Abstract
Introduction
High-resolution micro-ultrasound has the capability of imaging prostate cancer based on detecting alterations in ductal anatomy, analogous to multiparametric magnetic resonance imaging (mpMRI). This technology has the potential advantages of relatively low cost, simplicity, and accessibility compared to mpMRI. This multicenter, prospective registry aims to compare the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of mpMRI with high-resolution micro-ultrasound imaging for the detection of clinically significant prostate cancer.
Methods
We included 1040 subjects at 11 sites in seven countries who had prior mpMRI and underwent ExactVu micro-ultrasound-guided biopsy. Biopsies were taken from both mpMRI targets (Prostate Imaging-Reporting and Data System [PI-RADS] >3 and micro-ultrasound targets (Prostate Risk Identification using Micro-ultrasound [PRIMUS] >3). Systematic biopsies (up to 14 cores) were also performed. Various strategies were used for mpMRI target sampling, including cognitive fusion with micro-ultrasound, separate software-fusion systems, and software-fusion using the micro-ultrasound FusionVu system. Clinically significant cancer was those with Gleason grade group ≥2.
Results
Overall, 39.5% were positive for clinically significant prostate cancer. Micro-ultrasound and mpMRI sensitivity was 94% vs. 90%, respectively (p=0.03), and NPV was 85% vs. 77%, respectively. Specificities of micro-ultrasound and MRI were both 22%, with similar PPV (44% vs. 43%). This represents the initial experience with the technology at most of the participating sites and, therefore, incorporates a learning curve. Number of cores, diagnostic strategy, blinding to MRI results, and experience varied between sites.
Conclusions
In this initial multicenter registry, micro-ultrasound had comparable or higher sensitivity for clinically significant prostate cancer compared to mpMRI, with similar specificity. Micro-ultrasound is a low-cost, single-session option for prostate screening and targeted biopsy. Further larger-scale studies are required for validation of these findings.
Introduction
About 15% of men will be diagnosed with prostate cancer at some point during their lifetime.1 While mortality has improved substantially, attributed to early detection and improved treatment, it remains the second leading cause of cancer death in men.2 Accurate biopsy-derived histopathology is a key determinant in treatment selection, along with patient-physician shared decision-making.3 Most men with localized, low-risk disease are managed via active surveillance, while those with localized, higher-risk disease are treated with surgery, radiation, or focal therapy regimens.3 Accurate biopsy techniques are crucial for determining the optimal treatment path for each patient.
Historically, prostate cancer diagnosis has been predicated upon transrectal ultrasound (TRUS)-guided systematic biopsies initiated due to prostate-specific antigen (PSA) abnormal values and/or an abnormal prostate exam. The limitations of this approach are a high rate of clinically insignificant cancers, especially when performed solely for PSA abnormalities, as well as the potential for missing clinically significant cancer in 25–30% of biopsied patients.4
Recent randomized studies have demonstrated the superiority of multiparametric magnetic resonance imaging (mpMRI)-guided targeted biopsies in correctly identifying clinically significant cancer and reducing the rate of diagnosis of insignificant cancer.4–6 The European Association of Urology (EAU) currently recommends mpMRI imaging prior to all prostate biopsies, with the caveat that systematic biopsy is acceptable if mpMRI is unavailable.7 The National Comprehensive Cancer Network (NCCN) recommends MRI-assisted biopsy for patients with a prior negative systematic biopsy and clinical suspicion of cancer.8 This poses many challenges in terms of access, cost, and expertise. High-resolution 29 MHz micro-ultrasound, a novel imaging modality, aims to improve the diagnostic accuracy of prostate biopsy while maintaining the affordability and convenience of ultrasound. Micro-ultrasound operates at 29 MHz, compared to traditional ultrasound systems that operate at frequencies of 6–9MHz.9 The axial resolution is improved from 200 μm with conventional ultrasound to <70 μm with micro-ultrasound, with a similar improvement in lateral resolution due to 90 μm crystal spacing. This resolution is approximately the diameter of a prostatic duct and allows for the visualization of subtle changes in ductal anatomy associated with cancer. Diffusion-weighted imaging (DWI) is based upon measuring the random Brownian motion of water molecules within a voxel of tissue. Highly cellular tissues exhibit lower diffusion coefficients. Thus, both technologies identify changes associated with high-grade cancer. Real-time targeted biopsy can be performed, avoiding the need for a second procedure. A grading system, Prostate Risk Identification using Micro-ultrasound (PRI-MUS), analogous to the Prostate Imaging-Reporting and Data System (PI-RADS),10 has been developed to stratify micro-ultrasound images according to the risk of significant cancer.
