Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, imaging, magnetic resonance imaging, neurocritical care, neurology, point-of-care
Abstract
Objectives:
Patients in ICUs often require neuroimaging to rule out a wide variety of intracranial problems. CT may be available in the ICU itself, but MRI has greater sensitivity for many conditions that affect the brain. However, transporting patients who are on ventilators and other life-sustaining devices is a labor-intensive process and involves placing the patient at risk for adverse events. This is a report of portable MRI in a clinical setting.
Design:
This is a prospective, nonrandomized, observational study at one institution, utilizing a 0.064-T, self-shielding, portable MRI in ventilated patients in an ICU setting.
Setting:
Academic medical center.
Patients:
Nineteen patients with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 infection. Patients selected for imaging had any of the following: 1) unexplained encephalopathy or coma, 2) seizures, 3) focal neurologic deficit, or 4) abnormal head CT. Imaging was performed in each patient’s ICU room with a portable, self-shielding, 0.064-T MRI.
Interventions:
None.
Measurements and Main Results:
Among 19 patients, 20 MRI scans in seven ICUs were acquired between April 13, 2020, and April 23, 2020. No adverse events to patients or staff from MRI acquisition were reported. In 12 patients, abnormal findings were seen, which included increased fluid attenuated inversion recovery signal (n = 12), hemorrhage (n = 3), and diffusion-weighted imaging positivity (n =3). Imaging led to changes in clinical management in five patients.
Conclusions:
In this case series of patients, use of portable MRI has been found to be safe, feasible, and led to changes in clinical management based on imaging results. However, future studies comparing results with other imaging modalities are required to understand fully the extent of its clinical utility.
The use of portable, bedside imaging such as CT has been adopted by health systems to safely image patients in the ICU (1–3). However, studies of the diagnostic sensitivity of CT versus MRI show that MRI adds more value in patient evaluations due to its comparatively higher sensitivity to ischemia, encephalopathy, hemorrhage, and other diagnostic categories (4). Consequently, CT alone may not provide the necessary sensitivity required to diagnose brain abnormalities in critically ill patients. The use of a portable MRI presents an opportunity for clinicians to gain important information at the patient bedside in the ICU.
For this study, we imaged patients infected with the coronavirus disease of 2019 (Covid-19). This pandemic has infected over 6 million people worldwide since the first reported case in December 2019 in Wuhan City, China (5). It has been estimated that 10–12% of hospitalized patients require mechanical ventilation and intensive care (6, 7). Recent literature has reported cerebral infarction, acute necrotizing encephalopathy, and encephalitis in severely ill Covid-19 patients due to associated inflammatory-mediated thrombosis and disruption of the blood brain barrier (8–11). Neuroimaging for Covid-19 patients has several limitations, including the risks associated with patient transport and exposure to a larger patient population and hospital staff (3, 12–15).
We report on the safety and feasibility of utilizing portable MRI in the ICU setting and the clinical utility of this technology. The images acquired can facilitate more targeted treatment by medical providers. We have established a working protocol for bedside imaging in the ICU and provide an experience-based account of the utility of this point-of-care MRI.
MATERIALS AND METHODS
This is a prospective, nonrandomized, observational study of patients imaged with a portable MRI over 10 days in April 2020. Each patient had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Patients selected for neuroimaging with portable MRI were admitted to an ICU and had at least one of the following: 1) unexplained encephalopathy or coma, 2) seizures, 3) focal neurologic deficit, 4) abnormal head CT, or 5) elevated inflammatory markers in the blood or cerebrospinal fluid (CSF). At the time of use, the MRI device used in the study was approved under the Food and Drug Administration Emergency Use Authorizations pathway. The Northwell Health Institutional Review Board approved this study as minimal-risk research using data collected for routine clinical practice and waived the requirement for informed consent.
