Abstract
Introduction
We aimed to perform a systematic review and meta-analysis on the long-term durability, incidence of complications, and patient satisfaction outcomes in ileal conduit (IC) and orthotopic neobladder (ONB).
Methods
A systematic electronic literature search was performed in Medline, Embase, Cochrane Library, and Scopus using MeSH and free-text search terms “Urinary diversion” AND “Ileal conduit” AND “Neobladder.” The search concluded June 19, 2018. Inclusion criteria were those patients who had a cystectomy and required urinary diversion by either IC or neobladder.
Results
In total, 32 publications met the inclusion criteria. Data were available on 46 787 patients (n=36 719 for IC and n=10 068 for ONB). Meta-analyses showed that IC urinary diversions performed less favorably than ONB in terms of re-operation rates, Clavien-Dindo complications, and mortality rates; odds ratios (ORs) and 95% confidence intervals (CIs) were 1.76 (1.24, 2.50), p<0.01; 1.16 (1.09, 1.22), p<0.01; and 6.29 (5.30, 7.48), p<0.01, respectively. IC urinary diversion performed better than ONB in relation to urinary tract infection rates and ureteric stricture rates, OR and 95% CI 0.67 (0.58, 0.77), p<0.01; and 0.70 (0.55, 0.89), p<0.01, respectively.
Conclusions
Our results show that there is no significantly increased morbidity with ONB compared to IC. Selection of either urinary diversion technique should be based on factors such as tumor stage, comorbidities, surgical experience, and patient acceptance of postoperative sequalae.
Introduction
There are many conditions that necessitate removal of the urinary bladder using cystectomy.1 The most common indication is cancer of the urinary bladder but in some cases, cystectomy is indicated to treat benign disease such as interstitial cystitis.1 Cystectomy, therefore, requires replacement of the urinary bladder with a procedure known as urinary diversion.1 Urinary diversion is a form of urinary reconstruction and most commonly involves the use of a gastrointestinal (GI) segment to replace part or all of the function of the urinary bladder.1 An optimal bladder replacement should be able to hold large intravesical volumes while maintaining low pressure values in order to restore normal function and preserve the upper urinary tracts.1
Lifelong postoperative complications are common with any type of urinary diversion.1 These can be divided into three broad groups: 1) metabolic complications due to the intestinal segment’s resorptive capacity; 2) neuromechanical aspects, which affect storage volume and diversion compliance; and 3) technical-surgical complications, which result in postoperative morbidity.1
Ileal conduit (IC) has long been considered the gold standard for replacement of the urinary bladder. However, orthotopic neobladder (ONB) has a superior cosmetic appearance and better preservation of body image.2 The aim of this systematic review and meta-analysis is to perform a robust comparison of IC and ONB urinary diversion and to provide practitioners with a summary of the global trends for reconstructive preferences in urinary diversion.
Methods
Search strategy
This review was planned and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).3
A systematic electronic literature search was carried out in Medline, Embase, Cochrane Library, and Scopus. Using MeSH and free-text terms, the search strategy was: “Urinary diversion” AND “Ileal conduit” AND “Neobladder.” Titles and abstracts retrieved by the June 2018 search were screened independently by two authors (EB and ND), following the removal of duplicates. Where there was any uncertainty regarding inclusion, full-texts were retrieved and assessed for inclusion. Excluded studies were listed, with reasons given for their exclusion. Disagreements regarding the inclusion or exclusion of an article were resolved by discussion.
Eligibility criteria
Inclusion criteria were those patients who have had a cystectomy for any reason and required urinary diversion by either IC or ONB. Exclusion criteria were review articles, case reports, commentaries, letters, conference abstracts without sufficient outcome data and failure to meet the inclusion criteria.
Data extraction and outcomes
The following data were extracted from each study: author’s name, journal of publication, year of publication, country of origin, study type, total number of patients, and patient demographics (age, sex, body mass index [BMI]). Information regarding the outcomes listed below were recorded from each eligible study. The primary outcome measures were quality of life, measures of long-term durability (including re-operation, urinary tract infections [UTI], and ureteric stricture), postoperative morbidity, postoperative mortality, and length of stay. Secondary outcome measures were physiological changes, including active reflux, mucous, upper tract dilatation/hydronephrosis, renal scarring, metabolic changes, urinary stones, and health economics.
Statistical analysis
Data were presented as a mean ± standard deviation (SD) for continuous variables. Differences between outcomes measured were considered significant at p<0.05. Meta-analysis was performed with Review Manager Version 5.3 software.4 The Mantel-Haenszel model was used for meta-analysis of dichotomous data and the inverse variance model for meta-analysis of continuous data.5
Results
Eligible studies
In total, 2907 articles were identified. Following the removal of duplicates (n=1458), 1449 articles were screened, of which 1417 were excluded, as they did not meet the inclusion criteria. In total, 32 articles were included in the qualitative and quantitative analysis; see the PRISMA diagram in Fig. 1 for the flow of studies through the review and the reasons for which studies were excluded.
Fig 1.
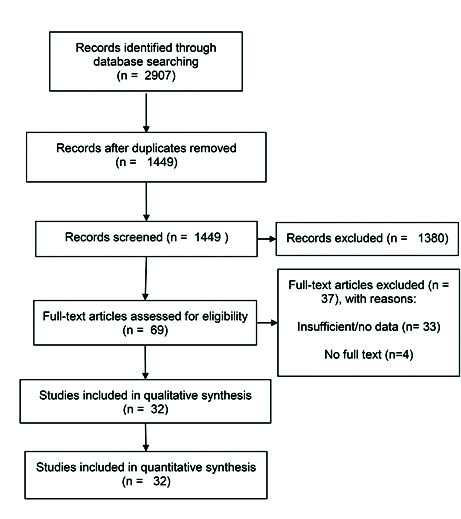
PRISMA diagram for the flow of studies through the review and the reasons for which studies were excluded.
