Abstract
Background
Hypocitraturia and hypercalciuria are the most prevalent risk factors in kidney stone formers (KSFs). Citrate supplementation has been introduced for metaphylaxis in KSFs. However, beyond its effects on urinary parameters and stone recurrence, only a few studies have investigated the impact of citrate on other metabolic pathways such as glucose or lipid metabolism.
Methods
We performed an observational study using data from the Swiss Kidney Stone Cohort. Patients were subdivided into two groups based on treatment with potassium citrate or not. The outcomes were changes of urinary risk parameters, haemoglobin A1c (HbA1c), fasting glucose, cholesterol and body mass index (BMI).
Results
Hypocitraturia was present in 19.3% of 428 KSFs and potassium citrate was administered to 43 patients (10.0%) at a mean dosage of 3819 ± 1796 mg/day (corresponding to 12.5 ± 5.9 mmol/ day). Treatment with potassium citrate was associated with a significantly higher mean change in urinary citrate (P = 0.010) and urinary magnesium (P = 0.020) compared with no potassium citrate treatment. Exogenous citrate administration had no effect on cholesterol, fasting glucose, HbA1c and BMI. Multiple linear regression analysis demonstrated no significant association of 1,25-dihydroxyvitamin D3 [1,25(OH)2 D3] levels with urinary citrate excretion.
Conclusion
Potassium citrate supplementation in KSFs in Switzerland resulted in a beneficial change of the urinary risk profile by particularly increasing anti-lithogenic factors. Fasting glucose, HbA1c, cholesterol levels and BMI were unaffected by potassium citrate therapy after 3 months, suggesting that potassium citrate is safe and not associated with unfavourable metabolic side effects. Lastly, 1,25(OH)2 D3 levels were not associated with urinary citrate excretion.
Keywords: 1,25(OH)2 D3; hypocitraturia; metaphylaxis; nephrolithiasis; urolithiasis
INTRODUCTION
Urolithiasis is common in developed countries, with a prevalence of 10%, and is associated with an increased risk for subsequent loss of kidney function [1–6] and cardiovascular disease [7]. The recurrence rate after the first stone episode is up to 40% in the first 5 years and 75% after 20 years [8]. The identification of risk factors is essential for the implementation of preventive therapy in kidney stone formers (KSFs) [9–11]. Urinary supersaturation of calcium oxalate or calcium phosphate has been discussed as a driving force for stone formation that consequently results in crystallization and lithogenesis [8, 12]. Hypercalciuria and hypocitraturia are the most important risk factors for stone formation, followed by hyperoxaluria and hyperuricosuria [11, 13–16].
Citrate is a strong inhibitor of crystallization [17, 18] and citrate supplementation has been shown to significantly increase urinary citrate excretion and decrease stone recurrence rates in hypocitraturic and normocitraturic KSFs [19, 20]. Citrate is administered orally, absorbed by the small intestine and filtered into urine, where it complexes and reduces the concentration of free calcium ions, thereby preventing calcium supersaturation. In addition, citrate supplementation increases urinary pH, which in turn further promotes urinary citrate excretion and prevents uric acid, calcium oxalate and cystine stone formation [13, 14, 18, 21]. The liver metabolizes a proportion of the orally administered citrate to acetyl coenzyme A (acetyl-CoA), which is a precursor of fatty acid and cholesterol synthesis. Endogenous citrate exerts a positive regulatory effect on lipogenesis and a negative regulatory effect on glycolysis [22–24]. Despite the widespread use of potassium citrate, no data are available regarding additional effects of oral citrate supplementation on glucose or lipid metabolism in KSFs.
Interestingly, there is evidence that vitamin D significantly reduces mitochondrial citrate metabolism (oxidation) in renal cells, resulting in increased urinary citrate excretion in rats [25]. Furthermore, 1,25-dihydroxyvitamin D3 [1,25(OH)2 D3] may modulate intracellular citrate metabolism and transport, which consequently leads to modified citrate excretion [13, 26]. In addition, vitamin D receptor gene polymorphisms may enhance the effects of 1,25(OH)2 D3 on citrate metabolism [27]. To date, it is not known whether there is an association between 1,25(OH)2 D3 levels and urinary citrate excretion in KSFs.
The aim of this study was to evaluate the impact of oral potassium citrate therapy on the urinary stone risk profile of KSFs in Switzerland and its effect on glucose and lipid metabolism. Additionally, we investigated if 1,25(OH)2 D3 levels correlate with urinary citrate excretion.
MATERIALS AND METHODS
Study design and study population
We performed an observational study using prospectively collected data from the Swiss Kidney Stone Cohort (SKSC) [28]. Our study has been approved by the Cantonal Ethics Committee Zurich and the local ethics committees of all participating centres.
Inclusion criteria included ≥18 years of age, any recurrent kidney stone former or first KSF with one or more of the following risk factors (first presentation at <25 years of age, positive family history, non–calcium oxalate kidney stones, gastrointestinal disease, osteoporosis, nephrocalcinosis, pregnancy, single kidney, gout, metabolic syndrome, diabetes mellitus type 1/2, bilateral or more than one kidney stones, chronic urinary tract infections, chronic kidney disease, kidney transplantation or having remaining kidney stones ≥3 months after therapy).
Study visits were performed at baseline, after 3 months and once annually up to 3 years according to the SKSC protocol. Data on physical activity, medical history, medication and demographic information were collected. All data were extracted from the study database (SLims 5.4.77) at baseline and after 3 months. Patients were subdivided into two groups based on treatment with potassium citrate (C; including potassium citrate or potassium citrate/hydrogen carbonate) or not (NC). Only citrate-naïve patients were included in this study.
Laboratory analyses were performed at Bioanalytica laboratories. Twenty-four-hour urine collections were collected with thymol and paraffin oil. The urinary pH value was measured by a pH electrode. Blood samples were obtained in the morning after an overnight fast. Stone composition was determined by X-ray diffractometry or infrared spectroscopy. If patients had several stones analysed, all analyses were included in the study.
Definitions
Hypocitraturia was defined as 24-h urinary citrate excretion <1.50, hypercalciuria as 24-h urinary calcium excretion >6.25 in females and >7.50 in males, hyperoxaluria as 24-h urinary oxalate excretion >0.5 and hyperuricosuria as 24-h urinary uric acid excretion >4.5 in females and >4.8 in males (all values in mmoles). Pure stones were defined as >95% of a single component.
Statistical analyses
Data are presented as mean ± standard deviation (SD) unless otherwise specified. Normal distribution was tested with the Shapiro–Wilk test and normality was assumed for sample sizes >30. Comparisons among the groups were performed using the chi-squared test and Fisher’s exact test for categorical variables and Student’s t-test for continuous variables. For comparisons, paired or unpaired parametric (Student’s t-test) and non-parametric tests (signed-rank tests) were used. The statistical significance threshold was set at P < 0.05 and all tests were two-sided. Statistical analyses were performed using R (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Characteristics of the study population
Patient characteristics are shown in Tables 1 and 5. A total of 428 participants were analysed, including 304 (71.0%) male subjects. The mean patient age was 47.2 ± 14.1 years. The mean body mass index (BMI) was 26.6 ± 4.7 kg/m2 and diabetes mellitus type 2 and type 1 were present in 43 (10.0%) and 3 (0.7%) patients, respectively. Of the patients, 41.8% were recurrent stone formers and stone composition was available in 37.2% of 934 reported calculi. A total of 177 (51.0%) stones were of mixed type, with 109 (61.6%) consisting of >50% calcium oxalate; 43.2% were pure calcium oxalate stones followed by pure apatite (2.6%), uric acid (2.0%) and other origins (1.2%). In the entire cohort, hypercalciuria was the most prevalent metabolic risk factor [113 patients (28.3%)], followed by hypocitraturia [78 patients (19.3%)], hyperuricosuria [40 patients (10.0%)] and hyperoxaluria [9 patients (2.2%); Figure 1].
Table 1.
