Abstract
To compare the relationship between background parenchymal enhancement (BPE) on contrast-enhanced spectral mammography (CESM), mammographic breast density (MBD), age, in the group with benign vs malignant breast lesions.
Four hundred thirty three non-high-risk patients from January 2018 to May 2019 were retrospectively analyzed. Patients were assigned into 4 groups: premenopausal benign lesions, premenopausal malignant lesions, postmenopausal benign lesions, and postmenopausal malignant lesions. The differences in CESM BPE and MBD between premenopausal benign lesions and premenopausal malignant lesions, between postmenopausal benign lesions and postmenopausal malignant lesions, between premenopausal and postmenopausal benign lesions, and between premenopausal and postmenopausal malignant lesions were evaluated. Pearson Chi-Squared test was used to analyze the differences between the above groups. Spearman rank correlation analysis was used to evaluate the correlations between BPE, MBD, and age. Multiple logistic regression was used to analyze the influencing factors of breast cancer. P < .05 was considered statistically significant.
There was no significant difference in CESM BPE or MBD of benign and malignant lesions regardless of premenopausal or postmenopausal status, but there was a significant difference in CESM BPE and MBD of premenopausal and postmenopausal patients regardless of the presence of benign or malignant lesions. The intensity of CESM BPE was positively correlated with MBD, and the intensity of CESM BPE and MBD were negatively correlated with age. Multiple logistic regression analysis showed that age was an influencing factor for breast cancer in both premenopausal and postmenopausal patients.
For non-high-risk women, CESM BPE and MBD were not correlated with benign or malignant breast lesions, and age was an influencing factor for breast cancer.
Keywords: breast cancer, CESM, BI-RADS, BPE, MBD
1. Introduction
Breast cancer is the most common malignant tumor in women. The number of new cases every year accounts for nearly 30% of female malignant tumors.[1] Imaging technology to assess the risk of breast cancer is important for clinical screening and diagnosis.
Mammography is the method of choice for breast screening. Also, it usually is the first method for breast lesion detection and diagnosis. However, the sensitivity of this method is limited, resulting in approximately 20% missed diagnoses of breast cancer.[2] Contrast-enhanced spectral mammography (CESM) is a new imaging technology based on digital mammography that uses a contrast agent for the examination. After intravenous injection of the contrast agent, high- and low-energy exposure is performed, and low-energy images and subtraction images are obtained after processing. CESM is characterized by its short examination time, low cost, and no noise, compared with MR, CESM is especially suitable for patients with pacemaker implantation and claustrophobia. Several studies have demonstrated that the accuracy of CESM diagnosis is comparable to that of breast magnetic resonance imaging (MRI), while its specificity is even better than that of MRI.[3,4] Similar to dynamic enhanced breast MRI, background parenchymal enhancement (BPE) can also be observed in CESM subtraction. There have been several studies on MRI BPE. BPE is affected by various factors[5] and was associated with breast cancer risk in some studies.[6,7] However, there are few studies on CESM BPE, and the relationship with benign or malignant breast lesions has not yet been reported. At the same time, the CESM low-energy image can display mammographic breast density (MBD). Although MBD is a predictor of breast cancer, the magnitude of risk is controversial.[8] This study evaluated the significant differences between CESM BPE and MBD in benign and malignant breast lesions, compared the relationship between CESM BPE, MBD, and age in the group with benign vs malignant lesions, analyzed the correlation between CESM BPE, MBD, and age, and analyzed the correlation between CESM BPE, MBD, and menopausal status.
2. Materials and methods
2.1. Study subjects
This was a retrospective study approved by the ethics committee of Yantai Yuhuangding Hospital, and informed consent was not required. The study period was from January 2018 to May 2019. Patient information was obtained from electronic medical records. All subjects were non-high-risk patients (no family history of breast cancer, no known gene mutation, no history of breast radiation, and no history of breast cancer diagnosis) with unilateral breast lesions. Patients with bilateral breast lesions and using hormone replacement therapy or hormonal contraception were excluded. In order to minimize the impact of lesions on CESM BPE, CESM BPE, and MBD of the unaffected breast were evaluated only in this study. All patients were assigned to 1 of 4 groups: premenopausal benign lesion, premenopausal malignant lesion, postmenopausal benign lesion, and postmenopausal malignant lesion groups. A total of 433 patients (all female, age range of 17–85 years, average age of 54 ± 11.6 years) were included in the study (Fig. 1). There were 97 cases of premenopausal benign lesions, 45 cases of premenopausal malignant lesions, 69 cases of postmenopausal benign lesions, and 222 cases of postmenopausal malignant lesions. The menstrual cycle phase was not recorded in this study. All pathological results are shown in Table 1. All patients did not receive surgical treatment before CESM.
