Abstract
Background
Acute kidney injury (AKI) is a common complication in patients during intensive care unit (ICU) admission. AKI is defined as an increase in serum creatinine (SCr) and/or a reduction in urine output. SCr is a marker of renal function with several limitations, which led to the search for biomarkers for earlier AKI detection. Our aim was to study the predictive value of plasma neutrophil gelatinase-associated lipocalin (NGAL) at admission as a biomarker for AKI progression during the first 48 h of ICU admission in an unselected, heterogeneous ICU patient population.
Methods
We conducted a prospective observational study in an academic tertiary referral ICU population. We recorded AKI progression in all ICU patients during the first 48 h of ICU admission in a 6-week period. Plasma NGAL was measured at admission but levels were not reported to the attending clinicians. As possible predictors of AKI progression, pre-existing AKI risk factors were recorded. We examined the association of clinical parameters and plasma NGAL levels at ICU admission with the incidence and progression of AKI within the first 48 h of the ICU stay.
Results
A total of 361 patients were included. Patients without AKI progression during the first 48 h of ICU admission had median NGAL levels at admission of 115 ng/mL [interquartile range (IQR) 81–201]. Patients with AKI progression during the first 48 h of ICU admission had median NGAL levels at admission of 156 ng/mL (IQR 97–267). To predict AKI progression, a multivariant model with age, sex, diabetes mellitus, body mass index, admission type, Acute Physiology and Chronic Health Evaluation score and SCr at admission had an area under the receiver operating characteristics (ROC) curve of 0.765. Adding NGAL to this model showed a small increase in the area under the ROC curve to 0.783 (95% confidence interval 0.714–0.853).
Conclusions
NGAL levels at admission were higher in patients with progression of AKI during the first 48 h of ICU admission, but adding NGAL levels at admission to a model predicting this AKI progression showed no significant additive value.
Keywords: AKI, biomarkers, creatinine, intensive care, NGAL
INTRODUCTION
Acute kidney injury (AKI) is a common complication in patients during intensive care unit (ICU) admission, with incidences up to 33% [1–3]. AKI is associated with increased mortality and increased incidence of chronic kidney disease (CKD) after recovery from AKI [2, 4–6]. The severity of AKI is associated with increased ICU mortality and the requirement for renal replacement therapy (RRT) [2, 7].
The main reason for early identification of patients at risk for AKI is the opportunity to intervene to prevent AKI progression. Early intervention may be successful in selected, homogeneous patient populations, for instance, after cardiac surgery [8, 9].
AKI is defined as an increase in serum creatinine (SCr) and/or a reduction in urine output as described by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [10]. SCr is a marker of renal function with several limitations. SCr is related to age, sex, diet and muscle mass and SCr rises only when ≥50% of glomerular filtration rate is lost [11]. Moreover, SCr needs to accumulate, leading to a delay between the renal insult and the AKI diagnosis [11, 12]. A reduction of urine output is more sensitive and can detect AKI earlier, but is less specific [3, 13, 14]. Furthermore, urine output is associated with patient outcome [15].
The disadvantages of SCr led to the search for new biomarkers for earlier AKI detection. Ideally these biomarkers are associated with the cause of AKI (e.g. sepsis, ischaemic- or toxicity-induced AKI) and guide potential therapeutic interventions [16]. Neutrophil gelatinase–associated lipocalin (NGAL), a 25-kDa protein, is one of these AKI biomarkers [17–21].
In animal studies, NGAL messenger RNA expression in the kidney and NGAL protein levels in plasma and in urine increase after ischaemic injury to the kidney [22, 23]. This NGAL protein increase is observed in humans as well, with increased levels in plasma and urine in septic AKI [24].
Earlier detection of AKI using biomarkers has been studied in ICU patients after cardiac surgery and in patients after abdominal surgery [8, 9]. In both studies, patients at risk for AKI are identified post-operatively and biomarker-guided interventions reduced AKI.
Our aim was to study the predictive value of NGAL at admission on AKI progression in an unselected, heterogeneous ICU patient population. Furthermore, we aimed to evaluate the differences in the predictive value of NGAL at admission using the KDIGO SCr and urine output criteria and as separate criteria.
MATERIALS AND METHODS
The aim of this study was to examine the association of NGAL plasma levels at ICU admission with the incidence and progression of AKI within 48 h during an ICU stay and to evaluate the utility of NGAL plasma levels at ICU admission in addition to a clinical model for the prediction of the incidence and progression of AKI within the first 48 h of an ICU stay.
Study design
We conducted a prospective observational study. The study was performed in an academic tertiary referral ICU population. Patients were included between 18 February and 31 March 2014. Plasma NGAL was measured at admission but levels were not reported to the attending clinicians. The need for informed consent was waived by the Institutional Review Board of our hospital (METc 2013-174).
Participants
All consecutive patients admitted to the ICU during the study period were included. If patients were admitted multiple times during the study period, only data from the first admission were used for the analysis. Patients with CKD (defined by a previously known SCr >177 µmol/L) and patients on chronic RRT were excluded from the study. Renal transplant recipients were also excluded from the study.
Data collection
We recorded AKI progression during the first 48 h of ICU admission in all ICU patients. For possible predictors of AKI progression during the first 48 h of ICU admission, age, sex, Acute Physiology and Chronic Health Evaluation (APACHE) IV score and admission type (medical or surgical, scheduled or emergency) were determined at ICU admission. In addition, the history of diabetes mellitus (DM) was recorded.
