Abstract
Background
The rapid spread of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) around the world has caused a global pandemic, infecting millions of individuals, with an unprecedented impact in health care systems worldwide. Healthcare workers are one of the risk groups that need to be well protected, due to their strategic role in patient management, presently and in prevention of healthcare needs for future outbreaks. Here, we present the results of the first SARS-CoV-2 seroprevalence study in the Northern Metropolitan Area of Barcelona, Spain.
Methods
IgG SARS-CoV-2 antibodies were analyzed in serum samples from 7563 healthcare workers of the Northern Metropolitan Area of Barcelona. Samples were collected after the first pandemic wave (from May 4th to May 22nd, 2020) and were analyzed by automated chemiluminescence assays. All samples were tested for IgG anti-S1/S2. Participant samples with negative or equivocal results but with analytical signals above the limit of detection and/or previously confirmed COVID-19 diagnosis were also tested for IgG anti-Nucleocapsid.
Results
A total of 779 of 7563 (10.3%) healthcare workers were positive for anti-SARS-CoV-2 IgG (specific for either S1/S2 or N antigens). No significant differences were observed between those working at primary care or at the reference hospital. Interestingly, among 341 participants with a confirmed COVID-19 diagnosis, 36 (10.55%) tested negative for SARS-CoV-2 IgG (both S1/S2 and recombinant N antigen).
Conclusion
Seroprevalence of anti-SARS-CoV-2 IgG in the healthcare workers of the North Metropolitan Area of Barcelona was higher than in the general population in the same geographical area. Safety measures have to be stressed in order to protect these essential workers from future pandemic waves.
Introduction
On January 2020, the World Health Organization (WHO) received information from the Chinese National Health Commission about an outbreak, which was highly suggestive to be associated with exposures in one market in Wuhan. Chinese authorities identified a new type of coronavirus, which was isolated on January 7th,2020. On March 11th, 2020, WHO declared SARS-CoV-2 as pandemic and on June 1st, 2020, more than 6 million total cases had already been reported worldwide [1]. Detection of viral RNA by rRT-PCR is currently the gold standard for diagnosis of SARS-CoV-2 infection. However, in most cases, it becomes almost undetectable 14 days post-symptom onset, although RNA persistence for longer periods does occur sometimes [2]. This phenomenon can occur in nasopharyngeal or oropharyngeal swabs but also in other samples such as saliva or lower respiratory tract samples. Saliva is easy and self-collected sample but, as well as oral or nasopharyngeal swabs, it may miss early infection. Other lower respiratory tract samples could also be employed, but may be limited to patients presenting with more severe disease. Nevertheless it seems that they have yielded the highest viral loads. SARS-CoV-triggered humoral S- and N-specific IgM response reached a peak within 4 weeks and was no more detectable 3 months post symptoms onset. The switch to IgG often occurred around day 14, and IgGs were detectable up to 36 months [3].
SARS-CoV-2 constituent proteins include spike (S), envelope (E), membrane (M), nucleocapsid (N), and other ones with still unknown functions. Both, S and N are the most immunogenic proteins of the virus. N protein is thought to participate in the replication and transcription of viral RNA and to interfere with cell cycle processes of host cells. S comprises two functional subunits responsible for binding to the host cell receptor (S1 subunit) and fusion of the viral and cellular membranes (S2 subunit) [4]. The receptor binding domain (RBD) of S1 protein mediates membrane fusion and may induce the generation of neutralizing antibodies in the host [5]. N protein is highly immunogenic in many coronaviruses, and abundantly expressed during infection [6]. In contrast S1 and S2 are specific of SARS-CoV-2, reducing the possibility of cross-reaction with other coronaviruses. Antibodies against N and S proteins are used to determine the primary and secondary immunological response to SARS-CoV-2 [7]. Primary humoral response is mediated by IgM and IgA antibodies that are already detectable after 7 days of the onset of the clinical symptoms and remain in serum for several weeks. The secondary immune response is mediated by IgG antibodies that are usually detectable after the second week and persist for months [3].
Sero-epidemiology is a powerful tool to understand how diseases spread in specific environments and may help to design and to monitor vaccination programs [8]. By using seroprevalence surveys, we can learn about the total number of people that have been infected, including those that might have missed diagnosis. These surveys can also help to estimate the percentage of the population that has not yet been infected, helping public health officials plan for future healthcare needs.