Figure 1.
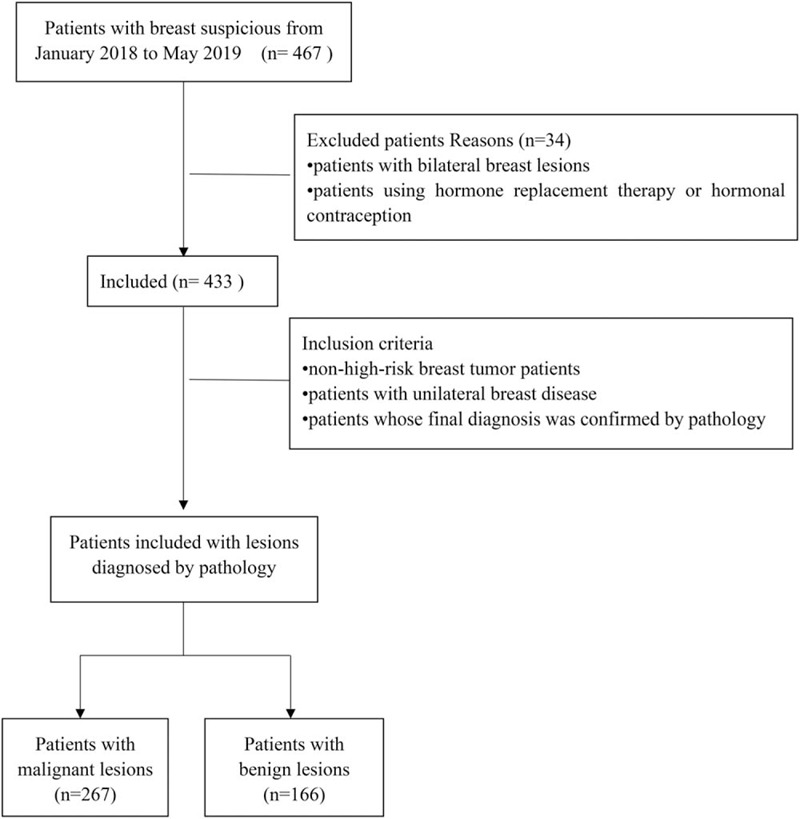
Patient inclusion and exclusion flowchart.
Table 1.
Pathological results of breast lesions.
| Pathologic diagnosis | Number of cases | |
| Encapsulated papillary carcinoma | 2 | |
| Encapsulated papillary carcinoma with invasive ductal carcinoma | 1 | |
| Ductal carcinoma in situ with mucinous carcinoma | 1 | |
| Intraductal papillary carcinoma | 2 | |
| Ductal carcinoma in situ with invasive ductal carcinoma | 5 | |
| Ductal carcinoma in situ | 16 | |
| Mlignant adenomyoepithelioma | 1 | |
| Malignant phyllodes tumor | 3 | |
| Intraductal carcinoma | 1 | |
| Metaplastic carcinoma | 2 | |
| Invasive ductal carcinoma | 119 | |
| Malignant | Invasive ductal carcinoma with Ductal carcinoma in situ | 81 |
| Invasive ductal carcinoma with invasive lobular carcinoma | 1 | |
| Invasive ductal carcinoma with intraductal carcinoma | 1 | |
| Invasive papillary carcinoma | 4 | |
| Invasive cribriform carcinoma | 1 | |
| Invasive lobular carcinoma | 8 | |
| Basal-like breast carcinoma | 1 | |
| Solid papillary carcinoma | 2 | |
| B-cell lymphoma | 1 | |
| Mucinous carcinoma | 7 | |
| Invasive carcinoma | 8 | |
| Epidermoid cyst with infection | 1 | |
| Hamartoma | 1 | |
| Catheter dilatation | 5 | |
| Intraductal papilloma | 28 | |
| Radial scar | 1 | |
| Inflammation | 10 | |
| Benign phyllodes tumor | 1 | |
| Fibrocystic breast disease | 5 | |
| Benign | Papilloma | 2 |
| Cyst | 2 | |
| Adenosis | 20 | |
| Adenosis with intraductal papilloma | 5 | |
| Adenosis with papilloma | 2 | |
| Adenosis with fibroadenoma | 10 | |
| Fibrouscystic breast disease with fibroadenoma | 1 | |
| Fibroadenoma | 71 | |
| Sclerosing adenosis | 1 |
2.2. Imaging technology