For the outcome, we recorded the incidence and severity of AKI based on SCr and urine output using the KDIGO definitions [10]. SCr was measured at admission and routinely each day. Urinary output (UO) was recorded hourly. The reference creatinine was based on the ideal SCr, which was calculated assuming a clearance of 75 mL/min/1.73 m2 using the Modification of Diet in Renal Disease formula. Furthermore, we recorded the need for RRT during ICU admission, length of ICU stay and ICU mortality.
Test methods
NGAL was measured in routinely collected lithium heparin plasma samples using the BioPorto NGAL Test (Bioporto Diagnostics, Hellerup, Denmark) in the Department of Laboratory Medicine on a Roche Modular P800 chemistry platform (Roche, Mannheim, Germany). According to the manufacturer, the NGAL test is validated for NGAL levels between 25 and 5000 µg/L. Overall, the coefficient of variation is 2.9% at a level of 206 µg/L and 2.3% at a level of 511 µg/L.
Outcome
The primary outcome was the progression of AKI during the first 48 h of ICU admission. We chose a 48-h window for AKI progression because of the fact that NGAL is suggested to detect AKI ~24–48 h earlier than SCr. Progression of AKI was defined as an increase of one or more KDIGO stages based on both SCr and UO criteria. Progression of AKI based on the separate components was based on an increase of one or more KDIGO stages on either SCr or UO.
Statistical analysis
Continuous variables were reported as means with standard deviations (SDs) or as medians with interquartile ranges (IQRs) depending on the distribution. Categorical data were presented in proportions. Associations were calculated as odds ratios (ORs) with 95% confidence intervals (CIs). Differences between groups were analysed using Student’s t-test, Mann–Whitney U test or the chi-square test as appropriate. A two-sided P-value <0.05 was considered statistically significant.
Associations of variables with AKI progression were analysed by univariate analysis. Age, sex and the presence of DM were included in the basic model (Model A) based on prior knowledge [25], regardless of their univariate association in the current study. NGAL was added to this basic model (Model B). Associations with P < 0.1 were used for entrance in the multivariable model (Model C). NGAL was also added to this model (Model D).
A post hoc analysis was performed to analyse the predictive value of NGAL at admission for AKI progression based on the separate criteria, SCr and urine output using the same variables in the separate models compared with AKI progression based on both criteria.
No data were imputed. Missing hourly UO data were replaced based on averages using the first value recorded after the missing hours. UO data were omitted from the analysis if all hourly UOs were missing. For patients discharged from the ICU within 48 h, only data during ICU admission were used (last observation carried forward method). Variables were assessed for collinearity. In case of strong correlation, only the one with the strongest univariate association was included in the multivariate model. The discriminative value of the models was analysed with receiver operating characteristics (ROC) curves. Calibration was analysed with the Hosmer–Lemeshow test. All analyses above were performed using SPSS (version 23; IBM, Armonk, NY, USA). Differences between the areas under the receiver operating characteristics (AUROCs curves were analysed using Delong’s test using the ROCCOMP command in Stata version 15 (StataCorp, College Station, TX, USA).
RESULTS
Participants
In the 6-week inclusion period, a total of 361 patients were included (Supplementary data, figure). The mean age was 60.5 ± 15.5 years and 224 (62%) patients were male. A total of 130 (36%) patients were admitted for medical reasons, 181 (50%) patients were admitted after scheduled surgery and 50 (14%) were admitted after emergency surgery. Twenty-five (6.9%) patients had a confirmed infection at admission. Overall, ICU survival was 89% (Table 1). A total of 20 patients died within 48 h of ICU admission and 46 patients were discharged within 48 h. It should be noted that mortality was higher in patients with higher maximum AKI severity (P < 0001).
Table 1.