Point-of-Care MRI
A portable, 0.064-T, MRI device (Hyperfine, Guilford, CT) was used to image patients at the bedside. Two horizontally oriented, permanent magnets form the two poles of the system (Fig. 1). The radiofrequency transmit coils and the receive coil are supported together on a platform inside the gantry (Fig. 1D). Presently, the MRI can acquire T1 weighted (T1W), T2 weighted (T2W), fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI) (along with the associated apparent diffusion coefficient [ADC]). The device operators were trained medical providers (neurosurgery residents and critical care fellows) who completed a brief demonstration on usability of the device and were trained in magnetic resonance (MR) safety considerations. During the actual scanning procedure, the operators did not require any additional support from MRI technologists. The device is Federal Communications Commission class A and Comité International Spécial des Perturbations Radioélectriques group 2 compliant. For further details regarding the portable MRI and image acquisition, please see the supplementary appendix (http://links.lww.com/CCX/A465). Image acquisition times per sequence can be found in Table 1.
Figure 1.
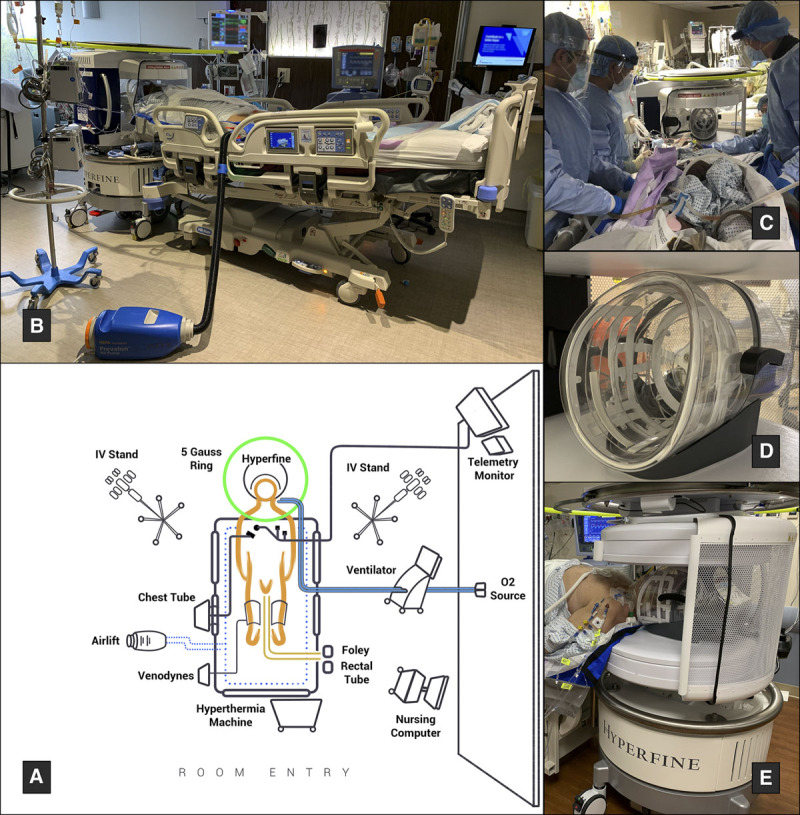
Portable MRI room setup. A, Key features of the portable MRI room setup, as demonstrated in a coronavirus disease 2019-ICU. B, The patient positioned for imaging. The bed is turned 90° relative to the ventilator and wall oxygen sources to minimize circuit disruptions. The bed is tilted in ~10° Trendelenburg. C, The mobility team moves the patient into the MRI gantry. D, The receive coil. E, The patient’s head secured in the transmit-receive coil.
TABLE 1.
Scanning Times for Each Sequence
| Sequence | Scan Time (min:s) | Standard MRI Scan Timesa (min:s) |
|---|---|---|
| Prescan calibration | 1:03 | 0:32 |
| Localizer | 0:18 | 0:15 |
| T1 weighted (axial) | 4:54 | 1:30 |
| T2W (axial) | 7:03 | 1:52 |
| Fluid attenuated inversion recovery (axial) | 9:31 | 3:53 |
| T2W (coronal) | 7:03 | 3:17b |
| Diffusion-weighed imaging + apparent diffusion coefficient map | 9:04 | 1:36 |
| Total | 38:56 | 12:55 |
T2W = T2 weighted.
aAcquisition time from sample patient scans acquired on a GE Medical Signa HDxt 1.5T scanner.
bAcquisition time from a partial brain volume. T2 coronal not routinely acquired for clinical purposes.