When considering undertaking a seroepidemiological study, it is important to choose the priority public health questions to which serology can contribute: a) the antigens/antibodies to be studied; b) the populations of interest; c) the best sampling method to provide a representative sample of those populations; d) the most appropriate laboratory assays and e) how data will be managed, analyzed and reported.
Spain is the country with the highest number of coronavirus infections among healthcare workers, according to available official data [9]. Knowing the prevalence of infection among healthcare workers is particularly important, since their role in the pandemic implies a risk of high exposition against this pathogen. Protective measures and safety protocols have been used in order to minimize the risk of healthcare workers. Seroprevalence studies will be useful to assess the effectiveness of these protocols and to design new strategies against potential new outbreaks. A first serological study led by ISGlobal and the Hospital Clinic of Barcelona, revealed that 11.2% of the hospital staff was infected by SARS-CoV-2 [10]. SARS-CoV-2 prevalence in the general population in the area of Barcelona has been estimated to be around 7.0% [11].
The Catalan Institute of Health (ICS) is the largest public health service company in Spain and is affiliated to the Department of Health of the Catalan Government. The ICS is formed by eight hospitals and about 300 primary care teams. It provides healthcare to almost six million users, a figure that represents 75% of the total number of people with healthcare rights in Catalonia.
In the Northern Metropolitan Area of Barcelona, the ICS provides primary care services to nearly 1,400,000 citizens in 71 municipalities. In this Metropolitan Area, Germans Trias i Pujol University Hospital, in Badalona, is the reference center for the high-complexity care of the 800,000 citizens of Barcelonès Nord and the Maresme and the basic general hospital of more than 200,000 residents from Badalona city and other surrounding municipalities. Primary care is provided by 66 teams and 37 support care units, working in a total of 84 Primary Care centers and 22 local clinics.
As this area of Barcelona had a high incidence of Coronavirus disease 2019 (COVID-19) cases, reporting more than 1000 cases/day for 15 consecutive days [12], it was important to determine the impact of the pandemics on the health care system clusters. Hence, in this study we analyzed the SARS-CoV-2 IgG seroprevalence in healthcare workers of the Northern Metropolitan Area of Barcelona, Spain.
Methods
Study design
Healthcare workers of the Northern Metropolitan Area of Barcelona, Spain were recruited in a prospective cross-sectional study. This work was reviewed and obtained the ethical approval by the Ethics Committee of our Institution, the German Trias Hospital. All participants gave written informed consent.
From May 4th to May 22nd, 2020, all healthcare workers of the ICS-Northern Metropolitan Area of Barcelona (n = 9315) were offered to have serum testing performed for SARS-CoV-2 IgG antibodies. This program was not offered as a research protocol but as a service to healthcare workers. According to official data [1], SARS-CoV-2 diagnosis peak in Spain was reached on April 1st 2020.
The participation in this study was voluntary; healthcare workers were neither selected for participation based on symptoms nor previous exposure to COVID-19. All individuals willing to participate fulfilled a brief epidemiological questionnaire and gave permission to access their clinical records. The questionnaire included demographic data, professional information, a direct question about if they have been diagnosed of COVID-19, or if they presented any of the most characteristic COVID-19 symptoms, such as cough, respiratory distress, fever, chills, headache, sore throat, anosmia, ageusia or asthenia. After sample analysis, the laboratory database was consulted in order to confirm which participants had a previous positive rRT-PCR result.
Laboratory analysis
Serum testing was conducted by the Regional Clinical laboratory using the quantitative SARS-CoV-2 S1/S2 IgG LIAISON® test (DiaSorin, Vercelli, Italy) on the LIAISON XL platform, following the manufacturer’s instructions. This test discriminates among negative (<12AU/mL; with 3.8 as the limit of IgG detection), equivocal (12.0–15.0 AU/mL) and positive (> 15.0 AU/mL) subjects.
