Abstract
Background
Prior studies have developed a chronic kidney disease–mineral and bone disorder (CKD-MBD) composite score based on combinations of calcium (Ca), phosphorus (P) and parathyroid hormone (PTH) that have been shown to be associated with an increased risk of clinical outcomes in the USA. We examined this association in a contemporary, international cohort of hemodialysis patients.
Methods
We studied 19 313 patients surviving ≥12 months in the Dialysis Outcomes and Practice Patterns Study Phases 3–5 (2005–15) from Europe, Canada and the USA. The CKD-MBD composite score was defined as the number of markers above target levels (P, 3.5–5.5 mg/dL; Ca, 8.4–10.2 mg/dL; PTH, 150–600 pg/mL). Using Cox models, we estimated hazard ratios (HRs) for death and a composite event (death or hospitalization), contrasting MBD 2/3 (2–3 parameters above target) with MBD 0 (all in target), adjusted for a disease risk score (DRS).
Results
MBD 2/3 above target was observed in 10–14% of patients across regions and was associated with greater DRS-adjusted mortality {HR 1.41 [95% confidence interval (CI) 1.10–1.82]} and composite events [HR 1.23 (95% CI 1.10–1.38)] in the USA compared with MBD 0; the mortality association was stronger for patients ≥ 65 years of age [HR 1.82 (95% CI 1.28–2.58)] compared with patients <65 years of age [HR 1.11 (95% CI 0.80–1.55)]. HRs observed in Canada and Europe were generally consistent but weaker. Estimates for MBD 2/3 outside target (above or below) were slightly lower in all regions.
Conclusions
Simultaneous consideration of Ca, P and PTH may help in identifying patients on dialysis with a higher risk of major clinical outcomes related to CKD-MBD.
Keywords: calcium, CKD-MBD, DOPPS, hemodialysis, parathyroid hormone, phosphorus
INTRODUCTION
Secondary hyperparathyroidism (SHPT) is present in a large proportion of patients with chronic kidney disease (CKD) receiving dialysis. SHPT is manifested by increased circulating levels of parathyroid hormone (PTH) and hyperplasia of the parathyroid glands [1–3]. SHPT is a common component of chronic kidney disease–mineral and bone disorder (CKD-MBD), a condition in which patients often present with high PTH and abnormal levels of calcium (Ca) and phosphorus (P). Several epidemiologic studies have shown that disordered bone and mineral metabolism are associated with adverse clinical outcomes, including fractures, vascular calcification and death among patients receiving dialysis [2, 4–9]. These analyses focused on evaluating clinical outcomes in relation to the independent effects of PTH, Ca and P. However, by evaluating the independent effects of CKD-MBD biochemical parameters on clinical outcomes, studies overlook the known interdependency between PTH, Ca and P that result from integrated physiologic processes central to the pathogenesis of CKD-MBD. Furthermore, in an attempt to achieve control in the care of patients with CKD-MBD, physicians frequently combine multiple therapies that may have divergent effects on individual laboratory parameters.
Block et al. [10] recently described an integrated approach for estimating the CKD-MBD risk of cardiovascular hospitalization or death among patients receiving hemodialysis (HD) based on the physiologic interdependency of PTH, Ca and P suggested by previous studies. This study categorized patients into 36 mutually exclusive combinations of PTH, Ca and P (referred to as ‘phenotypes’). Several phenotypes, notably those with target high or high PTH in addition to high Ca and/or P, were associated with a significantly higher risk of adverse clinical outcomes. These results highlighted the relation between the three CKD-MBD biochemical parameters and reinforced the importance of considering them together as opposed to separately.
Realizing the complexities and lack of clinical/practical utility of 36 phenotypes, the investigators subsequently defined a more simplistic yet highly prognostic approach in identifying patients at the highest risk of adverse clinical outcomes associated with MBD. Danese et al. [11] applied decision rules that both maximized the capture of patients who were at excess risk of cardiovascular hospitalization or death and minimized the capture of patients who were not, and found that a composite score based on having at least two of the CKD-MBD-associated biochemical parameters outside target performed best.