This study sought to compare the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of mpMRI with high-resolution micro-ultrasound in patients referred for biopsy who had a prior MRI.
Methods
This was a prospective registry that includes data from 11 urological centers in seven countries in North America and Europe. All sites have well-established prostate mpMRI programs with uro-radiologists with at least five years of experience interpreting prostate MRI. The urologists had at least five years’ experience performing TRUS prostate biopsy. The study represents the initial “real-life” experience with micro-ultrasound from those centers, which were early adopters of the technology.
Inclusion and exclusion criteria
Broad inclusion criteria were used to provide a real-world analysis in the clinical population of men presenting for biopsy. This includes men with suspected prostate cancer based on elevated PSA and/or abnormal digital rectal examination (DRE), with or without a suspicious mpMRI. A targeted biopsy procedure was performed for each subject, which included taking 2–3 cores from each mpMRI and micro-ultrasound target, as well as 12–14 systematic samples. A total of 1040 subjects were included in this analysis. Men were excluded if no mpMRI was performed prior to biopsy, no biopsy was performed, or biopsy was performed without sampling either modality’s targets.
Analysis
At all sites, 2–3 targeted samples from each target were taken for PI-RADS ≥3 or PRI-MUS11 ≥3 lesions. If the same site was identified on both modalities, this was biopsied using ultrasound guidance. In most cases, targeted biopsies (from both MRI- and ultrasound-identified abnormalities) were performed initially, followed by systematic biopsies. From the resultant pathology, clinically significant cancer was considered any Gleason grade (GG) ≥2. Patient-level sensitivities, specificities, PPVs, and NPVs were assessed for both micro-ultrasound and mpMRI, combined across all sites, as well as individually.
The primary endpoint of this registry was to determine patient-level sensitivity and specificity of each imaging modality to predict which men harbored significant prostate cancer. The benefit of MRI over systematic biopsy is about a 15% increase in the diagnosis of significant cancer. In this post-hoc analysis, we hypothesized that the lower 2.5th percentile of the difference between micro-ultrasound and mpMRI for GG ≥2 prostate cancer detection was greater than −7.5%, which maintains at least 50% of the benefit of MRI. Confidence intervals (CI) were calculated using the Jefferys technique, with exact calculation of difference p-values.12
Results
A total of 1040 patients were included, with median age of 67 years (interquartile range [IQR] 61–72) and PSA 7 ng/mL (5.1–10). Table 1 summarizes the demographics of the group. Twenty-two percent of the patients had a palpable lesion on DRE; 16% of ultrasound evaluations were non-suspicious (PRI-MUS 1–2), while 17% had an equivocal lesion (PRI-MUS 3) and 66% were suspicious or highly suspicious (PRI-MUS 4–5).
Table 1.
Patient demographics
| Overall | |
|---|---|
| N | 1040 |
| Age, median (IQR) | 67 (61–72) |
| PSA, median (IQR) | 7 (5.1–10) |
| DRE (positive) | 208 (128 NA) |
| Prostate volume (mL), median (IQR) | 38 (28–53) |
| Prior biopsy (positive/total) | 66/352 (281 NA) |
| Percentage equivocal imaging (PRI-MUS 3) | 17% |
| Percentage equivocal imaging (PI-RADS 3) | 19% |
DRE: digital rectal exam; IQR: interquartile range; PI-RADS: Prostate Imaging-Reporting and Data System; PRI-MUS: Prostate Risk Identification using Micro-ultrasound; PSA: prostatespecific antigen.
Table 2 lists the sites, indication for biopsy, mpMRI specifications, the fusion targeting system, and whether the ultrasound was performed blinded to the MRI. Most sites used clinical variables, including MRI, as the indication; one site only biopsied patients whose MRI showed a region of interest. Seven of the 11 sites used cognitive fusion; four sites used a fusion targeting system. Nine of the 11 sites were unblinded to the results of the MRI when the ultrasound was performed; two were blinded.
