Abstract
Nontuberculous mycobacteria (NTM) infection may interfere in the diagnosis and treatment of tuberculosis (TB) in TB-endemic regions. However, the population-based incidence of NTM disease and NTM–TB coinfection remains unclear.
We used Taiwan's National Health Insurance Research Database to identify new diagnoses of NTM disease and TB from 2005 to 2013 and calculated the incidence rate and the proportion of NTM–TB coinfection. The patients with NTM disease or TB were determined by the use of disease codes from International Classification of Diseases, Ninth Revision, Clinical Modification, laboratory mycobacterium examination codes, and antimycobacterial therapy receipts.
From 2005 to 2013, the age-adjusted incidence rate of NTM disease increased from 5.3 to 14.8 per 100,000 people per year and the age-adjusted incidence rate of NTM–TB coinfection was around 1.2 to 2.2 per 100,000 people per year. The proportion of NTM–TB coinfection among patients with confirmed TB was 2.8%. Male and older patients had a significantly higher incidence of NTM disease. The effects of urbanization and socioeconomic status (SES) on the incidences of TB and NTM disease were different. Rural living and lower SES were significantly associated with increasing the incidence of confirmed TB but not with that of NTM disease. For NTM disease, those living in the least urbanized area had significantly lower incidence rate ratio than in the highest urbanized area. The incidence of NTM–TB coinfection was higher in older patients and compared with patients aged < 45 years, the incidence rate ratio of the patients aged> 74 years was 12.5.
In TB-endemic Taiwan, the incidence of NTM disease increased from 2005 to 2013. Male gender and old age were risk factors for high incidence of NTM disease. SES did not have a significant effect on the incidence of NTM disease, but rural living was associated with lower incidence of NTM disease. In TB-endemic areas, NTM–TB coinfection could disturb the diagnosis of TB and treatment, especially in elderly patients.
Keywords: coinfection, incidence, nontuberculous mycobacteria, tuberculosis-endemic region, tuberculosis
1. Introduction
Over the past decades, the clinical isolation of nontuberculous mycobacteria (NTM) and the prevalence of NTM disease have increased worldwide.[1–7] The annual prevalence of NTM disease in North America and Australia ranges from 3.2 to 9.8 per 100,000 people per year; however, the population-based prevalence or incidence of NTM disease in East Asia has not been estimated.[8] For surveying the epidemiology of NTM disease, some studies investigate the microbiological isolation rate of NTM from laboratories; however, the prevalence rate of NTM disease cannot be estimated because of limited clinical information.[8,9] Other researchers used International Classification of Diseases codes, electronic clinical data and diagnostic information from integrated health care delivery systems to estimate the prevalence or incidence of NTM disease.[4,8] Even though the true rate could be underestimated in the integrated health care delivery systems, there is another advantage for this method, that is, it is able to estimate whether prevalence of NTM is affected by demographic features or comorbid conditions, which can be used to identify coinfection of NTM and tuberculosis (TB).
Both of NTM and TB cause pulmonary and extra-pulmonary disease, share similar clinical presentations and can coexist. The increasing isolation of NTM can interfere in the diagnosis of TB. For concerns of overdiagnosis of TB and potential under-treatment of NTM infections, understanding the distribution and clinical impact of NTM is of public health significance, particularly in TB-endemic regions. Few studies have investigated the incidence of NTM and NTM–TB coinfection in these regions.[5,6,10] In China, the overall rate of NTM isolated from mycobacterial culture-positive TB suspects was 5.9% between 2008 and 2012.[5] In Africa, of the individuals with presumptive TB, 15.1% and 0.2% were found to have NTM disease and NTM–TB coinfection, respectively.[11] However, the incidence of NTM disease and NTM–TB coinfection explored from a population-based survey is not investigated in East Asia and possible factors have an effect on the discrepancy of the incidence of NTM disease remains unclear.
In this study, we used the National Health Insurance (NHI) Research Database (NHIRD) of Taiwan to investigate the population-based incidence of NTM disease, the proportion of NTM–TB coinfection cases, and the association of the incidence of NTM disease and NTM–TB coinfection with demographic and socioeconomic characteristics in Taiwan, a TB-endemic region of East Asia.
2. Methods
2.1. Data source
The NHI program, which was launched on March 1, 1995 by the Taiwanese government, covers more than 99% of Taiwan's inhabitants. The NHIRD contains details regarding the ambulatory care, inpatient care, prescription data, medical procedures, and diagnostic codes of the beneficiaries of the NHI program. In this population-based cohort study, the Longitudinal Health Insurance Database 2005 (LHID 2005) was used as the data source.
2.2. Ethics statement
This study was approved by the Institutional Review Board of Chang Gung Medical Foundation (IRB No: 201700658B1).