In those cases in which a) IgG anti S1/S2 quantification was higher than the limit of detection (i.e. >3.8 AU/mL) but did not reach the limit of discrimination (i.e. <15 AU/mL) and/or b) when the healthcare workers answered the questionnaire saying that he or she had been diagnosed of COVID-19 but IgG anti S1/S2 where lower than 15 AU/ml, additional serological study was performed using a different antigen (N) as a target. In this case, a SARS-CoV-2 IgG test (Abbott Diagnostics, Sligo, Ireland) was run on an Architect i2000 platform (Fig 1). This test discriminates among negative (<1.4 Index (S/C)) and positive (≥1.4 Index (S/C) subjects.
Fig 1. Algorithm suited in rRT-PCR participants.
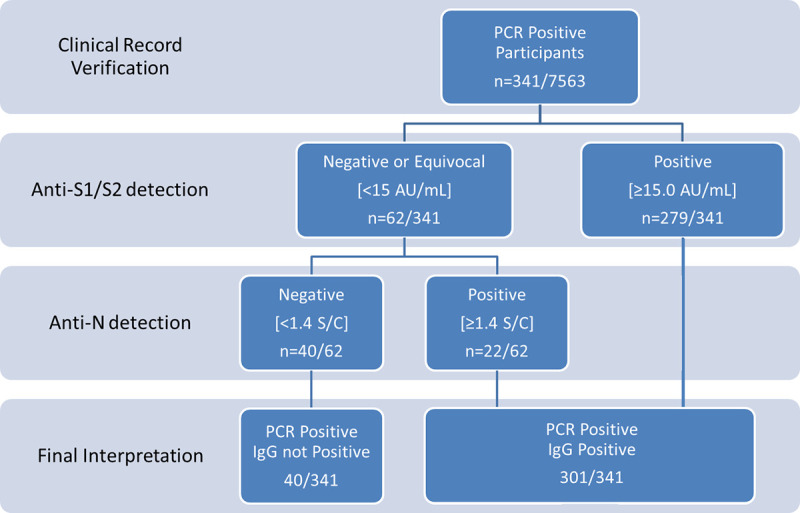
Positive and equivocal results were accompanied by a statement that the results did not indicate immunity to COVID-19 and healthcare workers should continue to wear full personal protective equipment. Participants with equivocal results were offered to be retested 4 weeks after the initial sample extraction.
Statistical analysis
Categorical variables were expressed as frequencies, while quantitative variables were expressed as the mean and standard deviation (SD). Qualitative variables were compared with Fisher’s exact test. These analyses were conducted with version 20 of SPSS.
Ethical considerations
As specified above, this program was not offered as a research protocol but as a service to ICS-Northern Metropolitan Area of Barcelona employees. All participants voluntarily accepted to participate and gave informed consent to review their health records.
Results
A total of 7563 healthcare workers of the Northern Metropolitan Area of Barcelona participated in the study. The participation rate was 81.2%.
Subjects’ characteristics are showed in Table 1. The mean age was 43.81 ± 12.43 years. 5746 participants were female (75.97%) and 1789 males (23.65%). From all of them, 4153 participants worked at Primary Care (54.91%) and 3410 (45.09%) at Germans Trias i Pujol Hospital, Tertiary Care.
Table 1. Demographic characteristics and antibody reactivity of participants.