Building on these prior studies, we leveraged the Dialysis Outcomes and Practice Patterns Study (DOPPS) to assess the association of the CKD-MBD composite score with clinical outcomes in an international population encompassing a variety of CKD-MBD management practices.
MATERIALS AND METHODS
Data source
The DOPPS is a prospective cohort study of adult in-center HD patients from >20 countries; details of the DOPPS study design have been published previously [12–14]. Briefly, a nationally representative sample of dialysis facilities is enrolled in each DOPPS country, followed by selection of a random sample of adult, prevalent HD patients at each participating center. The DOPPS study design and data collection instruments are uniform across countries and capture detailed longitudinal information from patient records, including patient demographics, disease history and comorbidities, dialysis treatment parameters, laboratory results, prescribed medications and hospitalizations. The present analyses utilize data from DOPPS Phase 3 (2005–08), Phase 4 (2009–11) and Phase 5 (2012–15) in Belgium, France (except DOPPS 5), Germany, Italy, Spain, Sweden, the UK (collectively hereafter ‘Europe’), Canada, and the USA.
Cohort identification
The study cohort included patients who survived at least 12 months after enrollment in their respective DOPPS phase. The length of this enrollment period was chosen to be consistent with prior studies [10, 11] and because the prevalence of SHPT increases with time on dialysis. Eligible patients additionally had to have at least one each of serum PTH, serum total Ca, and serum P laboratory values during a 4-month baseline period prior to their index date (defined as 12 months after DOPPS enrollment).
We excluded patients who had evidence of parathyroidectomy in the enrollment period or for whom clinical outcome data were not available. We also excluded 31 facilities with outlier values for facility mean of the MBD markers (Ca, P, and PTH) or facility event rates. Generally these corresponded to uncorrectable unit conversion errors or implausible variation due to critically low sample size within the facility. The total number of patients excluded for these reasons was 643 (3%).
Exposure and outcomes
The main exposure of interest was CKD-MBD composite score, defined as the number of MBD markers above target levels. Guided by prior studies [10, 11] and clinical practice guidelines [1, 15], we defined the following targets: PTH, 150–600 pg/mL; Ca, 8.4–10.2 mg/dL; P, 3.5–5.5 mg/dL. The composite score ranged from 0 (all markers within target) to 3 (all markers above target). Primary analyses assessed the relative rate of clinical outcomes between patients with two or three markers above range (MBD 2/3) and those with all markers within range (MBD 0). Distributions of CKD-MBD parameters and crude event rates are additionally provided for patients with only one marker above range (MBD 1). Sensitivity analyses used a CKD-MBD composite score defined using the number of MBD markers outside target levels (i.e. above or below) only. We also conducted a series of subgroup analyses to investigate effect modifiers of the CKD-MBD composite score with outcomes.
We assessed two outcomes: time to death and time to a composite event of death or first hospitalization. Follow-up for each patient began on the index date and ended at the earliest date of death (and/or hospitalization for composite outcome analyses), departure from the study facility for any reason, or 12 months (administrative censoring). For composite outcome (death or hospitalization) analyses, we excluded data from 39 facilities (1649 patients) for which hospitalization data were not reported.
Analytical modeling
We used Cox proportional hazards models to estimate associations between the CKD-MBD composite score and clinical outcomes. Analyses were conducted separately by geographic region. We used robust variance estimators to account for potential facility clustering effects and the proportional hazards assumption was not rejected in any model.
Unadjusted models included only the CKD-MBD composite score. Adjusted models used a summary disease risk score (DRS; parameterized as a restricted cubic spline) to control for confounding. In a sensitivity analysis, we also adjusted for MBD treatments, including dialysate Ca concentration (categorized using cut-points of 2.25, 2.75, and 3.25 mEq/L) and prescription of vitamin D (active or analog forms only), cinacalcet and phosphate binders. The four most common medication combinations comprised >10% of patients and were assigned to separate indicator variables. The remaining combinations were assigned to a fifth ‘other’ indicator.