2.3. Study design and population
LHID 2005 contains all the original claim data of 1,000,000 beneficiaries randomly sampled from the 2005 enrollment file of NHIRD and all the claims data collected from January 1, 1997, to December 31, 2013. Patients’ sociodemographic information, including age, sex, urbanization level, and socioeconomic status, was obtained from an enrollment data file in LHID 2005. The urbanization level was categorized by the population density of the residential area into 4 levels, with level 1 as the most urbanized and level 4 as the least urbanized. The enrollee category (EC) of all patients was used as a proxy measure of socioeconomic status (SES) and classified into four ranks: EC 1 (civil servants, full-time, or regularly paid personnel in governmental agencies and public schools, and self-employed people); EC 2 (employees of privately owned enterprises or institutions); EC 3 (other employee farmers’ or paid personnel, and members of the farmers’ or fishers’ associations); and EC 4 (substitute service draftees, members of low-income families, and veterans).[12,13] On average, the payroll-related amount for health insurance was highest for EC 1, followed by EC 2, EC 3, and EC 4. Accordingly, EC 1 is the highest SES and EC 4 is the lowest SES. From inpatient and outpatient claims data, we identified three groups of patients with NTM, suspected TB, and confirmed TB, who had a new disease diagnosis between 2005 and 2013 (Fig. 1). In Taiwan, TB is a disease of public concern. If a patient is clinically suspected of having TB, a doctor is mandatorily required to notify the Taiwan Centers for Disease Control and record the disease code of TB in the electronic medical system of the NHI. The diagnosis of confirmed TB is established based on final bacteriological results or the decision of a pulmonary or infectious disease physician after referring to the patient's clinical assessments, including chest imaging studies and treatment responses. A period of 1–2 months is mostly required to obtain the final bacteriological results. If the final bacteriological results confirm the diagnosis of TB, the disease code is added into the electronic medical system. By contrast, if the final results confirm the diagnosis of NTM, the physician excludes the disease code of TB and adds that of NTM into the system. If the final bacteriological results indicate no growth and no definitive clinical treatment responses are identified, then the physician also excludes the disease code of TB from the system. Base on the above, the definitions of TB and NTM disease were modified and used by the published literature.[14–16] Patients with suspected TB (A) were defined as those with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 010–018. Patients with confirmed TB (B) were defined as those should have: first, ICD-9-CM codes 010–018; secondary, at least 2 of mycobacteria laboratory examination codes were used for bacteriologic identification, including acid-fast smear (13006C/13025C), mycobacterial culture (13012C/13026C), acid-fast bacillus differentiation (13013C), tuberculin test (12106C), tuberculosis test (13024C), and bronchoscopy (28006C) results; third, received at least 1 prescription consisting of three or more than three anti-TB drugs including isoniazid (J04AC01), ethambutol (J04AD03), rifampin (J04AB02), pyrazinamide (J04AK01), amikacin (J01GB06), kanamycin (J01GB04), streptomycin (J01GA01), ciprofloxacin (J01MA02), ofloxacin (J01MA01), moxifloxacin (J01MA14), levofloxacin (J01MA12), prothionamide (J04AD01), clarithromycin (J01FA09), and thioridazine (N05AC02) for at least 120 days during a period of 180 days or any 1 fixed-dose compounds of Rifater (J04AM05) and Rifinah (J04AM02) used for at least 60 days during a 180-day period. Patients with NTM disease (C) were defined as those should have: first, an ICD-9-CM code for NTM disease (031.0, 031.1, 031.2, 031.8, and 031.9); second, the mycobacteria laboratory examination code criteria to be consistent with those for TB; third, received at least 2 drugs to treat NTM disease, including amikacin (J01GB06), cefoxitin (J01DC01), ciprofloxacin (J01MA02), clarithromycin (J01FA09), doxycycline (J01AA02), ethambutol (J04AK02), imipenem (J01DH51), levofloxacin (J01MA12), meropenem (J01DH02), minocycline (J01AA08), moxifloxacin (J01MA14), rifabutin (J04AB04), rifampin (J04AB02), tigecycline (J01AA12), and streptomycin (J01GA01).[16] The patients had confirmed TB and concomitant NTM disease were considered to have NTM–TB coinfection.
Figure 1.
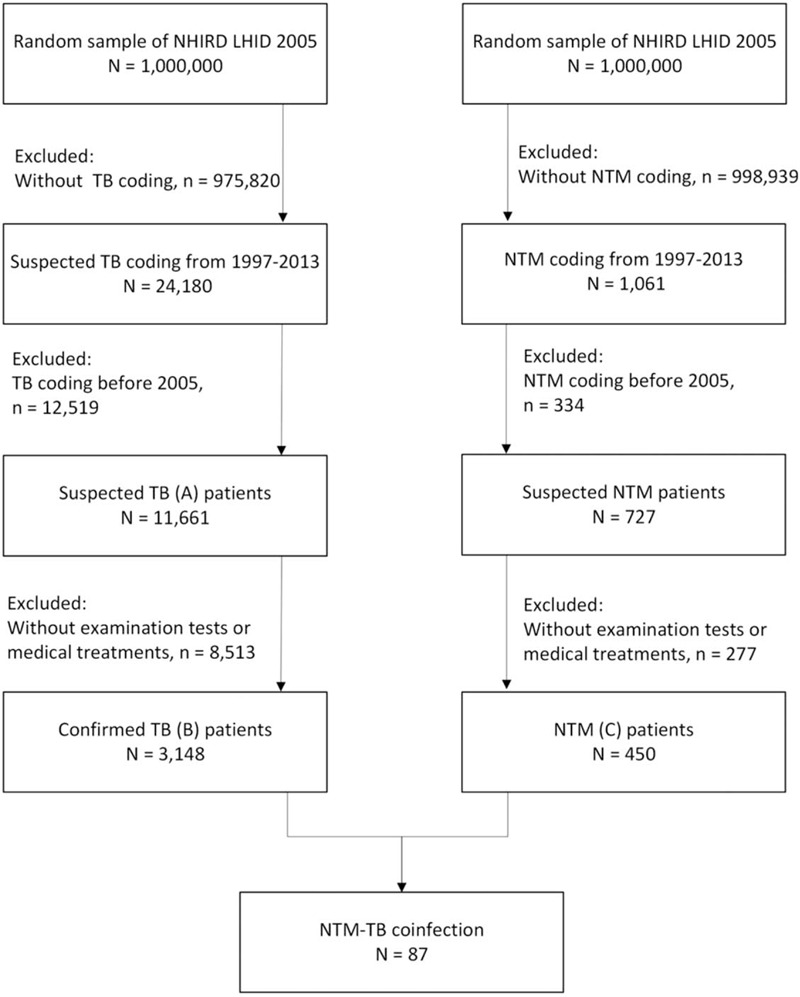
Flowchart of case selection. Patients with nontuberculous mycobacteria (NTM), suspected tuberculosis (TB), and confirmed TB from the Longitudinal Health Insurance Database (LHID) 2005, a data subset of the National Health Insurance Research Database (NHIRD) in Taiwan.