CESM was performed using the Senographe Essential all-digital mammography system (GE Healthcare, Inc, Princeton, USA). Iohexol (containing 350 mg/ml of iodine, Beilu Pharmaceutical Co. Ltd, Beijing, China) was used as the contrast agent at a dose of 1.5 ml/kg and injected into the upper arm vein through a high-pressure syringe at a flow rate of 3 ml/second. After injection for approximately 2 minutes, the imaging was projected in the following order: the craniocaudal (CC) view of the breast on the affected side, then the mediolateral oblique (MLO) view of the breast on the affected side, then the CC view of the breast on the unaffected side, and finally the MLO view of the breast on the unaffected side. The radiographic process of each patient was completed in 7 minutes. In radiography, a low-energy and a high-energy exposure was obtained continuously within 1.5 seconds of a compression. Two images, namely a low-energy image and a subtractive image, were obtained for each position on the workstation.
2.3. Imaging analysis
CESM BPE type and MBD (including CC and MLO views) of the unaffected breast were evaluated using a double-blind method by 2 radiologists with more than 10 years of experience in breast imaging. According to the Breast Imaging Reporting and Data System, MBD was divided into almost entirely fatty, scattered fibroglandular, heterogeneously dense, and extremely dense categories (Fig. 2). Because no widely accepted BPE classification criteria for CESM are available, the BPE classification criteria for MRI in the Breast Imaging Reporting and Data System were referred. CESM BPE was classified into minimal, mild, moderate, and marked (Fig. 3).
Figure 2.
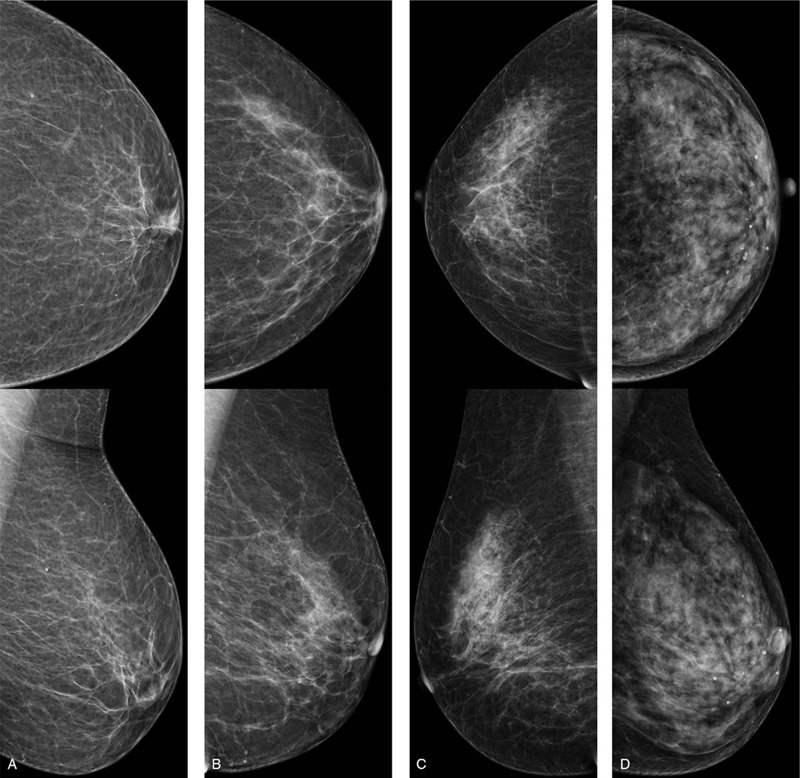
CC (top) and MLO (bottom) views showing examples of (a) almost entirely fatty, (b) scattered fibroglandular, (c) heterogeneously dense, (d) extremely dense breasts.