Data were available for 46 787 patients in the studies included in this review (n=36 719 for IC and n=10 068 for ONB). Study characteristics are summarized in Table 1. In total, there were 16 prospective case-control studies,6–21 one of which was a prospective case-control study with matched-pair analysis,20 and 16 retrospective case-control studies.2,22–36
Table 1.
Summary of studies included in the meta-analysis for ileal conduit and orthotopic neobladder
| Author (year) | Origin | Journal | Type of study | Level of evidence | IC (n) | ONB (n) |
|---|---|---|---|---|---|---|
| Abe et al, 2014 | Japan | Int J Urol | RCC | 3b | 493 | 175 |
| Aboumarzouk et al, 2014 | Poland | Cent European J Urol | PCC | 3b | 39 | 24 |
| Angulo et al, 2014 | Spain | Urology | PCC | 3b | 8 | 12 |
| Antonelli et al 2016 | Italy | Clin Genitourin Cancer | PCC | 3b | 85 | 85 |
| Belotti et al, 2012 | Italy | Anticancer Research | PCC | 3b | 223 | 111 |
| Cho et al, 2017 | Korea | Renal Failure | RCC | 3b | 33 | 62 |
| Collins et al, 2013 | Sweden | Eur Urol | PCC | 3b | 43 | 70 |
| De Nunzio et al, 2013 | Italy | Eur J Surg Oncol | PCC | 3b | 217 | 112 |
| Decaestecker et al, | 2016 | Belgium European Urology, Supplements | PCC | 3b | 40 | 32 |
| Erber et al, 2012 | Germany | IRSN Urol | RCC | 3b | 23 | 34 |
| Gburek et al, 1998 | USA | Journal of Urology | RCC | 3b | 66 | 66 |
| Gore et al, 2010 | USA | Journal of Urology | RCC | 3b | 1252 | 109 |
| Hofer et al, 2012 | USA | Journal of Urology | PCC | 3b | 245 | 63 |
| Jung et al, 2006 | Korea | Korean Journal of Urology | PCC | 3b | 29 | 19 |
| Kim et al, 2014 | Korea | Jpn J Clin Oncol | RCC | 3b | 161 | 147 |
| Mano et al, 2018 | Israel | Urology | RCC | 3b | 130 | 49 |
| Monn et al, 2014 | USA | Urologic Oncology: Seminars and Original Investigations | RCC | 3b | 139 | 55 |
| Nahar et al, 2018 | USA | Journal of Urology | RCC | 3b | 10197 | 692 |
| Navarro et al, 2008 | Chile | Urology | PCC | 3b | 17 | 37 |
| Nazmy et al, 2013 | USA | Journal of Urology | PCC | 3b | 67 | 91 |
| Nieuwenhuijzen et al, 2008 | The Netherlands | Eur Urol | PCC | 3b | 118 | 62 |
| Parekh et al, 2000 | USA | Urology | RCC | 3b | 81 | 117 |
| Popov et al, 2007 | Republic of Macedonia | Acta chirurgica lugoslavica | RCC | 3b | 32 | 52 |
| Prcic et al, 2017 | Bosnia and Herzegovina | Med Arch | PCC | 3b | 66 | 60 |
| Roghmann et al, 2013 | USA | Can Urol Assoc J | RCC | 3b | 18782 | 6282 |
| Roghmann et al, 2014 | Germany | Int J Urol | RCC | 3b | 349 | 186 |
| Roghmann et al, 2017 | Germany | Journal of Urology | PCC | 3b | 510 | 294 |
| Sherwani et al, 2009 | India | Int J Health Sci (Qassim) | PCC | 3b | 13 | 4 |
| Sogni et al, 2008 | Italy | Urology | RCC | 3b | 53 | 32 |
| Tan et al, 2017 | United Kingdom | Eur Urol Focus | PCC | 3b | 100 | 34 |
| Thulin et al, 2010 | Sweden | BJU Int | RCC | 3b | 190 | 180 |
| van Hemelrijck et al, 2013 | Sweden | BJU Int | RCC | 3b | 2918 | 720 |
IC: ileal conduit; ONB: orthotopic neobladder; PCC: prospective case-control; RCC: retrospective case-control.
Patient demographics (including patient age, male to female ratio, and patient BMI) were reported, if available; these are outlined in Tables 2 and 3. The mean patient age between the IC and ONB groups was significantly different, 69.65±5.84 in the IC group vs. 61.07±4.47 in the ONB group (95% confidence interval [CI] 8.44, 8.71, p <0.01), with patients undergoing IC urinary diversion being older overall. The mean BMI of the IC and ONB groups were significantly different, 25.7±4.6 in the IC group vs. 23.7±3.3 in the ONB group (95% CI 175, 2.25, p<0.01). The sex ratio in both groups was significantly different (11:2 male:female in the IC group vs. 11:1 male:female in the ONB group, p <0.01).
Table 2.
