional connectivity after concussion is associated with clinical recovery. Hum. Brain Mapp 40, 1211–1220. 10.1002/hbm.24440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M, 1989. A Glycine Site Associated with N‐Methyl‐d‐Aspartic Acid Receptors: Characterization and Identification of a New Class of Antagonists. J. Neurochem 10.1111/j.1471-4159.1989.tb01881.x [DOI] [PubMed] [Google Scholar]
- Klein AP, Tetzlaff JE, Bonis JM, Nelson LD, Mayer AR, Huber DL, Harezlak J, Mathews VP, Ulmer JL, Sinson GP, Nencka AS, Koch KM, Wu YC, Saykin AJ, DiFiori JP, Giza CC, Goldman J, Guskiewicz KM, Mihalik JP, Duma SM, Rowson S, Brooks A, Broglio SP, McAllister T, McCrea MA, Meier TB, 2019. Prevalence of Potentially Clinically Significant Magnetic Resonance Imaging Findings in Athletes with and without Sport-Related Concussion. J. Neurotrauma 36, 1776–1785. 10.1089/neu.2018.6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL, 2016. Sex differences in immune responses. Nat. Rev. Immunol 16, 626–638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A, Pekosz A, 2010. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis 10, 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay GM, Forrest CM, Stoy N, Christofides J, Egerton M, Stone TW, Darlington LG, 2006. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur. J. Neurol 13, 30–42. 10.1111/j.1468-1331.2006.01220.x [DOI] [PubMed] [Google Scholar]
- Manley G, Gardner AJ, Schneider KJ, Guskiewicz KM, Bailes J, Cantu RC, Castellani RJ, Turner M, Jordan BD, Randolph C, Dvořák J, Alix Hayden K, Tator CH, McCrory P, Iverson GL, 2017. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med 10.1136/bjsports-2017-097791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Bellgowan PS, Hanlon FM, 2015. Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci Biobehav Rev 49, 8–18. 10.1016/j.neubiorev.2014.11.016 [DOI] [PubMed] [Google Scholar]
- McCrea M, Meier T, Huber D, Ptito A, Bigler E, Debert CT, Manley G, Menon D, Chen J-K, Wall R, Schneider KJ, McAllister T, 2017. Role of advanced neuroimaging, fluid biomarkers and genetic testing in the assessment of sport-related concussion: a systematic review. Br. J. Sports Med 51, 919–929. 10.1136/bjsports-2016-097447 [DOI] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee HS, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC, 2013. The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64. 10.1093/brain/aws307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Drevets WC, Teague TK, Wurfel BE, Mueller SC, Bodurka J, Dantzer R, Savitz J, 2018. Kynurenic acid is reduced in females and oral contraceptive users: Implications for depression. Brain. Behav. Immun 67, 59–64. 10.1016/j.bbi.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, Bodurka J, Teague TK, Dantzer R, Savitz J, 2016a. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain. Behav. Immun 53, 39–48. 10.1016/j.bbi.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Giraldo-Chica M, España LY, Mayer AR, Harezlak J, Nencka AS, Wang Y, Koch KM, Wu YC, Saykin AJ, Giza CC, Goldman J, Difiori JP, Guskiewicz KM, Mihalik JP, Brooks A, Broglio SP, McAllister T, McCrea MA, 2020a. Resting-state fMRI metrics in acute sport-related concussion and their association with clinical recovery: A study from the NCAA-DOD CARE consortium. J. Neurotrauma 10.1089/neu.2019.6471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Lancaster MA, Mayer AR, Teague TK, Savitz J, 2017. Abnormalities in Functional Connectivity in Collegiate Football Athletes with and without a Concussion History: Implications and Role of Neuroactive Kynurenine Pathway Metabolites. J. Neurotrauma 34, 824–837. 10.1089/neu.2016.4599 [DOI] [PubMed] [Google Scholar]
- Meier TB, Nitta ME, Teague TK, Nelson LD, McCrea MA, Savitz J, 2020b. Prospective study of the effects of sport-related concussion on serum kynurenine pathway metabolites. Brain. Behav. Immun 10.1016/j.bbi.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Savitz J, Singh R, Teague TK, Bellgowan PSF, 2016b. Smaller Dentate Gyrus and CA2 and CA3 Volumes Are Associated with Kynurenine Metabolites in Collegiate Football Athletes. J. Neurotrauma 33, 1349–1357. 10.1089/neu.2015.4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt VC, Padgett CR, Jak AJ, 2019. A systematic review of sex differences in concussion outcome: What do we know? Clin. Neuropsychol 10.1080/13854046.2018.1508616 [DOI] [PubMed] [Google Scholar]
- Mijalkov M, Kakaei E, Pereira JB, Westman E, Volpe G, Alzheimer’s Disease Neuroimaging I, 2017. BRAPH: A graph theory software for the analysis of brain connectivity. PLoS One 12, e0178798 10.1371/journal.pone.0178798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, Contrepois K, Wang Q, Lee BA, Coronado M, Bernstein D, Snyder MP, Migaud M, Majeti R, Mochly-Rosen D, Rabinowitz JD, Andreasson KI, 2019. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol 10.1038/s41590-018-0255-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr CA, Albaugh MD, Watts R, Garavan H, Andrews T, Nickerson JP, Gonyea J, Hipko S, Zweber C, Logan K, Hudziak JJ, 2016. Neuroimaging Biomarkers of a History of Concussion Observed in Asymptomatic Young Athletes. J. Neurotrauma 33, 803–810. 10.1089/neu.2014.3721 [DOI] [PubMed] [Google Scholar]
- Perry VH, Holmes C, 2014. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol 10.1038/nrneurol.2014.38 [DOI] [PubMed] [Google Scholar]
- Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, Lerner A, Kher A, Kim SH, Khoury CC, Herzig SJ, Trovato ME, Simon-Tillaux N, Lynch MR, Thadhani RI, Clish CB, Khabbaz KR, Rhee EP, Waikar SS, Berg AH, Parikh SM, 2018. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat. Med 10.1038/s41591-018-0138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig J, Ellis MJ, Kornelsen J, Figley TD, Figley CR, Daunis-i-Estadella P, Mutch WAC, Essig M, 2020. Magnetic Resonance Imaging Biomarkers of Brain Connectivity in Predicting Outcome after Mild Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 10.1089/neu.2019.6623 [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH, 2010. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: Relationship to CNS immune responses and depression. Mol. Psychiatry 10.1038/mp.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Joliot M, Tzourio-Mazoyer N, 2015. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage 122, 1–5. 10.1016/j.neuroimage.2015.07.075 [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O, 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Sahm F, Oezen I, Opitz CA, Radlwimmer B, Von Deimling A, Ahrendt T, Adams S, Bode HB, Guillemin GJ, Wick W, Platten M, 2013. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res 10.1158/0008-5472.CAN-12-3831 [DOI] [PubMed] [Google Scholar]
- Savitz J, 2020. The kynurenine pathway: a finger in every pie. Mol. Psychiatry 10.1038/s41380-019-0414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, 2017. Role of kynurenine metabolism pathway activation in major depressive disorders, in: Current Topics in Behavioral Neurosciences. 10.1007/7854_2016_12 [DOI] [PubMed] [Google Scholar]
- Savitz J, Dantzer R, Meier TB, Wurfel BE, Victor TA, McIntosh SA, Ford BN, Morris HM, Bodurka J, Teague TK, Drevets WC, 2015a. Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology 62, 54–58. 10.1016/j.psyneuen.2015.07.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PSF, Bodurka J, Teague TK, Dantzer R, 2015b. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 40, 463–471. 10.1038/npp.2014.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PSF, Victor TA, Bodurka J, Teague TK, Dantzer R, 2015c. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain. Behav. Immun 46, 55–59. 10.1016/j.bbi.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Savitz J, Teague TK, Polanski DW, Mayer AR, Bellgowan PSF, Meier TB, 2016. Mood symptoms correlate with kynurenine pathway metabolites following sports-related concussion. J. Neurol. Neurosurg. Psychiatry 87, 670–675. 10.1136/jnnp-2015-311369 [DOI] [PubMed] [Google Scholar]
- Sinz EH, Kochanek PM, Heyes MP, Wisniewski SR, Bell MJ, Clark RSB, DeKosky ST, Blight AR, Marion DW, 1998. Quinolinic acid is increased in CSF and associated with mortality after traumatic brain injury in humans. J. Cereb. Blood Flow Metab 18, 610–615. 10.1097/00004647-199806000-00002 [DOI] [PubMed] [Google Scholar]
- Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F, 2002. Cerebral energetics and spiking frequency: The neurophysiological basis of fMRI. Proc. Natl. Acad. Sci. U. S. A 99, 10765–10770. 10.1073/pnas.132272199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgdrager FJH, Vermeiren Y, Van Faassen M, van der Ley C, Nollen EAA, Kema IP, De Deyn PP, 2019. Age- and disease-specific changes of the kynurenine pathway in Parkinson’s and Alzheimer’s disease. J. Neurochem 10.1111/jnc.14843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Saad ZS, 2013. FATCAT: (an efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain Connect 3, 523–535. 10.1089/brain.2013.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horn HJ, Scheenen ME, de Koning ME, Liemburg EJ, Spikman JM, van der Naalt J, 2017. The Default Mode Network as a Biomarker of Persistent Complaints after Mild Traumatic Brain Injury: A Longitudinal Functional Magnetic Resonance Imaging Study. J. Neurotrauma 34, 3262–3269. 10.1089/neu.2017.5185 [DOI] [PubMed] [Google Scholar]
- vom Steeg LG, Klein SL, 2016. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 12, e1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher KG, Eiferman DS, Godbout JP, 2015. Priming the Inflammatory Pump of the CNS after Traumatic Brain Injury. Trends Neurosci 10.1016/j.tins.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan EB, Frugier T, Lim CK, Heng B, Sundaram G, Tan M, Rosenfeld JV, Walker DW, Guillemin GJ, Morganti-Kossmann MC, 2015. Activation of the kynurenine pathway and increased production of the excitotoxin quinolinic acid following traumatic brain injury in humans. J. Neuroinflammation 12 10.1186/s12974-015-0328-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemek R, Barrowman N, Freedman SB, Gravel J, Gagnon I, McGahern C, Aglipay M, Sangha G, Boutis K, Beer D, Craig W, Burns E, Farion KJ, Mikrogianakis A, Barlow K, Dubrovsky AS, Meeuwisse W, Gioia G, Meehan WP 3rd, Beauchamp MH, Kamil Y, Grool AM, Hoshizaki B, Anderson P, Brooks BL, Yeates KO, Vassilyadi M, Klassen T, Keightley M, Richer L, DeMatteo C, Osmond MH, Pediatric Emergency Research Canada Concussion, T., 2016. Clinical Risk Score for Persistent Postconcussion Symptoms Among Children With Acute Concussion in the ED.[Erratum appears in JAMA. 2016 Jun 21;315(23):2624;]. JAMA. 10.1001/jama.2016.1203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


