Abstract
Background
Long-term outcomes of the Eurotransplant Senior Program (ESP) are urgently needed to improve selection criteria and allocation policies in the elderly.
Methods
We analysed patient and allograft outcomes of 244 ESP-kidney transplant recipients (KTRs) between 1999 and 2019 and assessed quality of living compared with 82 ESP-waitlisted dialysis patients using standardized short form-8.
Results
We observed 1-, 5- and 10-year patient survival of 91.7, 66.3 and 38.0%, respectively. Mortality risk factors included male gender (P = 0.006) and T-cell-mediated rejection (P < 0.001). Median patient survival of male ESP-KTRs was 80 versus 131 months for female ESP-KTRs (P = 0.006). 1-, 5- and 10-year death-censored allograft survival was 93.3, 82.6 and 70.4%. Risk factors included high body mass index (P < 0.001) and T-cell-mediated rejection (P < 0.001). After re-initiation of dialysis median patient survival was 58 months. Change of estimated glomerular filtration rate showed a mean decline of 2.3 and 6.8 mL/min at 5 and 10 years. Median physical and mental component scores of ESP-KTRs were 40.2 and 48.3, significantly higher compared with dialysis patients (P < 0.05). Of ESP-KTRs, 97.5% who underwent transplantation would again do so.
Conclusions
Long-term outcomes of ESP-KTRs ultimately support the effectiveness of an age-matched allocation system. Our data suggest that the survival advantage of women is maintained after kidney transplantation and calls for gender-specific care.
Keywords: elderly, gender gap, kidney transplantation, patient survival, TCMR
INTRODUCTION
To meet the needs of an ageing society and consider the increasing scarceness of organs, the Eurotransplant Senior Program (ESP) in conjunction with the Eurotransplant Kidney Allocation System was newly established on 4 January 1999, and performed in all Eurotransplant member countries (Austria, Belgium, Croatia, Germany, Hungary, Luxembourg, the Netherlands and Slovenia) [1, 2]. Organs from donors over the age of 65 years are allocated to recipients over the age of 65 years. Through a regional allocation system and by circumventing human leukocyte antigen (HLA) matching, a short cold ischaemia time (CIT) should be achieved, all with the intention to increase the number of elderly donors used for transplantation. So far, almost 10 000 ESP kidney transplant recipients (ESP-KTRs) were transplanted within the last 20 years [3].
By generating a shorter waiting time for the elderly on the transplant waiting list, previous reports suggest favourable short-term patient and kidney allograft outcomes and increased life expectancy of the ESP-KTR population [4–8]. Here, data from the ERA-EDTA registry support the finding, which even among ESP-KTRs life expectancy increases compared with those receiving dialysis, although the relative difference in the expected remaining lifetime increases compared with the age-matched general population [9].
Quite recently, the Dialysis Outcomes and Practice Pattern Study suggested that the gender gap in mortality, the survival advantage of women over men in the general population, is markedly diminished among end-stage kidney disease patients undergoing haemodialysis [10, 11]. Again, data from the ERA-EDTA registry support this finding in a European cohort that the gender gap in mortality almost disappeared among haemodialysis and peritoneal dialysis patients [9]. Although causality for the observed sex differences could not be provided by the study, it is highly suggestive to attribute this finding to end-stage kidney disease and dialysis therapy itself. Since most studies on elderly KTRs are limited to short-term data of only 5 years post-transplantation, any potential impact of kidney transplantation on the gender gap in mortality has been neglected so far. Therefore, long-term patient and kidney allograft outcomes of the ESP are urgently needed to address the benefit for male and female ESP-KTRs and to improve selection criteria, patient education and allocation policies.
Here, we tried to address the following questions: (i) what factors impact patient survival and death-censored allograft survival among ESP-KTRs? (ii) what impact does the kidney transplantation have on the gender gap in mortality? and (3) what impact does kidney transplantation have on quality of life compared with dialysis patients waitlisted within the ESP?
MATERIALS AND METHODS
Patients
This study was performed in compliance with the declaration of Helsinki. Informed written consent was obtained from all patients. We retrospectively examined 244 adult solitary KTRs of a first kidney allograft transplanted within the ESP at our single transplant centre at the Charité Campus Virchow Clinic between 1999 and 2019. Within the ESP kidneys from donors aged ≥65 years are allocated to recipients aged ≥65 years. To account for minimization of CIT, kidneys are preferably allocated locally. ESP allocation is performed by waiting time and blood group only but not HLA matching. Registered KTRs ≤65 years optionally enter the ESP at the age of 65 years by keeping their accumulated waiting time [7].
In addition, 102 dialysis patients waitlisted within the ESP at our centre at the Charité Campus Virchow Clinic were analysed.
Immunosuppressive therapy
Primary immunosuppression was a triple drug regimen with a calcineurin inhibitor (tacrolimus or cyclosporine), mycophenolate mofetil (MMF) or mycophenolic acid and steroids tapered to a maintenance dose of 4 mg methylprednisolone after 4 weeks. All patients received induction with an IL-2R antagonist (basiliximab or daclizumab), except presensitized KTRs. Presensitized KTRs received a lymphocyte-depleting agent (muromonab 3, anti-thymocyte globulin or alemtuzumab) for induction.
Diagnosis and treatment of acute allograft rejection
If acute rejection was suspected, then a kidney biopsy was performed and the rejection classified according to the current Banff classifications. Borderline changes and T-cell-mediated rejections Grades IA and IB were treated with intravenous steroids for 3–5 days. T-cell-mediated rejections grade Banff IIA, IIB or III were treated with a lymphocyte-depleting agent with/without steroid pulses.
Data collection procedures, aftercare and follow-up
Data collection included demographic, socio-demographic and medical characteristics. All data were obtained from the patient records and clinic database.