Table 2.
Performance metrics comparing mpMRI and micro-ultrasound
| A. For detection of GG ≥2 PCa (39% of cases) | |||||
|---|---|---|---|---|---|
|
| |||||
| Modality | Sensitivity | Specificity | PPV | NPV | |
| mpMRI | 90% (371/411) | 22% (136/629) | 43% (371/864) | 77% (136/176) | |
| Micro-ultrasound | 94% (386/411) | 22% (138/629) | 44% (386/877) | 85% (138/163) | |
| p (non-inferiority) | <0.001 | <0.001 | <0.001 | <0.001 | |
| p (superior) | 0.03 | 0.45 | 0.32 | 0.04 | |
|
| |||||
| B. For GG ≥3 (19% of cases) | |||||
|
| |||||
| Modality | Sensitivity | Specificity | PPV | NPV | |
|
| |||||
| mpMRI | 94% (145/154) | 17% (112/642) | 21% (145/675) | 93% (112/121) | |
| Micro-ultrasound | 93% (144/154) | 21% (136/642) | 22% (144/657) | 93% (136/146) | |
| p (non-inferiority) | <0.001 | <0.001 | <0.001 | <0.001 | |
| p (superior) | 0.59 | 0.06 | 0.43 | 0.41 | |
|
| |||||
| C. PPV by PI-RADS and PRI-MUS score | |||||
|
| |||||
| 3 | 4 | 5 | Unknown | 4 or 5 | |
|
| |||||
| MRI PI-RADS | 14% | 38% | 62% | 49% | 40% |
| US PRI-MUS | 19% | 39% | 61% | 46% | 42% |
GG: Gleason grade; mpMRI: multiparametric magnetic resonance imaging; NPV: negative predictive value; PCa: prostate cancer; PI-RADS: Prostate Imaging-Reporting and Data System; PRI-MUS: Prostate Risk Identification using Micro-ultrasound; PPV: positive predictive value; US: ultrasound.
Of the 1040 MRIs, 864 were positive (83%); of these, 364 (43%) showed significant cancer. Forty of 1040 patients (4%) had a negative MRI with significant cancer on biopsy. The false positive rate was 47%. A total of 877 of 1040 (84%) had a region of interest on micro-ultrasound; 25 had a negative micro-ultrasound with significant cancer on biopsy (2%). The false positive rate of micro-ultrasound was 47%. PPVs were similar between MRI and micro-ultrasound and varied with risk score. PI-RADS 3 lesions were positive for clinically significant prostate cancer in 14% (17/124) cases, while PI-RADS 4 were positive in 38% (132/351). PI-RADS 5 lesions were positive for clinically significant prostate cancer in 62% (120/193) of cases. PI-RADS scores were not available in 184 cases, with 91 cases of clinically significant prostate cancer (46%).
Prostate cancer was identified in 61% of patients (632/1040), GG 2 or higher prostate cancer in 39% (411/1040) of patients, and GG 3 or higher in 19% (154/803) of patients. Sites A and I did not differentiate between GG 2 and GG 3 cases and were not included in assessments of GG 3 or higher cancer. Significant variability in biopsy indication was evident between the enrolling centers, however, the resulting sensitivity difference of MRI and micro-ultrasound was consistent (Fig. 1 and Table 3). Overall, micro-ultrasound demonstrated a sensitivity of 94% (386/411) for predicting GG ≥2 prostate cancer, while mpMRI demonstrated a lower sensitivity of 90% (371/411) (p=0.03). Specificity was similar at 22% (138/629) for micro-ultrasound and 23% (136/629) for mpMRI (p<0.01 for non-inferiority) (Table 2).
Fig 1.
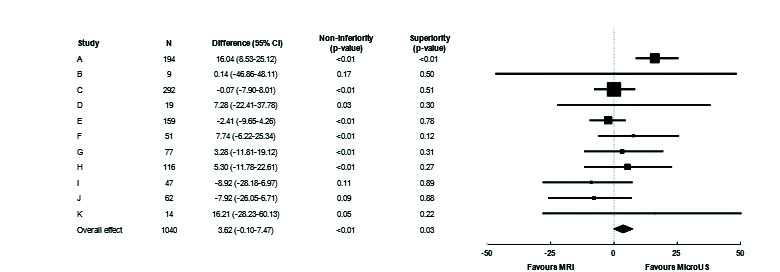
Forest plot demonstrating site-level sensitivity difference between micro-ultrasound (US) and multiparametric magnetic resonance imaging (MRI). Sensitivity difference overall was +3.6%, indicating superior sensitivity for micro-US (p=0.03). CI: confidence interval.
