The etiology of psoriasis is not fully understood, even though several genetic and environmental contributing factors have been suspected. 1 Evidence has accumulated that pathological autoimmune responses mediated via the IL‐23/IL‐17 axis underlie psoriatic skin inflammation. 1 Similar dermal activation of the Th17 immunity has also been observed in patients with metal hypersensitivity. 2 Metal hypersensitivity involves a T cell‐mediated, delayed‐type hypersensitivity reaction that causes local contact dermatitis and systemic cutaneous inflammation and is closely associated with a subset of inflammatory autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, and Sjögren's syndrome. 3 In addition, hypersensitivity to dental metals strongly correlates with cutaneous inflammatory conditions such as palmoplantar pustulosis, lichen planus, and dyshidrotic eczema. 4 , 5 , 6 These facts prompted us to assess the contribution of dental metal hypersensitivity to psoriasis. We conducted a case‐control retrospective study of the prevalence of hypersensitivity to zinc, gold, nickel, and palladium, which are the metals commonly used for dental alloys.
A total of 53 Japanese patients with psoriasis vulgaris (PV) and 22 age‐ and sex‐matched healthy controls (HC) were enrolled (PV: 20 males and 33 females, age range 22‐83 years, mean age [SD] 50.2 [14.4] years; HC: 7 males and 15 females, ages 12‐70 years, mean age [SD] 48.9 [13.7] years). All subjects were users of metallic dental implants and/or prostheses containing zinc, gold, nickel, and/or palladium as major constituents. Written informed consent was obtained from each participant. We employed the lymphocyte transformation test (LTT) to evaluate the sensitivity to the metals quantitatively. LTT was outsourced to SRL, Inc. (Tokyo, Japan). The stimulation index (SI) was calculated as SI = 100 × (cpm of [3H]thymidine incorporated into peripheral blood lymphocytes during stimulation with a metal species/cpm without stimulation). Any subject with an SI value of ≥180 was regarded as hypersensitive according to previous studies. 7
The majority of HC individuals (68.2%) were negative for sensitivity to any metals examined, and the rest (31.8%) were hypersensitive to only a single metal species (Figure 1A). In contrast, half (52.8%) of PV patients were hypersensitive to at least one metal species, and 30.2% were hypersensitive to more than one species. However, we found no significant difference in the overall prevalence of metal hypersensitivity between PV and HC (P = .129; odds ratio = 0.420; 95% confidence interval = 0.150‐1.19; Figure 1A). Furthermore, there was no significant difference in the prevalence of hypersensitivity to individual metals between PV and HC (Figure 1B). No significant difference was also observed in the mean SI value of each metal between PV and HC (Figure 1B).
FIGURE 1.
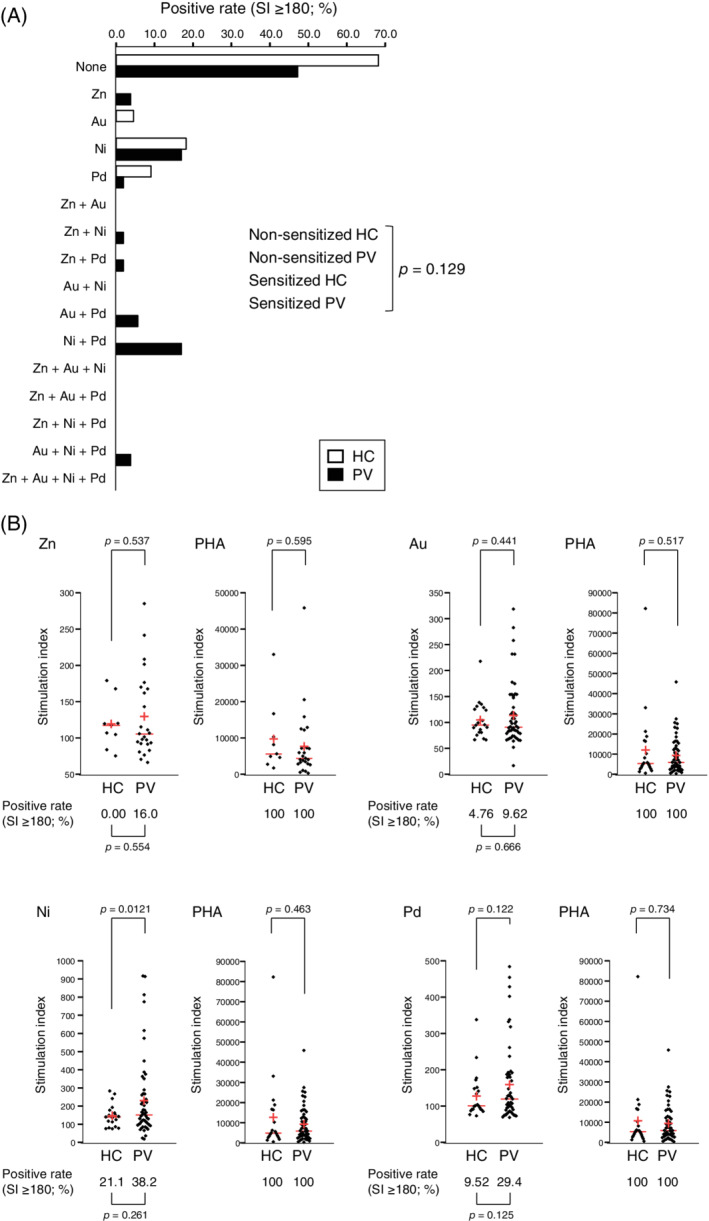
Prevalence and severity of sensitivity to dental metals in PV patients and healthy controls. (A) Prevalence of hypersensitivity to zinc, gold, nickel, and/or palladium (SI ≥ 180) in healthy controls (HC, open bar) and patients (PV, closed bar). (B) Distribution of SI values for HC and PV patients with respect to zinc, gold, nickel, and palladium. Phytohemagglutinin (PHA) was used as a positive control. Each red horizontal line and red cross corresponds to the median and the mean, respectively. LTT‐positive rates (SI ≥ 180) are shown below the bee swarms. No outliers were taken into account, and all collected data were subjected to a two‐tailed Fisher's exact test (A), two‐tailed Fisher's exact test with Bonferroni correction (B, LTT‐positive rates), and two‐tailed Welch's t‐test with Bonferroni correction (B, SI values). P values <.05 (A) and P values <.00625 (B) were regarded as statistically significant