Figure 3.
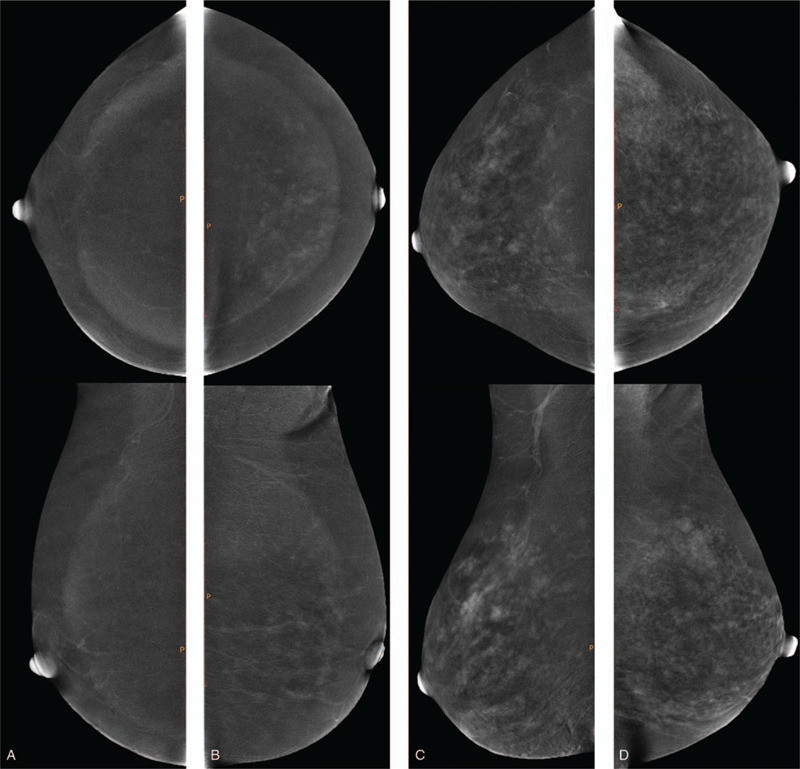
CC (top) and MLO (bottom) views showing examples of (a) minimal, (b) mild, (c) moderate and (d) marked BPE.
2.4. Cases with divergences in their classification were discussed to finally reach agreement.
The radiologists were blinded to the pathological results.
2.5. Statistical analysis
All measurements were the average of 2 observers. The consistency analysis of the 2 observers was tested by Kappa test. 0 < κ ≤ 0.4, 0.4 < κ < 0.75, and 0.75 ≤ κ < 1 were considered as poor, good, and excellent, respectively.
All patients were assigned to 1 of 4 groups: premenopausal benign lesion, premenopausal malignant lesion, postmenopausal benign lesion, and postmenopausal malignant lesion groups, avoiding potential bias due to hormone levels. MBD was divided into MBDa–b (almost entirely fatty and scattered fibroglandular) and MBDc–d (heterogeneously dense and extremely dense), and BPE was divided into BPE1–2 (minimal-to-mild enhancement) and BPE3–4 (moderate-to-marked enhancement). The differences in CESM BPE and MBD between premenopausal benign lesions and premenopausal malignant lesions, between postmenopausal benign lesions and postmenopausal malignant lesions, between premenopausal and postmenopausal benign lesions, and between premenopausal and postmenopausal malignant lesions were evaluated. Pearson Chi-Squared test was used to analyze differences between the above groups. Spearman rank correlation analysis was used to evaluate the correlations between BPE, MBD, and age. Multiple logistic regression was used to analyze the influencing factors of breast cancer. Categorical data were expressed in frequency (percentage), and measurement data were expressed in mean ± standard deviation. SPSS 19.0 statistical software (IBM, Armonk, NY, USA) was used for statistical analyses. P < .05 was considered statistically significant.
3. Results
3.1. Inter-observer agreements
The results of the 2 observers were consistent, with CESM BPE classification, 0.78, and MBD classification, 0.69.
3.2. BPE, MBD, and breast cancer
There was no significant difference in CESM BPE type between benign and malignant lesions in premenopausal patients or postmenopausal patients (P > .05) (Table 2).
Table 2.