Patient characteristics
| Variable | Overall (n = 361) | No AKI progression (n = 261) | AKI progression (n = 100) | P-value | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Available data, n (%) | Available data, n (%) | Available data, n (%) | |||||
| Age (years), mean ± SD | 60.5 ± 15.5 | 361 (100) | 59.8 ± 15.6 | 261 (100) | 62.3 ± 15.3 | 100 (100) | 0.17 |
| Sex, n (%) | 361 (100) | 261 (100) | 100 (100) | 0.14 | |||
| Male | 224 (62) | 168 (64) | 56 (56) | ||||
| Female | 137 (38) | 93 (36) | 44 (44) | ||||
| DM, n (%) | 361 (100) | 261 (100) | 100 (100) | 0.12 | |||
| No | 293 (81) | 217 (83) | 76 (76) | ||||
| Yes | 68 (19) | 44 (17) | 24 (24) | ||||
| APACHE, mean ± SD | 56 ± 29 | 349 (97) | 54 ± 28 | 253 (97) | 64 ± 29 | 96 (96) | 0.003 |
| Weight (kg), mean ± SD | 79.7 ± 16.4 | 361 (100) | 79.1 ± 16.2 | 261 (100) | 81.1 ± 16.9 | 100 (100) | 0.3 |
| BMI (kg/m2), mean ± SD | 26.2 ± 4.7 | 361 (100) | 25.9 ± 4.5 | 261 (100) | 27.2 ± 5 | 100 (100) | 0.01 |
| Creatinine at admission (µmol/L), median (IQR) | 73 (58–95) | 359 (99) | 73 (58–96) | 260 (99) | 76 (58–92) | 99 (99) | 0.94 |
| NGAL at admission (µmol/L), median (IQR) | 126 (84–214) | 339 (94) | 115 (81–201) | 245 (94) | 156 (97–267) | 94 (94) | 0.03 |
| Confirmed infection at admission, n (%) | 361 (100) | 261 (100) | 100 (100) | 0.97 | |||
| No | 226 (93) | 243 (93) | 93 (93) | ||||
| Yes | 25 (7) | 18 (7) | 7 (7) | ||||
| KDIGO stage admission, n (%) | 361 (100) | 261 (100) | 100 (100) | 0.008 | |||
| 0 | 297 (82) | 211 (81) | 86 (86) | ||||
| 1 | 29 (8) | 17 (7) | 12 (12) | ||||
| 2 | 19 (5) | 17 (7) | 2 (2) | ||||
| 3 | 16 (4) | 16 (6) | 0 (0) | ||||
| RRT during admission, n (%) | 19 (5) | 361 (100) | 10 (4) | 260 (99) | 9 (9) | 100 (100) | 0.05 |
| Admission type, n (%) | 361 (100) | 261 (100) | 0.03 | ||||
| Scheduled surgery | 181 (50) | 142 (54) | 39 (39) | ||||
| Unscheduled surgery | 50 (14) | 34 (13) | 16 (16) | ||||
| Medical | 130 (36) | 85 (33) | 45 (45) | ||||
| ICU survival, n (%) | 361 (100) | 261 (100) | 100 (100) | 0.405 | |||
| No | 39 (11) | 26 (10) | 13 (13) | ||||
| Yes | 322 (89) | 235 (90) | 87 (87) | ||||
| ICU LoS (calendar days), median (IQR) | 2 (2–5) | 361 (100) | 2 (2–3) | 261 (100) | 2 (2–8) | 100 (100) | <0.001 |
Overall and whether or not patients' develop AKI progression within 48 hours after ICU admission. LoS: length of stay.
AKI severity and plasma NGAL at admission
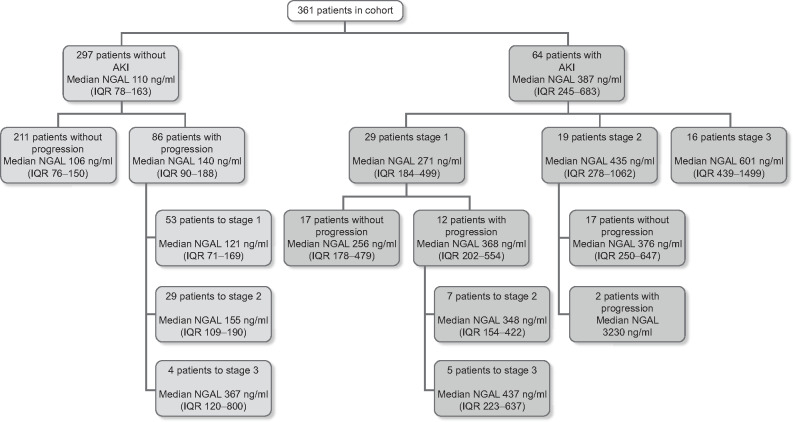
Median (IQR) SCr levels were 73 µmol/L (58–95) (Table 1) and AKI stage at admission was 0 in 297 patients (82%), Stage 1 in 29 patients (8%), Stage 2 in 19 patients (5.3%) and Stage 3 in 16 patients (4.4%) (Figure 1 and Table 2).
FIGURE 1.
Patients in the study with AKI stage at admission and progression during 48 h with median NGAL plasma levels at admission
Table 2.
Progression and regression of AKI during the first 48 h of ICU admission and median admission NGAL plasma levels
| Maximum AKI stage development during first 48 h |
|||||
|---|---|---|---|---|---|
| Number of patients, n | |||||
| NGAL at admission (ng/mL), median (IQR) | |||||
| AKI stage at admission | AKI 0 | AKI 1 | AKI 2 | AKI 3 | |
| AKI 0 | 211 | 53 | 29 | 4 | |
| 106 | 121 | 155 | 367 | ||
| (76–150) | (71–169) | (109–190) | (120–800) | ||
| AKI 1 | 10 | 7 | 7 | 5 | |
| 309 | 254 | 348 | 437 | ||
| (174–570) | (174–275) | (154–422) | (223–637) | ||
| AKI 2 | 1 | 6 | 10 | 2 | |
| 2076 | 365 | 376 | 3230 | ||
| (131–647) | (278–573) | ||||
| AKI 3 | 1 | 0 | 3 | 12 | |
| 589 | 1013 | 601 | |||
| (370–1499) | |||||
Green squares are patients with improvement of AKI levels during the first 48 h. Yellow squares are patients with no change in AKI levels during the first 48 h. Red squares are patients with deterioration in AKI levels during the first 48 h.
Median levels of NGAL at admission were 126 ng/mL (IQR 84–214) (Table 1). Median NGAL levels were different in patients without and with AKI at admission at 110 ng/mL (IQR 78–163) and 387 ng/mL (IQR 245–683), respectively (P < 0.001) (Figure 1 and Table 2).