We calculated the DRS based on prior established methods [10, 11, 16]. We first estimated coefficients for the DRS component variables for each outcome by region using a Cox model among the unexposed patients (i.e. MBD 0). Numerical variables were parameterized using restricted cubic splines and included age, Liu’s comorbidity index (described below), years with end-stage renal disease (ESRD; vintage), serum albumin, hemoglobin, body mass index (BMI), single-pool Kt/V and the number of patients dialyzing in the study facility. Categorical variables included male sex, black race (USA only due to the negligible number of patients in the other regions), data source (electronic health record versus other; USA only), primary cause of ESRD, smoking history (ever versus never smoked), hypertension, history of fracture, hospitalization within 120 days prior to the index date, DOPPS study phase and country (Europe only). Model coefficients were then multiplied by their respective covariate values for all study patients and summed (i.e. Xβ) to create the DRS for each patient.
The Liu comorbidity index [17] is a weighted sum of indicator variables corresponding to common comorbid conditions, developed and validated for use in an HD population. Possible scores range from 0 to 21 and include the following conditions: history of atherosclerotic heart disease and diabetes, each with weight 1; history of stroke, peripheral vascular disease, chronic obstructive pulmonary disease, gastrointestinal bleeding, dysrhythmia, other cardiac disease, liver disease and cancer, each with weight 2; and history of congestive heart failure, with weight 3.
Treatment of missing data
Missing data were minimal with all variables having <10% of values missing, except for single-pool Kt/V (19% overall; calculated only for patients dialyzing three times/week), smoking (52% overall; not available in US DOPPS facilities using electronic health record download) and hospitalization in the prior 120 days (18% overall; subject to facility-level availability of hospitalization data). To minimize the impact of missing data, we created 10 imputed data sets using IVEware [18]. Analyses were repeated for each imputed data set and results were combined using Rubin’s method as implemented in SAS PROC MIANALYZE (SAS Institute, Cary, NC, USA) [19].
RESULTS
The final analysis cohort (including 7078 patients with one or more CKD-MBD marker below target, which were only used for sensitivity analyses of MBD 2/3 outside target) consisted of 19 313 patients. Of the 12 235 patients in the primary analysis, 7577 were from the USA, 580 were from Canada, and 4078 were from Europe (Table 1). As anticipated, the distributions of ESRD primary cause, vitamin D use, and vitamin D route varied widely by region. The European sample had the highest mean age and years with ESRD, proportion of males, and median dialysate Ca concentrations at baseline. The Canadian sample had the lowest levels of serum albumin and cinacalcet use and the highest proportion of patients with a history of smoking. The US sample had not only the highest BMI and proportion hospitalized in the 120 days prior to the index date, but also the lowest mean age and years with ESRD. Patient characteristics stratified by CKD-MBD composite score are available in Supplementary data, Table S1.
Table 1.
Patient characteristics, by region: above target analysis sample
| Patient characteristic | Overall | USA | Canada | Europe |
|---|---|---|---|---|
| No. of patients | 12 235 | 7577 | 580 | 4078 |
|
| ||||
| Age (years), mean (SE) | 63.6 (14.8) | 62.7 (14.8) | 64.1 (14.8) | 65.3 (14.6) |
| Years with ESRD, median (IQR) | 3.1 (4.4) | 3.0 (4.2) | 3.2 (4.2) | 3.5 (4.9) |
| Male, % | 57 | 55 | 55 | 60 |
| Black race (USA only), % | – | 36 | – | – |