Baseline characteristics of stone formers with potassium citrate supplementation (Group C) compared with patients without supplementation (Group NC)
| Variables | Reference values | Total | NC | C | P-value |
|---|---|---|---|---|---|
| Patients, n (%) | – | 428 | 385 (90.0) | 43 (10.0) | – |
| Age (years) | – | 47.2 (14.1) | 47.2 (14.1) | 46.5 (14.5) | 0.731 |
| Gender (male), n % | – | 304 (71.0) | 276 (71.7) | 28 (65.1) | 0.469 |
| BMI (kg/m2) | – | 26.6 (4.7) | 26.5 (4.7) | 27.6 (4.7) | 0.187 |
| Received citrate therapy after baseline, n (%) | – | 43 (10.0) | – | – | – |
| Medical history, n (%) | |||||
| Hypertension | – | 83 (19.4) | 69 (17.9) | 14 (32.6) | 0.092 |
| Not available | – | 41 (9.6) | 41 (10.6) | 0 (0.0) | |
| Diabetes mellitus | – | 0.429 | |||
| Type 1 | – | 3 (0.7) | 3 (0.8) | 0 (0.0) | |
| Type 2 | – | 43 (10.0) | 36 (9.4) | 7 (16.3) | |
| Not available | – | 38 (8.9) | 38 (9.9) | 0 (0.0) | |
| IBD | – | 20 (4.7) | 18 (4.7) | 2 (4.7) | 0.999 |
| Not available | – | 44 (10.3) | 42 (10.9) | 2 (4.7) | |
| Blood parameters | |||||
| Creatinine (µmol/L) | 44–80 (f), 62–106 (m) | 76.0 (17.0) | 76.1 (17.2) | 75.3 (15.2) | 0.795 |
| eGFR (mL/min/1.73 m2) | – | 96.6 (19.4) | 96.5 (19.9) | 97.1 (15.5) | 0.854 |
| Glucose (mmol/L) | 3.9–5.6 | 5.4 (1.3) | 5.4 (1.3) | 5.5 (1.1) | 0.699 |
| HbA1c (%) | 4.4–5.7 | 5.5 (0.7) | 5.5 (0.7) | 5.5 (0.7) | 0.892 |
| Cholesterol (mmol/L) | <5.0 | 4.9 (1.2) | 5.0 (1.2) | 4.5 (0.9) | 0.016 |
| Calcium (mmol/L) | 2.09–2.54 | 2.4 (0.1) | 2.4 (0.1) | 2.3 (0.1) | 0.033 |
| Phosphate (mmol/L) | 0.87–1.45 | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.3) | 0.898 |
| 25(OH) D3 (nmol/L) | 75–140 | 53.7 (26.7) | 53.1 (26.0) | 57.8 (32.0) | 0.286 |
| 1,25(OH)2 D3 (pmol/L) | 47.7–190.3 | 103.5 (35.9) | 103.3 (35.3) | 105.2 (41.3) | 0.752 |
| PTH (ng/L) | 15.0–65.0 | 43.9 (24.8) | 44.6 (25.7) | 38.1 (14.7) | 0.105 |
| Serum bicarbonate (mmol/L) | 22.0–29.0 | 26.7 (2.7) | 26.7 (2.8) | 26.7 (2.5) | 0.943 |
| Potassium (mmol/L) | 3.3–4.5 | 4.3 (1.8) | 4.3 (1.9) | 4.1 (0.5) | 0.453 |
| Chloride (mmol/L) | 98–107 | 101.9 (2.8) | 101.9 (2.8) | 102.3 (2.6) | 0.299 |
| Sodium (mmol/L) | 136–145 | 141.2 (2.4) | 141.2 (2.4) | 141.2 (2.1) | 0.957 |
| Anion gap (mmol/L) | – | 12.6 (2.6) | 12.7 (2.6) | 12.3 (2.6) | 0.359 |
| 24-h urine collection parameters | |||||
| Volume (mL) | – | 1778.8 (797.9) | 1767.5 (790.3) | 1878.1 (865.4) | 0.395 |
| pH | – | 6.0 (1.2) | 6.0 (1.3) | 5.9 (0.7) | 0.339 |
| Creatinine (mmol/24 h) | 7.0–14.0 (f), 9.0–21.0 (m) | 12.9 (5.6) | 13.1 (5.8) | 11.6 (3.5) | 0.177 |
| Sodium (mmol/24 h) | 38.0–217.0 (f), 47.0–326.0 (m) | 162.3 (79.7) | 161.3 (75.3) | 170.5 (111.6) | 0.479 |
| Potassium (mmol/24 h) | 31.0–106.0 (f), 37.0–131.0 (m) | 58.7 (23.7) | 58.8 (24.4) | 58.0 (17.6) | 0.839 |
| Calcium (mmol/24 h) | <6.3 (f) , <7.5 (m) | 5.6 (3.2) | 5.8 (3.3) | 4.7 (2.5) | 0.039 |
| Phosphate (mmol/24 h) | 12.9–42.0 | 26.1 (10.4) | 26.3 (10.6) | 23.8 (8.4) | 0.144 |
| Magnesium (mmol/24 h) | 3.0–5.0 | 3.9 (1.8) | 4.0 (1.8) | 3.4 (1.7) | 0.071 |
| Citrate (mmol/24 h) | 1.0–6.5 | 2.7 (1.5) | 2.8 (1.5) | 2.4 (1.3) | 0.108 |
| Oxalate (mmol/24 h) | <0.5 | 0.207 (0.166) | 0.200 (0.122) | 0.263 (0.374) | 0.021 |
| Uric acid (mmol/24 h) | 1.2–5.9 | 3.1 (1.3) | 3.1 (1.3) | 2.9 (1.0) | 0.266 |
| Ammonium (mmol/24 h) | 10.0–107.0 | 19.2 (10.5) | 19.5 (10.7) | 16.5 (8.2) | 0.093 |
| Age at first kidney stone (years) | 36.6 (14.2) | 36.6 (14.1) | 36.6 (15.0) | 0.989 | |
| Stone composition, n (%) | |||||
| Calcium oxalate | – | 150 (43.2) | 132 (44.5) | 18 (36.0) | 0.337 |
| Apatite | – | 9 (2.6) | 9 (3.0) | 0 (0.0) | 0.368 |
| Uric acid | – | 7 (2.0) | 6 (2.0) | 1 (2.0) | 0.999 |
| Other | – | 4 (1.2) | 3 (1.0) | 1 (2.0) | 0.465 |
| Mixed | – | 177 (51.0) | 147 (49.5) | 30 (60.0) | 0.222 |
| Unknown | – | 595 | 526 | 69 | 0.250 |
Values are presented as mean (SD) unless stated otherwise. f, female; m, male.
Table 5.