Maximum AKI severity during first 48 h
Maximum AKI severity based on both criteria was Stage 0 in 211 patients (58%), Stage 1 in 70 patients (19%), Stage 2 in 53 patients (15%) and Stage 3 in 27 patients (7.5%) (Figure 1 and Table 2). Maximum AKI severity based on SCr was Stage 0 in 284 (79%) patients, Stage 1 in 27 patients (7.5%), Stage 2 in 25 patients (6.9%) and Stage 3 in 25 patients (6.9%)(Table 3).
Table 3.
Progression of AKI during the first 48 h of ICU admission based on KDIGO criteria for SCr or urine output alone
| AKI creatinine | AKI urine output | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | No progression (n = 337) | Progression (n = 24) | P-value | No progression (n = 248) | Progression (n = 32) | P-value |
| Age (years), mean ± SD | 60 ± 16 | 62 ± 13 | 0.59 | 60 ± 16 | 63 ± 15 | 0.35 |
| Sex, n (%) | 0.09 | 0.07 | ||||
| Male | 213 (63) | 11 (46) | 157 (63) | 15 (47) | ||
| Female | 124 (37) | 13 (54) | 91 (37) | 17 (53) | ||
| DM, n (%) | 0.42 | 0.24 | ||||
| No | 275 (82) | 18 (75) | 195 (79) | 28 (88) | ||
| Yes | 62 (18) | 6 (25) | 53 (21) | 4 (13) | ||
| APACHE, mean ± SD | 54 ± 28 | 85 ± 28 | <0.001 | 53 ± 26 | 75 ± 29 | <0.001 |
| Weight (kg), mean ± SD | 79.8 ± 16.4 | 78.1 ± 16.5 | 0.63 | 79.5 ± 16.3 | 76.6 ± 178 | 0.40 |
| BMI (kg/m2), mean ± SD | 26.3 ± 4.7 | 26.4 ± 4.6 | 0.88 | 26.1± 4.6 | 26 ± 5.6 | 0.87 |
| Creatinine at admission (µmol/L), median (IQR) | 71 (58–91) | 114 (92–141) | <0.001 | 70 (58–94) | 76 (56–127) | 0.4 |
| NGAL at admission (µmol/L), median (IQR) | 121 (82–194) | 266 (157–458) | <0.001 | 121 (85–214) | 170 (130–371) | 0.02 |
| Confirmed infection at admission, n (%) | 0.27 | 0.9 | ||||
| No | 315 (94) | 21 (88) | 231 (93) | 30 (94) | ||
| Yes | 22 (7) | 3 (13) | 17 (7) | 2 (6) | ||
| KDIGO stage admission, n (%) | <0.001 | 0.05 | ||||
| 0 | 284 (84) | 13 (54) | 208 (84) | 22 (69) | ||
| 1 | 20 (6) | 9 (38) | 17 (7) | 6 (19) | ||
| 2 | 17 (5) | 2 (8) | 13 (5) | 1 (3) | ||
| 3 | 16 (5) | 0 (0) | 10 (4) | 3 (9) | ||
| RRT during admission, n (%) | 11 (3.3) | 8 (33) | <0.001 | 11 (4) | 5 (16) | 0.01 |
| Admission type, n (%) | 0.03 | 0.01 | ||||
| Scheduled surgery | 175 (52) | 6 (25) | 129 (52) | 7 (22) | ||
| Unscheduled surgery | 44 (13) | 6 (25) | 35 (14) | 7 (22) | ||
| Medical | 118 (35) | 12 (50) | 84 (34) | 18 (56) | ||
| ICU survival, n (%) | 0.34 | 0.56 | ||||
| No | 35 (10) | 4 (17) | 23 (9) | 4 (13) | ||
| Yes | 302 (90) | 20 (83) | 225 (91) | 28 (88) | ||
| ICU LoS (calendar days), median (IQR) | 2 (2–4) | 7 (4–10) | 0.05 | 2 (2–5) | 7.5 (4–16) | <0.001 |
LoS: length of stay.
UO was available in 280 (78%) patients. Maximum AKI severity based on UO criteria was Stage 0 in 160 patients (44%), Stage 1 in 64 patients (18%), Stage 2 in 48 patients (13%) and Stage 3 in 8 patients (2.2%) (Table 3).
AKI progression in the first 48 h of admission
AKI progression based on the combined criteria was not present in 261 patients (72%) and was present in 100 patients (28%) (Table 1 and Figure 1). AKI progression based on SCr was not present in 337 patients (93%) and was present in 24 patients (6.6%) (Table 3). AKI progression based on UO criteria was not present in 248 patients (69%) and was present in 32 patients (8.9%). UO data were missing in 81 patients (22%) (Table 3).
Predictive value of plasma NGAL at admission for AKI progression
One hundred patients showed AKI progression (Table 1 and Figure 1). Patients without AKI progression during the first 48 h of ICU admission had median NGAL levels at admission of 115 ng/mL (IQR 81–201). Patients with AKI progression during the first 48 h of ICU admission had median NGAL levels at admission of 156 ng/mL (IQR 97–267; P = 0.03) (Table 1 and Figure 1).
The variables age, sex and DM were not statistically significantly associated with AKI progression. No collinearity between variables was observed. In univariate analyses, APACHE score, body mass index (BMI), admission type, SCr at admission and NGAL at admission were statistically significant associated with AKI progression in Table 4.
Table 4.