Patient Preparation for Imaging
Prior to completing any imaging, our institution-specific MRI screening form, adhering to 1.5-T MRI guidelines, was completed with the patient’s next of kin. Patients who did not pass the safety checklist were not imaged (the current MRI device is safe for use with biomedical devices that have been deemed safe for use with 1.5-T magnets and less). Patients who passed the safety checklist were then attended to by the MRI team (who transported and operated the device), which consisted of two trained neurosurgery residents or neurocritical care fellows. Several parameters were considered in every ICU where imaging was performed:
1) Bed positioning was relative to the patient ventilator. Limited movement of the ventilator, the joints and tubing, and the oxygen supply was important for patient and provider safety. To accommodate the MRI, the patient’s bed was either laterally shifted or turned 90° relative to the position of the ventilator to eliminate the risk of disrupting normal ventilator function (Fig. 1, A and B).
2) In certain patients, oxygenation was increased to 100% Fio2 during the imaging study to reduce the risk of desaturation during MRI acquisition.
3) Unnecessary equipment was removed from the patient room to create adequate space for the MRI to maneuver into an appropriate position.
4) Sedation or paralytics were titrated as needed before moving the patient into the correct position.
To assist in positioning and to reduce the risk of patient movement from the receive coil during image acquisition, the bed was raised and placed in around 10° of Trendelenburg position (Fig. 1B). The mobility team that consisted of a nurse and two to three providers assisted in positioning as needed (Fig. 1C). In these circumstances, an Air Lift (Prevalon, Stryker, Kalamazoo, MI) was placed under the bedsheets to assist in movement. Use of the blower function of the airlift was limited, as the excessive positive pressure could place medical providers at risk for infection. Using the bedsheet, the patient was then carefully moved into the gantry with the head secured safely in the coil (Fig. 1E). With the workflow refined, patient positioning could be completed in 15–20 minutes. Image acquisition was controlled with an iPad provided by the manufacturer.
Data Collection and Analysis
Data were prospectively collected from the patient’s electronic medical record (EMR). Patients’ MRI scans were reviewed in the picture archiving and communications systems (PACS). Two attending neurosurgeons and an attending neuroradiologist judged the images for diagnostic quality. Image quality was graded as a whole and by individual sequence on a scale of 1–5, with 1 being the best, 2–3 being of an adequate quality for interpretation, 4 being of an inadequate quality for interpretation though with some recognizable anatomy, and with 5 deemed uninterpretable. The grading criteria are outlined in Table 2. Intraclass correlation coefficient (ICC) estimates and their 95% CIs were calculated using the SPSS statistical package Version 26 (SPSS, Chicago, IL) based on a mean rating (k = 3), absolute agreement, and a two-way mixed-effects model.
TABLE 2.
Scale of Diagnostic Quality
| Grade | Description |
|---|---|
| 1 | Highest quality for clinical interpretation |
| 2 | Adequate quality for interpretation with minor limitations, sufficient for clinical interpretation |
| 3 | Major limitations with some minor clinical utility |
| 4 | Inadequate quality for interpretation but with recognizable anatomy |
| 5 | Uninterpretable |
RESULTS
Patient Characteristics
Twenty image sets were acquired in 19 patients in seven different ICUs over 10 days. An MRI was requested in 14 patients who recovered from their pulmonary disease, but who had an unexplained, persistent encephalopathy that prevented them from being extubated. Other indications included seizure, abnormal head CT, focal weakness, or vision changes (noted before intubation). Fifteen patients were male and the average age was 62.2 years (range 34–82). These known comorbidities associated with Covid-19 were observed: hypertension, diabetes mellitus, obesity, and cardiovascular disease. A head CT had been performed prior to the MRI in 14 patients: four patients had abnormal CT findings, including hemorrhage, infarct, and nonspecific edema. These data are summarized in Table 3.
TABLE 3.