Baseline characteristics of normocitraturic versus hypocitraturic KSFs
| Parameters | Normocitraturic | Hypocitraturic | P- value |
|---|---|---|---|
| Patients, n (%) | 326 (80.7) | 78 (19.3) | – |
| Age (years) | 47.3 (13.7) | 46.5 (15.8) | 0.658 |
| Gender (male), n (%) | 232 (71.2) | 53 (67.9) | 0.673 |
| BMI (kg/m2) | 26.9 (4.7) | 25.5 (5.1) | 0.030 |
| Received citrate therapy after baseline, n (%) | 28 (8.6) | 14 (17.9) | 0.026 |
| Medical history, n (%) | |||
| Hypertension | 62 (19.0) | 20 (25.6) | 0.296 |
| Not available | 29 (8.9) | 5 (6.4) | |
| Diabetes mellitus | – | – | 0.489 |
| Type 1 | 3 (0.9) | 0 (0.0) | |
| Type 2 | 31 (9.5) | 10 (12.8) | |
| Not available | 26 (8.0) | 6 (7.7) | |
| IBD | 10 (3.1) | 7 (9.0) | 0.056 |
| Not available | 32 (9.8) | 4 (5.1) | |
| Blood parameters | |||
| Creatinine (µmol/L) | 76.3 (16.8) | 76.0 (18.5) | 0.895 |
| eGFR (mL/min/1.73 m2) | 96.5 (19.8) | 96.2 (19.2) | 0.896 |
| Glucose (mmol/L) | 5.5 (1.3) | 5.3 (1.1) | 0.208 |
| HbA1c (%) | 5.5 (0.7) | 5.4 (0.7) | 0.934 |
| Cholesterol (mmol/L) | 4.9 (1.1) | 4.8 (1.2) | 0.476 |
| Calcium (mmol/L) | 2.4 (0.1) | 2.3 (0.1) | 0.008 |
| Phosphate (mmol/L) | 1.0 (0.2) | 1.0 (0.2) | 0.342 |
| 25(OH) D3 (nmol/L) | 53.9 (26.8) | 53.8 (26.9) | 0.985 |
| 1,25(OH)2 D3 (pmol/L) | 102.4 (35.2) | 106.2 (37.7) | 0.409 |
| PTH (ng/L) | 44.8 (26.7) | 40.3 (17.3) | 0.169 |
| Serum bicarbonate (mmol/L) | 26.9 (2.6) | 25.8 (3.2) | 0.003 |
| Potassium (mmol/L) | 4.4 (2.0) | 4.1 (0.3) | 0.395 |
| Chloride (mmol/L)} | 101.8 (2.7) | 102.5 (2.9) | 0.070 |
| Sodium (mmol/L) | 141.2 (2.4) | 141.0 (2.2) | 0.509 |
| Anion gap (mmol/L) | 12.5 (2.6) | 13.0 (2.9) | 0.233 |
| 24-h urine collection parameters | |||
| Volume (mL) | 1823.9 (762.9) | 1575.8 (912.8) | 0.014 |
| pH | 6.0 (0.6) | 5.9 (0.6) | 0.825 |
| Creatinine (mmol/24 h) | 13.4 (4.8) | 10.3 (4.7) | <0.001 |
| Sodium (mmol/24 h) | 171.9 (81.1) | 122.7 (61.3) | <0.001 |
| Potassium (mmol/24 h) | 62.4 (23.2) | 42.4 (18.2) | <0.001 |
| Calcium (mmol/24 h) | 6.1 (3.2) | 3.9 (2.8) | <0.001 |
| Phosphate (mmol/24 h) | 27.6 (10.1) | 19.7 (9.1) | <0.001 |
| Magnesium (mmol/24 h) | 2.6 (1.4) | 2.5 (1.5) | 0.617 |
| Citrate (mmol/24 h) | 3.2 (1.3) | 0.9 (0.4) | <0.001 |
| Oxalate (mmol/24 h) | 0.209 (0.122) | 0.201 (0.285) | 0.727 |
| Uric acid (mmol/24 h) | 3.2 (1.3) | 2.3 (0.9) | <0.001 |
| Ammonium (mmol/24 h) | 19.4 (8.6) | 18.6 (16.4) | 0.542 |
| Age at first kidney stone (years) | 36.8 (13.8) | 36.4 (15.9) | 0.859 |
| Stone composition, n (%) | |||
| Calcium oxalate | 113 (44.6) | 27 (34.6) | 0.173 |
| Apatite | 4 (1.6) | 5 (6.4) | 0.054 |
| Uric acid | 9 (3.5) | 0 (0.0) | 0.124 |
| Other | 5 (1.9) | 1 (1.3) | 0.999 |
| Mixed | 125 (48.4) | 45 (57.7) | 0.193 |
| Unknown | 462 | 111 | 0.196 |
Values are presented as mean (SD) unless stated otherwise.
FIGURE 1.
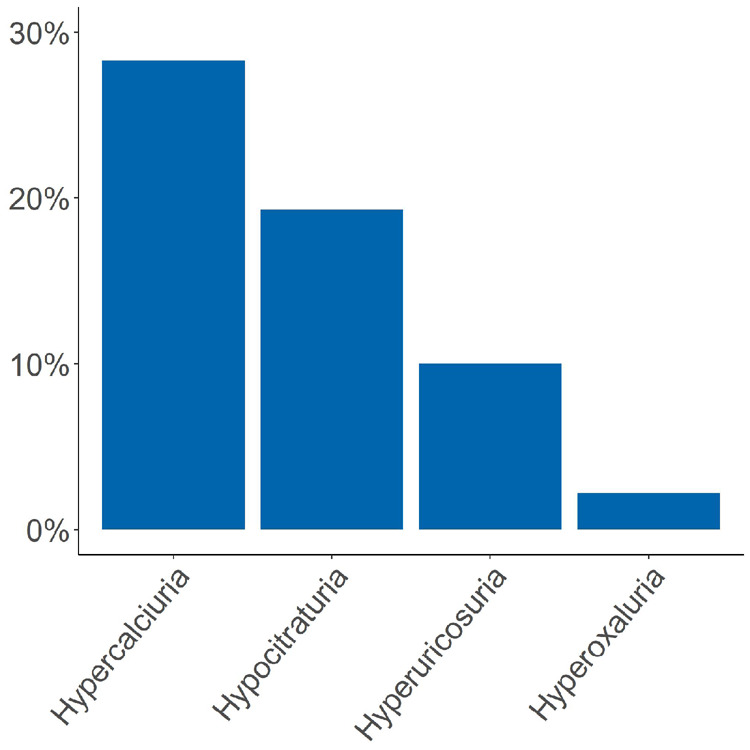
Frequency of urinary metabolic risk factors at baseline. Hypercalciuria was most frequent followed by hypocitraturia, hyperuricosuria and hyperoxaluria.
Ten per cent of stone formers were treated with potassium citrate
Potassium citrate was administered to 43 patients (10.0%) at a mean dosage of 3819 ± 1796 mg/day (corresponding to 12.5 ± 5.9 mmol/day; Table 1). Interestingly, only 14 (32.6%) of the 43 patients were hypocitraturic. Of the 43 citrate-treated patients, 28 (65.1%) were male, the median BMI was 27.6 ± 4.7 kg/m2 and diabetes mellitus type 2 was present in 7 (16.3%) patients, whereas diabetes mellitus type 1 was not present in this group. Only 30.2% were recurrent stone formers, and stone composition of potassium citrate-treated patients consisted of mixed type (60.0%), pure calcium oxalate (36.0%), uric acid (2.0%) and others (2.0%). In non-citrate-treated patients calculi consisted of mixed type (49.5%), pure calcium oxalate (44.5%), pure apatite (3.0%) and uric acid (2.0%). In this group, 1.0% of calculi were of other compositions and 41.7% of the patients were recurrent stone formers.
At baseline, serum cholesterol and calcium levels were markedly lower in citrate-treated patients. Also, urinary calcium excretion (5.8 ± 3.3 versus 4.7 ± 2.5 mmol/24 h) was lower in the potassium citrate group, whereas urinary oxalate excretion (0.2 ± 0.1 versus 0.3 ± 0.4 mmol/24 h) was higher (Table 1). There were no further differences regarding all other parameters at baseline between both patient groups.
Impact of potassium citrate supplementation on 24-h urine risk profile parameters
Blood and 24-h urinary parameters of all patients with or without potassium citrate supplementation are presented in Tables 2 and 3. Interestingly, parathyroid hormone (PTH) declined significantly in all patients (C: 38.1 ± 14.7 to 37.1 ± 14.8 ng/L, NC: 44.6 ± 25.7 to 40.4 ± 17.9 ng/L), whereas calcidiol only increased in patients not treated with potassium citrate (53.1 ± 26.0 to 58.8 ± 30.0 nmol/L). No relevant changes were found for all other blood parameters after potassium citrate therapy.
Table 2.