Associations of variables with AKI progression during admission analysed by univariate analysis
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Age | 1.01 (1–1.03) | 0.17 |
| Sex | 0.14 | |
| Male (reference) | 1 | |
| Female | 1.42 (0.89–2.27) | |
| DM | 0.12 | |
| No (reference) | 1 | |
| Yes | 1.56 (0.89–2.73) | |
| APACHE | 1.01 (1–1.02) | 0.004 |
| Weight (kg) | 1.01 (0.99–1.02) | 0.3 |
| BMI (kg/m2) | 1.06 (1.01–1.12) | 0.02 |
| SCr at admission | 1 (0.99–1) | 0.09 |
| NGAL at admission | 1 (1–1) | 0.77 |
| Confirmed infection at admission | 0.97 | |
| No (reference) | 1 | |
| Yes | 1.02 (0.41–2.51) | |
| KDIGO stage admission | 0.18 | |
| 0 (reference) | 1 | |
| 1 | 1.73 (0.79–3.78) | |
| 2 | 0.29 (0.07–1.28) | |
| 3 | 1 | not to be estimated |
| Admission type | 0.03 | |
| Scheduled surgery | 0.52 (0.31–0.86) | |
| Unscheduled surgery | 0.89 (0.44–1.78) | |
| Medical (reference) | 1 |
To analyse the predictive value of NGAL at admission for predicting AKI progression during the first 48 h of ICU admission, several multivariant models were tested. The basic model with age, sex and DM (Model A) had an AUROC of 0.572 (95% CI 0.503–0.640). The result of the Hosmer–Lemeshow goodness of fit test was 0.411. Adding NGAL to this, the basic model (Model B) had an AUROC of 0.574 (95% CI 0.503–0.644). The result of the Hosmer–Lemeshow goodness of fit test was 0.233 (Table 5). Comparing the two AUROC’s of Models A and B using Delong’s test showed no difference between the AUROCs (P = 0.67).
Table 5.
Predictive models for AKI progression based on SCr and urine output criteria
| Hosmer– Lemeshow goodness of fit test | AUROC (95% CI) | P-value | |
|---|---|---|---|
| Model A (age, sex and DM) | 0.411 | 0.572 (0.503–0.640) | 0.035 |
| Model B (age, sex and DM + NGAL) | 0.233 | 0.574 (0.503–0.644) | 0.036 |
| Model C (age, sex and DM + BMI + admission type + APACHE + SCr at admission) | 0.014 | 0.765 (0.701–0.831) | <0.001 |
| Model D (age, sex and DM + BMI + admission type + APACHE + SCr at admission + NGAL) | 0.045 | 0.783 (0.714–0.853) | <0.001 |
Model A is based on 361 patients. Model B is based on 339 patients. Model C is based on 349 patients. Model D is based on 327 patients.
The complete model with age, sex, DM, BMI, admission type, APACHE score and SCr at admission (Model C) had an AUROC of 0.765 (95% CI 0.701–0.831) and the Hosmer–Lemeshow goodness of fit test result was 0.014. Adding NGAL to this complete model (Model D) showed an increase in the AUROC to 0.783 (95% CI 0.714–0.853) and a Hosmer–Lemeshow goodness of fit test of 0.045 (Table 5). Comparing the two AUROCs of Models C and D using Delong’s test showed no difference between the AUROCs (P = 0.31) (Table 5). The ORs of the individual components of Model D are presented in Table 6.
Table 6.
ORs of the individual components of Model D
| Variable | OR (95% CI) |
|---|---|
| Age at ICU admission | 0.99 (0.95–1.02) |
| Sex (female) | 1.41 (0.53–3.73) |
| Diabetes | 1.12 (0.34–3.68) |
| BMI | 1.01 (0.92–1.11) |
| Admission type | |
| Medical (reference) | 1.33 (0.34–5.25) |
| Scheduled surgery | 2.03 (0.51–8.08) |
| Unscheduled surgery | |
| APACHE IV score | 1.03 (1.01–1.06) |
| SCr at admission | 1.00 (0.99–1.00) |
| NGAL at admission | 1.00 (1.00–1.00) |
For the comparison of Models C and D, the chi-squared of the Delong’s test yielded P = 0.31.
NGAL in Model D, P = 0.45.
Predictive value of plasma NGAL at admission for AKI progression based on SCr
The characteristics of patients with AKI progression based on SCr alone are presented in Table 3. The variables age and DM were not statistically significantly associated with AKI progression in univariate analysis (Supplementary data, Table S1A). No collinearity between variables was observed. In univariate analyses, sex, APACHE score, NGAL at admission, KDIGO stage at admission and admission type were statistically significantly associated with AKI progression (Supplementary data, Table S1A). The AUROCs and Hosmer–Lemeshow goodness of fit test results are presented in Supplementary data, Table S1B.
Predictive value of plasma NGAL at admission with AKI progression based on UO
The characteristics of patients with AKI progression based on UO alone are presented in Table 3. The variables age and DM were not statistically significantly associated with AKI progression in univariate analysis (Supplementary data, Table S1A). No collinearity between variables was observed. Sex, APACHE score, KDIGO stage at admission and admission type were statistically significantly associated with AKI progression in univariate analysis (Supplementary data, Table S1A). The AUROCs and Hosmer–Lemeshow goodness of fit test results are presented in Supplementary data, Table S1B.