| Characteristics | Participants n (%) | Positive SARS-CoV-2 IgG S1/S2 n (%) | Positive SARS-CoV-2 IgG S1/S2 or SARS-CoV-2 IgG N n (%)* | No. negative IgG S1/S2 (%) | No. negative IgG N (%) |
|---|---|---|---|---|---|
| Entire sample | 7563 (100) | 712 (9.41) | 779 (10.30) | 6801 (83.92) | 519/591**(87.82) |
| Sex | |||||
| Male | 1789 (23.65) | 181 (10.11) | 192 (10.73) | 1597 (89.27) | 106 (5.92) |
| Female | 5673 (75) | 522 (9.20) | 575 (10.13) | 5144 (90.68) | 407 (7.17) |
| NA | 101 (1.33) | 9 (9.73) | 12 (11.88) | 90 (89.11) | 6 (5.94) |
| Age | |||||
| 18–34 | 2025 (26.78) | 209 (23.36) | 228 (11.25) | 1806 (89.19) | 169 (8.34) |
| 35–54 | 3664 (48.45) | 330 (9) | 354 (9.61) | 3321 (90.64) | 241(6.58) |
| ≥55 | 1771 (23.42) | 179 (25.14) | 186 (10.50) | 1582 (89.33) | 103 (5.81) |
| NA | 103(1.36) | 9 (1.26) | 12 (11.43) | 92 (89.32) | 6 (5.82) |
| Location | |||||
| Primary Care | 4153 (54.91) | 381 (9.17) | 415 (9.99) | 3740 (90.06) | 283 (6.81) |
| Tertiary Care | 3410 (45.09) | 331 (9.71) | 364 (10.67) | 3061 (89.77) | 236 (6.92) |
| Health Care Job | |||||
| Nurse | 2243 (29.64) | 216 (9.62) | 239 (10,64) | 2016 (89.88) | 173 (7.71) |
| Physician | 1821 (24.08) | 192 (26.96) | 215 (11.70) | 1612 (87.70) | 126 (6.92) |
| Nursing Assistant | 832 (11) | 85 (11.93) | 116 (10.52) | 745 (89.54) | 55 (6.61) |
| Health Care Support Services | 429 (5.67) | 33 (4.63) | 33 (7.69) | 394 (91.84) | 27 (6.29) |
| Administrative Healthcare | 1181 (15.62) | 75 (10.53) | 77 (6.52) | 1097 (92.89) | 71 (6.01) |
| Other | 616 (8.14) | 51 (7.16) | 52 (8.44) | 562 (91.23) | 22 (3.57) |
| NA | 441 (5.83) | 60 (8.42) | 69 (15.65) | 375 (85.03) | 45 (10.220) |
| COVID Symptoms | |||||
| Yes | 3475 (45.95) | 523 (15.05) | 567 (16.32) | 2923 (63.61) | 232 (6.68) |
| No | 3646 (48.21) | 129 (3.53) | 143 (3.92) | 3502 (96.05) | 243 (6.66) |
| NA | 442 (5.84) | 60 (13.57) | 69 (5.61) | 376 (85.07) | 44 (9.95) |
| Previous COVID Diagnosis | |||||
| Yes | 385 (5.09) | 291 (75.58) | 313 (81.30) | 82 (21.29) | 21 (5.45) |
| No | 6740 (89.12) | 362 (5.37) | 398 (5.90) | 6346 (94.15) | 456 (6.76) |
| NA | 438 (5.79) | 59 (13.47) | 68 (15.52) | 373 (85.16) | 42 (9.59) |
| SARS-CoV-2 Confirmed rRT-PCR Positive | |||||
| Yes | 341 (4.51) | 279 (81.81) | 301 (88.26) | 50 (14.66) | 16 (4.69) |
| Hospital admitted for COVID | |||||
| Yes | 59 (0.78) | 46 (77.97) | 46 (77.97) | 13 (22.03) | 1 (1.69) |
| No | 7044 (93.14) | 605 (8.59) | 663 (9.41) | 6395 (90.79) | 474 (6.73) |
| NA | 460 (6.08) | 61 (13,26) | 70 (15.20) | 393 (85.43) | 44 (9.56) |
*N antigen determination was performed in 646 participants, according to the laboratory analysis algorithm.
** Total samples IgG S1/S2 (>3.80 AU/mL and <15 AU/mL) negative and processed by Abbott IgG N: 591. NA (Not answered).
A total of 712 out of the 7563 participants (9.41%) were positive for S1/S2 IgG; 6260 were lower than 3.80 AU/mL (82.7%). There were no differences related to age (p = 0.119) nor sex (p = 0.507). Administrative Healthcare workers showed the lowest percentage of positive samples (6.52%, p = 0.014) (Table 1). 3475 out of 7563 (45.95%) healthcare workers claimed to have suffered COVID-19 related symptoms. Among those 3475; 523 (15.05%) were positive for S1/S2 (Table 1).
A total of 385 (5.09%) of the total participants answered to the questionnaire that they had been diagnosed of COVID-19, 59 of them answered that they had been hospitalized. In 341 participants, a positive rRT-PCR was confirmed reviewing the medical records. Among those, 279 (81.82%) were positive for S1/S2 IgG.