Patient demographics from papers where age was reported as a mean ± standard deviation
| Author (year) | Age (conduit) | Age (neobladder) | Male/female (conduit) | Male/female (neobladder) | BMI (conduit) | BMI (neobladder) |
|---|---|---|---|---|---|---|
| Aboumarzouk et al, 2014 | 60±7.11 | 57±8.68 | 34/5 | 24/0 | 27.2±2.3 | 27.96±2 |
| Antonelli A, et al 2016 | 63±8.8 | 63.5±6.7 | 69/16 | 72/13 | NR | NR |
| Belotti et al, 2012 | 70.4±8.1 | 60.6±0.9 | 183/34 | 90/21 | 26.3±4.3 | 26.3±3.6 |
| Cho et al, 2017 | 69.5±8.1 | 64.5±8.6 | 23/10 | 52/10 | NR | NR |
| Collins et al, 2013 | 69.9±6.7 | 59.8±9.0 | 31/11 | 62/8 | 24.8±3.1 | 26.1±3.4 |
| De Nunzio et al, 2013 | 71±9.75* | 63±0.25* | NR | NR | 26.4±6* | 25±3.25* |
| Decaestecker et al, 2016 | 71±1.5* | 63±1.25* | 29/11 | 27/5 | 26±4.25* | 26±3.75* |
| Gburek B et al, 1998 | 69±11.75* | 62±12.75* | 66/0 | 62/4 | NR | NR |
| Hofer et al, 2012 | 69.7±3.75* | 59.7±15 | NR | NR | NR | NR |
| Jung et al, 2006 | 65.6±9.9 | 60.8±8.3 | NR | NR | NR | NR |
| Kim et al, 2014 | 67.1±8.9 | 59.4±9.4 | 115/32 | 156/5 | 23.6±3.3 | 24±3.1 |
| Monn et al, 2014 | 72.6±10 | 59.6±9 | 107/32 | 49/6 | NR | NR |
| Nahar et al, 2018 | 68.8±10.1 | 62.8±10 | 8835/1362 | 663/29 | NR | NR |
| Parekh et al, 2000 | 68±12.75* | 60±13.5* | 48/33 | 97/20 | NR | NR |
| Roghmann et al, 2013 | 69.6 | 60.8 | 81/19 | 91/9 | NR | NR |
| Sherwani et al, 2009 | 59 | 53.3 | NR | NR | NR | NR |
| Thulin et al, 2010 | 70.1 | 64.3 | 134/56 | 165/15 | NR | NR |
Estimated standard deviation based on the Range Rule of Thumb.
BMI: body mass index; NR: not reported.
Table 3.
Patient demographics from papers where age was reported as a median and range
| Author (year) | Age (conduit) | Age (neobladder) | Male/female (conduit) | Male/female (neobladder) | BMI (conduit) | BMI (neobladder) |
|---|---|---|---|---|---|---|
| Abe et al, 2014 | 70 (37–89) | 63 (25–86) | 364/129 | 164/11 | 23 (14.6–35.1) | 23.3 (16–31.5) |
| Angulo et al, 2014 | 74.5 (70–82.2) | 66 (61.5–75) 5/3 | 12/0 | 27.7 (23.2–31.8) | 27.3 (25.5–28.5) | |
| Erber et al, 2012 | 70 (64–75) | 62 (56–66) | 98/48 | 110/5 | NR | NR |
| Mano et al, 2018 | 72 (65–78) | 60 (53–65) | 112/18 | 43/6 | NR | NR |
| Nieuwenhuijzen et al, 2008 | 70 (46–85) | 62 (32–73) | 88/30 | 59/3 | NR | NR |
| Roghmann et al, 2014 | 72 (67–76) | 61 (55–67) | 256/93 | 158/28 | 27.3 (24.6–29.8) | 26.1 (23.8–29.2) |
| Sogni et al, 2008 | 78.9 (75–88) | 77.5 (75–82) | NR | NR | NR | NR |
| Tan et al, 2017 | 67.4 (60.4–74.3) | 54.5 (48.6–61.6) | 75/25 | 28/6 | 27.2 (23.4–31) | 27.3 (23–28.5) |
BMI: body mass index; NR: not reported.
Primary outcomes
Quality of life
Patient satisfaction, general measures of health status and disease specific measures of quality of life were not reported in a standardized manner across the studies. This precludes meaningful statistical analysis. Of the 32 included publications, five compared quality of life in patients with either diversion type.13,18,24,34,35 Using The European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire, 37 Navarro et al found a better acceptance in ONB vs IC.13 The scale used by the authors rates overall quality of life on a seven-point scale, where 1=very poor and 7=excellent.37 Sogni et al also used the QLQ-C30 questionnaire, as well as the bladder cancer-specific module EORTC QLQ-muscle-invasive bladder cancer module 30 (BLM 30)38 and found that the quality of life reported in both groups was comparable but with a non-significant higher quality of life rating seen in the ONB group.34 Erber et al also used the QLQ-C30 questionnaire and reported overall quality of life as 58±25.3 in the IC group and 72.3±19.5 in the ONB group.24 Sherwani et al used a simple satisfaction scale of “very good,” “good,” “poor” to compare quality of life between the two groups and reported higher ratings in the ONB group.18 Finally, Thulin et al had patients rate quality of life as “high,” “moderate,” or “low.”35 Of these, 68% of patients with an ONB reported their quality of life as “high” compared to 53% of IC recipients.
Measures of long-term durability
The measures of long-term durability included in this review were re-operation rates, UTI rates, and ureteric stricture rates. The meta-analysis of these outcomes are detailed in Fig. 2.
Fig. 2.
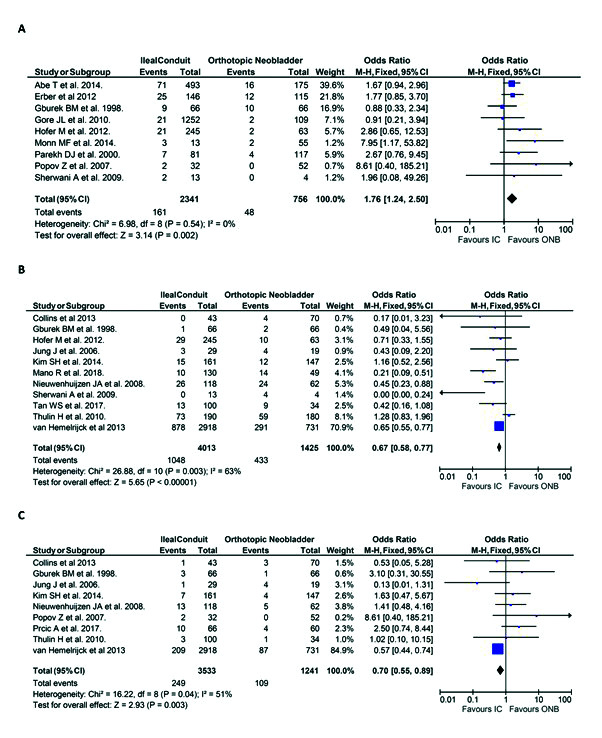
Primary outcomes in the ileal conduit (IC) vs. orthotopic neobladder (ONB) cohorts included in the review: (A) rate of re-operation; (B) incidence of urinary tract infection; and (C) incidence of ureteric stricture. CI: confidence interval.