DISCUSSION
In our study, NGAL levels at admission were higher in patients with progression of AKI during the first 48 h of ICU admission. Adding NGAL levels at admission to a model predicting AKI progression during the first 48 h of ICU admission, including patient age, sex, DM, BMI, admission type and SCr, showed no significant additive value.
Different from other studies, we analysed the additive value of NGAL to variables predicting AKI progression in all consecutive patients admitted to the ICU. Most studies analysed the univariate predictive value or isolated diagnostic accuracy of NGAL independent of other variables. This isolated diagnostic accuracy is less clinically relevant since diagnostic testing or clinical prediction at the bedside is not a univariate process.
Furthermore, the reported predictive value of plasma NGAL on AKI progression in ICU patients differs with the population studied, AKI definition used, the definition of the primary outcome and the timing of this outcome compared with our study. Haase-Fielitz et al. [26] analysed the predictive value of NGAL in AKI development in patients after cardiac surgery with cardiopulmonary bypass (CPB). They found an AUROC of 0.80 for NGAL at ICU admission and AKI development within 5 days. Tuladhar et al. [27] analysed patients after coronary surgery with CPB. They found an AUROC of 0.85 for NGAL for an increase in SCr of 0.44 µmol/L in the post-operative period. Parikh et al. [28] analysed patients with an a priori high risk for AKI after undergoing cardiac surgery. Their primary outcome was AKI development during the hospital stay. The authors added NGAL at ICU admission to a clinical prediction model, which increased the AUROC from 0.69 to 0.75. Koyner et al. [29] analysed the predictive value of NGAL for AKI progression in patients with an a priori high AKI risk after coronary surgery. These authors observed a modest effect in the prediction of AKI progression when adding NGAL at the time of first AKI diagnosis, with an increase in the AUROC from 0.75 to 0.80. Constantin et al. [30] analysed the diagnostic accuracy of NGAL at ICU admission for AKI using the Risk, Injury, Failure, Loss, End-stage kidney disease (RIFLE) definition in 88 general ICU patients. Sensitivity and specificity were optimal at a cut-off of 155 ng/mL with an AUROC of 0.92. In a comparable population with 632 patients using the creatinine RIFLE definition for AKI during the first week of ICU admission and a diagnostic approach, de Geus et al. [17] found a sensitivity of 0.91, a specificity of 0.50, a positive predictive value (PPV) of 0.15 and a negative predictive value (NPV) of 0.98 for NGAL at admission with a cut-off of 168 ng/mL. Cruz et al. [19] analysed the diagnostic accuracy of NGAL for AKI development in the next 48 h. They observed an AUROC of 0.78 in their 307 patients, with a sensitivity of 0.73, a specificity of 0.81, a PPV of 0.24 and an NPV of 0.97. Their post hoc logistic regression analysis showed that the APACHE score and NGAL at admission were significant predictors both in a univariate model and in a multivariate model. Royakkers et al. [31] studied the predictive value of NGAL for AKI development using a diagnostic approach within the next 48 h using the RIFLE definition based on both SCr and UO criteria. They selected patients with an expected stay of at least 48 h and found an AUC of 0.53. Pickering et al. [32] analysed the predictive value of NGAL at admission for AKI development within 7 days based on the KDIGO definitions using SCr and UO criteria and found an AUC of 0.79. Bagshaw et al. [18] showed the influence of sepsis on NGAL levels in ICU patients. The AUROC for AKI worsening was 0.71. Overall, different methods, primarily aimed at a diagnostic approach, have been used to analyse the value of NGAL to discriminate patients with and without AKI development or progression. Time in the ICU or during hospitalization and AKI definitions and criteria differ between studies. Limited data exist on the prognostic value of NGAL in general ICU populations, especially regarding the different criteria. The data presented in our current study extend this knowledge.
To improve the prediction of AKI progression during ICU admission, combinations of biomarkers might outperform single biomarkers. Using combinations of biomarkers related to different mechanistic causes of AKI might lead to better prediction of AKI progression in heterogeneous critically ill patient populations. This combination approach is applied to predict the progression of AKI after cardiac surgery. A combination of urine levels of kidney injury molecule-1 (KIM-1) and interleukin-18 is suggested to have a better predictive value than the single biomarkers [33]. Furthermore, NephroCheck (Astute Medical, San Diego, CA, USA) uses urine tissue inhibitor of metalloproteinases and insulin-like growth factor binding protein-7 levels to predict AKI.