Blood and 24-h urine parameters of patients with potassium citrate supplementation
| Baseline |
3 months |
||||
|---|---|---|---|---|---|
| Parameters | Value | n | Value | n | P-value |
| BMI (kg/m2) | 27.6 (4.7) | 40 | 27.8 (4.2) | 8 | 0.522 |
| Blood parameters | |||||
| Creatinine (µmol/L) | 75.3 (15.2) | 43 | 77.5 (18.0) | 43 | 0.154 |
| eGFR (mL/min/1.73 m2) | 97.1 (15.5) | 43 | 97.0 (16.5) | 43 | 0.932 |
| Glucose (mmol/L) | 5.5 (1.1) | 39 | 5.4 (1.1) | 39 | 0.321 |
| HbA1c (%) | 5.5 (0.7) | 43 | 5.5 (1.0) | 42 | 0.661 |
| Cholesterol (mmol/L) | 4.5 (0.9) | 43 | 4.5 (1.0) | 43 | 0.906 |
| Calcium (mmol/L) | 2.3 (0.1) | 42 | 2.3 (0.2) | 41 | 0.312 |
| Phosphate (mmol/L) | 1.0 (0.3) | 43 | 1.1 (0.3) | 43 | 0.311 |
| 25(OH) D3 (nmol/L) | 57.8 (32.0) | 43 | 58.3 (28.0) | 43 | 0.887 |
| 1,25(OH)2 D3 (pmol/L) | 105.2 (41.3) | 42 | 101.2 (32.1) | 42 | 0.516 |
| PTH (ng/L) | 38.1 (14.7) | 43 | 37.1 (14.8) | 43 | <0.001 |
| Serum bicarbonate (mmol/L) | 26.7 (2.5) | 41 | 27.4 (2.4) | 39 | 0.148 |
| Potassium (mmol/L) | 4.1 (0.5) | 43 | 4.2 (0.3) | 43 | 0.104 |
| Chloride (mmol/L) | 102.3 (2.6) | 43 | 102.7 (2.5) | 43 | 0.536 |
| Sodium | 141.2 (2.1) | 43 | 141.0 (2.1) | 43 | 0.565 |
| 24-h urine collection parameters | |||||
| Volume (mL) | 1878.1 (865.4) | 42 | 2076.4 (878.7) | 42 | 0.056 |
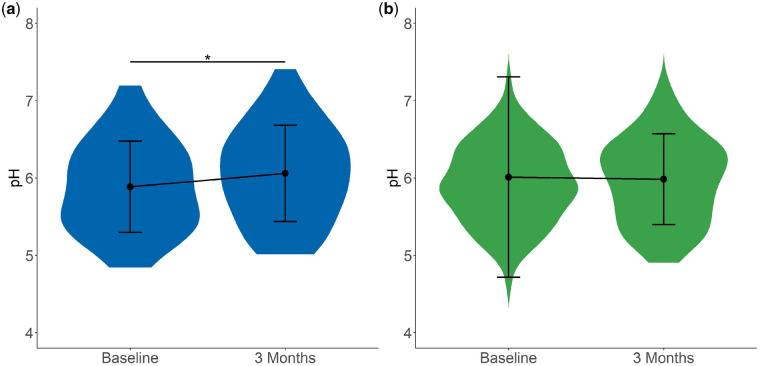
| pH | 5.9 (0.7) | 26 | 6.1 (0.6) | 40 | 0.022 |
| Creatinine (mmol/24 h) | 11.6 (3.5) | 42 | 12.74 (5.2) | 42 | 0.126 |
| Sodium (mmol/24 h) | 170.5 (111.6) | 42 | 169.0 (88.2) | 42 | 0.919 |
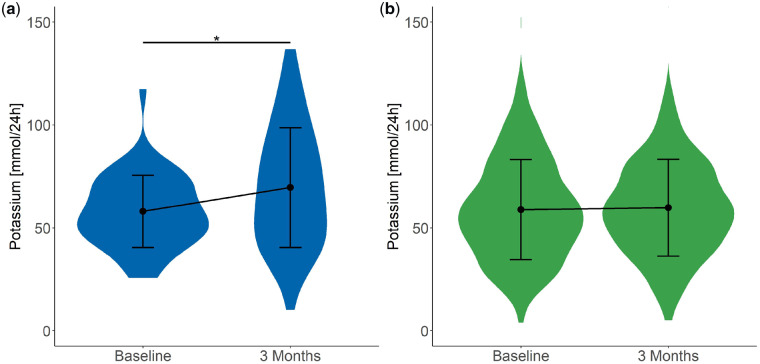
| Potassium (mmol/24 h) | 58.0 (17.6) | 42 | 69.6 (29.1) | 42 | 0.015 |
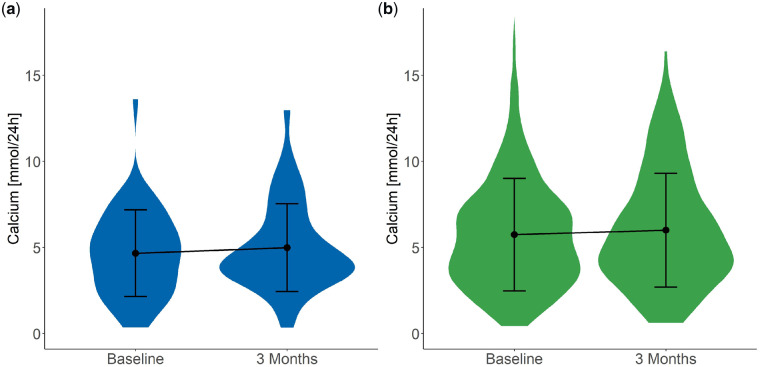
| Calcium (mmol/24 h) | 4.7 (2.5) | 42 | 5.0 (2.6) | 42 | 0.307 |
| Phosphate (mmol/24 h) | 23.8 (8.4) | 40 | 24.8 (10.6) | 40 | 0.587 |
| Magnesium (mmol/24 h) | 3.4 (1.7) | 42 | 4.3 (3.0) | 42 | 0.016 |
| Citrate (mmol/24 h) | 2.4 (1.3) | 42 | 3.3 (2.1) | 42 | <0.001 |
| Oxalate (mmol/24 h) | 0.263 (0.374) | 41 | 0.272 (0.253) | 42 | 0.688 |
| Uric acid (mmol/24 h) | 2.9 (1.0) | 42 | 3.0 (1.3) | 42 | 0.326 |
| Ammonium (mmol/24 h) | 16.5 (8.2) | 49 | 16.2 (9.2) | 37 | 0.575 |
Values are presented as mean (SD) unless stated otherwise.
Table 3.
Blood and 24-h urine parameters of patients without potassium citrate supplementation
| Baseline |
3 months |
||||
|---|---|---|---|---|---|
| Parameters | Value | n | Value | n | P-value |
| BMI (kg/m2) | 26.5 (4.7) | 336 | 26.8 (4.2) | 59 | 0.261 |
| Blood parameters | |||||
| Creatinine (µmol/L) | 76.1 (17.2) | 351 | 76.8 (18.1) | 200 | 0.837 |
| eGFR (mL/min/1.73 m2) | 96.5 (19.9) | 349 | 95.8 (21.4) | 198 | 0.932 |
| Glucose (mmol/L) | 5.4 (1.3) | 308 | 5.4 (1.3) | 178 | 0.890 |
| HbA1c (%) | 5.5 (0.7) | 338 | 5.6 (2.3) | 196 | 0.327 |
| Cholesterol (mmol/L) | 5.0 (1.2) | 348 | 5.0 (1.1) | 200 | 0.999 |
| Calcium (mmol/L) | 2.4 (0.1) | 345 | 2.4 (0.1) | 194 | 0.908 |
| Phosphate (mmol/L) | 1.0 (0.2) | 344 | 1.0 (0.2) | 193 | 0.740 |
| 25(OH) D3 (nmol/L) | 53.1 (26.0) | 342 | 58.8 (30.0) | 197 | 0.025 |
| 1,25(OH)2 D3 (pmol/L) | 103.3 (35.3) | 341 | 106.7 (41.5) | 191 | 0.696 |
| PTH (ng/L) | 44.6 (25.7) | 337 | 40.4 (17.9) | 194 | <0.001 |
| Serum bicarbonate (mmol/L) | 26.7 (2.8) | 281 | 27.0 (2.6) | 170 | 0.450 |
| Potassium (mmol/L) | 4.3 (1.9) | 350 | 4.2 (0.3) | 199 | 0.927 |
| Chloride (mmol/L) | 101.9 (2.8) | 350 | 100.2 (10.3) | 198 | 0.028 |
| Sodium | 141.2 (2.4) | 350 | 140.8 (3.0) | 199 | 0.299 |
| 24-h urine collection parameters | |||||
| Volume (mL) | 1767.5 (790.3) | 369 | 2131.5 (724.7) | 171 | <0.001 |
| pH | 6.0 (1.3) | 249 | 6.0 (0.6) | 135 | 0.707 |
| Creatinine (mmol/24 h) | 13.1 (5.8) | 365 | 14.1 (18.5) | 169 | 0.522 |
| Sodium (mmol/24 h) | 161.3 (75.3) | 368 | 156.2 (70.3) | 169 | 0.361 |
| Potassium (mmol/24 h) | 58.8 (24.4) | 368 | 59.7 (23.5) | 169 | 0.244 |
| Calcium (mmol/24 h) | 5.8 (3.3) | 368 | 6.0 (3.3) | 168 | 0.360 |
| Phosphate (mmol/24 h) | 26.3 (10.6) | 332 | 26.5 (11.8) | 145 | 0.903 |
| Magnesium (mmol/24 h) | 4.0 (1.8) | 368 | 4.0 (1.7) | 167 | 0.891 |
| Citrate (mmol/24 h) | 2.8 (1.5) | 362 | 2.9 (1.4) | 164 | 0.044 |
| Oxalate (mmol/24 h) | 0.200 (0.122) | 364 | 0.229 (0.108) | 166 | <0.001 |
| Uric acid (mmol/24 h) | 3.1 (1.3) | 368 | 3.0 (1.2) | 169 | 0.619 |
| Ammonium (mmol/24 h) | 19.5 (10.7) | 356 | 21.0 (12.2) | 149 | 0.384 |
Values are presented as mean (SD) unless stated otherwise.