Of the 32 studies included in this review, nine examined re-operation rates. The rate of re-operation was significantly greater in patients with an IC compared to patients undergoing ONB formation (odds ratio [OR] 1.76, 95% CI 1.24, 2.50, p<0.01) (Fig. 2A).
The incidence of UTI rates was reported in 11 studies. The incidence of UTI was significantly less in patients with an IC vs. patients with an ONB (n=1048/4013, 26.1% vs. n=433/1425, 30.4%, respectively; OR 0.67, 95% CI 0.58, 0.77, p<0.01) (Fig. 2B).
Ureteric stricture rates in both groups were reported in nine publications. The incidence of the ureteric stricture was statistically less significant in patients undergoing IC urinary diversion vs. patients with ONB (n=249/3533, 7.0% vs. n=109/1241, 8.8%, respectively; OR 0.70, 95% CI 0.55, 0.89, p<0.01) (Fig. 2C).
Complications
Postoperative morbidity, reported in 21 publications, was described using the Clavien-Dindo classification in 12 publications. 6,7,10,14,15,19–22,26,28,33,39
The incidence of postoperative morbidity was significantly higher in patients undergoing IC urinary diversion vs. those undergoing ONB urinary diversion (n=15659/25264, 61.9% vs. n=5102/8478, 60.1%, respectively; OR 1.16, 95% CI 1.09, 1.22, p<0.01) (Fig. 3A). Subgroup analysis of patients who suffered Clavien-Dindo 1–2 (minor) complications showed that patients undergoing IC were less likely to suffer a minor complication than those undergoing ONB urinary diversion, although this was not statistically significant (n=1016/1802, 56.4% vs. n=573/1029, 55.7%, respectively; OR 0.89, 95% CI 0.75, 1.06, p=0.21) (Fig. 3B). Subgroup analysis of those who suffered Clavien-Dindo 3–5 (major) complications showed that patients with IC were significantly more likely to suffer a major complication than those with ONB (n=375/1802, 20.8% vs. n=184/1029, 17.9%, respectively; OR 1.25, 95% CI 1.02, 1.53, p=0.03) (Fig. 3C).
Fig. 3.
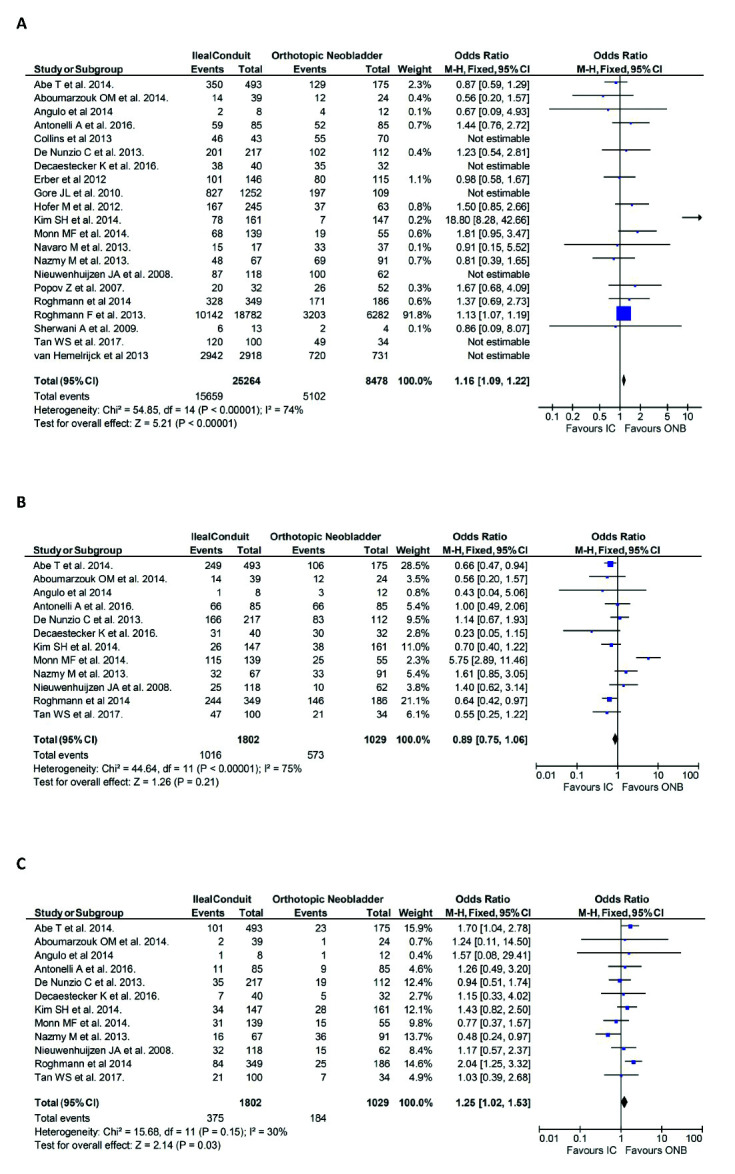
Complications in the ileal conduit (IC) vs. orthotopic neobladder (ONB) cohorts reported in 21 publications of the review: (A) incidence of postoperative morbidity; (B) Clavien-Dindo 1–2 (minor) complications; (C) Clavien-Dindo 3–5 (major) complications. CI: confidence interval.