Demographic and Clinical Characteristics
| Variables | Result (n = 19) |
|---|---|
| Age, yr | |
| Mean | 62.2 ± 11.99 |
| Median (min–max) | 67 (34–82) |
| Sex, n | |
| Male | 15 |
| Female | 4 |
| Coexisting conditions, n | |
| Hypertension | 15 |
| Diabetes mellitus | 5 |
| Obese (BMI > 30) | 11 |
| Obese (BMI > 35) | 7 |
| Cardiovascular history | 7 |
| C-reactive protein, mg/dL | |
| Average | 16.98 ± 16.00 |
| Range | 2.77–59.80 |
| > 5 mg/dL | 15 |
| Ferritin, ng/mL | |
| Average | 1566 ± 1784.99 |
| Range | 52–6500 |
| > 900 ng/mL | 10 |
| d-dimer, ug/mL | |
| Average | 2.23 ± 2.76 |
| Range | 0.002–13.20 |
| > 0.5 ug/mL | 18 |
| > 0.1 ug/mL | 12 |
| Prior imaging, n | |
| Normal CT | 10 |
| Abnormal CT | 4 |
| Indication for scan, n | |
| Unexplained encephalopathy | 14 |
| Seizure | 1 |
| Focal motor deficit | 1 |
| Diplopia | 1 |
| Abnormal prior CT | 4 |
BMI = body mass index.
Feasibility
Upon the introduction of this new technology, there was an immediate, significant demand for bedside imaging from critical care providers. The MRI was successfully used in seven ICUs on six floors of the hospital, each with a unique floor plan that required individual attention. There were no cases of inaccessibility. In one patient, the MRI was incomplete, as we had not positioned his head entirely into the transmit-receive coil during the first scan. The scan was redone using the mobility team and an air lift with the bed in a slight Trendelenburg position. Images took 39 minutes to acquire and the average bedside time from start to finish was 90 minutes per patient. One to four patients were successfully scanned per day. This was the practical limit during the peak pandemic period due to limited personnel available at our institution. A significantly larger number of patients could be scanned with a dedicated MRI team.
Safety
No patients or staff experienced any adverse events. Throughout the scanning process, patients remained in their room connected to all necessary lines and mechanical ventilation. There were no inadvertent line disconnections or extubations. Providers were able to request, order, and immediately review images via the hospital’s EMR and PACS resources. Critical care physicians and nurses were able to continue with routine care during image acquisition. All staff involved in mobilizing the patient wore the required personal protective equipment and minimized their time spent in the room.
Demonstrated MRI Findings
Table 4 summarizes the imaging findings of the 19 patients scanned at our institution, 12 of whom had an abnormal MRI. Findings included abnormal FLAIR signal (n = 12), DWI positivity (n = 3), hemorrhage (n = 3), and periventricular white matter changes (n = 12). Four of the 12 patients with abnormal MR images had an unremarkable head CT prior to our investigation.
TABLE 4.
Portable MRI Findings
| Results | All Completed Scans (n = 20) |
|---|---|
| MRI findings, n | |
| Abnormal MRI (overall) | 12 |
| Increased FLAIR signal | 12 |
| Hemorrhage | 3 |
| Abnormal FLAIR in thalamus | 3 |
| Periventricular white matter changes | 12 |
| Hyperintensity on diffusion imaging | 3 |
| MRI findings after normal HCT | 4 |
| MRI findings with no prior HCT | 5 |
| Change in management after MRI | 5 |
FLAIR = fluid attenuated inversion recovery, HCT = head computer tomography.
Two neurosurgeons and a neuroradiologist reviewed the 20 image acquisitions and assessed their utility in clinical practice. This process yielded 60 grades for each sequence and for the entire MRI study. The general MRI quality was graded to be adequate for interpretation in 51 out of 60 evaluations (mean: 2.75 ± 0.88; median: 3). Regarding individual sequences, images were deemed to be adequate for interpretation (or better) as follows: 53 out of 60 FLAIR (mean: 2.19 ± 0.98; median: 2); 53 out of 60, T1W (mean: 2.6 ± 0.98; median: 3); 52 out of 60, T2W (mean: 2.47 ± 0.99; median: 2); and 45 out of 60, T2W coronal (mean: 2.88 ± 0.99, median: 3). However, only 16 out of 60 of the DWI evaluation grades were consistent with sufficient quality (mean: 4.13 ± 1.02, median: 4) for interpretation. Interrater reliability as measured by the ICC was calculated to be moderate (ICC = 0.65 with 95% CI = 0.42–0.78).