| Primary ESRD cause, % | – | – | – | – |
| Diabetes | 36 | 43 | 36 | 22 |
| Glomerulonephritis | 14 | 11 | 21 | 20 |
| Hypertension | 25 | 30 | 18 | 18 |
| Other | 24 | 15 | 26 | 40 |
| BMI (kg2/m2), mean (SE) | 27.9 (6.6) | 28.9 (7.0) | 28.2 (6.8) | 26.2 (5.3) |
| Albumin (g/dL), mean (SE) | 3.8 (0.4) | 3.9 (0.3) | 3.6 (0.4) | 3.8 (0.4) |
| Hemoglobin (g/dL), mean (SE) | 11.3 (1.1) | 11.1 (1.0) | 11.2 (1.1) | 11.6 (1.1) |
| spKt/V, mean (SE) | 1.6 (0.3) | 1.6 (0.3) | 1.5 (0.3) | 1.6 (0.3) |
| Liu comorbidity indexa, median (IQR) | 4.0 (5.0) | 4.0 (6.0) | 4.0 (5.0) | 3.0 (5.0) |
| Hypertension, %a | 88 | 91 | 92 | 86 |
| History of smoking, %a | 49 | 44 | 54 | 49 |
| History of fracture, %a | 7 | 6 | 8 | 7 |
| Hospitalized in prior 120 days, % | 32 | 34 | 21 | 28 |
| Patient count in facility, median (IQR) | 72 (57) | 70 (64) | 101 (110) | 73 (42) |
| Patient in LDO facility (USA only), % | 77 | |||
| Serum phosphorus (mg/dL), mean (SE) | 5.3 (1.2) | 5.3 (1.2) | 5.3 (1.2) | 5.2 (1.2) |
| >5.5 mg/dL, % | 36 | 37 | 35 | 34 |
| Serum total calcium (mg/dL), mean (SE) | 9.2 (0.5) | 9.2 (0.5) | 9.2 (0.5) | 9.2 (0.5) |
| >10.2 mg/dL, % | 3 | 3 | 3 | 4 |
| Mean PTH (pg/mL), mean (SE) | 456 (402) | 467 (411) | 516 (415) | 427 (379) |
| Median PTH (pg/mL), median (IQR) | 342 (277) | 350 (275) | 385 (364) | 322 (269) |
| >600 pg/mL, % | 19 | 19 | 27 | 17 |
| IV vitamin D use, % | 59 | 81 | 8 | 26 |
| Oral vitamin D use, % | 21 | 9 | 58 | 38 |
| Cinacalcet use, % | 25 | 28 | 9 | 23 |
| Phosphate binder use, % | 86 | 86 | 92 | 85 |
| Dialysate calcium (mEq/L), median (IQR) | 2.5 (0.0) | 2.5 (0.0) | 2.5 (0.0) | 3.0 (0.5) |
Comorbidities in USA reported for non-LDO facilities. Liu et al. [17] comorbidity index possible range is 0–21.
LDO, large dialysis organization (>1000 facilities). Europe includes Belgium, France, Germany, Italy, Spain, Sweden and the UK.
Overall, a CKD-MBD composite score above target was distributed as 55, 34 and 12% for MBD 0, MBD 1, and MBD 2/3, respectively (Table 2). MBD 2/3 prevalence was slightly higher in Canada (14%) compared with Europe (10%) and the USA (12%). Among MBD 2/3 patients, the most common pairs of markers above target were PTH and P (88% overall, 83–90% by region), followed by P and Ca (8% overall, 3–12% by region). Among MBD 1 patients, P was most commonly above target in all three regions (72%), followed by PTH (24%). A CKD-MBD composite score defined as outside target ranged from 28 to 39% for MBD 0 and 19 to 24% for MBD 2/3 by region. Differences in mean biochemical marker levels between MBD 2/3 and MBD 0 tended to be larger in the USA compared with Europe (Table 3).
Table 2.
Distribution of CKD-MBD composite scores, overall and by region
| Above target |
Outside targeta |
|||||||
|---|---|---|---|---|---|---|---|---|
| CKD-MBD composite score | Overall | USA | Canada | Europe | Overall | USA | Canada | Europe |
| No. of patients | 12 235 | 7577 | 580 | 4078 | 19 313 | 10 699 | 1046 | 7568 |
|
| ||||||||
| MBD 0 | 55 | 54 | 50 | 56 | 35 | 39 | 28 | 30 |
| MBD 1 | 34 | 33 | 35 | 35 | 41 | 40 | 43 | 44 |
| PTH | 24 | 23 | 37 | 23 | 39 | 33 | 49 | 45 |
| Ca | 4 | 3 | 2 | 6 | 14 | 15 | 16 | 13 |
| P | 72 | 74 | 61 | 71 | 47 | 52 | 35 | 41 |
| MBD 2 | 11 | 12 | 14 | 10 | 21 | 19 | 24 | 23 |
| PTH, P | 88 | 90 | 89 | 83 | 61 | 60 | 56 | 62 |
| PTH, Ca | 4 | 3 | 9 | 5 | 14 | 11 | 21 | 15 |
| P, Ca | 8 | 7 | 3 | 12 | 25 | 28 | 24 | 22 |
| MBD 3 | 1 | 1 | 1 | 1 | 4 | 3 | 5 | 4 |
Values indicate % of patients (for MBD rows) or % of patients in group (MBD 1 and MBD 2 subdistributions). Subdistributions for MBD 1 and MBD 2 indicate which CKD-MBD markers are above or outside target. Europe includes Belgium, France, Germany, Italy, Spain, Sweden and the UK. CKD-MBD marker targets used: PTH, 150–600 pg/mL; Ca, 8.4–10.2 mg/dL; P, 3.5–5.5 mg/dL.