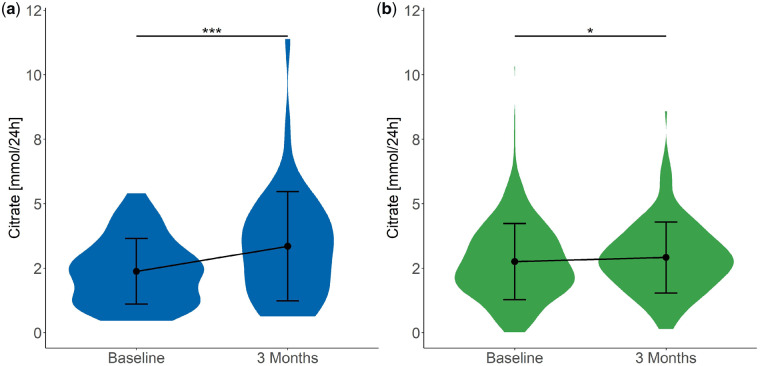
As expected, both urinary citrate (Figure 2) and potassium (Figure 3) excretion increased significantly (2.4 ± 1.3 to 3.3 ± 2.1 mmol/24 h and 58.0 ± 17.6 to 69.6 ± 29.1 mmol/24 h, respectively) after potassium citrate supplementation. In addition, a significant increase in urinary pH was detected (from 5.9 ± 0.7 to 6.1 ± 0.6; Figure 4), whereas urinary calcium excretion (Figure 5) did not change after potassium citrate therapy. In patients who were not receiving potassium citrate, both urinary citrate excretion (from 2.8 ± 1.5 to 2.9 ± 1.4 mmol/24 h) and 24-h urine volume increased significantly (Figure 6).
FIGURE 2.
Plot of 24-h urinary citrate excretion at baseline and 3 months. Plot shows the mean ± SD. (a) In Group C, 24-h citrate excretion increased significantly between baseline and 3 months. ***P < 0.001. (b) In Group NC, 24-h citrate excretion increased significantly between baseline and 3 months. *P < 0.05.
FIGURE 3.
Plot of 24-h urinary potassium excretion at baseline and 3 months. Plot shows the mean ± SD. (a) In Group C, 24-h potassium excretion increased significantly between baseline and 3 months. *P < 0.05. (b) In Group NC, 24-h potassium did not show changes.
FIGURE 4.
Plot of 24-h urine pH at baseline and 3 months. Plot shows the mean ± SD. (a) In Group C, 24-h urine pH increased significantly between baseline and 3 months. *P < 0.05. (b) In Group NC, 24-h urine pH did not show changes.
FIGURE 5.
Plot of 24-h urinary calcium excretion at baseline and 3 months. Plot shows the mean ± SD. (a) In Group C, 24-h urinary calcium excretion did not show changes. (b) In Group NC, 24-h urinary calcium excretion did not show changes.
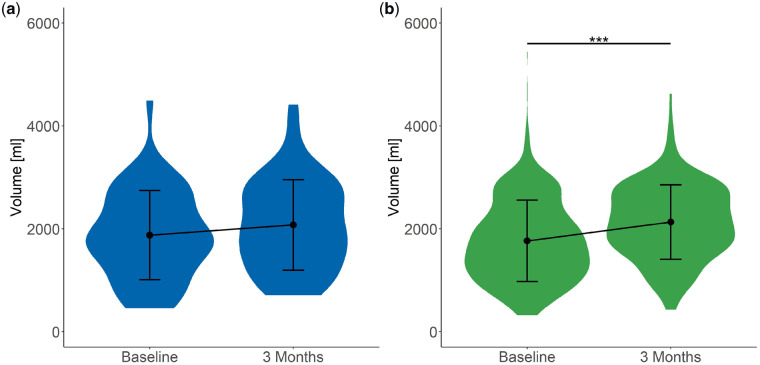
FIGURE 6.
Plot of 24-h urine volume at baseline and 3 months. Plot shows the mean ± SD. (a) In Group C, 24-h urine volume did not show changes. (b) In Group NC, 24-h urine volume increased significantly between baseline and 3 months. ***P < 0.001.
We additionally performed between-group analyses to compare the changes in blood and urinary parameters between both groups. Notably, the mean change of citrate excretion was significantly higher in patients who were treated with potassium citrate (1.0 ± 1.7 mmol/24 h) compared with the NC group (0.2 ± 1.3 mmol/24 h; Table 4). The comparison of urinary pH between both groups demonstrated a trend towards a higher change in the citrate group when compared with the NC group. Taken together, citrate supplementation resulted in a beneficial change of urinary parameters in hypo- and normocitraturic KSFs.
Table 4.
Mean change of blood and urine parameters of patients with potassium citrate supplementation (Group C) compared with patients without supplementation (Group NC)
| Parameters | Mean change C | Mean change NC | P-value |
|---|---|---|---|
| BMI (kg/m2) | 0.6 (2.4) | 0.2 (1.4) | 0.695 |
| Creatinine (µmol/L) | 2.2 (9.8) | 0.1 (6.9) | 0.194 |
| eGFR (mL/min/1.73 m2) | −0.1 (7.1) | −0.1 (9.8) | 0.980 |
| Glucose (mmol/L) | −0.1 (0.6) | 0.0 (1.3) | 0.323 |
| HbA1c (%) | 0.0 (0.5) | 0.2 (2.4) | 0.282 |
| Cholesterol (mmol/L) | 0.0 (0.6) | 0.0 (0.6) | 0.914 |
| Calcium (mmol/L) | 0.0 (0.2) | 0.0 (0.1) | 0.341 |
| Phosphate (mmol/L) | 0.1 (6.4) | −2.0 (7.5) | 0.105 |
| 25(OH) D3 (nmol/L) | 0.6 (26.1) | 4.2 (25.5) | 0.417 |
| 1,25(OH)2 D3 (pmol/L) | −4.2 (41.0) | 1.3 (44.6) | 0.449 |
| PTH (ng/L) | −1.0 (10.5) | −4.2 (22.8) | 0.167 |
| Serum bicarbonate (mmol/L) | 0.7 (2.8) | 0.2 (2.6) | 0.308 |
| Potassium (mmol/L) | 0.1 (0.5) | 0.0 (0.3) | 0.109 |
| Chloride (mmol/L) | 0.3 (3.2) | −1.7 (10.5) | 0.029 |
| Sodium | −0.2 (2.6) | −0.2 (3.3) | 0.983 |
| Volume (mL) | 227.6 (740.9) | 290.0 (716.0) | 0.628 |
| pH | 0.2 (0.48) | 0.0 (0.63) | 0.066 |
| Creatinine (mmol/24 h) | −0.4 (3.4) | −1.2 (8.3) | 0.361 |
| Sodium (mmol/24 h) | 1.9 (122.0) | −6.5 (91.4) | 0.680 |
| Potassium (mmol/24 h) | 12.6 (31.7) | 2.5 (27.5) | 0.066 |
| Calcium (mmol/24 h) | 0.3 (2.1) | −0.3 (3.5) | 0.170 |
| Phosphate (mmol/24 h) | 1.0 (10.9) | −0.1 (12.9) | 0.598 |
| Magnesium (mmol/24 h) | 0.9 (2.3) | 0.0 (1.8) | 0.020 |
| Citrate (mmol/24 h) | 1.0 (1.7) | 0.2 (1.3) | 0.010 |
| Oxalate (mmol/24 h) | 0.013 (0.198) | 0.041 (0.129) | 0.385 |
| Uric acid (mmol/24 h) | 0.2 (1.3) | −0.1 (1.5) | 0.271 |
| Ammonium (mmol/24 h) | 0.0 (6.1) | 1.0 (13.3) | 0.539 |
Values are presented as mean (SD) unless stated otherwise.