2.4. Statistical analysis
The annual incidence during the period 2005 to 2013 was calculated as the rate of new cases divided by the yearly overall population from the LHID 2005. The incidence rate ratio (IRR) of suspected TB, confirmed TB, NTM disease, and NTM–TB coinfection for various age groups, sexes, urbanization levels, and SES were calculated. Poisson regression was used to assess the IRR and the corresponding 95% confidence intervals of suspected TB, confirmed TB, NTM disease, and NTM–TB coinfection. In addition, all statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Changes in the annual incidence of NTM disease and NTM–TB coinfection in TB-endemic regions
During 2005–2013, 11,661 patients with suspected TB, 3,148 patients with confirmed TB, 450 patients with NTM disease, and 87 patients with NTM–TB coinfection were identified (Fig. 1). The annual incidence rates of suspected TB, confirmed TB, NTM, and NTM–TB coinfection are listed in Table 1. The age-adjusted incidence rate of NTM disease increased from 5.3 per 100,000 people per year in 2005 to 14.8 per 100,000 in 2013. By contrast, the age-adjusted incidence rate of confirmed TB decreased from 63.4 per 100,000 people per year in 2005 to 50.1 per 100,000 in 2013 (Fig. 2). The age-adjusted incidence rate of NTM–TB coinfection was around 1.2 to 2.2 per 100,000 people per year from 2005 to 2013, respectively. Furthermore, 2% of patients with suspected TB were diagnosed with NTM disease, and 2.8% of patients with confirmed TB were diagnosed with NTM–TB coinfection (See Table, Supplement Content, which illustrates the proportions of NTM disease in suspected TB and NTM–TB coinfection in patients with confirmed TB).
Table 1.
The incidence of tuberculosis, nontuberculous mycobacteria disease, and nontuberculous mycobacteria–tuberculosis coinfection during 2005–2013.
| suspected TB | confirmed TB | NTM | NTM-TB coinfection | |||||||||
| Year | N | incidence rate | 95% CI | N | incidence rate | 95% CI | N | Incidence rate | 95% CI | N | Incidence rate | 95% CI |
| Total | 11661 | 3148 | 450 | 87 | ||||||||
| 2005 | 1955 | 359.3 | 343.5–375.8 | 356 | 63.4 | 57.0–70.5 | 34 | 5.3 | 3.6–7.8 | 8 | 1.2 | 0.5–2.7 |
| 2006 | 1825 | 341.1 | 325.6–357.4 | 379 | 68.4 | 61.7–75.8 | 45 | 7.6 | 5.6–10.4 | 13 | 0.0 | 0.0–0.0 |
| 2007 | 1506 | 284.3 | 270.0–299.4 | 369 | 67.5 | 60.9–75.0 | 44 | 7.6 | 5.5–10.4 | 12 | 2.2 | 1.3–3.9 |
| 2008 | 1261 | 239.8 | 226.5–253.9 | 383 | 71.6 | 64.7–79.3 | 43 | 7.9 | 5.8–10.7 | 10 | 1.5 | 0.7–3.2 |
| 2009 | 1116 | 220.4 | 207.6–233.9 | 369 | 70.6 | 63.7–78.3 | 45 | 8.4 | 6.3–11.4 | 13 | 2.0 | 1.0–4.0 |
| 2010 | 1156 | 228.6 | 215.4–242.7 | 362 | 69.5 | 62.5–77.2 | 43 | 8.2 | 6.0–11.1 | 9 | 1.7 | 0.9–3.4 |
| 2011 | 1003 | 199.6 | 187.1–213.0 | 339 | 66.4 | 59.5–74.0 | 51 | 10.0 | 7.6–13.2 | 9 | 1.6 | 0.8–3.3 |
| 2012 | 994 | 202.6 | 189.8–216.3 | 340 | 66.5 | 59.4–74.3 | 68 | 13.6 | 10.6–17.3 | 10 | 1.8 | 0.9–3.7 |
| 2013 | 845 | 176.2 | 164.2–189.1 | 251 | 50.1 | 44.0–57.1 | 77 | 14.8 | 11.6–18.9 | 3 | ||
Figure 2.