At the time of data assessment, patient death or kidney allograft loss, 153 KTRs (62.7%) were treated quarterly in our transplant centre. Ninety-one KTRs (37.3%) were followed by local nephrologists or general practitioners only. Data on KTRs who were followed by local nephrologists only were provided by those local nephrologists. Follow-up rates were high with a 1-, 5-, 10- and 15-year follow-up rate at the time of data analysis of 99.2, 97.9, 95.6 and 92.3%, respectively. Only 16 of 244 ESP-KTRs (6.6%) were lost to follow-up at any time after transplantation. Two ESP-KTRs were lost within 1 year post-transplant, another six ESP-KTRs were lost within 5 years post-transplant, another five ESP-KTRs were lost within 10 years post-transplant and three ESP-KTRs were lost after 15 years post-transplant. After kidney allograft loss, ESP-KTRs were followed for death on dialysis. At the time of data analysis, follow-up rate was 100%.
Questionnaire-based survey on health-related quality of life
The German version of the internationally standardized short form-8 (SF-8) questionnaire was used to evaluate health-related quality of life. The SF-8 questionnaire was sent in July 2017 to all KTRs transplanted within the ESP who are alive with a functioning allograft. In addition, the SF-8 questionnaire was sent to 102 patients on dialysis waitlisted within the ESP. KTRs and dialysis patients returned the completed questionnaire via a pre-stamped envelope. Non-respondents were reminded by a second letter 4 weeks later and/or contacted by phone. Response rate of KTRs within the ESP was 77.4% (72/93 ESP-KTRs). Response rate of dialysis patients waitlisted within the ESP was 81.4% (83/102 dialysis patients).
The SF-8 scores ranging from 0 (complete dissatisfaction) to 100 (full satisfaction) for eight domains: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional and mental health. The correlated physical component score (PCS) and mental component score (MCS) were computed by the providers software.
In addition to the SF-8 questionnaire, all KTRs were assessed, if they would again decide to undergo kidney transplantation.
Statistical methods
Statistical tests were performed using SPSS Version 23 (SPSS, Chicago, IL, USA). For comparisons of study groups, two-sided Mann–Whitney U-test for non-parametric independent samples was used. Clinical characteristics were compared across groups using Fisher’s exact test categorical variables. Outcomes were measured with Kaplan–Meier models and overall strata comparisons measured by log-rank tests. Stepwise regression was performed to select variables that approached statistical significance in the univariate analysis. A P < 0.10 was used for selection. Multivariate Cox regression was performed for selected variables. Box plots show median and interquartile range. The whiskers are drawn down to the 10th and up to the 90th percentile. Two-sided P < 0.05 were considered statistical significant.
RESULTS
Clinical characteristics of ESP-KTRS
A total of 244 ESP-KTRs of a first kidney allograft were analysed. Patient characteristics are shown in Table 1. Median follow-up of ESP-KTRs was 48 months (range 0–189 months), during which 107 ESP-KTRs died (43.8%) and 44 returned to dialysis (18.0%). Remaining 93 ESP-KTRs with a functioning kidney allograft were assessed by the questionnaire-based survey on health-related quality of life.
Table 1.
Clinical characteristics of all ESP-KTRs and male ESP-KTRs versus female ESP-KTRs
| Characteristics | Total (n = 244) | Male ESP-KTRs (n = 150) | Female ESP-KTRs (n = 94) | P-value |
|---|---|---|---|---|
| Age (years)a | 67 (65–79) | 67 (65–79) | 68 (65–77) | 0.470 |
| Male sex, n (%) | 150 (61) | 148 (100) | 96 (100) | – |
| Donor age (years)a | 71 (65–89) | 71 (65–89) | 72 (65–85) | 0.925 |
| Donor male sex, n (%) | 105 (43) | 70 (47) | 35 (37) | 0.184 |
| Donor serum creatinine (mg/dL)a | 0.88 (0.37–2.10) | 0.94 (0.37–2.10) | 0.97 (0.40–2.06) | 0.536 |
| KDPIa,b | 97 (51–100) | 97 (51–100) | 97 (67–100) | 0.630 |
| Kidney donor risk index (KDRI)a,b | 1.92 (1.01–3.73) | 1.91 (1.01–3.73) | 1.95 (1.18–2.91) | 0.480 |
| Causes of ESRD, n (%) | 0.119 | |||
| Glomerulonephritis | 37 (15) | 21 (14) | 16 (17) | |
| Diabetic nephropathy | 35 (14) | 25 (17) | 10 (11) | |
| Nephroangiosclersosis | 38 (16) | 28 (19) | 10 (11) | |