Table 3.
Detailed results per site
| Site | Multiparametric MRI | Micro-ultrasound | n | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| True positive | True negative | False positive | False negative | True positive | True negative | False positive | False negative | ||
| A | 70 | 46 | 64 | 14 | 84 | 13 | 97 | 0 | 194 |
| B | 3 | 1 | 5 | 0 | 3 | 0 | 6 | 0 | 9 |
| C | 97 | 22 | 163 | 10 | 97 | 44 | 141 | 10 | 292 |
| D | 9 | 7 | 1 | 2 | 10 | 2 | 6 | 1 | 19 |
| E | 76 | 5 | 76 | 2 | 74 | 12 | 69 | 4 | 159 |
| F | 20 | 9 | 20 | 2 | 22 | 5 | 24 | 0 | 51 |
| G | 24 | 13 | 38 | 2 | 25 | 9 | 42 | 1 | 77 |
| H | 29 | 27 | 53 | 7 | 31 | 24 | 56 | 5 | 116 |
| I | 19 | 3 | 25 | 0 | 17 | 8 | 20 | 2 | 47 |
| J | 21 | 1 | 40 | 0 | 19 | 21 | 20 | 2 | 62 |
| K | 3 | 2 | 8 | 1 | 4 | 0 | 10 | 0 | 14 |
| Total | 371 | 136 | 493 | 40 | 386 | 138 | 491 | 25 | 1040 |
While clear site-level variability is seen on targeting percentage and accuracy, only 3 sites (B, I, J) failed to achieve non-inferior sensitivity on their own. Aggregate results demonstrate superior sensitivity for micro-ultrasound (p=0.03) and non-inferior specificity (p<0.01). MRI: magnetic resonance imaging.
Fig. 1 shows a Forest plot of sensitivity by site. This was relatively consistent between sites, whether blinded or not. The overall sensitivity of micro-ultrasound was 3.6% higher than MRI; the p-value for non-inferiority was <0.001 and for superiority was 0.03. Fig. 2 shows the Forest plot of specificity. There was substantial variation between sites. Micro-ultrasound specificity was 0.3% higher than MRI, with p<0.001 for non-inferiority and p=0.45 for superiority.
Fig. 2.
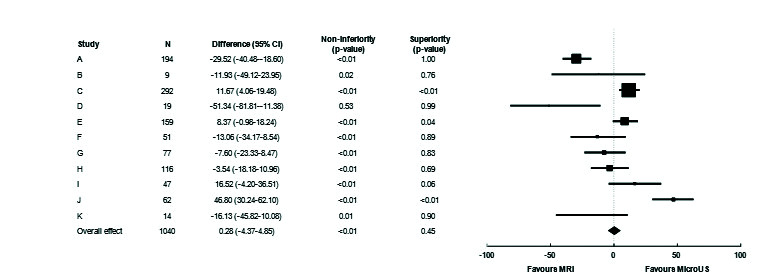
Forest plot demonstrating site-level specificity difference between micro-ultrasound (US) and multiparametric magnetic resonance imaging (MRI). Specificity difference was +0.3% overall, indicating non-inferiority for micro-US (p<0.01), however, significant variability was noted between sites depending on biopsy population and user targeting habits. CI: confidence interval.
All but three groups (all with <65 subjects) performed within the non-inferiority margin individually, suggesting robust inter-site performance. A sensitivity analysis was further conducted by removing each of the groups to determine whether the overall conclusion still held. P-values for non-inferiority were consistently significant for both sensitivity and specificity (<0.001–0.025 for both). Superiority p-values varied considerably for specificity, as expected given the widely varying performances between sites (0.01–0.73), however, sensitivity was consistently superior (<0.01 in all cases).
Discussion
The advent of prostate cancer imaging with mpMRI has dramatically altered the approach to prostate cancer diagnosis. Targeted biopsies result in more significant cancer and less insignificant cancer being diagnosed.