Sensitivity analysis.
Table 3.
Mean values of MBD biochemical parameters, by region and CKD-MBD composite score
| Above target |
Outside targeta |
|||||
|---|---|---|---|---|---|---|
| Parameter | Region | MBD 0 | MBD 1 | MBD 2/3 | MBD 1 | MBD 2/3 |
| Calcium (mg/dL) | USA | 9.14 | 9.20 | 9.36 | 9.04 | 8.77 |
| Canada | 9.12 | 9.14 | 9.32 | 8.96 | 8.67 | |
| Europe | 9.13 | 9.21 | 9.52 | 9.05 | 8.85 | |
| Phosphorus (mg/dL) | USA | 4.61 | 5.97 | 6.78 | 5.27 | 6.15 |
| Canada | 4.56 | 5.78 | 6.58 | 5.00 | 5.84 | |
| Europe | 4.53 | 5.96 | 6.62 | 4.96 | 5.41 | |
| PTH (pg/mL) | USA | 315 | 487 | 1077 | 374 | 660 |
| Canada | 324 | 569 | 1060 | 358 | 525 | |
| Europe | 303 | 476 | 935 | 297 | 388 | |
Sensitivity analysis.
Europe includes Belgium, France, Germany, Italy, Spain, Sweden and the UK. CKD-MBD marker targets used: PTH, 150–600 pg/mL; Ca, 8.4–10.2 mg/dL; P, 3.5–5.5 mg/dL.
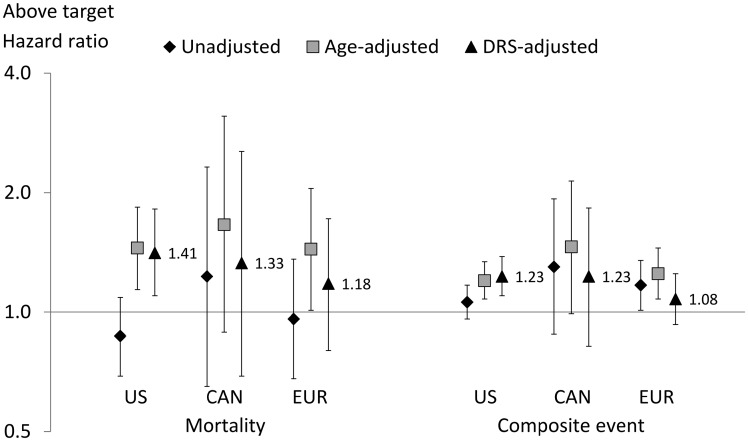
We observed 1205 deaths (unadjusted rates 0.13–0.16 by region) and 5734 composite events (unadjusted rates for first event 0.86–0.97 by region). For mortality, the mean follow-up duration for the US sample (0.72 years) was slightly lower than for the Canada (0.81) and Europe (0.81) samples. For MBD 2/3 compared with MBD 0, unadjusted hazard ratios (HRs) were 0.87–1.23 by region for mortality and 1.06–1.30 by region for the composite event (Figure 1). HRs by region were substantially higher in models adjusted for age alone, but further adjustment for the DRS (which included age) attenuated the increase in Canada and Europe. In the USA, DRS-adjusted HRs were 1.41 for mortality [95% confidence interval (CI) 1.10–1.82] and 1.23 for the composite event (95% CI 1.10–1.38). Smaller adjusted HRs were observed in Canada [HR 1.33 (95% CI 0.69–2.54) and HR 1.23 (95% CI 0.82–1.83)] and Europe [HR 1.18 (95% CI 0.80–1.72) and HR 1.08 (95% CI 0.93–1.25)] for mortality and the composite event, respectively. Additional adjustment for MBD treatment yielded no substantial differences.