| Polycystic kidney disease | 30 (12) | 19 (13) | 11 (12) | |
| Uropathy | 16 (7) | 6 (4) | 10 (11) | |
| Other or undetermined | 89 (36) | 51 (34) | 38 (40) | |
| Diabetes mellitus, n (%) | 82 (33) | 52 (35) | 30 (32) | 0.679 |
| Cold ischemia time (h:min)a | 8:28 (3:04–19:35) | 8:16 (3:30–17:56) | 8:30 (3:04–19:35) | 0.480 |
| BMI (kg/m2)a | 25.6 (16.1–40.2) | 25.8 (16.1–37.0) | 25.2 (17.2–40.1) | 0.490 |
| BMI >30 kg/m2, n (%) | 41 (17) | 24 (16) | 17 (18) | 0.726 |
| Time on dialysis (months)a | 52 (7–152) | 54 (7–152) | 52 (9–93) | 0.670 |
| 4 (4) | ||||
| <12 | 9 (4) | 5 (3) | ||
| 12–36 | 70 (29) | 49 (33) | 21 (22) | |
| 36–60 | 72 (30) | 36 (24) | 36 (38) | |
| >60 | 93 (38) | 60 (40) | 33 (35) | |
| CMV seropositivity, n (%) | 160 (65) | 92 (61) | 68 (72) | 0.097 |
| Total HLA mismatcha | 4 (0–6) | 4 (0–6) | 4 (0–6) | 0.013 |
| 2 HLA-A MM, n (%) | 84 (34) | 55 (37) | 29 (30) | 0.407 |
| 2 HLA-B MM, n (%) | 154 (63) | 101 (67) | 53 (56) | 0.102 |
| 2 HLA-DR MM, n (%) | 119 (49) | 84 (56) | 35 (37) | 0.006 |
| 4–6 HLA MM, n (%) | 180 (73) | 118 (79) | 62 (66) | 0.036 |
| Induction immunosuppression, n (%) | ||||
| IL2-R-antagonist | 237 (97) | 148 (99) | 89 (95) | 0.111 |
| T-cell depletion | 7 (3) | 2 (1) | 5 (5) | 0.111 |
| Maintenance immunosuppression, n (%) | ||||
| Tacrolimus/MMF/steroids | 162 (66) | 96 (64) | 66 (70) | 0.333 |
| Cyclosporin/MMF/steroids | 78 (31) | 52 (35) | 26 (28) | 0.263 |
| mTOR-based regime | 4 (2) | 2 (1) | 2 (2) | 0.640 |
| Outcomes | ||||
| DGF, n (%) | 110 (45) | 77 (51) | 33 (35) | 0.017 |
| Acute cellular rejection, n (%) | 82 (33) | 51 (34) | 31 (33) | 0.890 |
| Borderline | 25 (10) | 14 (9) | 11 (11) | 0.665 |
| IA/IB | 33 (14) | 22 (15) | 11 (11) | 0.568 |
| IIA/IIB/III | 24 (10) | 15 (10) | 9 (10) | 1 |
| Cancer, n (%) | 24 (10) | 16 (11) | 8 (8) | 0.663 |
| Causes of death, n (%) | 0.329 | |||
| Cardiovascular disease | 22 (9) | 17 (11) | 5 (5) | |
| Infection | 16 (7) | 8 (5) | 8 (8) | |
| Cancer | 17 (7) | 12 (8) | 5 (5) | |
| Other or undetermined | 53 (22) | 33 (22) | 20 (21) | |
| Causes of allograft loss, n (%) | 0.751 | |||
| Primary non-function | 9 (4) | 5 (3) | 4 (4) | |
| Rejection | 5 (2) | 2 (1) | 3 (3) | |
| Chronic allograft nephropathy | 8 (3) | 4 (3) | 4 (4) | |
| Infection | 5 (2) | 4 (3) | 1 (1) | |
| Other or undetermined | 18 (7) | 9 (6) | 9 (10) | |
| Death with functioning allograft, n (%) | 108 (44) | 70 (47) | 38 (40) | 0.357 |
| PCSa | 40.2 (16.8–62.5) | 39.8 (23.6–60.7) | 40.6 (16.8–62.5) | 0.995 |
| MCSa | 48.3 (21.1–62.5) | 49.1 (27.0–59.9) | 48.1 (21.1–62.5) | 0.977 |
Median (range).
KDPI and KDRI calculated from donor age, donor height, donor weight, donor ethnicity, donor history of hypertension, donor history of diabetes, cause of death, donor serum creatinine, donor hepatitis C status and donation after circulatory death. The KDRI expresses the relative risk of kidney graft failure compared with the median kidney donor in the USA. The KDPI maps the KDRI onto a cumulative percentage scale so that a KDPI expresses a higher risk of graft failure compared with those donors with a lower KDPI.
For comparison, quality of life of 102 dialysis patients waitlisted within the ESP was analysed. Median age of dialysis patients was 69 years (range 65–81 years), 68 of 102 (66.7%) were males, median time on dialysis at the time of analysis was 49 months (range 11–82 months).
Patient and kidney allograft outcomes among ESP-KTRs
Patient survival
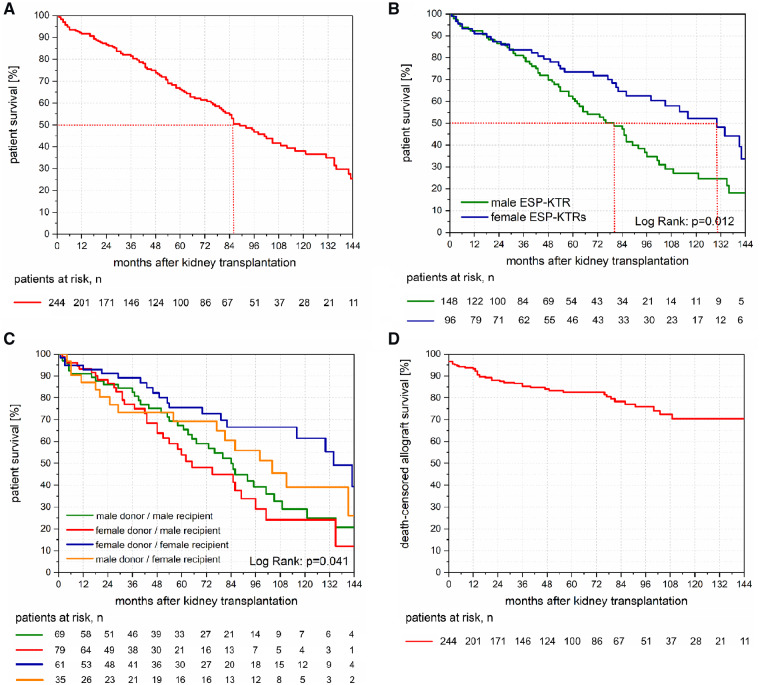
Among all ESP-KTRs, we observed 1-, 5- and 10-year patient survival of 91.7, 66.3 and 38.0%, respectively (Figure 1A). Median patient survival of ESP-KTRs was 86 months (Figure 1A), and median age of death of ESP-KTRS was 74 years (range 65–86 years). Upon multivariate analysis, male gender was identified as the only independent mortality risk factor at the time of transplantation [odds ratio (OR) = 1.777, 95% confidence interval (CI) 1.181–2.672; P = 0.006]. Median patient survival of male ESP-KTRs was 80 months versus 131 months for female ESP-KTRs (Figure 1B). Median age of death of male ESP-KTRS was 72 years (range 65–86 years) compared with female ESP-KTRs with 76 years (range 65–82 years). Donor–recipient gender mismatch showed inferior patient survival for both male and female KTRs. Differences, however, did not reach statistical significance (Figure 1C).
FIGURE 1.