However, mpMRI has several limitations. It is a relatively expensive technology, there is a significant educational learning curve, and it involves a second visit for the fusion targeted biopsy. There is potential toxicity associated with gadolinium. mpMRI misses about 15% of significant cancers.13,14 Many care management facilities globally face challenges in providing access to MRI for all patients in whom it is indicated. Further, MRI interpretation is complex and subject to variability. In a study of the concordance by expert genitourinary (GU) radiologists, the kappa for peripheral zone prostate lesions was only 0.59.15 Fusion targeting introduces another potential source of error, whether performed cognitively or with an additional fusion biopsy system. Some men have a relative or absolute contraindication to MRI, including claustrophobia, prosthetic implants, pacemakers, or renal failure precluding gadolinium.16 Modifications to MRI, including the use of bi-parametric MRI, will address some of these issues, including gadolinium-related toxicity and cost.17
High-resolution micro-ultrasound offers the benefit of a comparatively inexpensive, simple technology, with imaging and biopsy performed as a single procedure. It does not require contrast. Ultrasound skills are widely diffused in the urological and radiological community, and the learning curve for micro-ultrasound imaging appears to be short. In one study, the area under the curve flattened after 15 cases.18 The capital cost of the device is similar to other high-end ultrasound machines or MRI/ultrasound fusion devices, a fraction of the capital cost of MRI. The footprint is similar to conventional ultrasound. The only patients in whom the procedure is not possible are those with anal stenosis or absence post-abdominal perineal resection.
The sensitivity of micro-ultrasound in this registry for significant prostate cancer was superior to MRI. The specificity trended non-significantly below that of MRI. The specificity likely is strongly related to the learning curve. Acquiring the confidence to exclude certain borderline abnormalities takes more experience identifying abnormalities. It is likely that with further experience and validation, the specificity will improve.
Limitations
This was a real-world registry in 11 centers in seven countries of a new technology; thus, the results incorporate learning curves and significant variability between centers. Data was collected prospectively, but there was not a uniform protocol. Details of the methodological variation between sites are listed in Supplementary Table 1. Conventional 12-core systematic biopsies were not performed in most patients due to the inclusion of micro-ultrasound targets within the systematic samples and adjustment of systematic positions to reflect tissue variations observed on micro-ultrasound. Thus, the performance of micro-ultrasound compared to systematic biopsies could not be determined. Variation in the approach to borderline lesions was substantial. For example, site A targeted all borderline lesions comprehensively, and achieved a high sensitivity at the expense of specificity. Site C did not enroll or schedule patients for biopsy unless they had a visible lesion on MRI, potentially missing MRI-negative, micro-ultrasound-positive cases. Despite this, the sensitivity at their site was 94% compared to MRI. The number of cores per target was not standardized. Seven of the 11 sites were unblinded to the MRI when the ultrasound was performed, introducing an important source of bias in the interpretation of the ultrasound. However, results between the blinded and unblinded sites were similar. Only men with a prior mpMRI undergoing biopsy were enrolled, and data on patients who were excluded due to no prior MRI was not available for comparison. Current studies where the ultrasound annotation is performed blinded to the MRI are ongoing. This was a comparison to mpMRI, without a gold standard of surgical pathology; therefore, the known inaccuracies of biopsy grading could not be avoided. The PRI-MUS system used for ultrasound grading has not been validated.
Conclusions
This registry, the first large-scale analysis of the initial multicenter experience with micro-ultrasound, has demonstrated comparable metrics to mpMRI with respect to sensitivity, specificity, NPV, and PPV. This technology is an appealing alternative to MRI in the initial evaluation of men at risk for prostate cancer. Additional studies are warranted to further validate this technology.
Supplementary Information
Supplementary Table 1.