Mortality
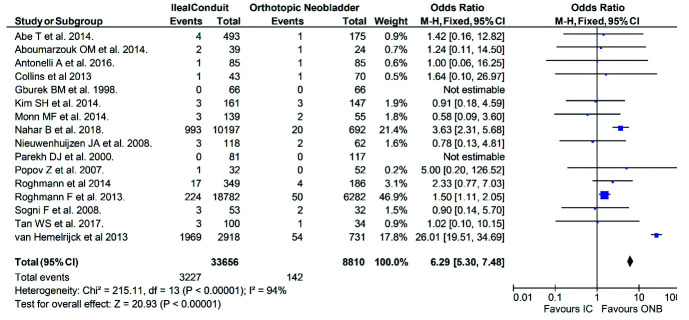
Postoperative mortality was reported in 21 publications. The mortality rate in patients with IC urinary diversion was significantly higher than that of patients undergoing ONB (n=3227/33656, 9.6% vs. n=142/8810, 1.6%, respectively; OR 6.29, 95% CI 5.30, 7.48, p<0.01) (Fig. 4).
Fig. 4.
Postoperative mortality in the ileal conduit (IC) vs. orthotopic neobladder (ONB) cohorts reported in 21 publications of the review. CI: confidence interval.
Length of stay
Seven publications reported length of stay IC and ONB groups as a mean ± SD (Fig. 5). Length of stay was shorter in the IC group compared to the orthotopic neobladder group (17.56±8.61 days vs. 19.93±7.85 days, respectively), with a mean difference of −0.74 (95% CI −1.30, −0.18, p<0.01].
Fig. 5.
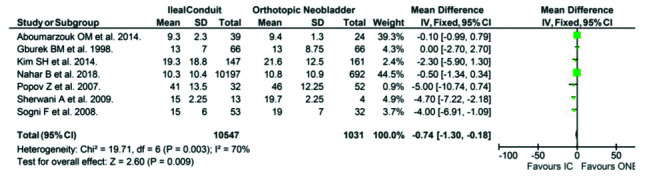
Length of stay in the ileal conduit (IC) vs. orthotopic neobladder (ONB) cohorts reported in 7 publications of the review.
Eleven other publications reported length of stay as median and range and these are outlined in Table 4.
Table 4.
Studies where length of stay is reported as median and range
| Author (year) | Ileal conduit | Orthotopic neobladder | ||
|---|---|---|---|---|
|
| ||||
| n | Median (range) | n | Median (range) | |
| Abe et al, 2014 | 493 | 39 (3–257) | 175 | 42 (18–364) |
| Angulo et al, 2014 | 8 | 9.5 (8–11) | 12 | 8.5 (7.2–10.7) |
| Antonelli et al, 2016 | 85 | 17 | 85 | 21 |
| Belotti et al, 2012 | 223 | 20 (16–24) | 111 | 24 (20–29) |
| Collins et al, 2013 | 43 | 9 (6–142) | 70 | 9 (4–78) |
| Decaestecker et al, 2016 | 40 | 10 (5–36) | 32 | 11 (6–39) |
| Monn et al, 2014 | 139 | 8 (6–10) | 55 | 7 (6–8) |
| Nieuwenhuijzen et al, 2008 | 118 | 17 (6–53) | 62 | 15 (8–44) |
| Parekh et al, 2000 | 81 | 8 (5–60) | 117 | 7 (5–28) |
| Roghmann et al, 2014 | 349 | 19 (16–24) | 70 | 9 (17–23) |
| Tan et al, 2017 | 100 | 10 (8–15.5) | 34 | 11 (8.5–14) |
Secondary outcomes
Physiological changes
For the purpose of this review, physiological changes following urinary diversion were defined as: active reflux, upper tract dilation or hydronephrosis, mucous, metabolic changes, urinary stones, and renal scarring.
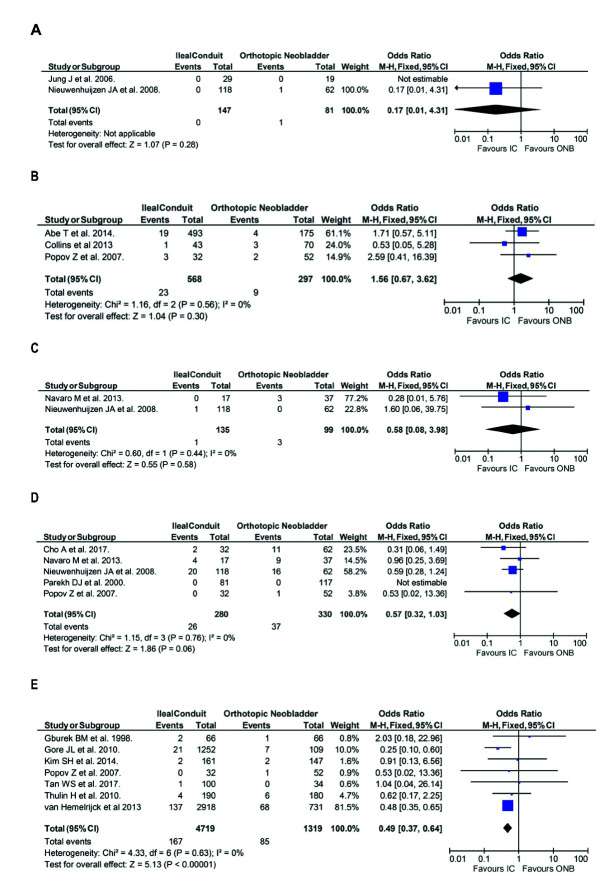
The incidence of active reflux was reported in two of the 32 included publications and showed that patients undergoing IC urinary diversion were at lower risk of active reflux than those undergoing ONB, although this was not statistically significant (n=0/147 vs. n=1/81, respectively; OR 0.17, 95% CI 0.01, 4.31, p=0.28) (Fig. 6A).