Comparison of CESM BPE type benign and malignant lesions.
| CESM BPE | Total | Benign | Malignant | χ2 | P |
| Premenopausal | 0.009 | .924 | |||
| BPE1-2 | 108 | 74 (76.29) | 34 (75.56) | ||
| BPE3-4 | 34 | 23 (23.71) | 11 (24.44) | ||
| Postmenopausal | 0.475 | .491 | |||
| BPE 1-2 | 282 | 66 (95.65) | 216 (97.30) | ||
| BPE 3-4 | 9 | 3 (4.35) | 6 (2.70) |
There was no significant difference in MBD between benign and malignant lesions in premenopausal patients or postmenopausal patients (P > .05) (Table 3).
Table 3.
Comparison of MBD between benign and malignant lesions.
| MBD | Total | Benign | Malignant | χ2 | P |
| Premenopausal | 0.467 | .494 | |||
| MBD a-b | 21 | 13 (13.40) | 8 (17.78) | ||
| MBD c-d | 121 | 84 (86.60) | 37 (82.22) | ||
| Postmenopausal | 2.359 | .125 | |||
| MBDa-b | 187 | 39 (56.52) | 148 (66.67) | ||
| MBDc-d | 104 | 30 (43.48) | 74 (33.33) |
3.3. CESM BPE and MBD and menopausal status
In both benign and malignant lesions, the proportion of BEP3–4 type in premenopausal patients was significantly higher than that in postmenopausal patients (P < .05) (Table 4).
Table 4.
Comparison in CESM BPE type between premenopausal and postmenopausal patients.
| CESM BPE | Total | Premenopausal | Postmenopausal | χ2 | P |
| Benign | 11.444 | .001 | |||
| BPE 1-2 | 140 | 74 (76.29) | 66 (95.65) | ||
| BPE 3-4 | 26 | 23 (23.71) | 3 (4.35) | ||
| Malignant | 29.667 | .000 | |||
| BPE 1-2 | 250 | 34 (75.56) | 216 (97.30) | ||
| BPE 3-4 | 17 | 11 (24.44) | 6 (2.70) |
In both benign and malignant lesions, the proportion of MBDc–d in premenopausal patients was significantly higher than that in postmenopausal patients (P < .05) (Table 5).
Table 5.
Comparison of MBD between premenopausal and postmenopausal patients.
| MBD | Total | Premenopausal | Postmenopausal | χ2 | P |
| Benign | 34.848 | .000 | |||
| MBD a-b | 52 | 13 (13.40) | 39 (56.52) | ||
| MBD c-d | 114 | 84 (86.60) | 30 (43.48) | ||
| Malignant | 36.817 | .000 | |||
| MBD a-b | 156 | 8 (17.78) | 148 (66.67) | ||
| MBD c-d | 111 | 37 (82.22) | 74 (33.33) |
3.4. Odds ratio of malignant lesions
Multivariate logistic regression analysis was performed using disease type as a dependent variable, and age and CESM BPE and MBD as independent variables. The result showed that age (ORpre=1.477, ORpost = 1.079) was an independent factor for malignant lesions in both premenopausal and postmenopausal patients, with statistical significance (P < .05). It can be assumed that the probability of malignant lesions increases with age. CESM BPE and MBD were not independent influencing factors of the lesion type (P > .05) (Table 6).
Table 6.
Logistic regression analysis of influencing factors of malignant lesions.
| Factor | B | SE | Wald χ2 | P | OR | 95% CI |
| Premenopausal | ||||||
| Age | 0.390 | 0.085 | 21.190 | .000 | 1.477 | 1.251–1.743 |
| MBD | 0.200 | 0.492 | 0.165 | .684 | 1.222 | 0.466–3.206 |
| CESM BPE | −0.246 | 0.283 | 0.760 | .383 | 0.782 | 0.449–1.360 |
| Postmenopausal | ||||||
| Age | 0.076 | 0.024 | 10.408 | .001 | 1.079 | 1.030–1.131 |
| MBD | 0.085 | 0.211 | 0.160 | .689 | 1.088 | 0.719–1.647 |
| CESM BPE | −0.330 | 0.262 | 1.585 | .208 | 0.719 | 0.430–1.202 |
3.5. Correlation analysis
In all the patients, the average age of BPE3–4 patients was significantly younger than that of BPE1–2 patients (P < .05) (Table 7).
Table 7.