Clinical Utility
The MRI results led to a change in management in five patients, including the diagnosis and treatment of venous thrombosis, cerebral infarction (patient illustration 1), and the involvement of palliative care (patient illustration 2). In the case of patient illustration 1, the portable MRI confirmed the presence of ischemic strokes and the patient was started on antiplatelet medical therapy (Fig. 2). In the case of patient illustration 2, the portable MRI revealed extensive abnormalities in the bilateral basal ganglia and left parietal region consistent with new hemorrhagic infarctions (Fig. 3). After discussing the MRI results, the family requested that any further aggressive medical management be withheld and the patient later expired. Three patients underwent a lumbar puncture as a result of the MRI. The CSF was unremarkable with the exception of one patient having an elevated protein and IgG/albumin ratio. CSF results did not lead to a change in management. At the time of imaging, we were unable to test for SARS-CoV-2 RNA in the CSF samples.
Figure 2.
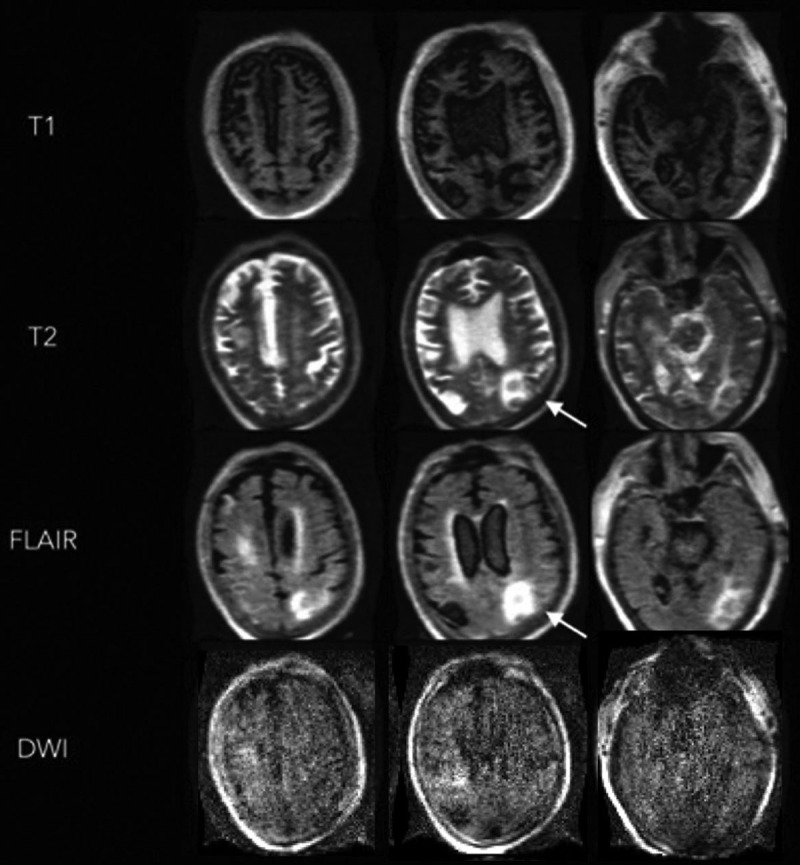
Portable MRI of a critically ill patient with coronavirus disease 2019 (patient illustration 1). A 74-year-old male with a past medical history of obesity, hypertension, and diabetes was admitted with respiratory failure and was subsequently found to have severe acute respiratory syndrome coronavirus 2. The patient had a prolonged ICU course complicated by acute respiratory distress syndrome, acute kidney injury, and upper gastrointestinal bleed. A noncontrast head CT was performed after the patient failed to make a meaningful neurologic recovery despite being stabilized systemically. This revealed multifocal edema in bilateral occipital and parietal lobes, and a right superior cerebellar hemorrhage without evidence of hydrocephalus. Portable MRI-revealed diffuse atrophic changes are seen on the T1 weighted images. Hyperintense T2 weighted and fluid attenuated inversion recovery (FLAIR) signals are noted in the left parietooccipital region, without increased diffusion-weighted imaging (DWI) signal.
Figure 3.