Fig. 6.
Secondary outcomes in the ileal conduit (IC) vs. orthotopic neobladder (ONB) cohorts included in the review. Incidence of (A) active reflux; (B) upper tract dilatation/hydronephrosis; (C) mucous production; (D) metabolic change; (E) urinary stones. CI: confidence interval.
The incidence of upper tract dilatation/hydronephrosis was reported in three of the 32 included publications. Analysis shows that patients with IC urinary diversion were more likely to have hydronephrosis than those with ONB (n=23/568, 4.0% vs. n=9/297, 3.0%, respectively; OR 1.56, 95% CI 0.67, 3.62, p=0.30) (Fig. 6B), although again, not statistically significant.
The incidence of mucous production was less in patients with IC than those with ONB (n=1/135, 0.7% vs n=3/99, 3%, respectively: OR 0.58, 95% CI 0.08, 3.98, p=0.58) (Fig. 6C). This was not statistically significant.
The incidence of metabolic change is less in patients with an IC vs. ONB (n=26/280, 4.5% vs. n=37/330, 11.2%, respectively: OR 0.57, 95% CI 0.32, 1.03, p=0.06) (Fig. 6D). This finding was also not statistically significant.
The incidence of urinary stones was lower in patients with IC urinary diversion compared to ONB (n=167/4719, 3.5% vs. n=85/1319, 6.4%, respectively; OR 0.49, 95% CI 0.37, 0.64, p<0.01) (Fig. 6E). This was statistically significant.
There were no data reported in any of the included studies regarding the incidence of renal scarring in patients with either urinary diversion.
Health economics
None of the studies included in this review examined or made any comment on the economic impact of either IC or ONB urinary diversion, precluding a comparison of cost of intervention or assessment of cost-benefit relationship.
Discussion
This study is a comprehensive review comparing IC and ONB urinary diversions. The choice of urinary diversion has significant implications for both the patient in terms of their future health and quality of life, and for the surgeon and their methods. The choice of which urinary diversion to use depends on many factors, such as surgical skill, urethral disease, or patient acceptance.1 It is, therefore, imperative that a thorough comparison is made of the outcomes of IC and ONB to provide practitioners with a comprehensive summary of the data to aid surgical and patient decision-making.
Our review of the literature demonstrates that there is an overall preference towards IC urinary diversion and a tendency for this type of diversion to be performed in older patients. We also showed a higher re-operation rate and rate of postoperative morbidity and mortality in those patients who underwent an IC urinary diversion. ONB urinary diversions, however, performed worse in terms of UTI, ureteric stricture, and urinary stone rates.
In this study, comparison of patient age in the two groups showed that patients with IC urinary diversion were significantly older than those patients undergoing ONB diversion. It can be reliably assumed that older patients have greater comorbidities so interpretation of results may be affected by this finding. Younger patients, likely with fewer comorbidities, tend to have a ONB diversion, possibly due to the widely accepted belief that ONB has a greater risk of perioperative complications due to its technical complexity.2 This is, therefore, likely to confound data relating to postoperative complication rates in each group. The larger numbers of IC performed in these studies compared to ONB demonstrates the preference for IC as the choice of urinary diversion. This is likely to be multifactorial, as addressed previously, including patient preference, surgical skill, and other patient factors, such as age or comorbidity.
From the included publications, there was a better acceptance and quality of life in patients with ONB diversions than those with IC.40 However, it is worth bearing in mind that each type of urinary diversion has inherently different challenges associated with it.6 According to meta-analysis, there is a higher rate of UTI in the ONB group, potentially as ONB often requires self-catheterization, which comes with the associated risk of bacterial inoculation.35 Meta-analysis also demonstrated a significantly higher risk of uretero-ileal stricture in patients with an ONB than those with an IC urinary diversion, possibly due to the use of an anti-reflux mechanism in uretero-intestinal anastomosis; however, an anti-reflux mechanism was only used for ONB in two of the publications included in this review. This highlights the importance of forming a low-pressure reservoir.1
The studies included for analysis in this review focused predominantly on reporting perioperative data regarding IC urinary diversion and ONB, revealing a dearth of information on long-term outcomes of these two types of urinary diversion. This may explain the paucity of evidence relating to the long-term complications of urinary diversion.1 Meta-analysis of those publications that reported postoperative morbidity using Clavien-Dindo shows a significantly higher morbidity in IC compared to ONB.6,7,10,14,15,19–22,26,28,33 This is potentially explained by noting that within this systematic review, patients undergoing IC tended to be older and have higher-grade tumors, which may increase the risk of death independent of diversion type. It must also be considered that minor (Clavien-Dindo 1–2) complications may be under-reported, given that many of the publications included in this review were retrospective case controls.27
Meta-analysis of mortality rates between IC and ONB urinary diversion showed a significantly increased risk of death in those patients undergoing IC diversion. However, patients undergoing IC tend to be older and have higher-grade tumours, which may increase the risk of death independent of diversion type.2
Meta-analysis of mean ± SD of length of stay demonstrated a significantly longer length of stay in patients undergoing ONB. Length of stay is sometimes dependent on the practice of individual institutions and, thus, it cannot be assumed that ONB urinary diversion always results in an increased length of stay; still, it is an important consideration when deciding which type of urinary diversion to use.
With the exception of stones (which were significantly more likely in ONB), meta-analysis of the physiological changes considered in this review showed no statistically significant results. Nonetheless, consideration of physiological changes, such as hydronephrosis, vesicoureteric reflux, mucous production, metabolic changes, and urinary stones, is important when deciding between IC and ONB, particularly in patients with pre-existing conditions.