(A) Kaplan–Meier plot of patient survival of ESP-KTRs. ESP-KTRs show a median patient survival after kidney transplantation of 86 months. (B) Kaplan–Meier plot of patient survival between male ESP-KTRs and female ESP-KTRs. Male ESP-KTRs showed significantly inferior long-term patient survival compared with female ESP-KTRs (P = 0.012). While no differences were observed for the first 2.5 years post-transplantation, median patient survival after kidney transplantation was 80 months for male ESP-KTRs versus 131 months for female ESP-KTRs. (C) Kaplan–Meier plot of patient survival between donor–recipient gender mismatch groups. No differences are shown for patient survival between male recipients from male donors versus male recipients (Log rank: P = 0.390), and female recipients from female donors versus female recipients from male donor (Log rank: P = 0.188). (D) Kaplan–Meier plot of death-censored allograft survival of ESP-KTRs. ESP-KTRs show primary non-function in 3.7% of cases and a 5- and 10-year death-censored allograft survival of 83 and 70%. (E) Kaplan–Meier plot of death-censored allograft survival between male ESP-KTRs and female ESP-KTRs. No differences are shown for death-censored allograft survival between male ESP-KTRs and female ESP-KTRs. (F) Kaplan–Meier plot of death-censored allograft survival between donor–recipient gender mismatch groups. No differences are shown for death-censored allograft survival between donor–recipient gender mismatch groups. (G) Kaplan–Meier plot of uncensored allograft survival of ESP-KTRs. ESP-KTRs show a median uncensored kidney allograft survival of 73 months. (H) Kaplan–Meier plot of uncensored allograft survival between male ESP-KTRs and female ESP-KTRs. Male ESP-KTRs show a tendency for inferior uncensored allograft survival compared with female ESP-KTRs with a median of 61 versus 82 months.
FIGURE 1.
continued.
Using Cox regression with the time of T-cell-mediated rejection as the time-dependent covariate showed a significantly inferior patient survival with a hazard ratio (HR) of 2.293 (95% CI 1.451–3.623; P = 0.001) among ESP-KTRs with T-cell-mediated rejection compared with ESP-KTRs without T-cell-mediated rejection. Upon univariate analysis, neither overall HLA-mismatch nor HLA-DR mismatch was associated with patient survival (Supplementary data, Table S1).
Kidney allograft survival
Among all ESP-KTRs, we observed that 1-, 5- and 10-year death-censored allograft survival was 93.3, 82.6 and 70.4%, respectively (Figure 1D). Upon multivariate analysis, high body mass index (BMI) was identified as the only independent risk factor for allograft loss (OR = 1.096, 95% CI 1.044–1.150; P < 0.001). No differences were observed for death-censored kidney allograft survival between male and female ESP-KTRs (P = 0.572; Figure 1E) or donor–recipient gender mismatch (P = 0.346; Figure 1F). Using Cox regression with the time of T-cell-mediated rejection as the time-dependent covariate showed a significantly inferior death-censored kidney allograft survival with an HR of 2.064 (95% CI 1.356–4.634; P = 0.019) among ESP-KTRs with T-cell-mediated rejection compared with ESP-KTRs without T-cell-mediated rejection.
Upon univariate analysis, neither overall HLA-mismatch nor HLA-DR mismatch was associated with an increased risk of T-cell-mediated rejection (P = 0.561; P = 0.736), nor inferior death-censored kidney allograft survival (Supplementary data, Table S2).
Twenty-eight of 244 ESP-KTRs (11.5%) developed donor-specific antibodies (DSAs) after kidney transplantation. Median time of first occurrence of DSA was 16 months after kidney transplantation (range 1–117 months). No ESP-KTR showed kidney allograft loss due to antibody-mediated rejection in further follow-up.
Overall, uncensored kidney allograft survival among all ESP-KTRs was 85.5, 54.7 and 26.7% at 1-, 5- and 10-years, respectively (Figure 1G). Median uncensored kidney allograft survival of male ESP-KTRs was 60 months versus 81 months for female ESP-KTRs (0 = 0.055; Figure 1H).
Kidney allograft function
Nine of 244 ESP-KTRs (4%) showed primary non-function and 110 of 244 ESP-KTRs (45%) showed delayed graft function (DGF). Male ESP-KTRs were more likely to show DGF compared with female ESP-KTRs (P = 0.017; Table 1). Upon univariate analysis, two HLA-DR mismatch was not associated with an increased risk of DGF (P = 0.090). However, two HLA-DR mismatch among male ESP-KTRs was associated with an increased risk of DGF with an HR of 1.675 (95% CI 1.018–2.756; P = 0.042).
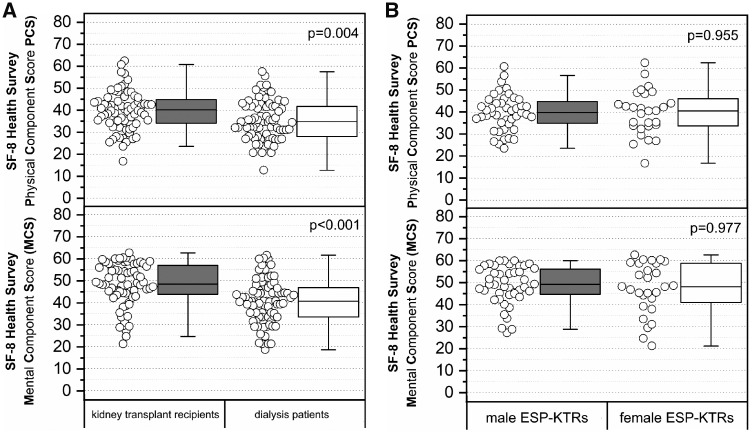
Median estimated glomerular filtration rate (eGFR) of all ESP-KTRs at 12 months post-transplantation was 39.7 mL/min (range 13.6–87.0 mL/min). The mean annual decline in eGFR independent from kidney allograft loss was 0.4 mL/min. Change of eGFR showed a mean decline of 2.3 and 6.8 mL/min at 5- and 10-years post-transplantation (Figure 2A). Median eGFR of male ESP-KTRs was 38.7 mL/min range (range 13.6–87.0 mL/min) compared with 41.7 mL/min (range 15.9–79.2 mL/min) among female ESP-KTRs (P = 0.868). No differences were observed with respect to the decline of eGFR between male and female ESP-KTRs (Figure 2B). No differences were observed for median eGFR and decline of eGFR at any time post-transplantation with respect to donor–recipient gender mismatch (P > 0.05).