Comparison of age of patients with different breast CESM BPE types.
| CESM BPE | Total | Age (years) | t | P |
| Total | 7.442 | .000 | ||
| BPE 1-2 | 390 | 55.06 ± 11.44 | ||
| BPE 3-4 | 43 | 44.86 ± 8.14 |
Spearman rank correlation analysis showed that there was a significant negative correlation between all patients’ age and CESM BPE (r = −0.386, P < .001).
In all the patients, the average age of MBDc–d patients was significantly younger than that of MBDa–b patients (P < .05) (Table 8).
Table 8.
Comparison of age between different MBD patients.
| MBD | Total | Age (years) | t | P |
| Total | 12.675 | .000 | ||
| MBD a-b | 208 | 60.30 ± 9.09 | ||
| MBD c-d | 225 | 48.26 ± 10.56 |
Spearman rank correlation analysis showed that there was a significant negative correlation between all patients’ age and MBD (r = −0.608, P < .001).
In all patients, the proportion of breast density of MBDc–d in patients with BPE1–2 type was significantly lower than that in patients with BPE3–4 type (P < .05) (Table 9).
Table 9.
Comparison of MBD of patients with different breast CESM BPE types.
| CESM BPE | |||||
| MBD | Total | BPE 1-2 | BPE 3-4 | χ2 | P |
| Total | 28.696 | .000 | |||
| MBD a-b | 208 | 204 (52.31) | 4 (9.30) | ||
| MBD c-d | 225 | 186 (47.69) | 39 (90.70) | ||
Spearman rank correlation analysis showed that there was a significant positive correlation between all patients’ MBD and CESM BPE (r = 0.318, P < .001).
4. Discussion
The results of this study indicated that the intensity of CESM BPE and MBD were negatively correlated with age, and the intensity of CESM BPE was positively correlated with MBD. In both premenopausal and postmenopausal patients, the intensity of CESM BPE and MBD were not related to benign or malignant breast lesions. Age was an influencing factor for breast cancer. The intensity of CESM BPE and MBD were higher in premenopausal patients than in postmenopausal patients.
Bennani-Baiti et al highlighted that the degree of MRI BPE in non-high-risk patients had no correlation with breast cancer risk,[9] possibly due to the difference in breast structure between non-high-risk patients and high-risk patients. For example, BRCA1/2 mutations may result in significant histological remodeling;[10] In patients who have suffered genotoxic damage to tissue as a result of radiation or chemotherapy, other genetic mechanisms contribute to tissue vulnerability and may lead to the formation of cancerous lesions upon cell growth stimuli or tissue activation.[11] In a meta-analysis study, Thompson et al reported that the degree of MRI BPE was related with breast cancer risk in high-risk patients. The degree of MRI BPE was not significantly correlated with breast cancer risk in non-high-risk or general-risk patients.[12] At present, there are few studies on CESM BPE. Here, no significant correlation was found between the degree of CESM BPE and benign or malignant breast lesions. Savaridas et al reported that the intensity of CESM BPE was correlated with MBD and MRI BPE, suggesting that CESM BPE might be a risk indicator of breast cancer.[13] However, our study did not support this conclusion. The possible reasons are as follows. First, the study population was different. This study focused on non-high-risk patients, but some previous studies did not distinguish the degree of breast cancer risk in patients. Second, it was related to CESM image acquisition time. The time of MRI BPE was defined as 90 seconds after contrast agent injection, but the time of CESM image acquisition was within 7 minutes (there might be differences between medical institutions), and the intensity of BPE gradually increased over time,[14] which may weaken the difference in the intensity of CESM BPE between benign and malignant lesions.
MBD is recognized as a breast cancer risk marker, and the risk of breast cancer increases with MBD.[15,16] However, most studies have focused on Caucasian women. Asian women have higher MBD, but a lower incidence of breast cancer than Caucasian women.[17] Furthermore, the relationship between MBD and breast cancer risk in Asian women may be different from that of Caucasian women. Maskarinec et al reported that the correlation between MBD and breast cancer risk in Japanese women was statistically weak compared with Caucasian women and Hawaiian women.[18] The results of this study showed no significant correlation between MBD and benign or malignant breast lesions in Asian women, regardless of premenopausal or postmenopausal status, possibly due to several reasons. First, it may be related to the study population. All the patients in this study were Chinese women. Compared with Western women, Asian women have smaller breasts and are more likely to be evaluated as having dense breast tissue.[19] The correlation between breast cancer risk and MBD is lower in Asian women. Second, the subjects in this study were all non-high-risk patients, and breast structure is different in high-risk and non-high-risk patients,[9] which might affect the research findings.