In samples from healthcare workers claiming to have been previously diagnosed of COVID-19 but S1/S2 IgG where lower than 15 AU/ml (= 94 out of 385; 24.41%) an additional serological test to determine anti-N was performed. Twenty two of these 94 samples (23.4%) showed positivity to IgG against the nucleocapsid antigen. Also, samples from healthcare workers in which S1/S2 IgG quantification was higher than the limit of detection (>3.8 AU/mL) but did not reach the limit of discrimination (<15 AU/mL) (n: 591) were also tested to determine N antibodies. Sixty three of these samples (10.65%) showed positivity to anti-N IgG.
Taking into account the detection of anti-N IgG antibodies, the seroprevalence of the entire sample increased to 10.30% (n: 779/7563). Accordantly, the percentage of positive SARS-CoV-2 IgG in healthcare workers with a previous positive rRT-PCR increased after analyzing the presence of IgG anti-Nucleocapsid, finally been of 88.26% (301/341). However, 36 of these 341 healthcare workers having a previous positive rRT-PCR were negative for both tests and 4 were equivocal for anti-S IgG and negative for anti-N IgG.
Discussion
Serological assays play an important role in the knowledge of the impact and development of the COVID-19 pandemic, especially in healthcare workers. These data are useful to answer two questions: 1) the exposure of our healthcare workers to SARS-CoV-2 during the crisis and 2) the analysis of the humoral response in a population with a low proportion of hospitalized individuals.
To know the seroprevalence of our healthcare workers compared to our local population is important to assess the efficiency of the safety protocols established to protect our colleagues. Spain is known to have established a strict lockdown in the country’s general population since the pandemic was declared on March 13, 2020. Consistently, a higher seroprevalence among healthcare workers was expected, since this collective has been in contact with infected patients during more than three months, in an outstanding professional task. SARS-CoV-2 IgG seroprevalence in the Barcelona area has been estimated to be at around 7.0%, data obtained, interestingly, in an almost perfect timing match with our study [10]. Our results among healthcare workers were slightly higher, but not as concerning as they could have been expected without the application of strict protocols.
In relation to the analysis of the humoral response, the study of a whole population of individuals comprising all types of cases, including mild forms, can be very useful, since most published serology data are referred to hospitalized patients. Serological data of rRT-PCR positive but mild symptomatic patients are scarce and this information is important for a better understanding of this infection [13]. A 10.55% of diagnosed patients tested negative for IgGs against SARS-CoV-2. Thirty-nine of the healthcare workers with confirmed positive rRT-PCR required hospitalization. Only, one of the hospitalized rRT-PCR positive did not show discriminative SARS-CoV-2 IgG either S1/S2 or anti-nucleocapsid. This patient was clinically classified as a mild COVID-19. These results underline the relevance of characterizing broad cohorts of patients, and not only the ones with most relevant clinical manifestations.
Few seroprevalence studies of healthcare workers have been published so far and those have various outcomes depending on diverse factors, other than those specific for the analytical techniques. Age, gender, type of institution, participant specific tasks and incidence in general population of each geographical area are highly variable among published studies. For instance, seroprevalence of 316 healthcare workers of a tertiary hospital in Germany was 1.6% [14], while another German study, in a large neurological center, reported a seroprevalence of 2.7% [15]. Besides, SARS-CoV-2 incidence in Germany has been reported to be lower than in Spain [1] and this fact alone can bias any data comparison. A study performed in Italy, a country with a high burden of COVID-19, more similar to Spain, and performed with the same primary analytic platform showed similar global data to the ones of the present work (447 positive/3985 participants; 11.2%) [16], but, contrary to us, it showed significant differences regarding age and sex Also, published data from hospital workers in France reported a seroprevalence of 12.1%, with no significant differences between healthcare workers and those in administrative positions [17]. However, different exposure to the virus at work may lead to significant differences. For example, a study aimed to all individuals working in a hospital in Belgium reported a seroprevalence of 6.4% [18], while another study in the same country, but designed to assess the prevalence in healthcare workers highly exposed to the virus, obtained a prevalence of 12.1% [19]. In Barcelona, another study led by IS Global from 583 workers at the Clinic Hospital gave a seroprevalence of 11.3% considering a pool of antigens and the three immunoglobulin isotypes (IgG+ IgA+ IgM) using an in house test based on Luminex platform [10]. Our results analyzing only IgG specific levels are very similar to the last one, which was performed in a close geographical area.