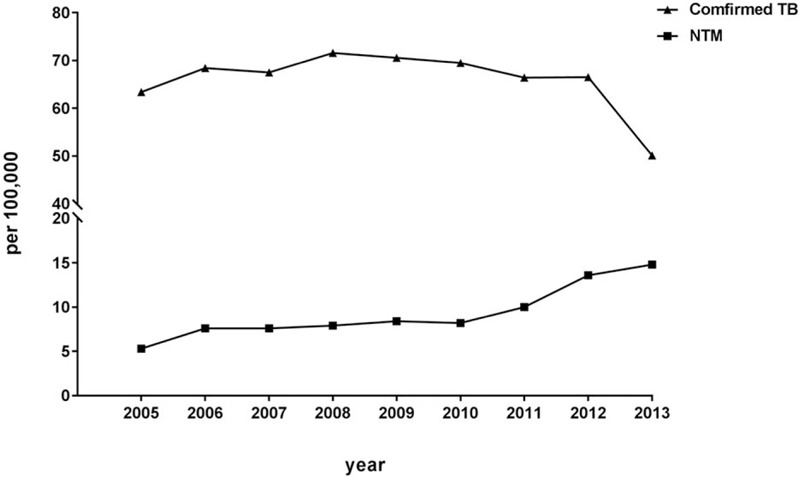
Annual age-adjusted incidence rate of nontuberculous mycobacteria (NTM) disease and confirmed tuberculosis (TB) during 2005-2013.
3.2. Incidence of NTM disease and NTM–TB coinfection according to demographic characteristics
The demographic characteristics of the patients with confirmed TB, NTM disease, and NTM–TB coinfection are shown in Table 2. The incidence rate of confirmed TB (96.5 vs 43.4 per 100,000 people per year) and NTM disease (10.9 vs 6.5 per 100,000 people per year) was higher in men than in women, and the IRR of the men was 2.2 for confirmed TB and 1.7 for NTM disease, respectively (P < .001 for both). In addition, the incidence rate of confirmed TB and NTM disease increased with age. Compared with patients aged < 45 years, the IRR of the patients aged > 74 years with confirmed TB and NTM disease was 11.8 and 20.3, respectively (P < .001 for both). In respect to SES, lower the payroll-related amount for health insurance, which is the higher EC rank to represent the lower SES, was significantly associated with increasing the incidence of confirmed TB but not with that of NTM disease. In addition, rural living was also significantly associated with increasing the incidence of confirmed TB. But for NTM disease, those living in the least urbanized area had significantly lower IRR than in the highest urbanized area (P = .043). Male predominance was observed among the patients with NTM–TB coinfection, and the IRR of the men with NTM–TB coinfection was 1.9 (P < .001). The older patients had a higher incidence of NTM–TB coinfection, and the IRR of the patients with NTM–TB coinfection aged > 74 years was 12.5 compared with that of the patients aged < 45 years (P < .001). Urbanization and SES had no statistical effect on the incidence of NTM–TB coinfection.
Table 2.
The incidence of tuberculosis, nontuberculous mycobacteria disease, and nontuberculous mycobacteria–tuberculosis coinfection according to demographic and socioeconomic characteristics adjusted by gender, age, urbanization level, and socioeconomic status.
| confirmed TB | NTM | NTM-TB coinfection | |||||||||||||
| N | Incidence rate | 95% CI | IRR | P value | N | Incidence rate | 95% CI | IRR | P value | N | Incidence rate | 95% CI | IRR | P value | |
| Total | 3148 | 64.7 | 61.4–68.2 | 450 | 8.4 | 7.3–9.7 | 87 | 1.5 | 1.1–2.1 | ||||||
| Gender | |||||||||||||||
| Female | 997 | 43.4 | 40.3–46.7 | 1 | 171 | 6.5 | 5.4–7.9 | 1 | 31 | 1.1 | 0.7–1.7 | 1 | |||
| Male | 2151 | 96.5 | 91.4–101.8 | 2.2 | <.001 | 279 | 10.9 | 9.3–12.8 | 1.7 | <.001 | 56 | 2.1 | 1.4–3.0 | 1.9 | <.001 |
| Age | |||||||||||||||
| <45 | 917 | 15.8 | 14.6–17.0 | 1 | 102 | 1.4 | 1.1–1.8 | 1 | 19 | 0.3 | 0.2–0.5 | ||||
| 45-64 | 933 | 47.7 | 44.2–51.5 | 3 | <.001 | 132 | 5.8 | 4.7–7.1 | 4 | <.001 | 31 | 1.3 | 0.8–2.1 | 5 | <.001 |
| 65-74 | 630 | 125.2 | 114.7–136.6 | 7.9 | <.001 | 108 | 20.4 | 16.4–25.4 | 14.1 | <.001 | 24 | 4.4 | 2.7–7.1 | 16.5 | <.001 |
| >74 | 668 | 186.1 | 171.0–202.5 | 11.8 | <.001 | 108 | 29.4 | 23.7–36.4 | 20.3 | <.001 | 13 | 3.3 | 1.8–6.1 | 12.5 | <.001 |
| Urbanization level∗ | |||||||||||||||
| 1 | 726 | 52.7 | 48.7–57.1 | 1 | 132 | 10 | 8.3–12.1 | 1 | 32 | 2.2 | 1.5–3.3 | ||||
| 2 | 1344 | 55.7 | 52.3–59.4 | 1.1 | .224 | 214 | 9.4 | 8.1–11.0 | 0.9 | .601 | 35 | 1.4 | 0.9–2.1 | 0.6 | .06 |
| 3 | 659 | 87 | 78.3–96.7 | 1.3 | <.001 | 74 | 8.1 | 6.3–10.3 | 0.8 | .146 | 13 | 1.3 | 0.7–2.3 | 0.6 | .091 |
| 4 | 419 | 87 | 78.3–96.7 | 1.7 | <.001 | 30 | 6.6 | 4.5–9.6 | 0.7 | .043 | 7 | 1.3 | 0.6–2.9 | 0.6 | .231 |
| EC† | |||||||||||||||
| 1 | 197 | 49.3 | 42.7–56.8 | 1 | 33 | 7 | 4.9–10.0 | 1 | 5 | 1 | 0.4–2.4 | ||||
| 2 | 1066 | 56.4 | 52.6–60.3 | 1.1 | .084 | 184 | 8.7 | 7.3–10.4 | 1.2 | .244 | 34 | 1.5 | 1.0–2.2 | 1.5 | .406 |
| 3 | 1398 | 73.9 | 70.0–78.1 | 1.5 | <.001 | 168 | 8.5 | 7.2–10.0 | 1.2 | .32 | 36 | 1.8 | 1.2–2.5 | 1.8 | .227 |
| 4 | 487 | 85.4 | 77.5–94.0 | 1.7 | <.001 | 65 | 9.6 | 7.3–12.6 | 1.4 | .145 | 12 | 2 | 1.1–3.7 | 2 | .185 |