Of note, we observed that the prognostic value of NGAL for AKI progression is different for AKI progression based on SCr compared with either the combined criteria or the UO criteria. In this study, we show that the prognostic value of NGAL for AKI progression based on the SCr criterion overall appears to be better. We previously described heterogeneity in AKI prognosis, uncovered with the use of SCr and UO criteria [3]. In this current cohort, we show that there is a difference in plasma NGAL at admission depending on which AKI progression KDIGO criteria are fulfilled in patients. It is important to appreciate that of all the patients, 29 had AKI Stage 1 at admission, and of the patients without AKI at admission, 53 progressed to AKI Stage 1. As AKI based on UO criteria has a different prognosis than more severe forms of AKI, this relatively mild AKI might have a different mechanism than the more severe forms of AKI [33]. Patients meeting the KDIGO UO only AKI criteria have lower plasma NGAL at admission (170 µmol/L) than patients meeting the SCr criteria (NGAL 266 µmol/l) for AKI progression (Table 3). This might reflect different underlying pathophysiological mechanisms, while in the less severe AKI pre-renal azotaemia might be an important factor. Multiple mechanisms play a role in the development of AKI in critically ill patients [34]. In a histopathological study of patients who died in the ICU with septic AKI, no single uniform pathophysiological renal change was observed [35]. Creatinine is one of the biomarkers reflecting the loss of renal function. Decreased UO may be the result of renal function loss. However, a healthy kidney will also retain water if hypovolaemia is present. Biomarkers reflecting a specific pathophysiological mechanism, such as NGAL, might change only if that mechanism is involved. NGAL is also produced by white blood cells and is increased in inflammation without AKI [18, 21, 36]. Adding additional specific pathophysiological biomarkers and patient clinical and haemodynamic variables obtained by physical examination and critical care ultrasound might help in further subphenotyping different AKI populations [37]. As an example of such an approach, the study of Neyra et al. [38] investigated 106 adults undergoing cardiac surgery with CPB to study the utility of combining biomarkers of kidney function loss (serum cystatin C) and kidney tubular damage (urine NGAL and KIM-1) for the prediction of post-cardiac surgery AKI. This relatively small homogeneous patient study found that combining biomarkers improved the prediction of in-hospital AKI following cardiac surgery. This clinical and laboratory-based subphenotyping might be a step forward to more precise therapy for AKI patients.
Our study has several strengths and limitations. The strength of this current study is the relatively large, heterogeneous ICU patient cohort. By including this population, we established the additive prognostic accuracy of NGAL at admission for a general ICU population. This increases the external validity of the results of this study. It should be noted that this unselected heterogeneous ICU patient cohort comes with an ICU mortality of 11%, while previous ICU mortality for a similar unselected group was 5.6% [3], while a selection of only acute admissions in the same ICU had a higher 90-day mortality of 33% in the AKI group and 18% in the non-AKI group [39]. We also used both SCr and UO in defining KDIGO AKI; thus we were able to establish that the predictive value of NGAL differs when applied to different patient groups. The first limitation is that we have missing data in UO criteria that may have led to selection bias. Since most of the patients with missing values had no AKI based on SCr, the risk of missing AKI because of a lack of UO data is less likely. A second limitation is that patients who were discharged before the 48-h endpoint were considered not to have developed AKI in the ward. This was confirmed for SCr, but AKI based on UO criteria could not be determined in these patients. A third limitation is that our study was underpowered to determine whether NGAL plasma levels were predictive in the subgroup of patients with AKI progression without AKI at admission (86 patients; Figure 1). A fourth limitation is that UO was not always registered every hour, but sometimes every 2 or 3 h. This replacement procedure for missing hourly UO data, based on averages using the first value recorded after the missing hours, might have missed some ‘zero urine’ output. This also could have led to misclassification of some patients. Fifth, the fact that we have not taken ‘competing risk of death’ into account may influence the results. Of the 13 patients who died within the first 48 h of admission, only 1 patient showed AKI progression. AKI progression might have been hindered by this early death. A sixth limitation is that in our study we used ‘ideal’ SCr as a reference for the KDIGO AKI definition. This might induce bias in baseline kidney function and the best baseline would be to use creatinine values measured 7–365 days before admission [40]. Unfortunately, this was not available in most of our patients. Also, other surrogates for baseline renal function affect the classification of AKI [41]. Despite this baseline kidney function uncertainty, we think our data are relevant for AKI researchers and clinicians, as many other studies use this ideal SCr method, making comparison with other studies possible.
In conclusion, our study indicates that in a heterogeneous ICU population, the additive predictive value of NGAL plasma levels for AKI progression in the first 48 h of ICU admission is not significant. We plan future studies aimed at clinical and laboratory-based subphenotyping of AKI patients.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Igor van der Weide for his support with the database.
FUNDING
Funding was provided by the University Medical Center Groningen from 'Healthy ageing pilots’ (to J.G.Z.).