A total of 3646 participants answering the epidemiological survey claimed to be fully asymptomatic. Among those, 143 (3.92%) did test positive for IgG anti SARS-CoV-2. As social restrictions are being eased, characterizing asymptomatic infected individuals is crucial to understand how the disease is spreading [20]. As our cohort of subjects is notably large, we cannot discard that a small proportion of these results could be false positives. In this context, our primary IgG test, anti S1/S2, displays a specificity of 98.5%, according to the manufacturer. Even taking these values into account, there is still a high likelihood that most of the asymptomatic individuals that tested positive were infected.
In our study, we observed that 10.55% of the individuals that theoretically had passed COVID19 infection did not present a discriminative level of IgG using two different groups of antigens (S and N). Any of both methods do not guarantee a 100% analytical sensitivity. LIAISON® SARS-CoV-2 S1/S2 IgG displays a sensitivity of 86.8–99.5%, according to the manufacturer. Abbott Architect IgG claims a sensitivity of 95.89–100%, but external evaluations have reported it to be around 93% and dropping along the days of evolution of the illness [21]. Besides, other explanations can arise. The first one could be that some individuals that reported symptoms or previous rRT-PCR positive were not really infected. This explanation was discarded after reviewing their personal records. A second possibility could be that samples were obtained during the first 10 days of infection, when it is well known that most of the IgG are not detectable [3]. This was also discarded as all those patients had positive rRT-PCR from at least 23 days before the serum sampling. Another possible explanation would be that infection occurred, but the innate immune system eliminated the virus, not allowing to organize a relevant specific response. Finally, there are a percentage of individuals who do not show detectable antibodies for unknown reasons. All this serves as a reminder that individual protective measures should never be discontinued, regardless of symptomatology.
Another point of discussion is the putative protective value of antibodies against reinfection. Even considering tests as highly sensitive, much about protective immunity is unknown [4, 6], however it seems that spike glycoprotein antibody tests will be preferred as a target for further studies related with the neutralizing antibodies [13].
Our study has several limitations. No other immunoglobulin isotypes were analyzed, and it cannot be discarded that some individuals might be positive for IgA or IgM and the moment of blood extraction. Moreover, not all antigens have been tested in all individuals. Only those with a report of disease or with detectable but non-positive SARS-CoV-2 IgG S1/2 were analyzed for both N and S antigens. The population studied was not randomly selected as a representation of diverse demographic characteristics, since it included every healthcare worker in the area willing to participate. This fact limits the possibility of comparing data with other seroprevalence studies like ENE-COVID.
Nevertheless, some strong points can be considered in the current study. First, the large number of individuals tested in a clearly restricted sanitary area suffering a high impact of the pandemic. Second, the study set-up, in the context of a high throughput diagnostic laboratory, showing the technical viability of testing high number of patients in a short period. Third, the concordance of the results with reported infection and/or rRT-PCR results from the staff.
Interestingly, this study opens new research perspectives, as it has identified a group of individuals in which, despite of having suffered COVID19 infection, not detectable antibodies were found. Further analysis considering broader antibodies isotypes as well as cellular responses need to be implemented in routine bases to better characterize these population.
In summary, we report that seroprevalence of anti-SARS-CoV-2 IgG antibodies in the healthcare workers of the Nord Metropolitan Area of Barcelona gives was slightly increased in comparison with the general population in the same geographical area and similar to other referent hospitals in Barcelona. Interestingly a similar prevalence was observed in Primary care and Hospital workers, but in both settings, administrative positions showed the lowest seroprevalence.