4. Discussion
In this population-based retrospective cohort study of TB-endemic region, we found that the annual incidence rate of NTM disease increased from 5.3 per 100,000 people per year in 2005 to 14.8 per 100,000 in 2013. By contrast, the incidence rate of confirmed TB decreased from 63.4 per 100,000 people per year in 2005 to 50.1 per 100,000 in 2013. During the study period, the incidence of NTM–TB coinfection was around 1.2 and 2.2 per 100,000 people per year. 2% of patients with suspected TB were diagnosed with NTM disease, and 2.8% of patients with confirmed TB were diagnosed with NTM–TB coinfection. The incidence of NTM disease was significantly higher in male and older patients but lower in rural living. However, SES did not significantly affect the incidence of NTM disease.
The incidence and prevalence of NTM disease have varied in various populations and have increased worldwide.[3,7,17,18] In North America, a population-based investigation indicated that the incidence rate of pulmonary NTM disease increased from 4.8 per 100,000 people per year in 2007 to 5.6 per 100,000 in 2012.[7] In Europe, the incidence rate of NTM disease increased from 5.6 per 100,000 to 7.6 per 100,000 in England, Wales and Northern Ireland between 2007 and 2012.[19] In Australia, the incidence rate of pulmonary NTM disease increased from 2.2 per 100,000 of the population in 1999 to 3.2 per 100,000 in 2005.[3] Before the current study, there was lack of a large-scale and precise investigation for the incidence of NTM disease in East Asia. A study conducted in Japan evaluated the medical records of 11 hospitals and indicated that the incidence rate of NTM lung disease increased from 5.9 per 100,000 of the population in 2005 to 10.1 per 100,000 in 2009.[17] In Korea, Park and colleagues used the NHI database to investigate the incidence of NTM disease and found that the incidence of NTM infection was increased from 2003 to 2016. The age-adjusted incidence was 1.0 per 100,000 of the population in 2003 and 17.9 per 100,000 in 2016.[20] The incidence rates of NTM disease are close to our findings in Taiwan. However, in the study, a single diagnostic code was used to establish the diagnosis of NTM that the true incidence rates of NTM disease in Korea might be lower than those in Taiwan. Lai and colleagues used the database of a university hospital in northern Taiwan to identify NTM isolates from clinical specimens and estimate the incidence of NTN disease and showed that the incidences of NTM disease increased from 2.7 per 100,000 inpatients and outpatients in 2000 to 10.2 per 100,000 in 2008.[21] In this study, we find that the incidence rate of NTM disease in 2008 is 7.9 per 100,000 people per year, which is lower than the findings of Lai's study. In the study, because there was not a clear disease-free period and the incidence data was estimated from laboratory NTM isolates without other clinical assessments, the incidence of NTM disease could be uncertain and overestimated. In addition, for the study was conducted in a tertiary-care center and lack of population-based inferences, the results might not reflect the overall situation in Taiwan. In the present study, we used the NHIRD, which covers the medical records of almost all of Taiwan's residents, to investigate the incidence of NTM disease in the general population. For validating the disease diagnosis, we used three criteria of coding for NTM disease, mycobacteria laboratory examinations, and medication treatment. In addition, we excluded patients with the aforementioned disease codes before 2005 from the cohort to evaluate the incidence in accordance with the frequency of new cases occurring in the defined population over a specified time period. To compare with previous studies, the incidence of NTM disease investigated in this study is more precise and can represent the estimation of the general population.