FIGURE 1.
HRs for MBD 2/3 above target versus MBD 0, by region. CAN, Canada; DRS, disease risk score; EUR, Europe. Europe includes Belgium, France, Germany, Italy, Spain, Sweden and the UK. Composite outcome defined as death or hospitalization. DRS adjustment variables include age, Liu’s comorbidity index, years with ESRD (vintage), serum albumin, hemoglobin, BMI, single-pool Kt/V, number of patients dialyzing in the study facility, male sex, black race (USA only), data source (electronic health record versus other; USA only), primary cause of ESRD, smoking history (ever versus never smoked), hypertension, history of fracture, hospitalization within 120 days prior to the index date, DOPPS study phase and country (Europe only).
In the USA, the mortality HR for MBD 2/3 compared with MBD 0 was greater for patients ≥ 65 years of age [HR = 1.82 (95% CI 1.28–2.58)] than for patients <65 years of age [HR 1.11 (95% CI 0.80–1.55)] (Supplementary data, Table S2), but no difference was detected for the composite event (P = 0.1) (Supplementary data, Table S3). No differences were observed in the USA between black and non-black patients (P = 0.5 for mortality, P = 0.7 for composite event) and no differences within region were detected for history of fracture (P = 0.3–0.6 for mortality, P = 0.1–0.8 for composite event).
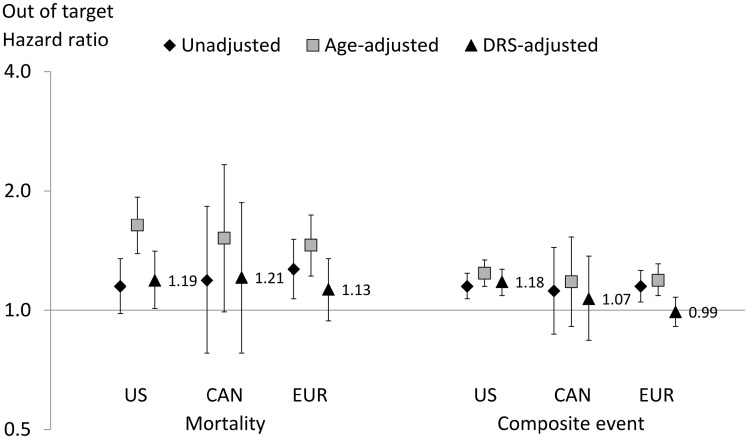
In a sensitivity analysis defining MBD 2/3 based on markers outside target and again comparing to MBD 0, we observed lower HRs for mortality in the USA [HR 1.19 (95% CI 1.01–1.41)], Canada [HR 1.21 (95% CI 0.78–1.87)] and Europe [HR 1.13 (95% CI 0.94–1.35)] (Figure 2). Slightly lower HR estimates were also observed for the composite event in the USA [HR 1.18 (95% CI 1.09–1.27)], Canada [HR 1.07 (95% CI 0.84–1.37)] and Europe [HR 0.99 (95% CI 0.91–1.08)].
FIGURE 2.
HRs for MBD 2/3 outside target versus MBD 0, by region. CAN, Canada; DRS, disease risk score; EUR, Europe. Europe includes Belgium, France, Germany, Italy, Spain, Sweden and the UK. Composite outcome defined as death or hospitalization. DRS adjustment variables include age, Liu’s comorbidity index, years with ESRD (vintage), serum albumin, hemoglobin, body mass index, single-pool Kt/V, number of patients dialyzing in the study facility, male sex, black race (USA only), data source (electronic health record versus other; USA only), primary cause of ESRD, smoking history (ever versus never smoked), hypertension, history of fracture, hospitalization within 120 days prior to the index date, DOPPS study phase and country (Europe only) .