FIGURE 2.
(A) Change of eGFR (using the chronic kidney disease (CKD)-EPI creatinine equation) of ESP-KTRs with respect to baseline eGFR at 1 year post-transplantation. Change of eGFR showed a mean decline of 2.3 and 6.8 mL/min at 5- and 10-years post-transplantation. (B) Change of eGFR (using the CKD-EPI creatinine equation) of male versus female ESP-KTRs with respect to baseline eGFR at 1 year post-transplantation. No differences were observed between male and female ESP-KTRs at any time (P > 0.05). KTRs who lost their kidney allograft were represented in the year they lost their kidney allograft, but not thereafter. Boxes show the quartiles and medians, whiskers show the minimum and maximum values.
Patient survival after kidney allograft loss
Among ESP-KTRs who showed kidney allograft loss, median patient survival after re-initiation of dialysis treatment was 58 months (range: 0–152 months; Figure 3A). ESP-KTRs with kidney allograft loss <12 months after transplantation showed a median patient survival of 40 months compared with 82 months among ESP-KTRs with kidney allograft loss >12 months after transplantation (P = 0.020; Figure 3B). No differences were observed for patient survival after kidney allograft loss between male and female ESP-KTRs (P = 0.583; Figure 3B). None of those ESP-KTR with kidney allograft loss underwent retransplantation.
FIGURE 3.
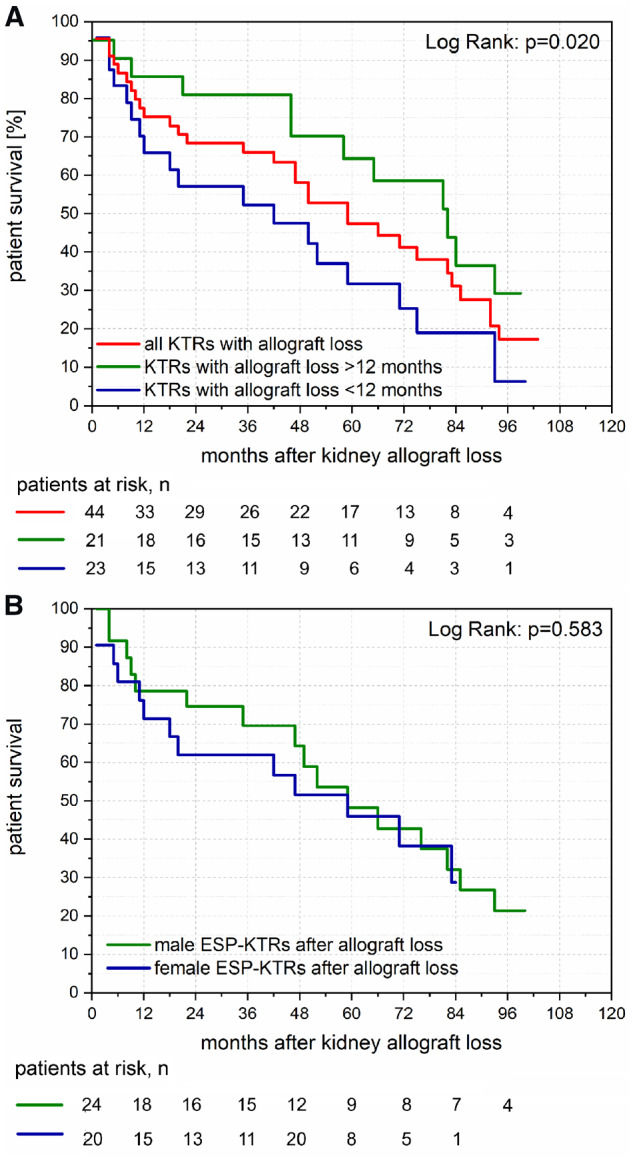
(A) Kaplan–Meier plot of patient survival after kidney allograft loss. Median patient survival after kidney allograft loss was 58 months. ESP-KTRs with kidney allograft loss <12 months post-transplantation showed inferior patient survival compared with ESP-KTRs with kidney allograft loss >12 months post-transplantation. (B) Kaplan–Meier plot of patient survival after kidney allograft loss between male ESP-KTRs and female ESP-KTRs. No differences are shown for patient survival after kidney allograft loss between male ESP-KTRs and female ESP-KTRs.
Quality of life of ESP-KTRs compared with waitlisted dialysis patients
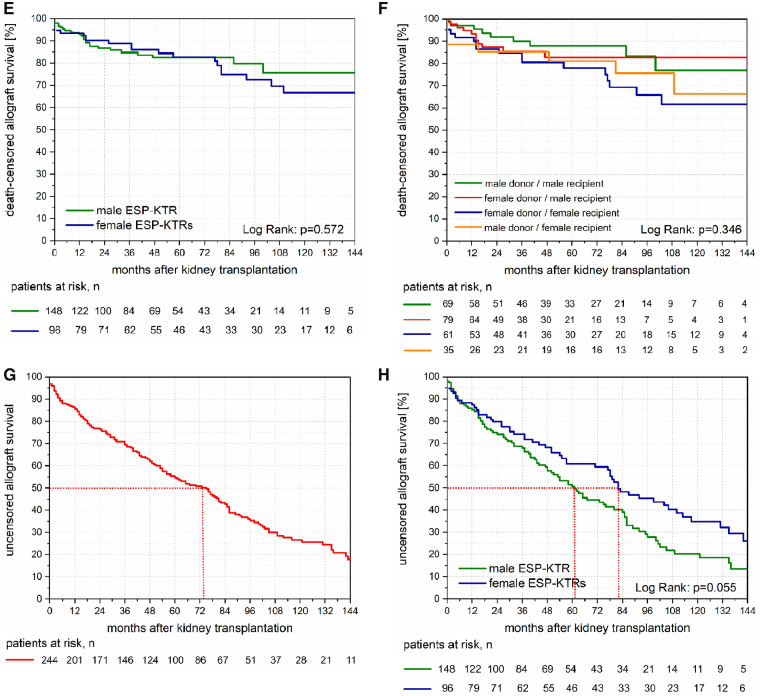
PCS of ESP-KTRs were significantly higher compared with dialysis patients waitlisted within the ESP (Figure 4A; P = 0.004). Similarly, MCSs of ESP-KTRs were significantly higher compared but dialysis patients waitlisted within the ESP (Figure 4A; P < 0.001).