Sex, age, geographical location, and SES have been shown to associate with the prevalence or incidence of NTM disease.[22,23] However, sex distribution with respect to NTM disease remains controversial.[8] In this population-based cohort study, we discovered male predominance for NTM disease, which is consistent with the findings of studies exploring the incidence of pulmonary NTM disease in North America.[7,24] Studies that have suggested substantial female predominance for NTM disease have not been population-based cohort studies and have generally been restricted to patients without predisposing conditions such as chronic obstructive pulmonary disease (COPD)[25,26] The recent population-based cohort study from Korea showed that the incidence of NTM infection in 2016 was about 2.5 times higher in women than that in men. However, the proportions of COPD and bronchiectasis in female and male patients were not clear.[20] Comorbidities such as COPD and bronchiectasis are considered host risk factors for NTM disease.[27] Studies have also reported a more frequent diagnosis of COPD among men with pulmonary NTM and bronchiectasis among women with pulmonary NTM.[28–30] The frequency of comorbidities by sex can lead to discrepancies in the effect of sex on NTM disease. Regarding the effect of age on NTM disease, advanced age is strongly associated with pulmonary NTM.[31] Recently, an incidence survey of pulmonary NTM conducted in the United States indicated that incidence increased with age; it was more than 25 per 100,000 in patients aged ≥80 years.[7] In TB-endemic East Asia, we also observed that the incidence of NTM disease increased with age; the incidence rate of the patients aged > 74 years was 29.4 per 100,000 people per year, and the IRR of NTM disease among the patients aged > 74 years was 20.3-fold higher than that among patients aged < 45 years. In respect to the relationship of urbanization with NTM disease, the patients with disease due to Mycobacterium kansasii (M kansasii) infection were significantly more likely to come from urban than rural areas and there was a higher incidence of M avium complex in the population living in urban communities.[32,33] Cassidy et al. further elucidated urban living could be a risk factor of NTM disease after adjusting for differences in age and sex distribution.[31] Their findings are consistent with the results of the current study that there is a significantly lower incidence of NTM disease in the population living in rural areas. The supposed reason is that most urban areas lean on municipal water systems which are reservoirs providing opportunity for biofilm formation that promotes NTM growth.[34,35] Although a diagnostic bias is concerned that persons living in urban areas had more chance to seek medical care and be diagnosed with NTM disease. However, the higher incidence of confirmed TB in the population living in rural areas is also noted in this study that does not support the diagnostic bias theory.
In TB-endemic countries, NTM–TB coinfection is not infrequent and disturbs the diagnosis of TB.[36] In Zambia, Africa, the results of a nationwide survey of the prevalence of NTM revealed that the proportion of NTM–TB coinfection was 0.2%.[11] In India, South Asia, Singh et al. used molecular methods and identified NTM in 17.6% of suspected multidrug-resistant pulmonary TB cases and in 12.4% of suspected extrapulmonary tuberculosis cases.[37] Another comprehensive meta-analysis study that investigated the prevalence of NTM infections in Iran showed that the proportion of NTM infections was 10.2% among culture-positive TB cases.[38] The results of our population-based cohort study showed that 2.8% of the patients with confirmed TB harbored NTM infections. Furthermore, we observed that the IRR of NTM–TB coinfection was higher in older patients than in younger patients. These findings suggest that clinicians should be vigilant regarding possible NTM–TB coinfection, particularly when an older patient is diagnosed with TB.
This study has several limitations of concern. First, since the sample population used in the present study was a fixed cohort, the disease incidence may be overestimated. For this concern, we used Poisson regression to estimate the incidence, adjusted for age. Besides, we obtained the population incidences of TB reported on the Taiwan CDC for verification. The national TB incidences in 2005 and 2013 are 72.5 and 49.4 per 100,000, respectively, similar to those of the present study.[39] Second, for validating the diagnosis, the incidence was determined by using three rigorous criteria to survey the patients with NTM disease from the NHIRD; thus, the actual incidence of NTM disease could have been underestimated. Our findings may be better interpreted as treated incidence. Third, for making a diagnosis of NTM disease, clinical, radiographic, and microbiologic criteria are equally important. The study used at least 2 of mycobacteria laboratory examination codes for bacteriologic identification, but that cannot represent the accurate bacteriologic results. However, the initiation of therapy for NTM disease is decided by a physician based on the diagnosis of NTM disease and potential risks and benefits of therapy for individual patients.[40] We utilized the criteria of receiving at least 2 drugs for NTM disease, which represents the judgment from the physician to verify the diagnosis of NTM disease.
5. Conclusion
In TB-endemic Taiwan, the incidences of NTM gradually increased in the last twenty years. Male and older patients had a higher incidence of NTM disease. NTM–TB coinfection could disturb the diagnosis of TB and treatment, especially in elderly patients. For the number of people aged ≥60 years projected to grow by 56% worldwide in the next ten years,[41] the increasing incidence of NTM disease and NTM–TB coinfection in older patients should be recognized as a crucial public health concern in TB-endemic areas.