The prevalence of metal sensitization in patients with psoriasis has been investigated in patch test studies, yielding controversial results. 8 , 9 , 10 Some of the previous studies have demonstrated that patients with psoriasis have a significantly lower prevalence of metal hypersensitivity than healthy individuals, 8 , 9 but others have shown comparable prevalence between psoriasis patients and healthy controls. 10 Our result from an in vitro LTT study supports the latter conclusion—at least with this subset of dental metal allergens. This conclusion suggests that psoriasis and metal hypersensitivity are caused by unrelated immune mechanisms, even though metal hypersensitivity is closely associated with other inflammatory autoimmune or cutaneous inflammatory diseases. The relatively small sample size may limit the conclusion, and further studies with larger cohorts are needed to determine causality and generalizability more firmly.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
ETHICS STATEMENT
This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All procedures were reviewed and approved by the Ethics Committees of Takanawa Clinic (approval number: 2018‐2). A signed informed consent form was obtained from each participant prior to inclusion in this study.
CONFLICT OF INTEREST
Yasunari Kageyama, Koichi Aida, Kimihiko Kawauchi, and Masafumi Morimoto are employees of Takanawa Clinic. Tetsu Akiyama and Tsutomu Nakamura have advisory roles in conducting clinical research in Takanawa Clinic and receive advisory fees from Takanawa Clinic.
AUTHOR CONTRIBUTIONS
Conceptualization: Yasunari Kageyama, Tetsu Akiyama, Tsutomu Nakamura
Formal Analysis: Tsutomu Nakamura
Investigation: Yasunari Kageyama, Koichi Aida, Kimihiko Kawauchi, Masafumi Morimoto
Project Administration: Yasunari Kageyama
Resources: Yasunari Kageyama, Koichi Aida, Kimihiko Kawauchi, Masafumi Morimoto
Supervision: Yasunari Kageyama, Tetsu Akiyama, Tsutomu Nakamura
Visualization: Tsutomu Nakamura
Writing—Original Draft Preparation: Tsutomu Nakamura
Writing—Review & Editing: Yasunari Kageyama, Koichi Aida, Kimihiko Kawauchi, Masafumi Morimoto, Tetsu Akiyama
All authors have read and approved the final version of the manuscript.
The corresponding author, Tsutomu Nakamura, had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Kageyama Y, Aida K, Kawauchi K, Morimoto M, Akiyama T, Nakamura T. Dental metal hypersensitivity in patients with psoriasis vulgaris: A case‐control study using an in vitro quantitative sensitivity test. Health Sci Rep. 2020;4:e223 10.1002/hsr2.223
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci. 2019;20:4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Samelko L, Caicedo MS, Jacobs J, Hallab NJ. Transition from metal‐DTH resistance to susceptibility is facilitated by NLRP3 inflammasome signaling induced Th17 reactivity: implications for orthopedic implants. PLoS One. 2019;14:e0210336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjørklund G, Dadar M, Aaseth J. Delayed‐type hypersensitivity to metals in connective tissue diseases and fibromyalgia. Environ Res. 2018;161:573‐579. [DOI] [PubMed] [Google Scholar]
- 4. Kouno M, Nishiyama A, Minabe M, et al. Retrospective analysis of the clinical response of palmoplantar pustulosis after dental infection control and dental metal removal. J Dermatol. 2017;44:695‐698. [DOI] [PubMed] [Google Scholar]
- 5. Müller S. Oral lichenoid lesions: distinguishing the benign from the deadly. Mod Pathol. 2017;30:S54‐S67. [DOI] [PubMed] [Google Scholar]
- 6. Nishizawa A. Dyshidrotic eczema and its relationship to metal allergy. Curr Probl Dermatol. 2016;51:80‐85. [DOI] [PubMed] [Google Scholar]
- 7. Kano Y, Hirahara K, Mitsuyama Y, Takahashi R, Shiohara T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy. 2007;62:1439‐1444. [DOI] [PubMed] [Google Scholar]
- 8. Bangsgaard N, Engkilde K, Thyssen JP, et al. Inverse relationship between contact allergy and psoriasis: results from a patient‐ and a population‐based study. Br J Dermatol. 2009;161:1119‐1123. [DOI] [PubMed] [Google Scholar]
- 9. Claßen A, Buhl T, Schubert S, et al. The frequency of specific contact allergies is reduced in patients with psoriasis. Br J Dermatol. 2019;180:315‐320. [DOI] [PubMed] [Google Scholar]
- 10. Alwan W, Lynch M, McFadden J, White IR, Banerjee P. Patch testing in patients with psoriasis: results of a 30‐year retrospective study. Br J Dermatol. 2018;178:559‐560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.