FIGURE 4.
(A) PCS and MCS between 80 ESP-KTRs and 83 patients on dialysis waitlisted within the ESP. ESP-KTRs showed significantly superior PCS and MCS compared with patients on dialysis waitlisted within the ESP. (B) PCS and MCS between 42 male ESP-KTRs and 28 female ESP-KTRs. No differences are observed for PCS and MCS between male ESP-KTRs and female ESP-KTRs.
According to the PCS and MCS of an age-matched general population norm [12] assessed with the SF-8, ESP-KTRs were classified into those with PCS/MCS above and below average (Table 2). No factors were identified that were associated with impairment of physical and mental quality of life.
Table 2.
Clinical characteristics of ESP-KTRs divided into ESP-KTRs with PCS/MCS above versus below average
| Characteristics | PCS above averagea (n = 22) | PCS below averagea (n = 50) | P-value | MCS above averageb (n = 30) | MCS below averageb (n = 42) | P-value |
|---|---|---|---|---|---|---|
| Age at the time of the survey (years)c | 76 (69–83) | 75 (67–85) | 0.589 | 76 (68–81) | 75 (67–85) | 0.692 |
| Male sex, n (%) | 13 (60) | 31 (62) | 1 | 18 (60) | 26 (62) | 1 |
| Donor age (years)c | 70 (65–82) | 71 (65–89) | 0.823 | 71 (65–89) | 81 (65–85) | 0.810 |
| Time from transplantation (months)c | 71 (7–192) | 69 (6–172) | 0.869 | 68 (6–173) | 72 (7–192) | 0.914 |
| Aftercare in the transplant centre, n (%) | 20 (91) | 43 (86) | 0.712 | 26 (87) | 37 (88) | 1 |
| Distance to transplant centre (km)c | 30 (7–179) | 87 (1–485) | 0.290 | 124 (4–186) | 45 (1–485) | 0.533 |
| Time to transplant centre (min)c | 39 (18–160) | 84 (11–269) | 0.224 | 89 (13–151) | 51 (11–269) | 0.472 |
| Time on dialysis (months)c | 35 (11–91) | 53 (7–152) | 0.302 | 47 (7–105) | 51 (9–142) | 0.066 |
| CAPD, n (%) | 4 (18) | 6 (12) | 0.482 | 4 (13) | 6 (14) | 1 |
| Causes of ESRD, n (%) | 0.821 | 0.805 | ||||
| Glomerulonephritis | 5 (23) | 10 (20) | 8 (27) | 7 (17) | ||
| Diabetic nephropathy | 2 (10) | 5 (10) | 2 (7) | 5 (12) | ||
| Nephroangiosclersosis | 5 (23) | 7 (14) | 5 (17) | 7 (17) | ||
| Polycystic kidney disease | 2 (10) | 10 (20) | 5 (17) | 7 (17) | ||
| Uropathy | 1 (5) | 4 (8) | 1 (3) | 4 (11) | ||
| Other or undetermined | 7 (32) | 14 (28) | 9 (30) | 12 (29) | ||
| BMI (kg/m2)c | 23 (19–32) | 25 (18–36) | 0.340 | 27 (20–36) | 24 (18–32) | 0.066 |
| BMI >30 kg/m2, n (%) | 3 (14) | 3 (6) | 0.361 | 4 (13) | 2 (5) | 0.227 |
| Initial hospital stay (days)c | 21 (7–61) | 20 (9–115) | 0.561 | 20 (11–61) | 22 (7–115) | 0.982 |
PCS divided into ESP-KTRs with PCS above/below average according to the mean of the German population aged >70 years (mean PCS = 43.3).
MCS divided into ESP-KTRs with MCS above/below average according to the mean of the German population aged >70 years (mean MCS = 51.4).
Median (range).
No differences were observed for PCS and MCS of male versus female ESP-KTRs (Figure 4B; P = 0.955; P = 0.977).
Seventy-eight of 80 ESP-KTRs (97.5%) who underwent successful transplantation would again do so. Reasons for dialysis patients to be waitlisted within the ESP included dependence from dialysis (73.5%), hope for better physical capacity (51.0%), hope for longer life expectancy (49.0%) and hope for less medication (24.5%).
DISCUSSION
With a persisting organ shortage and a soaring number of elderly patients awaiting kidney transplantation, an urgency to optimize allocation of elderly kidney allografts with respect to patient and allograft survival has arisen. In the past, short-term outcomes of the ESP have proven to be quite successful in achieving this goal without compromising the outcome for the individual KTR [5, 7, 13–15]. However, despite all the applause for the short-term ESP outcomes, burning questions arise with respect to the long-term outcomes in this cohort. To elucidate this matter, we for the first time show results of two decades of ESP-KTRs followed at our centre.