Hypocitraturia is associated with lower serum bicarbonate levels in KSFs
Patients were divided into two groups according to urinary citrate excretion (Table 5): Group 1, normocitraturic and Group 2, hypocitraturic. A total of 78 patients (19.3%) presented with hypocitraturia at baseline (Figure 1), of which 14 (17.9%) were treated with potassium citrate. As expected, significantly more hypocitraturic patients received potassium citrate therapy after baseline (17.9% versus 8.6%). Inflammatory bowel disease (IBD) showed a trend of greater prevalence in hypocitraturic patients (P = 0.056), suggesting increased urinary citrate reabsorption in response to enteric bicarbonate losses. Furthermore, serum bicarbonate levels were significantly lower in hypocitraturic patients (25.8 ± 3.2 versus 26.9 ± 2.6 mmol/L), indicating renal tubular acidosis or enteric bicarbonate loss as the potential underlying cause. Serum calcium was significantly higher in normocitraturic patients (2.4 ± 0.1 mmol/L) compared with hypocitraturic patients (2.3 ± 0.1 mmol/L), with no differences found for 25-hydroxyvitamin D3, 1,25(OH)2 D3 and PTH.
At baseline, 24-h urine volume was markedly higher in normocitraturic patients compared with hypocitraturic patients (1823.9 ± 762.9 versus 1575.8 ± 912.8 mL/24 h). Interestingly, urinary calcium (6.1 ± 3.2 versus 3.9 ± 2.8 mmol/24 h), potassium (62.4 ± 23.2 versus 42.4 ± 18.2 mmol/24 h), phosphate (27.6 ± 10.1 versus 19.7 ± 9.1 mmol/24 h), uric acid (3.2 ± 1.3 versus 2.3 ± 0.9 mmol/24 h) and sodium (171.9 ± 81.1 versus 122.7 ± 61.3 mmol/24 h) were also significantly higher in normocitraturic patients at baseline (Table 5).
Citrate supplementation had no impact on fasting glucose, HbA1c, cholesterol and BMI
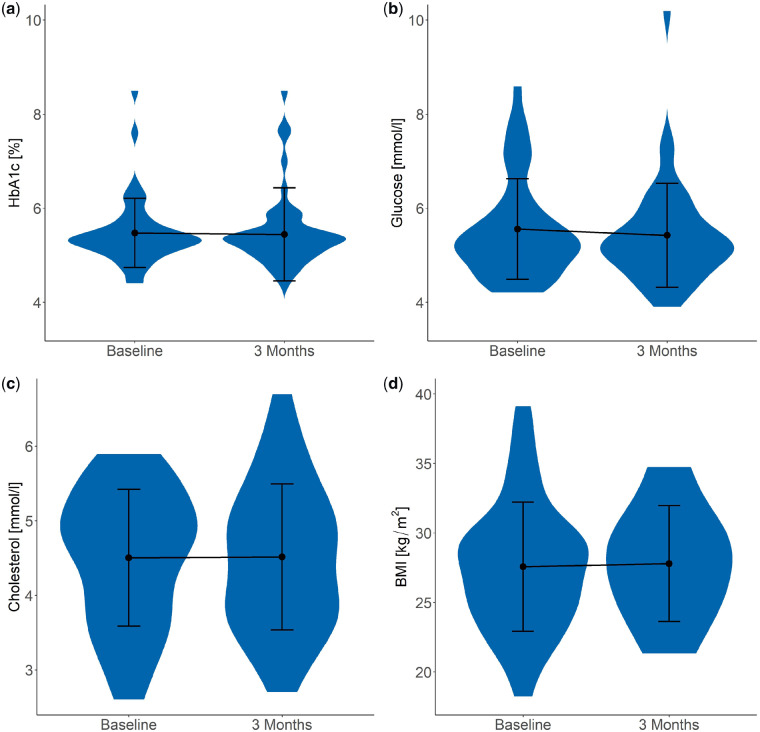
Endogenous citrate has been reported to have a positive regulatory effect on lipogenesis and a negative regulatory effect on glycolysis, but it is unknown if there is also an effect of oral citrate supplementation on glucose and lipid parameters in KSFs. In our analysis, no significant changes in fasting glucose (5.5 ± 1.1 to 5.4 ± 1.1 mmol/L), HbA1c (5.5 ± 0.7 to 5.5 ± 1.0%), cholesterol (4.5 ± 0.9 to 4.5 ± 1.0 mmol/L) and BMI (27.6 ± 4.7 to 27.8 ± 4.2 kg/m2) were found in potassium citrate–treated stone formers after 3 months (Figure 7 and Table 2). Similarly, fasting glucose, HbA1c, cholesterol and BMI were unchanged after 3 months in stone formers who were not receiving potassium citrate (Table 3).
FIGURE 7.
(a) HbA1c, (b) fasting glucose, (c) cholesterol and (d) BMI at baseline and 3 months of patients receiving potassium citrate therapy (Group C). Plot shows the mean ± SD. No significant changes were observed.
Serum 1,25(OH)2 D3 levels were not associated with urinary citrate excretion
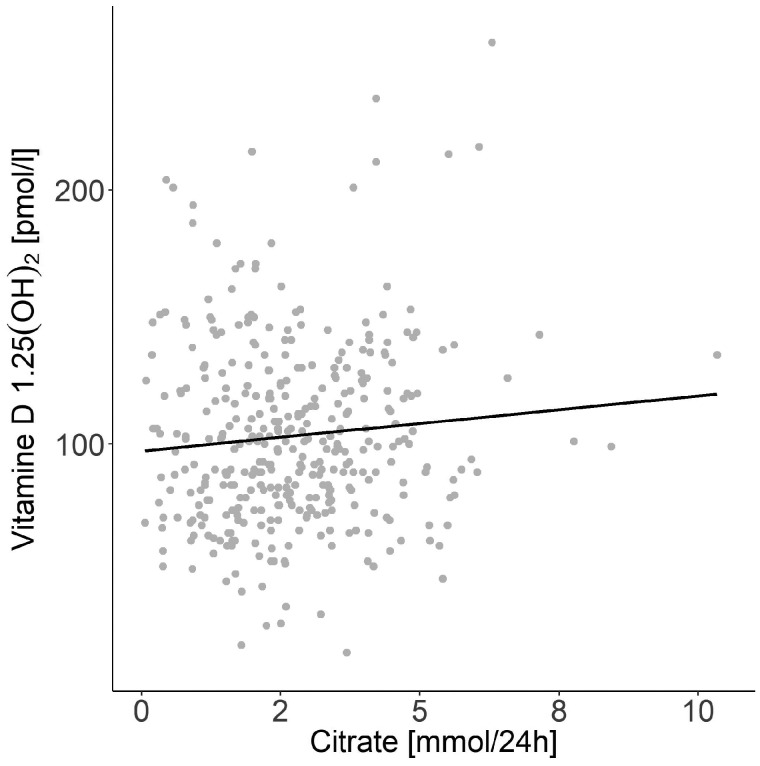
The active form of vitamin D, 1,25(OH)2 D3, has been reported to increase urinary citrate excretion [25]. We performed multiple linear regression analyses to predict urinary citrate excretion based on 1,25(OH)2 D3 (Model 1, Figure 8) and 1,25(OH)2 D3 and estimated glomerular filtration rate (eGFR; Model 2). No significant regression was found for either model.