A cost-benefit comparison is a crucial aspect of assessing any intervention and there seems to be a complete lack of any such analysis in contemporary literature. This is certainly an aspect of urinary diversion that requires further study. In countries where universal or socialized healthcare does not exist, the type of urinary diversion a patient receives may depend on their ability to pay for this type.41
The main limitation of this meta-analysis is that the studies included for analysis consist of retrospective or prospective case-control studies. Other limitations include the small sample sizes contained within most publications, limiting the generalizability of the findings of this review and the non-standard reporting of outcomes. Thirdly, some data points were presented as median and range, which precluded any analysis regarding these figures. Lastly, there is the potential for significant selection bias in all publications in that those undergoing ONB have lower-stage tumors and would, therefore, have better postoperative outcomes in terms of recovery and mortality rates.20
However, this is a very robust analysis involving large numbers of patients with extensive followup, using standardized questionnaires, and involving data from multiple institutions. This analysis also includes international publications and so is representative of global trends for reconstructive preferences in urinary diversion.
Conclusions
This systematic review and meta-analysis does not support the widely held perception that ONB is associated with increased risk of postoperative morbidity, however, the reason for this may be multifactorial. Our findings demonstrated that ONB was associated with a lower rate of major (Clavien-Dindo 3–5) complications than the IC. However, larger cohort studies are required to reach a definitive conclusion as to which type of diversion is superior. Our results also reinforce that the selection of urinary diversion should be based on careful preoperative counselling, taking into account patient factors (such as tumor stage and comorbidities), surgical skill, and patient acceptance of the sequalae of either type of urinary diversion.
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Wein AJ, Kavoussi LR, Partin AW, et al. Campbell-Walsh urology. Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 2.Gburek BM, Lieber MM, Blute ML. Comparison of Studer ileal neobladder and ileal conduit urinary diversion with respect to perioperative outcome and late complications. J Urol. 1998;160:721–3. doi: 10.1016/S0022-5347(01)62767-8. [DOI] [PubMed] [Google Scholar]
- 3.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Int Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 4.Review Manager (RevMan) [program]. 5.3 version. Copenhagen: The Nordic Cochrance Centre: The Cochrane Collaboration; 2014. [Google Scholar]
- 5.Davis NF, Quinlan MR, Poyet C, et al. Miniaturized percutaneous nephrolithotomy vs. flexible ureteropyeloscopy: A systematic review and meta-analysis comparing clinical efficacy and safety profile. World J Urol. 2018;36:1127–38. doi: 10.1007/s00345-018-2230-x. [DOI] [PubMed] [Google Scholar]
- 6.Aboumarzouk OM, Drewa T, Olejniczak P, et al. Laparoscopic radical cystectomy: Neobladder or ileal conduit, debate still goes on. Centr Eur J Urol. 2014;67:9–15. doi: 10.5173/ceju.2014.01.art2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angulo JC, Caceres F, Cabrera PM, et al. Two-port laparoscopic radical cystectomy with reusable umbilical system: a feasibility study. Urology. 2014;84:1088–93. doi: 10.1016/j.urology.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Belotti S, Antonelli A, Bastiani N, et al. Role of urinary diversion on complication rate after radical cystectomy: Retrospective study on a single-centre cohort of 407 consecutive patients. Anticancer Research. 2012;32:1917–9. [Google Scholar]
- 9.Collins JW, Tyritzis S, Nyberg T, et al. Robot-assisted radical cystectomy: Description of an evolved approach to radical cystectomy. Eur Urol. 2013;64:654–63. doi: 10.1016/j.eururo.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 10.De Nunzio C, Cindolo L, Leonardo C, et al. Analysis of radical cystectomy and urinary diversion complications with the Clavien classification system in an Italian real-life cohort. Eur J Urol. 2013;39:792–8. doi: 10.1016/j.ejso.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Hofer M, Bhalani V, Kundu S, et al. Complications of cystectomy and urinary diversions from the national surgical quality improvement program. J Urol. 2012;187:e712. doi: 10.1016/j.juro.2012.02.1780. [DOI] [Google Scholar]
- 12.Jung JU, Sohn DW, Cho YH. Alteration in renal function for patients with ileal conduit and ileal orthotopic neobladder. Kor J Urol. 2006;47:1065–8. doi: 10.4111/kju.2006.47.10.1065. [DOI] [Google Scholar]
- 13.Navarro M, Tagle R, Montes C, et al. Comparison of clinical factors, surgical complications, functional results and quality of life between four different urinary diversions. Urology. 2013;82:S324. [Google Scholar]
- 14.Nazmy M, Wilson T, Yuh B, et al. Complications of robotic assisted radical cystectomy by diversion type. J Urol. 2013;189:e724. doi: 10.1016/j.juro.2013.02.2890. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwenhuijzen JA, de Vries RR, Bex A, et al. Urinary diversions after cystectomy: The association of clinical factors, complications, and functional results of four different diversions. Eur Urol. 2008;53:834–42. doi: 10.1016/j.eururo.2007.09.008. Discussion 42–4. [DOI] [PubMed] [Google Scholar]
- 16.Prcic A, Begic E. Complications after ileal urinary derivations. Med Arch. 2017;71:320–4. doi: 10.5455/medarh.2017.71.320-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roghmann F, Von Landenberg N, Schmidt J, et al. Evaluation of postoperative complications after radical cystectomy using the comprehensive complication index. J Urol. 2017;197:e722. doi: 10.1016/j.juro.2017.02.1673. [DOI] [Google Scholar]