DISCUSSION
In this study we evaluated the association of CKD-MBD composite score with clinical outcomes in the international DOPPS sample. In the USA, patients with two or more CKD-MBD biochemical parameters above target (MBD 2/3) were found to have increased rates of mortality and the composite endpoint of mortality and all-cause hospitalization compared with patients with all three biochemistries in target (MBD 0). Slightly weaker associations were observed for patients in Canada and Europe and for composite score defined as outside target. The direction and magnitudes of these associations are consistent with prior studies conducted using similar methodology among patients treated by a large US dialysis organization. Thus our findings extend the generality of those provider-specific findings to a broader HD population in the USA, Canada, and Europe. Additionally, the current analysis reflects the most contemporary data of biochemical parameter levels across multiple countries and highlights that in the USA there was improved control of Ca and P. Similar patterns were not as evident for PTH, which may represent an area for improvement in the management of MBD.
Approximately 10–14% of patients within each region had MBD 2/3 above target and 20–24% had MBD 2/3 outside target (data not shown). We elected to focus on levels above recommended targets because hallmarks of SHPT are elevated levels of PTH, Ca and P, and previous studies suggest that the impact of the CKD-MBD composite score on clinical outcomes is driven largely by levels above target compared with below target. Patients with levels for these biochemical parameters below the guidelines would generally include those experiencing pharmacodynamic effects of the various medicines used to treat SHPT, a potentially different subpopulation. It is reassuring, however, that the effects observed for ‘outside target’ analyses did not differ materially from the ‘above target’ analyses, suggesting that below target levels for these parameters did not confer additional risk from a CKD-MBD standpoint.
Differences in CKD-MBD management or other clinical practices that were not accounted for in our DRS model adjustment may contribute to understanding the differences in our results by region. For example, SHPT in the USA was typically managed using intravenous (IV) paricalcitol or IV doxercalciferol during the study period, whereas SHPT in Canada and Europe was typically managed using oral calcitriol or oral alfacalcidol [20]. Thus the effects of region and vitamin D route and preparation cannot easily be separated in our analysis. Interestingly, the US-DOPPS Practice Monitor data point to a resurgent use of oral calcitriol in HD facilities operated by large dialysis organizations in place of or in addition to the more commonly used IV therapies [20]. Such a shift in practice has the potential to impact CKD-MBD biochemistry levels in US patients, and resultant effects on clinical outcomes must be monitored carefully. This recent trend in the USA may also present an improved opportunity to study international CKD-MBD practices in the future by reducing the influence of confounding due to differences in vitamin D route of administration.
We also examined several subgroups of patients for which the CKD-MBD composite score may indicate a greater risk of clinical outcomes (Supplementary data, Table S2). While PTH levels are typically lower in elderly patients [8], we found higher mortality rates among patients ≥ 65 years of age compared with younger patients across all regions. This result is consistent with subgroup analyses reported by the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events trial investigators [21]. Elderly HD patients may have undiagnosed osteoporosis and may therefore benefit from more individualized CKD-MBD care (including less aggressive PTH management) than younger patients [22]. Although black patients typically have higher PTH levels than non-black patients [20, 23], our results are also consistent with several studies [9, 24] showing that race is not a significant moderator of PTH and mortality outcomes.
The DOPPS provides a unique opportunity to expand prior work to assess the association of the CKD-MBD composite score with clinical outcomes in an international setting. The common data collection protocol based on the medical record provides a high degree of uniformity across international regions, and the high proportion of electronic health record–based extracts in the USA ensure the accuracy of data collection. However, specific data elements were incomplete or unavailable in some facilities, requiring imputation. In addition, the categorization of CKD-MBD parameters may obscure important differences in CKD-MBD severity, potentially limiting the generalizability of our results. For example, the underlying differences in PTH and P levels between MBD 2/3 versus MBD 0 patients in the USA are larger than those in Europe (Table 3) and may have contributed to the stronger outcome signal in the USA.