Summary of registry sites with methodological variations
| Site identifier | Clinic name | Location | Indication for biopsy | mpMRI specifications | mpMRI targeting system | Blinded or unblinded MRI |
|---|---|---|---|---|---|---|
| A | Urología Clínica, Clínica IMQ Zorrotzaurre | Bilbao, Spain | Clinical variables, including MRI | b-value ≥1400 no ERC | Cognitive fusion (micro-US-guided) | Unblinded |
| B | Urology of Virginia, Eastern Virginia Medical School | Virginia Beach, U.S. | Clinical variables, including MRI | 3T Toshiba Titan no ERC b-value 2000 | Cognitive fusion (micro-US-guided) | Blinded |
| C | Instituto Clinico Humanitas | Rozzano, Italy | Suspicious mpMRI only | 1.5T and 3T | Biojet robotic fusion | Blinded |
| D | Glickman Urological Institute, Cleveland Clinic | Cleveland, U.S. | Clinical variables, including MRI | 3T Siemens Skyra no ERC | Uronav software fusion | Unblinded |
| E | Charité Universitätsmedizin | Berlin, Germany | Clinical variables, including MRI | 3T with pelvic phased array coil no ERC | Hitachi software fusion | Unblinded |
| F | Groupe Urologie Saint-Augustin | Bordeaux, France | Clinical variables, including MRI | 1.5T and 3T | Cognitive fusion (micro-US-guided) | Unblinded |
| G | Sunnybrook Hospital | Toronto, Canada | Clinical variables, including MRI | Siemens and Phillips 3T, no ERC | Cognitive fusion (micro-US-guided) | Unblinded |
| H | Polyclinique Reims-Bezannes | Bezannes, France | Clinical variables, including MRI | 1.5T and 3T | Cognitive fusion (micro-US-guided) | Unblinded |
| I | Institut Mutualiste Montsouris | Paris, France | Clinical variables, including MRI | 3T no ERC | Cognitive fusion (micro-US-guided) | Unblinded |
| J | Ordensklinikum | Linz, Austria | Clinical variables, including MRI | 1.5T and 3T | FusionVu micro-USguided | Unblinded |
| K | Carolina Urologic Research Center | Myrtle Beach, U.S. | Clinical variables, including MRI | 1.5T and 3T | Cognitive fusion (micro-US-guided) | Unblinded |
MRI: magnetic resonance imaging; US: ultrasound.
Acknowledgement/funding
The Terry Fox Foundation provided funding for the Sunnybrook ExactVue imaging system. The authors would like to thank Brian Wodlinger for management of the registry and statistical assistance. This registry was provided by Exact Imaging, Toronto Canada; statistical support was provided by Brian Wodlinger, Exact Imaging, Toronto, ON, Canada.
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.National Cancer Institute. [Accessed June 1, 2020];Cancer stat facts: Prostate cancer. Available at: https://seer.cancer.gov/statfacts/html/prost.html. [Google Scholar]
- 2.Centers for Disease Control and Prevention. [Accessed June 1, 2020];U.S. cancer statistics data visualizations tool. Available at: https://www.cdc.gov/cancer/uscs/dataviz/index.htm. [Google Scholar]
- 3.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Prostate cancer. 2019. Version 4. Available at: https://www.nccn.org.
- 4.Rouvière O, Puech P, Renard-Penna R, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicenter, paired diagnostic study. Lancet Oncol. 2018;2045:1–10. doi: 10.1016/S1470-2045(18)30569-2. [DOI] [PubMed] [Google Scholar]
- 5.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate cancer diagnosis. N Engl J Med. 2018;378:1767–77. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet. 2017;6736:1–8. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 7.Mottet N, Cornford P, van den Bergh RCN, et al. EAU Annual Congress. Amsterdam: EAU Guidelines Office, Arnhem, The Netherlands; 2020. EAU guidelines: Prostate cancer. [Google Scholar]
- 8.NCCN clinical practice guidelines in oncology V1.2019 Prostate cancer early detection recommendations. Natl Compr Cancer Network, Inc; 2019. Available at: https://www.nccn.org. [Google Scholar]