Because some studies have shown that CESM BPE is not affected by the menstrual cycle, the menstrual cycle phase was not recorded in this study.[13] The current study proved that MBD and CESM BPE were not related to benign or malignant breast lesions, and age was an influencing factor of breast cancer, which was consistent with the results of Bennani-Baiti et al.[9] In this study, for non-high-risk patients, MRI BPE, fibroglandular tissue (FGT) were not related to breast cancer risk, and age was an independent risk factor for breast cancer. The intensity of CESM BPE was positively correlated with MBD, which is consistent with the result of Savaridas et al.[13] Our study found that the intensity of CESM BPE and MBD in premenopausal patients was higher than that in postmenopausal patients, and there was a negative correlation between CESM BPE and MBD and age, indicating that both CESM BPE and MBD were affected by endogenous hormone levels, and CESM BPE and MBD were only indicators of age.
Our study proved that for non-high-risk women, CESM BPE and MBD were not correlated with benign or malignant breast lesions, increased CESM BPE intensity and MBD may not indicate an increased likelihood of breast cancer.
We acknowledge several limitations to our study. First, this study is a retrospective study with a relatively small sample size and may have sample bias. Second, the phase of the menstrual cycle was not recorded in this study. Although some studies have indicated that CESM BPE is not affected by the menstrual cycle,[13] the results of this study may be more accurate if the menstrual cycle phase is recorded. Third, the evaluation of CESM BPE is subjective. Quantitative analysis of CESM BPE will obtain more accurate results.
5. Conclusion
For non-high-risk women, regardless of premenopausal or postmenopausal status, CESM BPE and MBD have no correlations with benign or malignant breast lesions, and age is an influencing factor for breast cancer. The intensity of CESM BPE and MBD in premenopausal patients are higher than those in postmenopausal patients. The intensity of CESM BPE is positively correlated with MBD, and the intensity of CESM BPE and MBD are negatively correlated with age, while CESM BPE and MBD are only indicators of age. This is a preliminary study and further research is needed.
Acknowledgments
We thank H. Nikki March, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author contributions
Conceptualization: Liangliang Yu, Yongtao Wang, Dong Xing, Qianqian Chen.
Data curation: Yongtao Wang, Dong Xing, Peiyou Gong.
Formal analysis: Liangliang Yu, Qianqian Chen.
Investigation: Yongtao Wang, Dong Xing.
Methodology: Dong Xing, Peiyou Gong.
Project administration: Liangliang Yu, Dong Xing.
Software: Yongtao Wang, Peiyou Gong.
Supervision: Yongtao Wang, Peiyou Gong.
Validation: Dong Xing.
Visualization: Dong Xing, Peiyou Gong.
Writing – original draft: Liangliang Yu, Yongtao Wang, Dong Xing.
Writing – review & editing: Qianqian Chen, Yongbin Lv.
Footnotes
Abbreviations: BI-RADS = Breast Imaging Reporting and Data Syste, BPE= background parenchymal enhancement, CC= craniocaudal, CESM= contrast-enhanced spectral mammography, MBD= mammographic breast density, MLO= mediolateral obliquem.
How to cite this article: Yu L, Wang Y, Xing D, Gong P, Chen Q, Lv Y. Background parenchymal enhancement on contrast-enhanced spectral mammography does not represent an influencing factor for breast cancer: a preliminary study. Medicine. 2020;99:52(e23857).
LY, YW, and DX contributed equally to this work.
The authors declare that they have no competing interests.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Value in parentheses indicates percentage.
Value in parentheses indicates percentage.
Value in parentheses indicates percentage.
Value in parentheses indicates percentage.
Value in parentheses indicates percentage.