CONCLUSIONS
In summary, this analysis of international DOPPS data supports prior work highlighting the association of the CKD-MBD composite score and clinical outcomes in US HD patients. Consistent but weaker associations were observed in Europe and Canada. Our findings suggest that simultaneous consideration of MBD parameters in the management of CKD-MBD may help with identifying patients with a higher risk of adverse outcomes and who may benefit from more directed therapeutic intervention.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Shauna Leighton, a medical editor at Arbor Research Collaborative for Health, for providing editorial assistance.
FUNDING
This manuscript was directly supported by Amgen. Global support for the ongoing DOPPS programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx.
CONFLICT OF INTEREST STATEMENT
P.J.D., K.C., and B.D.B. are employees of Amgen. The other authors have nothing to disclose.
REFERENCES
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 Suppl 3): S1–S201 [PubMed] [Google Scholar]
- 2. Kalantar-Zadeh K, Kuwae N, Regidor DL et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006; 70: 771–780 [DOI] [PubMed] [Google Scholar]
- 3. St Peter WL, Li Q, Liu J et al. Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol 2009; 4: 354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Danese MD, Kim J, Doan QV et al. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 2006; 47: 149–156 [DOI] [PubMed] [Google Scholar]
- 5. Jadoul M, Albert JM, Akiba T et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int 2006; 70: 1358–1366 [DOI] [PubMed] [Google Scholar]
- 6. Goodman WG, Goldin J, Kuizon BD et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000; 342: 1478–1483 [DOI] [PubMed] [Google Scholar]
- 7. Raggi P, Boulay A, Chasan-Taber S et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 2002; 39: 695–701 [DOI] [PubMed] [Google Scholar]
- 8. Floege J, Kim J, Ireland E et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 2011; 26: 1948–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tentori F, Wang M, Bieber BA et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 2015; 10: 98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Block GA, Kilpatrick RD, Lowe KA et al. CKD–mineral and bone disorder and risk of death and cardiovascular hospitalization in patients on hemodialysis. Clin J Am Soc Nephrol 2013; 8: 2132–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danese MD, Halperin M, Lowe KA et al. Refining the definition of clinically important mineral and bone disorder in hemodialysis patients. Nephrol Dial Transplant 2015; 30: 1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young EW, Goodkin DA, Mapes DL et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int 2000; 57(Suppl 74): S74–S81 [DOI] [PubMed] [Google Scholar]
- 13. Pisoni RL, Gillespie BW, Dickinson DM et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 2004; 44: 7–15 [DOI] [PubMed] [Google Scholar]
- 14. Robinson B, Fuller D, Zinsser D et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor: rationale and methods for an initiative to monitor US bundled dialysis payment system. Am J Kidney Dis 2011; 57: 822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int 2009; 76(Suppl 113): S1–S130 [DOI] [PubMed] [Google Scholar]
- 16. Glynn RJ, Gagne JJ, Schneeweiss S. Role of disease risk scores in comparative effectiveness research with emerging therapies. Pharmacoepidemiol Drug Saf 2012; 21: 138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Huang Z, Gilbertson DT et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 2010; 77: 141–151 [DOI] [PubMed] [Google Scholar]
- 18. Raghunathan TE, Lepkowski JM, Van Hoewyk J. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodol 2001; 27: 85–95 [Google Scholar]
- 19. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, 1987 [Google Scholar]
- 20.Arbor Research Collaborative for Health. DOPPS Practice Monitor http://www.dopps.org/DPM (27 July 2018, date last accessed)
- 21. Parfrey PS, Drüeke TB, Block GA et al. The effects of cinacalcet in older and younger patients on hemodialysis: the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Clin J Am Soc Nephrol 2015; 10: 791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagner J, Jhaveri KD, Rosen L et al. Increased bone fractures among elderly United States hemodialysis patients. Nephrol Dial Transplant 2014; 29: 146–151 [DOI] [PubMed] [Google Scholar]
- 23. Gupta A, Kallenbach LR, Zasuwa G et al. Race is a major determinant of secondary hyperparathyroidism in uremic patients. J Am Soc Nephrol 2000; 11: 330–334 [DOI] [PubMed] [Google Scholar]
- 24. Scialla JJ, Parekh RS, Eustace JA et al. Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol 2015; 42: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.