First, T-cell-mediated rejection has been identified as the only independent risk factor that impacts both patient survival and death-censored kidney allograft survival among ESP-KTRs. Principally, in the ESP-KTR cohort, avoiding T-cell-mediated rejection must be weighed against over immunosuppression, escalating the risk of severe infectious complications. Previous studies suggest that the impact of T-cell-mediated rejection on kidney allograft loss is aggravated among ESP-KTRs due to an impaired ability to repair allograft injury in the elderly kidney with reduced nephron number [16, 17]. Both immediate infectious complications after T-cell-mediated rejection treatment and associations of more impaired kidney allograft function with cardiovascular complications may explain rejection-related deaths in this cohort [18]. Previously, combined matching for HLA-DR antigens has been proposed to be implemented in the ESP in avoidance of increased risk of T-cell-mediated rejection [7, 19]. Although our data did not show HLA-DR mismatches impacting T-cell-mediated rejection, kidney allograft survival or patient survival in the long-term, our data indicate an association of HLA-DR mismatches and delayed allograft function. Due to the small sample size of our single-centre analysis, however, definite conclusions cannot be drawn from our results. Therefore, new biomarkers need to be implemented in this patient cohort to allow individualized risk stratification to reduce T-cell-mediated rejection rates and infectious complications due to over immunosuppression.
The finding that BMI was identified as a risk factor for kidney allograft loss remains interesting and suggests the need for adequate donor nephron mass with respect to the recipient’s metabolic demand. To what extent allocation of lower kidney donor profile index (KDPI) donors to higher BMI recipients and higher KDPI donors to lower weight recipients, to account for differences in recipient metabolic demand, may ultimately impact kidney allograft survival in the elderly needs to be addressed in upcoming studies.
Secondly, compared with the age-matched expected remaining lifetime in the general German population, our data suggest an inferior expected remaining lifetime of ESP-KTRs. Here, data from the ERA-EDTA registry again support this finding [9]. In the general population, however, women show a survival advantage over men with an expected remaining lifetime of about 21.0 years compared with 17.8 years at the age of 65 years [20]. This gender gap in mortality appears to be present also after kidney transplantation in the elderly with a median life expectancy of 72 years among male ESP-KTRs and 76 years among female ESP-KTRS, and a median expected remaining lifetime of 6.7 years among male ESP-KTRs compared with 10.9 years among female ESP-KTRs. Previous studies on kidney transplantation in the elderly suggest a substantial improvement of patient survival among KTRs compared with dialysis patients [21–23]. However, the impact of chronic kidney disease, end-stage kidney disease and reconstitution of kidney function by transplantation on sex-dependent issues remains scarcely described. Very recent data on patient survival of male versus female dialysis patients suggest that the mortality advantage of women in the general population is markedly diminished and even cancelled among dialysis patients [9, 20, 24, 25]. This data suggest that the gender gap in mortality is impacted at least in part by a status of end-stage renal disease with associated complications of uraemia and extra-renal manifestations of chronic kidney disease. Therefore, the gender gap in mortality that is observed in our study of ESP-KTRs suggests sex-dependent issues that return together with reconstitution of kidney function. It has been suggested in previous studies that the explanation of the gender gap in mortality should be based on both biological factors such as hormones, autoimmunity and genetics, and behavioural factors [26, 27]. More importantly, it has been observed recently that males and females basically sufller from the same diseases leading to death but that females typically experience them later in life [26]. This finding leads to the hypothesis that sex differences in the onset of most common life-threatening diseases such as cardiovascular diseases, infections and cancer are reversed by end-stage kidney disease and may return after kidney transplantation. At the behavioural side, the more frequent utilization of healthcare services and stronger adherence to medical care among females may come more to fruition after kidney transplantation than during dialysis treatment.
Thirdly, ESP-KTRs show a higher quality of living compared with dialysis patients waitlisted within the ESP and even achieve quality of living scores of the general population. Interestingly, the improvement of quality of living seems to apply for all ESP-KTRs even in the very long-term after transplantation, independent of any distinct patient characteristics. Most dialysis patients select kidney transplantation with the hope of improving their quality of living, and recipients of successful transplantation consistently report a better quality of living [28, 29]. Physical well-being is significantly better among ESP-KTRs and may be related to improvements of complications of uraemia that are typically not reversed fully by dialysis treatment as anaemia, peripheral and autonomic neuropathy and metabolic and electrolyte disorders [28–32]. More interestingly, the impact of transplantation on mental well-being appears to be more pronounced among ESP-KTRs. This becomes obvious with the distribution of MCS being negatively skewed among ESP-KTRs compared with dialysis patients. This result may be attributed to independence from dialysis, better physical capacity and hope for longer life expectancy.
Our study has several strengths. We describe the longest follow-up at present of ESP-KTRs almost exclusively maintained on calcineurin inhibitor, MMF and methylprednisolone and with very high follow-up rates due to predominantly centre-provided aftercare. We are able to show for the first time a restored gender gap in mortality among KTRs who underwent kidney transplantation in the elderly. Limitations of our analysis include the single-centre approach, the relatively small sample size and the inability to provide any causality for the observed sex difference in mortality.
To sum it up, long-term outcomes of ESP-KTRs over two decades ultimately support the effectiveness of the ESP allocation system for the use of elderly organ donors with respect to patient survival, allograft survival and quality of living. The original principle of decreasing CIT by neglecting HLA matching, however, gets questioned, since HLA-DR mismatches appear to be at least associated with a higher incidence of DGF, whereas CIT goals of <8 h were not met in the majority of the cases. The presence of the gender gap in mortality suggests sex differences as an important factor for treating CKD patients in the elderly. If a change in kidney allocation such that kidneys with a longer anticipated allograft survival are preferentially allocated to women may improve outcomes remains questionable. However, since very recent efforts to optimize kidney allocation focus on estimated post-transplant survival [33], our findings need to be evaluated in further studies.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully thank Anett Sefrin and Cordula Giesler and all the members of the Department of Nephrology who participated in the research of this study.