References
- [1].Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–36. Epub 2011 Jun 17. PMID: 21685461. [DOI] [PubMed] [Google Scholar]
- [2].Jong RA, Yaffe MJ, Skarpathiotakis M, et al. Contrast-enhanced digital mammography: initial clinical experience. Radiology 2003;228:842–50. Epub 2003 Jul 24. PMID: 12881585. [DOI] [PubMed] [Google Scholar]
- [3].Li L, Roth R, Germaine P, et al. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions. Diagn Interv Imaging 2017;98:113–23. Epub 2016 Sep 26. PMID: 27687829. [DOI] [PubMed] [Google Scholar]
- [4].Lewin J. Comparison of Contrast-Enhanced Mammography and Contrast-Enhanced Breast MR Imaging. Magn Reson Imaging Clin N Am 2018;26:259–63. Epub 2018 Feb 21. PMID: 29622130. [DOI] [PubMed] [Google Scholar]
- [5].Giess CS, Yeh ED, Raza S, et al. Background parenchymal enhancement at breast MR imaging: normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 2014;34:234–47. PMID: 24428293. [DOI] [PubMed] [Google Scholar]
- [6].King V, Brooks JD, Bernstein JL, et al. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Telegrafo M, Rella L, Stabile Ianora AA, et al. Breast MRI background parenchymal enhancement (BPE) correlates with the risk of breast cancer. Magn Reson Imaging 2016;34:173–6. [DOI] [PubMed] [Google Scholar]
- [8].Price ER, Hargreaves J, Lipson JA, et al. The California Breast Density Information Group: a collaborative response to the issues of breast density, breast cancer risk, and breast density notification legislation. Radiology 2013;269:887–92. [DOI] [PubMed] [Google Scholar]
- [9].Bennani-Baiti B, Dietzel M, Baltzer PA. MRI background parenchymal enhancement is not associated with breast cancer. PLoS One 2016;11:e0158573.Erratum in: PLoS One. 2016; 11(9):e0162936. PMID: 27379395; PMCID: PMC4933349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fridlich R, Annamalai D, Roy R, et al. BRCA1 and BRCA2 protect against oxidative DNA damage converted into double-strand breaks during DNA replication. DNA Repair (Amst) 2015;30:11–20. Epub. 2015 Mar 17. PMID: 25836596; PMCID: PMC4442488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bhatia S. Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer 2015;121:648–63. Epub 2014 Oct 29. PMID: 25355167; PMCID: PMC4339370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thompson CM, Mallawaarachchi I, Dwivedi DK, et al. The association of background parenchymal enhancement at breast MRI with breast cancer: a systematic review and meta-analysis. Radiology 2019;292:552–61. Epub 2019 Jun 25. PMID: 31237494. [DOI] [PubMed] [Google Scholar]
- [13].Savaridas SL, Taylor DB, Gunawardana D, et al. Could parenchymal enhancement on contrast-enhanced spectral mammography (CESM) represent a new breast cancer risk factor? Correlation with known radiology risk factors. Clin Radiol 2017;72: 1085.e1-1085.e9. doi: 10.1016/j.crad.2017.07.017. Epub 2017 Sep 1. PMID: 28870431. [DOI] [PubMed] [Google Scholar]
- [14].D’Orsi CJ, Sickles EA, Mendelson EB, et al. The American College of Radiology's Breast Imaging Reporting and Data System. China: Peking University Medical Press; 2013. [Google Scholar]
- [15].Boyd NF, Lockwood GA, Martin LJ, et al. Mammographic densities and breast cancer risk. Breast Dis 1998;10:113–26. PMID: 15687568. [DOI] [PubMed] [Google Scholar]
- [16].Boyd NF, Martin LJ, Yaffe MJ, et al. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 2011;13:223.Epub 2011 Nov 1. PMID: 22114898; PMCID: PMC3326547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol 2001 Oct;30:959–65. Erratum in: Int J Epidemiol. 2003 Jun;32(3):479. PMID: 11689504. [DOI] [PubMed] [Google Scholar]
- [18].Maskarinec G, Pagano I, Lurie G, et al. Mammographic density and breast cancer risk: the multiethnic cohort study. Am J Epidemiol 2005 Oct 15;162:743–52. Epub 2005 Sep 8. PMID: 16150892. [DOI] [PubMed] [Google Scholar]
- [19].Maskarinec G, Pagano I, Chen Z, et al. Ethnic and geographic differences in mammographic density and their association with breast cancer incidence. Breast Cancer Res Treat 2007;104:47–56. [DOI] [PubMed] [Google Scholar]