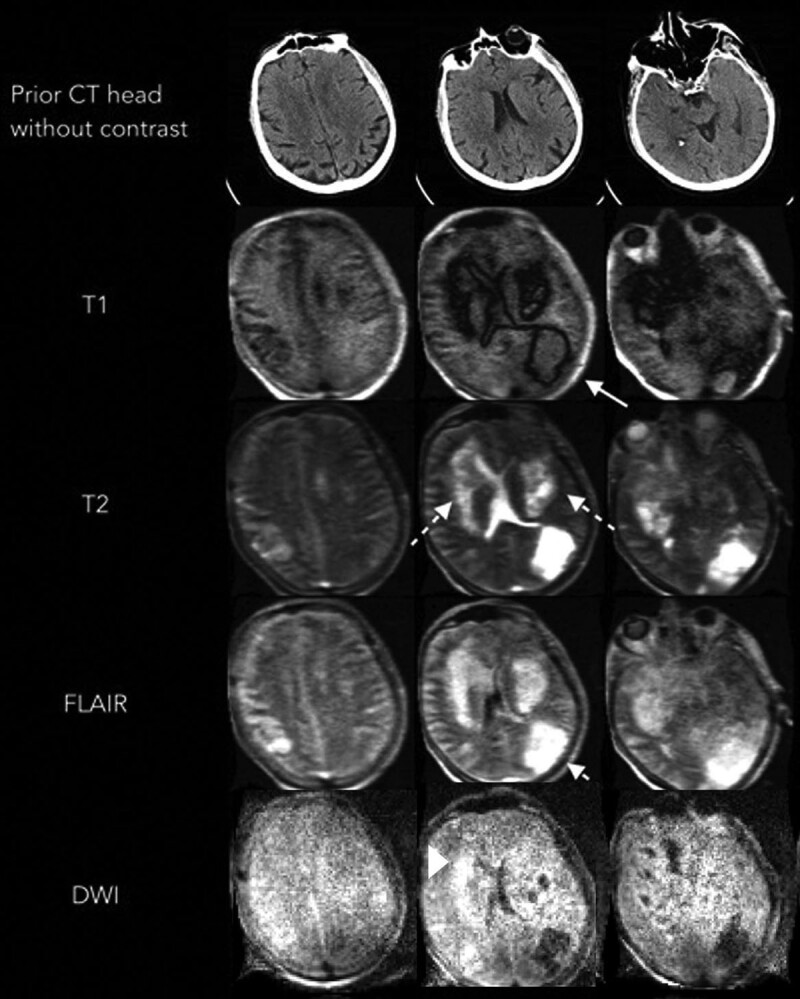
Portable MRI of a critically ill patient with coronavirus disease 2019 (patient illustration 2). A 60-year-old male with a history of coronary artery disease, prior coronary artery bypass grafting on antiplatelet medication, hypertension, and obesity presented with severe hypoxia and was subsequently diagnosed with severe acute respiratory syndrome coronavirus 2. The patient had a prolonged ICU course complicated by seizure, acute respiratory distress syndrome, cardiac arrhythmias requiring anticoagulation, and acute kidney injury requiring hemodialysis. A head CT performed for a poor neurologic examination despite being off sedation revealed no acute findings. Nine days later, a portable MRI demonstrated new areas of low T1 weighted signal surrounding isointense changes in the left parietal occipital region (solid arrow). Extensive high signal was noted on T2 weighted and fluid attenuated inversion recovery (FLAIR) sequences (solid arrow) in the bilateral basal ganglia and left parietal lobe. A concurrent area of restricted diffusion is seen in the basal ganglia on diffusion weighted imaging (DWI). Local mass effect was noted on the left lateral ventricle.
DISCUSSION
We chose to introduce this system for the imaging of patients who are critically ill with Covid-19 because of the understanding that the CNS can be affected by this disease and that scanning these patients in a diagnostic MR imager is often difficult, if not impossible. In our initial patient population, portable MRI was demonstrated to be safe and feasible, while limiting hospital exposure of patients and staff, and minimizing disruption of ICU care.