- 9.Rohrbach D, Wodlinger B, Wen J, et al. High-frequency quantitative ultrasound for imaging prostate cancer using a novel micro-ultrasound scanner. Ultrasound Med Biol. 2018:441341–54. doi: 10.1016/j.ultrasmedbio.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Turkbey B, Choyke PL. Pirads 2.0: What is new? Diagnostic Interv Radiol. 2015;21:382–4. doi: 10.5152/dir.2015.15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghai S, Eure G, Fradet V, et al. Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: Creation of the micro-ultrasound protocol for prostate risk identification. J Urol. 2016;196:562–9. doi: 10.1016/j.juro.2015.12.093. [DOI] [PubMed] [Google Scholar]
- 12.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16:101–33. doi: 10.1214/ss/1009213286. [DOI] [Google Scholar]
- 13.Stabile A, Giganti F, Emberton M, et al. MRI in prostate cancer diagnosis: Do we need to add standard sampling? A review of the last 5 years. Prostate Cancer Prostatic Dis. 2018;21:473–87. doi: 10.1038/s41391-018-0071-8. [DOI] [PubMed] [Google Scholar]
- 14.Padhani AR, Haider MA, Villers A, et al. Multiparametric magnetic resonance imaging for prostate cancer detection : What we see and what we miss. Eur Urol. 2019;75:721–2. doi: 10.1016/j.eururo.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Rosenkrantz AB, Ginocchio LA, Cornfeld D, et al. Interobserver reproducibility of the PI-RADS version 2 lexicon: A multicenter study of six experienced prostate radiologists. Radiology. 2016;280:793–804. doi: 10.1148/radiol.2016152542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Layne KA, Dargan PI, Archer JRH, et al. Gadolinium deposition and the potential for toxicological sequelae – a literature review of issues surrounding gadolinium-based contrast agents. Br J Clin Pharmacol. 2018;84:2522–34. doi: 10.1111/bcp.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter KK, King A, Galgano SJ, et al. Financial implications of biparametric prostate MRI. Prostate Cancer Prostatic Dis. 2020;23:88–93. doi: 10.1038/s41391-019-0158-x. [DOI] [PubMed] [Google Scholar]
- 18.Hyndman M, Pavlovich C, Eure G, et al. Prospective validation of PRI-MUSTM, the Prostate Risk Identification using Micro-Ultrasound protocol for real-time detection of prostate cancer using high-resolution micro-ultrasound imaging. American Urology Association; San Francisco, USA: 2018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Summary of registry sites with methodological variations
| Site identifier | Clinic name | Location | Indication for biopsy | mpMRI specifications | mpMRI targeting system | Blinded or unblinded MRI |
|---|---|---|---|---|---|---|
| A | Urología Clínica, Clínica IMQ Zorrotzaurre | Bilbao, Spain | Clinical variables, including MRI | b-value ≥1400 no ERC | Cognitive fusion (micro-US-guided) | Unblinded |
| B | Urology of Virginia, Eastern Virginia Medical School | Virginia Beach, U.S. | Clinical variables, including MRI | 3T Toshiba Titan no ERC b-value 2000 | Cognitive fusion (micro-US-guided) | Blinded |
| C | Instituto Clinico Humanitas | Rozzano, Italy | Suspicious mpMRI only | 1.5T and 3T | Biojet robotic fusion | Blinded |
| D | Glickman Urological Institute, Cleveland Clinic | Cleveland, U.S. | Clinical variables, including MRI | 3T Siemens Skyra no ERC | Uronav software fusion | Unblinded |
| E | Charité Universitätsmedizin | Berlin, Germany | Clinical variables, including MRI | 3T with pelvic phased array coil no ERC | Hitachi software fusion | Unblinded |
| F | Groupe Urologie Saint-Augustin | Bordeaux, France | Clinical variables, including MRI | 1.5T and 3T | Cognitive fusion (micro-US-guided) | Unblinded |
| G | Sunnybrook Hospital | Toronto, Canada | Clinical variables, including MRI | Siemens and Phillips 3T, no ERC | Cognitive fusion (micro-US-guided) | Unblinded |
| H | Polyclinique Reims-Bezannes | Bezannes, France | Clinical variables, including MRI | 1.5T and 3T | Cognitive fusion (micro-US-guided) | Unblinded |
| I | Institut Mutualiste Montsouris | Paris, France | Clinical variables, including MRI | 3T no ERC | Cognitive fusion (micro-US-guided) | Unblinded |
| J | Ordensklinikum | Linz, Austria | Clinical variables, including MRI | 1.5T and 3T | FusionVu micro-USguided | Unblinded |
| K | Carolina Urologic Research Center | Myrtle Beach, U.S. | Clinical variables, including MRI | 1.5T and 3T | Cognitive fusion (micro-US-guided) | Unblinded |
MRI: magnetic resonance imaging; US: ultrasound.