- 18.Sherwani Afak Y, Wazir BS, Hamid A, et al. Comparative study of various forms of urinary diversion after radical cystectomy in muscle invasive carcinoma urinary bladder. Int J Health Sci. 2009;3:3–11. [PMC free article] [PubMed] [Google Scholar]
- 19.Tan WS, Lamb BW, Tan MY, et al. In-depth critical analysis of complications following robot-assisted radical cystectomy with intracorporeal urinary diversion. Eur Urol Focus. 2017;3:273–9. doi: 10.1016/j.euf.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Antonelli A, Belotti S, Cristinelli L, et al. Comparison of perioperative morbidity of radical cystectomy with neobladder vs. ileal conduit: A matched pair analysis of 170 patients. Clin Genitourin Cancer. 2016;14:244–8. doi: 10.1016/j.clgc.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Decaestecker K, Vanmarsnille T, Vandamme E, et al. Neobladder vs. ileal conduit in robot-assisted radical cystectomy (RARC): Results from a high-volume academic center. Eur Urol Suppl. 2016;15:227–8. doi: 10.1016/S1569-9056(16)15185-1. [DOI] [Google Scholar]
- 22.Abe T, Takada N, Shinohara N, et al. Comparison of 90-day complications between ileal conduit and neobladder reconstruction after radical cystectomy: A retrospective multi-institutional study in Japan. Int J Urol. 2014;21:554–9. doi: 10.1111/iju.12357. [DOI] [PubMed] [Google Scholar]
- 23.Cho A, Lee SM, Noh JW, et al. Acid-base disorders after orthotopic bladder replacement: Comparison of an ileal neobladder and an ileal conduit. Renal Failure. 2017;39:379–84. doi: 10.1080/0886022X.2017.1287733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erber B, Schrader M, Miller K, et al. Morbidity and quality of life in bladder cancer patients following cystectomy and urinary diversion: A single-institution comparison of ileal conduit vs. orthotopic neobladder. ISRN Urol. 2012;2012 doi: 10.5402/2012/342796. 342796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gore JL, Gilbert SM, Lai J, et al. Downstream consequences of urinary diversion. J Urol. 2010;183:e21–2. doi: 10.1016/j.juro.2010.02.097. [DOI] [Google Scholar]
- 26.Kim SH, Yu A, Jung JH, et al. Incidence and risk factors of 30-day early and 90-day late morbidity and mortality of radical cystectomy during a 13-year follow-up: A comparative propensity-score matched analysis of complications between neobladder and ileal conduit. Jap J Clin Oncol. 2014;44:677–85. doi: 10.1093/jjco/hyu051. [DOI] [PubMed] [Google Scholar]
- 27.Mano R, Goldberg H, Stabholz Y, et al. Urinary tract infections after urinary diversion – different occurrence patterns in patients with ileal conduit and orthotopic neobladder. Urology. 2018;16:87–92. doi: 10.1016/j.urology.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 28.Monn MF, Kaimakliotis HZ, Cary KC, et al. Short-term morbidity and mortality of Indiana pouch, ileal conduit, and neobladder urinary diversion following radical cystectomy. Urol Oncol. 2014;32:1151–7. doi: 10.1016/j.urolonc.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Nahar B, Koru-Sengul T, Prakash NS, et al. Comparison of readmission and short-term mortality rates between different types of urinary diversion in patients undergoing radical cystectomy. J Urol. 2017;197:e726. doi: 10.1016/j.juro.2017.02.1683. [DOI] [PubMed] [Google Scholar]
- 30.Parekh DJ, Gilbert WB, Koch MO, et al. Continent urinary reconstruction versus ileal conduit: A contemporary single-institution comparison of perioperative morbidity and mortality. Urology. 2000;55:852–5. doi: 10.1016/S0090-4295(99)00619-6. [DOI] [PubMed] [Google Scholar]
- 31.Popov Z, Stavridis A, Lekovski L, et al. Urinary diversion: 30 years’ experience of a single center in Republic of Macedonia. Acta chirurgica Iugoslavica. 2007;54:49–55. doi: 10.2298/ACI0704049P. [DOI] [PubMed] [Google Scholar]
- 32.Roghmann F, Becker A, Trinh QD, et al. Updated assessment of neobladder utilization and morbidity according to urinary diversion after radical cystectomy: A contemporary US-population-based cohort. Can Urol Assoc J. 2013;7:E552–60. doi: 10.5489/cuaj.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roghmann F, Trinh QD, Braun K, et al. Standardized assessment of complications in a contemporary series of European patients undergoing radical cystectomy. Int J Urol. 2014;21:143–9. doi: 10.1111/iju.12232. [DOI] [PubMed] [Google Scholar]
- 34.Sogni F, Brausi M, Frea B, et al. Morbidity and quality of life in elderly patients receiving ileal conduit or orthotopic neobladder after radical cystectomy for invasive bladder cancer. Urology. 2008;71:919–23. doi: 10.1016/j.urology.2007.11.125. [DOI] [PubMed] [Google Scholar]
- 35.Thulin H, Steineck G, Kreicbergs U, et al. Hygiene and urinary tract infections after cystectomy in 452 Swedish survivors of bladder cancer. BJU Int. 2010;105:1107–17. doi: 10.1111/j.1464-410X.2009.08909.x. [DOI] [PubMed] [Google Scholar]
- 36.van Hemelrijck M, Thorstenson A, Smith P, et al. Risk of in-hospital complications after radical cystectomy for urinary bladder carcinoma: Population-based followup study of 7608 patients. BJU Int. 2013;112:1113–20. doi: 10.1111/bju.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 38.Group EQoLS. EORTC QLQ - BLM30. 1994 [Google Scholar]
- 39.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutta SC, Chang SC, Coffey CS, et al. Health-related quality of life assessment after radical cystectomy: Comparison of ileal conduit with continent orthotopic neobladder. J Urol. 2002;168:164–7. doi: 10.1016/S0022-5347(05)64853-7. [DOI] [PubMed] [Google Scholar]
- 41.Moeen AM, Safwat AS, Gadelmoula MM, et al. Health-related quality of life after urinary diversion. Which technique is better? J Egyp Natl Cancer Inst. 2018;30:93–7. doi: 10.1016/j.jnci.2018.08.001. [DOI] [PubMed] [Google Scholar]