There are numerous safety advantages to portable MRI. The use of portable CT, compared with conventional CT, has fewer complications, requires less time and resources, and provides immediate diagnostic results for treatment interventions (1). Furthermore, portable imaging removes the risks associated with intrahospital transport, along with reducing the transmission risks associated with environmental exposure (3, 12, 14–16). In our initial experience, we found that these same advantages applied to the use of portable MRI in the Covid ICUs. Although it is possible to diagnose hemorrhages and larger, older ischemic strokes on portable CT scans, portable MRI offers the distinct advantage of the ability to visualize these areas of injury on different weighted sequences, theoretically adding significant diagnostic benefit in clinical interpretation. However, this is based on the evidence extrapolated from comparisons of conventional MRI and CT, and is simply speculative at this time. Additionally, the cost-saving benefits of portable MRI cannot be ignored. Besides the benefit of significantly lower costs of the scanner, there are healthcare costs associated with transporting critically ill patients on isolation precautions to and from the MRI scanner. These costs will vary among various institutions, and this is a topic for future studies.
To determine feasibility, we performed MRI scans on patients in a variety of ICU settings. With critical care providers quick to integrate the MRI scanning protocol into their workflow, we brought this technology to areas beyond traditional critical care units. We imaged patients in a number of rapidly built ICUs as a result of the Covid-19 surge, including the postanesthesia care unit and interventional short stay units converted into Covid ICUs. Despite the challenges of navigating these spaces, we were able to complete safely up to four scans a day with no adverse events or disruptions to critical care workflow. The patient preparation process, as outlined in the Materials and Methods section, was a result of an iterative process that took place during the 10 days of scanning. Challenges we faced early on included fitting, positioning, and mobilizing larger patients within the gantry, excessive patient movement during scans, and long preparation times. It was through this iterative process that we started standardizing our approach and improving our efficiency. We found the imaging quality to be adequate in detecting the presence or absence of brain abnormalities in nearly all of the acquisitions. FLAIR sequences demonstrated the highest quality, with DWI scoring the lowest.
There are limited data on the neurologic complications and MRI findings in patients with Covid-19. Mao et al (8) found that 36.4% of patients had neurologic manifestations, including ischemic stroke, hemorrhage, and impaired consciousness. Li et al (17) reported the occurrence rate of stroke in hospitalized patients with Covid-19 to be 5%. Stroke has also been reported to be the initial presenting symptom in a small series of young patients (18). In addition to cerebrovascular disease, case reports have described patients with encephalitis and abnormal findings on MRI (9, 10). Some investigators recently proposed that a hypercoagulable state and/or excessive immune response are the direct result of the systemic manifestations of Covid-19 infection (8, 9, 17, 19). Timely diagnosis of CNS pathologies related to this disease provides a window of opportunity to guide treatment and prevent clinical deterioration that is associated with advanced Covid-19. In this series, 12 of 19 patients had abnormal findings on MRI. Most often, these were FLAIR signal changes, but restricted diffusion and hemorrhage were also noted. These images also prompted further investigation with serological testing or lumbar puncture, when appropriate. In fact, the utility of normal findings on MRI should not be undervalued. We found that a normal MRI allowed providers to narrow or shift differentials, and served as a valuable tool for shared decision-making with families with regard to goals of care and prognostication. Due to the lack of comparison with standard-of-care imaging, the possibility of missed findings on normal scans obtained on the portable MRI was accounted for during clinical decision-making and goals-of-care discussions.
Limitations of this study include the small sample size and the learning curve associated with the early use of new technology, particularly during a pandemic. Throughout the study, the strategy and logistics to perform portable MRI (such as patient-positioning, unique ICU considerations, and device transport) were continually improved. The study also lacks comparison with current standard-of-care imaging devices, such as portable CT and conventional MRI due to the constraints of the pandemic. This limits the conclusions about its clinical utility but provides promising preliminary data about the feasibility, safety, and expected utility in a clinical setting. Additionally, strategies to enhance the quality of imaging were tested throughout the process (e.g., use of various physiologic monitoring devices to reduce interference, evaluation of ventilator associated motion artifacts, and use of machine learning algorithms to enhance quality of postprocessed images). However, these are beyond the scope of this study and its current use in a clinical setting, and are actively under investigation. The inability to do contrasted MRI is a technical limitation currently under investigation by the manufacturer and clinical researchers. Furthermore, a large number of the DWI sequences and ADC maps were deemed nondiagnostic due to artifact and/or poor signal to noise. Another limitation of the current device is its capability to image only the brain and foot (not imaged in this article). Future work targeted in developing newer sequences as well as coils to expand its use to other anatomical locations is underway.