FIGURE 8.
Scatterplot of 24-h urinary citrate excretion at baseline against 1,25(OH)2 D3 at baseline in all patients. The black line depicts the linear regression.
DISCUSSION
We performed an analysis including 428 participants of the SKSC to address the impact of citrate supplementation on urinary parameters, glucose and lipid metabolism and association of 1,25(OH)2 D3 and urinary citrate excretion. To the best of our knowledge, this is the first study investigating potential metabolic side effects of potassium citrate metaphylaxis in this population.
At baseline, 19.3% of the cohort patients presented with hypocitraturia. In addition to hypercalciuria, hypocitraturia has been reported as the most common risk factor in KSFs [15–17, 29]. Accordingly, hypercalciuria and hypocitraturia were highly prevalent in our cohort. Potassium citrate was administered to 43 patients (10.0%), with only 14 patients (32.6%) presenting hypocitraturic. However, potassium citrate has been recommended not only for patients with hypocitraturia, but also with hypercalciuria, complete or incomplete distal renal tubular acidosis, chronic diarrheal states, uric acid or cystine stones and osteopenia/osteoporosis [18, 30].
The recommended dose of citrate supplementation according to the European Association of Urology is 14–36 mmol/day (4500–11 600 mg/day) [31]. In our cohort, potassium citrate was prescribed at a mean dosage of 3819 ± 1796 mg/day, corresponding to 12.5 ± 5.9 mmol potassium citrate/day. There are several possible explanations for the rather low and variable prescribed dosages in this cohort. Although evaluation of the patients was performed according to a highly standardized protocol, indication and type of therapy were the responsibility of the treating physician. Thus we cannot exclude that some physicians prescribed lower dosages, for instance, to avoid gastrointestinal side effects or because patients declined to increase the dose of potassium citrate.
Supplementation with potassium citrate resulted in a significant increase of both urinary citrate and potassium excretion, confirming patient adherence to therapy. These findings are very important since compliance has been reported to be limited by gastrointestinal side effects, unpleasant salty taste, costs and/or high pill burden [32, 33]. We could further confirm adherence by a parallel increase in urinary pH. All these findings have been reported by other authors following citrate therapy in KSFs [18, 34, 35]. However, most studies have described a greater percentage increase of citrate, potassium or pH when compared with our cohort. In addition, several studies have described a significant decrease in urinary calcium excretion following citrate supplementation [18, 34–36]. A retrospective study by Hermann et al. [34] demonstrated that citrate therapy led to a significant increase in urinary pH as well as urinary citrate and potassium excretion in normo- and hypocitraturic patients. Robinson et al. [35] analysed the effect of citrate in hypocitraturic patients after 6 months and detected a significant increase of urinary pH, citrate and potassium excretion. Both studies and another recent study from Song et al. [18] additionally demonstrated that citrate therapy also resulted in a significant decrease in urinary calcium. In our study, we observed no significant changes of urinary calcium after citrate therapy. Three different mechanisms have been discussed to decrease urinary calcium excretion by administration of citrate [18]. First, therapy with potassium citrate results in systemic alkalinization and thereby a reduction of bone turnover, which could consequently lead to lower glomerular filtration of calcium, resulting in lower urinary calcium levels. Second, citrate binds calcium in the gastrointestinal tract, resulting in lower intestinal calcium reabsorption into blood and thus lower urinary calcium excretion. Finally, the alkaline-sensitive TRPV5 channels in the distal convoluted tubule seem to be more active at higher urinary pH and thus enhance reabsorption of urinary calcium from the tubular fluid [37].
However, in our study, PTH levels were significantly lower after citrate supplementation. PTH, directly and indirectly, activates TRPV5 channels [38]. Thus a reduction in PTH, as has been shown in our cohort, may lead to less TRPV5 activity and therefore less renal calcium uptake, providing a potential explanation for the lack of change in urinary calcium excretion. In addition, compared with the retrospective study of Song et al. [18], urinary potassium excretion increased by 22% in our cohort versus 56% in theirs. This indicates lower doses and/or less compliance with potassium citrate therapy in our cohort, diminishing the effect of citrate on urinary calcium excretion. Lastly, hypocitraturic patients demonstrated significantly lower urinary calcium and phosphate levels at baseline that may explain no further significant decline after potassium citrate treatment in these patients. Taken together, in our study, we demonstrated that potassium citrate supplementation resulted in a beneficial change of urinary risk profile parameters by particularly increasing the anti-lithogenic factors in urine.
Endogenous citrate has been reported to be involved in several metabolic pathways, including a positive regulatory effect on lipogenesis and a negative regulatory effect on glycolysis [22–24]. More precisely, citrate inhibits the downstream enzyme phosphofructokinase 1, which converts fructose 6-phosphate and adenosine triphosphate to fructose 1,6-bisphosphate and adenosine diphosphate. By inhibiting this step, less acetyl-CoA is produced from glucose, which may lead to less fatty acid and cholesterol synthesis. In mice, a combined citrate and sucrose diet has resulted in altered glucose metabolism, indicating insulin resistance in these animals and no changes of lipid parameters [23]. Notably, despite the widespread use of potassium citrate, no data exist on the potential impact of citrate on glucose or lipid metabolism in humans and, in particular, on KSFs. Thus we used fasting glucose, HbA1c, cholesterol levels and BMI as surrogate markers for glucose and lipid metabolism from KSFs who were subjected to potassium citrate therapy. After 3 months of citrate supplementation, no changes in fasting glucose, HbA1c, cholesterol levels or BMI were found in our study.
Higher serum 1,25(OH)2 D3 levels in stone formers are associated with higher urinary calcium and phosphate excretion and consequently a greater risk of kidney stone formation [39, 40]. Thus we were interested to know whether there is an association between 1,25(OH)2 D3 levels and urinary citrate excretion in KSFs. In our analysis, no significant regression between calcitriol and urinary citrate excretion was found.
We would like to acknowledge that our study has several limitations. In this study, data collection was performed at baseline and at 3 months because only at these time points was sample size sufficient for statistical analysis. Thus we might have missed effects that occur and may be detected only after a longer treatment period. According to the nature of the study, several biases may have been present, such as bias in indication and dosage of potassium citrate therapy, as well as bias in diet and adherence to physician’s recommendations (e.g. advice on fluid or salt intake) and to potassium citrate therapy. Furthermore, a substantial number of patients who were treated with potassium citrate were normocitraturic and, importantly, the majority of the hypocitraturic patients did not receive citrate supplementation. Moreover, additional medication was not documented comprehensively in the patient data forms. However, we could demonstrate a significant increase in urinary potassium, citrate and pH, most probably excluding a lack of patient adherence to citrate intake in our cohort.
Thus further randomized controlled trials with larger sample sizes and a well-designed intervention are required to allow confirmation of our data and to fully elucidate the various effects of potassium citrate on different metabolic pathways in KSFs.
CONCLUSION
In conclusion, potassium citrate supplementation resulted in a beneficial change of the urinary risk profile by increasing anti-lithogenic factors in urine. In addition, fasting glucose, HbA1c, cholesterol levels and BMI were unaffected by potassium citrate therapy after 3 months, suggesting that potassium citrate may be safe and not associated with unfavourable metabolic side effects. Lastly, there was no anti-lithogenic effect of calcitriol on urinary risk profile of KSFs since 1,25(OH)2 D3 levels were not associated with urinary citrate excretion.
ACKNOWLEDGEMENTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the responsible cantonal ethics committee of Zürich, Switzerland (2017-00371). All participants signed the informed consent form of the Swiss Kidney Stone Cohort (2013-0330), which allows further use of data for research purposes.
The authors are thankful to all participating patients, study nurses and the study coordinator of the Swiss Kidney Stone Cohort. They thank Dr Fankhauser from the Division of Urology (University Hospital Zürich) for his valuable comments.
FUNDING
The Swiss Kidney Stone Cohort is sponsored by the National Center of Competence in Research <<NCCR-Kidney.CH>>. No additional funding was received for this specific work.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Vupputuri S, Soucie JM, McClellan W et al. History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol 2004; 14: 222–228 [DOI] [PubMed] [Google Scholar]
- 2. Shoag J, Halpern J, Goldfarb DS et al. Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J Urol 2014; 192: 1440–1445 [DOI] [PubMed] [Google Scholar]
- 3. Rule AD, Bergstralh EJ, Melton LJ 3rd et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 2009; 4: 804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El-Zoghby ZM, Lieske JC, Foley RN et al. Urolithiasis and the risk of ESRD. Clin J Am Soc Nephrol 2012; 7: 1409–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zisman AL, Evan AP, Coe FL et al. Do kidney stone formers have a kidney disease? Kidney Int 2015; 88: 1240–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keddis MT, Rule AD. Nephrolithiasis and loss of kidney function. Curr Opin Nephrol Hypertens 2013; 22: 390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferraro PM, Taylor EN, Eisner BH et al. History of kidney stones and the risk of coronary heart disease. JAMA 2013; 310: 408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moe OW. Kidney stones: pathophysiology and medical management. Lancet 2006; 367: 333–344 [DOI] [PubMed] [Google Scholar]
- 9. Scales CD Jr, Smith AC, Hanley JM et al. Prevalence of kidney stones in the United States. Eur Urol 2012; 62: 160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lewandowski S, Rodgers AL. Idiopathic calcium oxalate urolithiasis: risk factors and conservative treatment. Clin Chim Acta 2004; 345: 17–34 [DOI] [PubMed] [Google Scholar]
- 11. Eisner BH, Sheth S, Dretler SP et al. Abnormalities of 24-hour urine composition in first-time and recurrent stone-formers. Urology 2012; 80: 776–779 [DOI] [PubMed] [Google Scholar]
- 12. Worcester EM, Coe FL. Calcium kidney stones. N Engl J Med 2010; 363: 954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol 2009; 11: 134–144 [PMC free article] [PubMed] [Google Scholar]
- 14. Bos S, Nap RR, Wouters RS et al. Hypocitraturia: a common but not well-known cause of nephrolithiasis. Neth J Med 2014; 72: 545–547 [PubMed] [Google Scholar]
- 15. Seeger H, Kaelin A, Ferraro PM et al. Changes in urinary risk profile after short-term low sodium and low calcium diet in recurrent Swiss kidney stone formers. BMC Nephrol 2017; 18: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferraro PM, Robertson WG, Johri N et al. A London experience 1995–2012: demographic, dietary and biochemical characteristics of a large adult cohort of patients with renal stone disease. QJM 2015; 108: 561–568 [DOI] [PubMed] [Google Scholar]
- 17. Phillips R, Hanchanale VS, Myatt A et al. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst Rev 2015; 10: CD010057. doi: 10.1002/14651858.CD010057.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song Y, Hernandez N, Shoag J et al. Potassium citrate decreases urine calcium excretion in patients with hypocitraturic calcium oxalate nephrolithiasis. Urolithiasis 2016; 44: 145–148 [DOI] [PubMed] [Google Scholar]
- 19. Barcelo P, Wuhl O, Servitge E et al. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol 1993; 150: 1761–1764 [DOI] [PubMed] [Google Scholar]
- 20. Ettinger B, Pak CY, Citron JT et al. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol 1997; 158: 2069–2073 [DOI] [PubMed] [Google Scholar]
- 21. Spivacow FR, Negri AL, Polonsky A et al. Long-term treatment of renal lithiasis with potassium citrate. Urology 2010; 76: 1346–1349 [DOI] [PubMed] [Google Scholar]
- 22. Dashty M. A quick look at biochemistry: carbohydrate metabolism. Clin Biochem 2013; 46: 1339–1352 [DOI] [PubMed] [Google Scholar]
- 23. Leandro JG, Espindola-Netto JM, Vianna MC et al. Exogenous citrate impairs glucose tolerance and promotes visceral adipose tissue inflammation in mice. Br J Nutr 2016; 115: 967–973 [DOI] [PubMed] [Google Scholar]
- 24. Iacobazzi V, Infantino V. Citrate—new functions for an old metabolite. Biol Chem 2014; 395: 387–399 [DOI] [PubMed] [Google Scholar]
- 25. Simpson DP. Citrate excretion: a window on renal metabolism. Am J Physiol 1983; 244: F223–F234 [DOI] [PubMed] [Google Scholar]
- 26. Mossetti G, Vuotto P, Rendina D et al. Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med 2003; 253: 194–200 [DOI] [PubMed] [Google Scholar]
- 27. Tang J, Chonchol MB. Vitamin D and kidney stone disease. Curr Opin Nephrol Hypertens 2013; 22: 383–389 [DOI] [PubMed] [Google Scholar]
- 28. Roth B, Bonny O. The Swiss Kidney Stone Cohort: an observational study to unravel the cause of renal stone formation. Eur Urol Focus 2017; 3: 7–9 [DOI] [PubMed] [Google Scholar]
- 29.Osther PJS. Epidemiology of kidney stones in the European Union In: Talati J, Tiselius HG, Albala D, YE Z (eds). Urolithiasis. London: Springer, 2012 [Google Scholar]
- 30. Gambaro G, Croppi E, Coe F et al. Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: a consensus statement. J Nephrol 2016; 29: 715–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skolarikos A, Straub M, Knoll T et al. Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol 2015; 67: 750–763 [DOI] [PubMed] [Google Scholar]
- 32. Mechlin C, Kalorin C, Asplin J et al. Splenda® improves tolerance of oral potassium citrate supplementation for prevention of stone formation: results of a randomized double-blind trial. J Endourol 2011; 25: 1541–1545 [DOI] [PubMed] [Google Scholar]
- 33. Tracy CR, Pearle MS. Update on the medical management of stone disease. Curr Opin Urol 2009; 19: 200–204 [DOI] [PubMed] [Google Scholar]
- 34. Herrmann U, Schwille PO, Schwarzlaender H et al. Citrate and recurrent idiopathic calcium urolithiasis. A longitudinal pilot study on the metabolic effects of oral potassium sodium citrate administered as short-, medium- and long-term to male stone patients. Urol Res 1992; 20: 347–353 [DOI] [PubMed] [Google Scholar]
- 35. Robinson MR, Leitao VA, Haleblian GE et al. Impact of long-term potassium citrate therapy on urinary profiles and recurrent stone formation. J Urol 2009; 181: 1145–1150 [DOI] [PubMed] [Google Scholar]
- 36. Fabris A, Lupo A, Bernich P et al. Long-term treatment with potassium citrate and renal stones in medullary sponge kidney. Clin J Am Soc Nephrol 2010; 5: 1663–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bonny O, Rubin A, Huang CL et al. Mechanism of urinary calcium regulation by urinary magnesium and pH. J Am Soc Nephrol 2008; 19: 1530–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ko B. Parathyroid hormone and the regulation of renal tubular calcium transport. Curr Opin Nephrol Hypertens 2017; 26: 405–410 [DOI] [PubMed] [Google Scholar]
- 39. Shakhssalim N, Gilani KR, Parvin M et al. An assessment of parathyroid hormone, calcitonin, 1, 25 (OH)2 vitamin D3, estradiol and testosterone in men with active calcium stone disease and evaluation of its biochemical risk factors. Urol Res 2011; 39: 1–7 [DOI] [PubMed] [Google Scholar]
- 40. Taylor EN, Hoofnagle AN, Curhan GC. Calcium and phosphorus regulatory hormones and risk of incident symptomatic kidney stones. Clin J Am Soc Nephrol 2015; 10: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]