Acknowledgments
The authors wish to thank the Health Information and Epidemiology Laboratory (CLRPG6G0041) for their comments and assistance in data analysis. This study was supported by a grant from Chang Gung Memorial Hospital, Chia-Yi Branch, and was based on the National Health Insurance Research Database provided by the Central Bureau of National Health Insurance, the Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institute
Authors’ information: 1Division of Pulmonary infection and critical care, Department of Pulmonary and Critical Care Medicine Chang Gung Memorial Hospital, Chiayi, Taiwan. 2Graduate Institute of Clinical Medicine Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan. 3Department of Traditional Chinese Medicine, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan, ROC. 4Health Information and Epidemiology Laboratory of Chang Gung Memorial Hospital, Chiayi, Taiwan, ROC. 5School of Traditional Chinese Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan, ROC. 6Department of Psychiatry, Wan-Fang Hospital & School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan. 7Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Chang Gung Memorial Hospital-Kaohsiung Medical Center. 8Department of Respiratory Care, College of Medicine, Chang Gung University, Taoyuan, Taiwan. 9School of Occupational Therapy, Chung Shan Medical University, Taichung, Taiwan. 10Department of Psychiatry, Chiayi Chang Gung Memorial Hospital, Chiayi, Taiwan, ROC. 11Department of Public Health, College of Health Science, Kaohsiung Medical University, Kaohsiung, Taiwan. 12Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan. 13Department of Psychiatry, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
Author contributions
CKL, YHY, and VCHC conceived and designed the study. YHY prepared datasets and statistical analyses MLL, YHT, MJH, YCL, and TNW supervised the study. CKL and YLC wrote the draft of the manuscript and all authors contributed substantially to the revision of the manuscript. All the authors read and approve the last version of the manuscript. VCHC responsibility for the investigation as a whole.
Conceptualization: Chin-Kuo Lin, Vincent Chin-Hung Chen.
Data curation: Chin-Kuo Lin, Yao-Hsu Yang, Vincent Chin-Hung Chen.
Formal analysis: Chin-Kuo Lin, Vincent Chin-Hung Chen.
Funding acquisition: Vincent Chin-Hung Chen.
Investigation: Chin-Kuo Lin, Vincent Chin-Hung Chen.
Methodology: Chin-Kuo Lin, Yao-Hsu Yang, Yi-Chen Lee, Tsu-Nai Wang, Vincent Chin-Hung Chen.
Project administration: Vincent Chin-Hung Chen.
Resources: Vincent Chin-Hung Chen.
Software: Vincent Chin-Hung Chen.
Supervision: Ying-Huang Tsai, Meng-Jer Hsieh, Vincent Chin-Hung Chen.
Validation: Vincent Chin-Hung Chen.
Visualization: Vincent Chin-Hung Chen.
Writing – original draft: Chin-Kuo Lin, Yao-Hsu Yang.
Writing – review & editing: Yao-Hsu Yang, Mong-Liang Lu, Yi-Chen Lee, Tsu-Nai Wang, Yi-Lung Chen, Vincent Chin-Hung Chen.
Supplementary Material
Footnotes
Abbreviations: COPD = chronic obstructive pulmonary disease, ICD-9-CM = International Classification of Diseases, Ninth Revision Clinical Modification, IRR = incidence rate ratio, LHID 2005 = longitudinal health insurance database 2005, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NTM = nontuberculous mycobacteria, SES = socioeconomic status, TB = tuberculosis.
How to cite this article: Lin CK, Yang YH, Lu ML, Tsai YH, Hsieh MJ, Lee YC, Wang TN, Chen YL, Chen VH. Incidence of nontuberculous mycobacterial disease and coinfection with tuberculosis in a tuberculosis-endemic region: a population-based retrospective cohort study. Medicine. 2020;99:52(e23775).
This work was supported in part by the Ministry of Science and Technology, Taiwan, ROC (MOST 102-2314-B-040-004-MY3), and Chang Gung Medical Foundation, Chiayi Chang Gung Memorial Hospital, Taiwan, ROC (CMRPG6E0261, CMRPG6E02612, CMRPG6E02613).
The authors have no conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
Supplemental digital content is available for this article.
Incidence rate: per 100,000 people per year; The data was adjusted by age.
Incidence rate: per 100,000 people per year; IRR: incidence rate ratio.
The urbanization level was categorized by the population density of the residential area into 4 levels, with level 1 as the most urbanized and level 4 as the least urbanized.
EC: enrollee category, as a proxy measure of socioeconomic status and the payroll-related amount for health insurance was highest for EC 1, followed by EC 2, EC 3, and EC 4.