Supporting information
(TIF)
(PDF)
Acknowledgments
The authors would like to thank all participants and all nurses and laboratory technicians involved in this study, for their help in specimen collection, specimen processing and for their outstanding work during this pandemic.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. [cited 17 Jun 2020]. Available: https://covid19.who.int/
- 2.To KKW, Tsang OTY, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20: 565–574. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siracusano G, Pastori C, Lopalco L. Humoral Immune Responses in COVID-19 Patients: A Window on the State of the Art. Front Immunol. 2020;11: 1049 10.3389/fimmu.2020.01049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181: 281–292.e6. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang MS, Lu YT, Ho ST, Wu CC, Wei TY, Chen CJ, et al. Antibody detection of SARS-CoV spike and nucleocapsid protein. Biochem Biophys Res Commun. 2004;314: 931–936. 10.1016/j.bbrc.2003.12.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayanan K, Chen C-J, Maeda J, Makino S. Nucleocapsid-Independent Specific Viral RNA Packaging via Viral Envelope Protein and Viral RNA Signal. J Virol. 2003;77: 2922–2927. 10.1128/jvi.77.5.2922-2927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petherick A. Developing antibody tests for SARS-CoV-2.The Lancet, Volume 395, Issue 10230, 1101–1102. 10.1016/S0140-6736(20)30788-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutts FT, Hanson M. Seroepidemiology: an underused tool for designing and monitoring vaccination programmes in low- and middle-income countries Tropical Medicine and International Health. Blackwell Publishing Ltd; 2016. pp. 1086–1098. 10.1111/tmi.12737 [DOI] [PubMed] [Google Scholar]
- 9.García-Fernández L, Romero-Ferreiro V, López-Roldán PD, Padilla S, Calero-Sierra I, Monzó-García M, et al. Mental health impact of COVID-19 pandemic on Spanish healthcare workers. Psychol Med. 2020; 1–3. 10.1017/S0033291720002019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jiménez A, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020. July 8;11(1):3500 10.1038/s41467-020-17318-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollán Marina Blanco Faustino et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. The Lancet, Volume 396, Issue 10250, 535–544. 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.COVID-19 Evolución pandemia en España. [cited 6 Nov 2020]. Avalilable: https://cnecovid.isciii.es/covid19/#provincias.
- 13.Ni L, Ye F, Cheng M-L, Feng Y, Deng Y-Q, Zhao H, et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020. 10.1016/j.immuni.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korth J, Wilde B, Dolff S, Anastasiou OE, Krawczyk A, Jahn M, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020. July;128:104437 10.1016/j.jcv.2020.104437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt S. B., Grüter L., Boltzmann M., & Rollnik J. D. (2020). Prevalence of serum IgG antibodies against SARS-CoV-2 among clinic staff. PloS one, 15(6), e0235417 10.1371/journal.pone.0235417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandri MT, Azzolini E, Torri V, Carloni S, Tedeschi M, Castoldi M, et al. IgG serology in health care and administrative staff populations from 7 hospital representative of different exposures to SARS-CoV-2 in Lombardy, Italy. medRxiv. 2020; 2020.05.24.20111245. 10.1101/2020.05.24.20111245 [DOI] [Google Scholar]
- 17.Mesnil M, Joubel K, Yavchitz A, Miklaszewski N, Devys JM. Seroprevalence of SARS-Cov-2 in 646 professionals at the Rothschild Foundation Hospital (ProSeCoV study) [published online ahead of print, 2020 Aug 27]. Anaesth Crit Care Pain Med. 2020; S2352-5568(20)30171-5. 10.1016/j.accpm.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steensels D., Oris E., Coninx L., Nuyens D., Delforge M. L., Vermeersch P., et al. (2020). Hospital-Wide SARS-CoV-2 Antibody Screening in 3056 Staff in a Tertiary Center in Belgium. JAMA, 324(2), 195–197. 10.1001/jama.2020.11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin C., Montesinos I., Dauby N., Gilles C., Dahma H., Van Den Wijngaert S., et al. (2020). Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk healthcare workers and hospital staff. The Journal of hospital infection, 106(1), 102–106. 10.1016/j.jhin.2020.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi M, Yokoe DS, Havlir D V. Asymptomatic transmission, the achilles’ heel of current strategies to control Covid-19. New England Journal of Medicine. Massachussetts Medical Society; 2020. pp. 2158–2160. 10.1056/NEJMe2009758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahase E. Covid-19: Two antibody tests are “highly specific” but vary in sensitivity, evaluations find. BMJ. 2020;369: m2066 10.1136/bmj.m2066 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript.