AUTHORS’ CONTRIBUTIONS
J.K., I.v.d.H., J.Z. and M.v.M. were involved in conception, design, analysis and interpretation of data, drafting and revising the article, providing intellectual content of critical importance and approval of the version to be published. E.K., W.D. and R.W. were involved in revising the article, providing intellectual content of critical importance and approval of the version to be published; J.E.K. was involved in supervising measurement of plasma NGAL, revising the article, providing intellectual content of critical importance and approval of the version to be published.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
REFERENCES
- 1. Gammelager H, Christiansen CF, Johansen MB et al. One-year mortality among Danish intensive care patients with acute kidney injury: a cohort study. Crit Care 2012; 16: R124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nisula S, Kaukonen KM, Vaara ST et al. ; FINNAKI Study Group. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med 2013; 39: 420–428 [DOI] [PubMed] [Google Scholar]
- 3. Koeze J, Keus F, Dieperink W et al. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol 2017; 18: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coca SG, Yusuf B, Shlipak MG et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009; 53: 961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wald R, Quinn R, Luo J et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009; 302: 1179–1185 [DOI] [PubMed] [Google Scholar]
- 6. Linder A, Fjell C, Levin A et al. Small acute increases in serum creatinine are associated with decreased long term survival in the critically ill. Am J Respir Crit Care Med 2014; 189: 1075–1081 [DOI] [PubMed] [Google Scholar]
- 7. Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 2007; 35: 1837–1843 [DOI] [PubMed] [Google Scholar]
- 8. Meersch M, Schmidt C, Hoffmeier A et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017; 43: 1551–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gocze I, Jauch D, Gotz M et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg 2018; 267: 1013–1020 [DOI] [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; 2: 1–138. [Google Scholar]
- 11. Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 2009; 20: 2305–2313 [DOI] [PubMed] [Google Scholar]
- 12. Uchino S. Creatinine. Curr Opin Crit Care 2010; 16: 562–567 [DOI] [PubMed] [Google Scholar]
- 13. Wlodzimirow KA, Abu-Hanna A, Slabbekoorn M et al. A comparison of RIFLE with and without urine output criteria for acute kidney injury in critically ill patients. Crit Care 2012; 16: R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaara ST, Parviainen I, Pettila V et al. Association of oliguria with the development of acute kidney injury in the critically ill. Kidney Int 2016; 89: 200-208 [DOI] [PubMed] [Google Scholar]
- 15. Macedo E, Malhotra R, Bouchard J et al. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int 2011; 80: 760–767 [DOI] [PubMed] [Google Scholar]
- 16. Murray PT, Mehta RL, Shaw A et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 2014; 85: 513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Geus HR, Bakker J, Lesaffre EM et al. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med 2011; 183: 907–914 [DOI] [PubMed] [Google Scholar]
- 18. Bagshaw S, Bennett M, Haase M et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med 2010; 36: 452–461 [DOI] [PubMed] [Google Scholar]
- 19. Cruz DN, de CM, Garzotto F et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med 2010; 36: 444–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hjortrup PB, Haase N, Treschow F et al. Predictive value of NGAL for use of renal replacement therapy in patients with severe sepsis. Acta Anaesthesiol Scand 2015; 59: 25–34 [DOI] [PubMed] [Google Scholar]
- 21. Martensson J, Bell M, Oldner A et al. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med 2010; 36: 1333–1340 [DOI] [PubMed] [Google Scholar]
- 22. Supavekin S, Zhang W, Kucherlapati R et al. Differential gene expression following early renal ischemia/reperfusion. Kidney Int 2003; 63: 1714–1724 [DOI] [PubMed] [Google Scholar]
- 23. Mishra J, Ma Q, Prada A et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003; 14: 2534–2543 [DOI] [PubMed] [Google Scholar]
- 24. Mori K, Lee HT, Rapoport D et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 2005; 115: 610–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson T, Quan S, Cheema K et al. Risk prediction models for acute kidney injury following major noncardiac surgery: systematic review. Nephrol Dial Transplant 2016; 31: 231–240 [DOI] [PubMed] [Google Scholar]
- 26. Haase-Fielitz A, Bellomo R, Devarajan P et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery-a prospective cohort study. Crit Care Med 2009; 37: 553–560 [DOI] [PubMed] [Google Scholar]
- 27. Tuladhar SM, Puntmann VO, Soni M et al. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol 2009; 53: 261–266 [DOI] [PubMed] [Google Scholar]
- 28. Parikh CR, Coca SG, Thiessen-Philbrook H et al. ; for the TRIBE-AKI Consortium. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011; 22: 1748–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koyner JL, Garg AX, Coca SG et al. ; for the TRIBE-AKI Consortium. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 2012; 23: 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Constantin JM, Futier E, Perbet S et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care 2010; 25: 176. [DOI] [PubMed] [Google Scholar]
- 31. Royakkers AA, Bouman CS, Stassen PM et al. Systemic and urinary neutrophil gelatinase-associated lipocalins are poor predictors of acute kidney injury in unselected critically ill patients. Crit Care Res Pract 2012; 2012: 712695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pickering JW, Endre ZH. The clinical utility of plasma neutrophil gelatinase-associated lipocalin in acute kidney injury. Blood Purif 2013; 35: 295–302 [DOI] [PubMed] [Google Scholar]
- 33. Arthur JM, Hill EG, Alge JL et al. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int 2014; 85: 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gomez H, Ince C, De Backer D et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014; 41: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aslan A, van den Heuvel MC, Stegeman CA et al. Kidney histopathology in lethal human sepsis. Crit Care 2018; 22: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vanmassenhove J, Glorieux G, Lameire N et al. Influence of severity of illness on neutrophil gelatinase-associated lipocalin performance as a marker of acute kidney injury: a prospective cohort study of patients with sepsis. BMC Nephrol 2015; 16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hiemstra B, Eck RJ, Koster G et al. Clinical examination, critical care ultrasonography and outcomes in the critically ill: cohort profile of the Simple Intensive Care Studies-I. BMJ Open 2017; 7: e017170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neyra JA, Hu MC, Minhajuddin A et al. Kidney tubular damage and functional biomarkers in acute kidney injury following cardiac surgery. Kidney Int Rep 2019; 4: 1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wiersema R, Koeze J, Hiemstra B et al. ; SICS Study Group. Associations between tricuspid annular plane systolic excursion to reflect right ventricular function and acute kidney injury in critically ill patients: a SICS-I sub-study. Ann Intensive Care 2019; 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siew ED, Ikizler TA, Matheny ME et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 2012; 7: 712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siew ED, Matheny ME, Ikizler TA et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 2010; 77: 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.