References
- [1].Marras TK, Chedore P, Ying AM, et al. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997 2003. Thorax 2007;62:661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].van Ingen J, Bendien SA, de Lange WC, et al. Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region, The Netherlands. Thorax 2009;64:502–6. [DOI] [PubMed] [Google Scholar]
- [3].Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis 2010;16:1576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adjemian J, Olivier KN, Seitz AE, et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012;185:881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu J, Zhang Y, Li J, et al. Increase in nontuberculous mycobacteria isolated in Shanghai, China: results from a population-based study. PloS One 2014;9:e109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chiang CY, Yu MC, Yang SL, et al. Surveillance of tuberculosis in Taipei: the influence of nontuberculous mycobacteria. PloS one 2015;10:e0142324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Henkle E, Hedberg K, Schafer S, et al. Population-based incidence of pulmonary nontuberculous mycobacterial disease in oregon 2007 to 2012. Ann Am Thor Soc 2015;12:642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 2015;36:13–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013;42:1604–13. [DOI] [PubMed] [Google Scholar]
- [10].Hoza AS, Mfinanga SG, Rodloff AC, et al. Increased isolation of nontuberculous mycobacteria among TB suspects in Northeastern, Tanzania: public health and diagnostic implications for control programmes. BMC Res Notes 2016;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chanda-Kapata P, Kapata N, Klinkenberg E, et al. Non-tuberculous mycobacteria (NTM) in Zambia: prevalence, clinical, radiological, and microbiological characteristics. BMC Infect Dis 2015;15:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen CY, Liu CY, Su WC, et al. Factors associated with the diagnosis of neurodevelopmental disorders: a population-based longitudinal study. Pediatrics 2007;119:e435–43. [DOI] [PubMed] [Google Scholar]
- [13].Tsai MS, Lin MH, Lee CP, et al. Chang gung research database: a multi-institutional database consisting of original medical records. Biomed J 2017;40:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee CH, Lee MC, Lin HH, et al. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PloS one 2012;7:e37978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liao TL, Lin CH, Shen GH, et al. Risk for mycobacterial disease among patients with rheumatoid arthritis, Taiwan, 2001-2011. Emerg Infect Dis 2015;21:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yeh JJ, Wang YC, Lin CL, et al. Nontuberculous mycobacterial infection is associated with increased respiratory failure: a nationwide cohort study. PloS one 2014;9:e99260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ide S, Nakamura S, Yamamoto Y, et al. Epidemiology and clinical features of pulmonary nontuberculous mycobacteriosis in Nagasaki, Japan. PloS one 2015;10:e0128304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ringshausen FC, Wagner D, de Roux A, et al. Prevalence of nontuberculous mycobacterial pulmonary disease, Germany, 2009-2014. Emerg Infect Dis 2016;22:1102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shah NM, Davidson JA, Anderson LF, et al. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007-2012. BMC Infect Dis 2016;16:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park SC, Kang MJ, Han CH, et al. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: a nationwide population-based study. BMC Pulm Med 2019;19:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lai CC, Tan CK, Chou CH, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg Infect Dis 2010;16:294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med 2002;23:553–67. [DOI] [PubMed] [Google Scholar]
- [23].Chou MP, Clements AC, Thomson RM. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect Dis 2014;14:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marras TK, Mehta M, Chedore P, et al. Nontuberculous mycobacterial lung infections in Ontario, Canada: clinical and microbiological characteristics. Lung 2010;188:289–99. [DOI] [PubMed] [Google Scholar]
- [25].Prince DS, Peterson DD, Steiner RM, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med 1989;321:863–8. [DOI] [PubMed] [Google Scholar]
- [26].Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med 2015;36:1–1. [DOI] [PubMed] [Google Scholar]
- [28].Lewis AG, Jr, Dunbar FP, Lasche EM, et al. Chronic pulmonary disease due to atypical mycobacterial infections. Am Rev Respir Dis 1959;80:188–99. [DOI] [PubMed] [Google Scholar]
- [29].Carruthers KJ, Edwards FG. Atypical mycobacteria in Western Australia. Am Rev Respir Dis 1965;91:887–95. [DOI] [PubMed] [Google Scholar]
- [30].Billinger ME, Olivier KN, Viboud C, et al. Nontuberculous mycobacteria-associated lung disease in hospitalized persons, United States, 1998-2005. Emerg Infect Dis 2009;15:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cassidy PM, Hedberg K, Saulson A, et al. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009;49:e124–9. [DOI] [PubMed] [Google Scholar]
- [32].Ahn CH, Lowell JR, Onstad GD, et al. A demographic study of disease due to Mycobacterium kansasii or M intracellulare-avium in Texas. Chest 1979;75:120–5. [DOI] [PubMed] [Google Scholar]
- [33].du Moulin GC, Sherman IH, Hoaglin DC, et al. Mycobacterium avium complex, an emerging pathogen in Massachusetts. J Clin Microbiol 1985;22:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].September SM, Brozel VS, Venter SN. Diversity of nontuberculoid Mycobacterium species in biofilms of urban and semiurban drinking water distribution systems. Appl Environ Microbiol 2004;70:7571–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fan C, Kao CF, Liu YH. Quantitative characterization of organic diffusion using an analytical diffusion-reaction model and its application to assessing BOD removal when treating municipal wastewater in a plug flow reactor. Water Res 2017;121:329–37. [DOI] [PubMed] [Google Scholar]
- [36].Gopinath K, Singh S. Non-tuberculous mycobacteria in TB-endemic countries: are we neglecting the danger? PLoS Negl Trop Dis 2010;4:e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jain S, Sankar MM, Sharma N, et al. High prevalence of non-tuberculous mycobacterial disease among non-HIV infected individuals in a TB endemic country--experience from a tertiary center in Delhi, India. Pathog Glob Health 2014;108:118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nasiri MJ, Dabiri H, Darban-Sarokhalil D, et al. Prevalence of non-tuberculosis mycobacterial infections among tuberculosis suspects in Iran: systematic review and meta-analysis. PloS One 2015;10:e0129073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Taiwan Centers for Disease Control. Data & Statistics: Tuberculosis statistics (Monitor). Available at: https://daily.cdc.gov.tw/stoptb/CareMagList.aspx?funid=1. [Google Scholar]
- [40].Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- [41].United Nations, Department of Economic and Social Affairs, Population Division (2015). World Population Ageing. 2015:(ST/ESA/SER.A/390). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