The authors feel the study provides exciting data regarding the use of a point-of-care MR imager. However, much work needs to be done to allow further implementation in a clinical setting. Future studies aimed at optimizing and improving acquisition and postprocessed images (e.g., ADC), addition of newer sequences, and the ability to perform scans with contrast agents are underway. In addition, we are planning a study comparing the portable MRI images to images acquired using CT and 1.5- and 3-T MRI.
CONCLUSIONS
Advanced neuroimaging with a portable, self-shielding MRI is a safe and feasible method for imaging patients in an ICU setting. Preliminary imaging data from this series of patients have demonstrated clinical utility for treatment and decision-making in patient populations; however, future studies comparing results with other modalities are warranted to understand fully its clinical impact. Although circumstances surrounding the pandemic at the time led us to image patients with Covid-19, this technique can be applied to any ICU patient whose care requires imaging of the brain.
ACKNOWLEDGMENTS
The authors thank Hyperfine (Guilford, CT) for providing the point-of-care MRI system used in this study as well as Jonathan Rothberg, PhD, and the engineering team behind it, including Brian Welch, PhD, MBA, Samantha By, PhD, and Harry Hu, PhD. We also thank Erik Chapman (Sylvan Lake, MI) for the medical illustration and the Northwell Covid-19 Research consortium for providing the resources and facilitating parts of this research.
Supplementary Material
Footnotes
None of the authors have any commercial interest with Hyperfine, the manufacturer of the portable MRI. The senior engineers of Hyperfine were asked to review the article for the accuracy of technical descriptions of the device.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Gunnarsson T, Theodorsson A, Karlsson P, et al. Mobile computerized tomography scanning in the neurosurgery intensive care unit: Increase in patient safety and reduction of staff workload. J Neurosurg. 2000; 93:432–436 [DOI] [PubMed] [Google Scholar]
- 2.Peace K, Wilensky EM, Frangos S, et al. The use of a portable head CT scanner in the intensive care unit. J Neurosci Nurs. 2010; 42:109–116 [DOI] [PubMed] [Google Scholar]
- 3.Mossa-Basha M, Medverd J, Linnau KF, et al. Policies and guidelines for COVID-19 preparedness: Experiences from the University of Washington. Radiology. 2020; 296:E26–E31 [DOI] [PubMed] [Google Scholar]
- 4.Algethamy HM, Alzawahmah M, Young GB, et al. Added value of MRI over CT of the brain in intensive care unit patients. Can J Neurol Sci. 2015; 42:324–332 [DOI] [PubMed] [Google Scholar]
- 5.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020; 20:533–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. SSRN Electron J. 2020; 77:683–690 [Google Scholar]
- 9.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020; 94:55–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poyiadji N, Shahin G, Noujaim D, et al. : COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020; 296:E119–E120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-art review. J Am Coll Cardiol. 2020; 75:2950–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liew MF, Siow WT, Yau YW, et al. Safe patient transport for COVID-19. Crit Care. 2020; 24:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan L, Wang L, Huang X. How to face the novel coronavirus infection during the 2019-2020 epidemic: The experience of Sichuan Provincial People’s Hospital. Intensive Care Med. 2020; 46:573–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papson JP, Russell KL, Taylor DM. Unexpected events during the intrahospital transport of critically ill patients. Acad Emerg Med. 2007; 14:574–577 [DOI] [PubMed] [Google Scholar]
- 15.Zuchelo LTS, Chiavone PA. Transporte intra-hospitalar de pacientes sob ventilação invasiva: Repercussões cardiorrespiratórias e eventos adversos [Internet]. J Bras Pneumol. 2009; 35:367–37419466275 [Google Scholar]
- 16.Lovell MA, Mudaliar MY, Klineberg PL. Intrahospital transport of critically ill patients: Complications and difficulties. Anaesth Intensive Care. 2001; 29:400–405 [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Wang M, Zhou Y, et al. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. SSRN Electron J. 2020; 5:279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020; 382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020; 87:18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