AUTHORS’ CONTRIBUTIONS
T.S. contributed to research design, writing of the paper and performance of the research and data analysis. N.M.O. was involved in writing of the paper. P.R. performed the research design.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Langer RM, Cohen B, Rahmel A. History of Eurotransplant. Transplant Proc 2012; 44: 2130–2131 [DOI] [PubMed] [Google Scholar]
- 2. https://www.eurotransplant.org/cms/index.php?page=esp (20 August 2019, date last accessed)
- 3. https://www.eurotransplant.org/cms/index.php?page=annual_reports (20 August 2019, date last accessed)
- 4. Smits JM, Persijn GG, van Houwelingen HC et al. Evaluation of the eurotransplant senior program. The results of the first year. Am J Transplant 2002; 2: 664–670 [DOI] [PubMed] [Google Scholar]
- 5. Fritsche L, Hörstrup J, Budde K et al. Old-for-old kidney allocation allows successful expansion of the donor and recipient pool. Am J Transplant 2003; 3: 1434–1439 [DOI] [PubMed] [Google Scholar]
- 6. Lloveras J, Arcos E, Comas J et al. A paired survival analysis comparing hemodialysis and kidney transplantation from deceased elderly donors older than 65 years. Transplantation 2015; 99: 991–996 [DOI] [PubMed] [Google Scholar]
- 7. Frei U, Noeldeke J, Machold-Fabrizii V et al. Prospective age-matching in elderly kidney transplant recipients—a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant 2008; 8: 50–57 [DOI] [PubMed] [Google Scholar]
- 8. Boesmueller C, Biebl M, Scheidl S et al. Long-term outcome in kidney transplant recipients over 70 years in the Eurotransplant Senior Kidney Transplant Program: a single center experience. Transplantation 2011; 92: 210–216 [DOI] [PubMed] [Google Scholar]
- 9. Kramer A, Pippias M, Noordzij M et al. The European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: a summary. Clin Kidney J 2018; 1: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hecking M, Bieber BA, Ethier J et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med 2014; 11: e1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cobo G, Hecking M, Port FK et al. Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin Sci 2016; 130: 1147–1163 [DOI] [PubMed] [Google Scholar]
- 12. Beierlein V, Morfeld M, Bergelt C et al. Messung der gesundheitsbezogenen Lebensqualität mit dem SF-8 (article in German). Diagnostica 2012; 58: 145–153 [Google Scholar]
- 13. Fabrizii V, Kovarik J, Bodingbauer M et al. Long-term patient and graft survival in the eurotransplant senior program: a single-center experience. Transplantation 2005; 80: 582–589 [DOI] [PubMed] [Google Scholar]
- 14. Giessing M, Fuller TF, Friedersdorff F et al. Outcomes of transplanting deceased-donor kidneys between elderly donors and recipients. J Am Soc Nephrol 2009; 20: 37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peters-Sengers H, Berger SP, Heemskerk MB et al. Stretching the limits of renal transplantation in elderly recipients of grafts from elderly deceased donors. J Am Soc Nephrol 2017; 28: 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Fijter JW. An old virtue to improve senior programs. Transpl Int 2009; 22: 259–268 [DOI] [PubMed] [Google Scholar]
- 17. de Fijter JW, Mallat MJ, Doxiadis II et al. Increased immunogenicity and cause of graft loss of old donor kidneys. J Am Soc Nephrol 2001; 12: 1538–1546 [DOI] [PubMed] [Google Scholar]
- 18. Doyle SE, Matas AJ, Gillingham K et al. Predicting clinical outcome in the elderly renal transplant recipient. Kidney Int 2000; 57: 2144–2150 [DOI] [PubMed] [Google Scholar]
- 19. Halleck F, Khadzhynov D, Liefeldt L et al. Immunologic outcome in elderly kidney transplant recipients: is it time for HLA-DR matching? Nephrol Dial Transplant 2016; 31: 2143–2149 [DOI] [PubMed] [Google Scholar]
- 20.Statistisches Bundesamt. Lebenserwartung - Durchschnittliche Lebenserwartung nach Geschlecht und vollendetem Alter. https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/ Sterbefaelle-Lebenserwartung/_inhalt.html (20 August 2019, date last accessed)
- 21. Rao PS, Merion RM, Ashby VB et al. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation 2007; 83: 1069–1074 [DOI] [PubMed] [Google Scholar]
- 22. Ojo AO, Hanson JA, Meier-Kriesche H et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 2001; 12: 589–597 [DOI] [PubMed] [Google Scholar]
- 23. Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol 2005; 16: 1859–1865 [DOI] [PubMed] [Google Scholar]
- 24. Carrero JJ, de Jager DJ, Verduijn M et al. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol 2011; 6: 1722–1730 [DOI] [PubMed] [Google Scholar]
- 25. Villar E, Remontet L, Labeeuw M et al. Effect of age, gender, and diabetes on excess death in end-stage renal failure. J Am Soc Nephrol 2007; 18: 2125–2134 [DOI] [PubMed] [Google Scholar]
- 26. Carrero JJ, de Mutsert R, Axelsson J et al. Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant 2011; 26: 270–276 [DOI] [PubMed] [Google Scholar]
- 27. Schünemann J, Strulik H, Trimborn T. The gender gap in mortality: how much is explained by behavior? J Health Econ 2017; 54: 79–90 [DOI] [PubMed] [Google Scholar]
- 28. Lepeytre F, Dahhou M, Zhang X et al. Association of sex with risk of kidney graft failure differs by age. J Am Soc Nephrol 2017; 28: 3014–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oniscu GC, Brown H, Forsythe JL. How old is old fortransplantation? Am J Transplant 2004; 4: 2067–2074 [DOI] [PubMed] [Google Scholar]
- 30. Keith DS, Demattos A, Golconda M et al. Effect of donor recipient age match on survival after first deceased donor renal transplantation. J Am Soc Nephrol 2004; 15: 1086–1091 [DOI] [PubMed] [Google Scholar]
- 31. Jassal SV, Krahn MD, Naglie G et al. Kidney transplantation in the elderly: a decision analysis. J Am Soc Nephrol 2003; 14: 187–196 [DOI] [PubMed] [Google Scholar]
- 32. Foss A, Heldal K, Scott H et al. Kidneys from deceased donors more than 75 years perform acceptably after transplantation. Transplantation 2009; 87: 1437–1441 [DOI] [PubMed] [Google Scholar]
- 33. Bae S, Massie AB, Thomas AG et al. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor–recipient combination. Am J Transplant 2019; 19:425–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.