Abstract
Background
Obese kidney allograft recipients have worse results in kidney transplantation (KT). However, there is lack of information regarding the effect of body mass index (BMI) variation after KT. The objective of the study was to evaluate the effects of body weight changes in obese kidney transplant recipients.
Methods
In this study we used data from the Catalan Renal Registry that included KT recipients from 1990 to 2011 (n = 5607). The annual change in post-transplantation BMI was calculated. The main outcome variables were delayed graft function (DGF), estimated glomerular filtration rate (eGFR) and patient and graft survival.
Results
Obesity was observed in 609 patients (10.9%) at the time of transplantation. The incidence of DGF was significantly higher in obese patients (40.4% versus 28.3%; P < 0.001). Baseline obesity was significantly associated with worse short- and long-term graft survival (P < 0.05) and worse graft function during the follow-up (P < 0.005). BMI variations in obese patients did not improve eGFR or graft or patient survival.
Conclusions
Our conclusion is that in obese patients, decreasing body weight after KT does not improve either short-term graft outcomes or long-term renal function.
Keywords: epidemiology, graft function, kidney transplantation, obesity, survival analysis
INTRODUCTION
According to data from World Health Organization, the prevalence of obesity, described as a body mass index (BMI) ≥30 kg/m2, has increased in the general population from 5% to 10% in men and from 8% to 14% in women from 1980 to 2008. Moreover, in 2008, 35% of adult patients (>20 years old) presented as overweight (BMI ≥25 kg/m2) [1]. Obesity is recognized by the American Heart Association as a major cardiovascular risk factor, it is frequently associated with other cardiovascular risk factors and it is strongly related to metabolism disorders. Weight gain is related to elevation of arterial pressure, atherosclerosis and insulin resistance and has a negative effect on lipoprotein metabolism [2].
As in the general population, there has been an incremental increase of obesity in the end-stage renal disease (ESRD) population and also in kidney transplant candidates during the last decade, going from 6% to 11% in the Netherlands and from 26% to 34% in the USA [3].
The best renal replacement therapy (RRT) in patients with ESRD in terms of patient survival is kidney transplantation (KT). Although complications associated with obesity in renal transplantation are well proved [4, 5], some studies have shown a similar survival benefit with transplantation compared with dialysis in obese and non-obese patients [5, 6]. This benefit disappeared in patients with a BMI >41 kg/m2 [7]. Evidence from the published literature [8] shows better short-term patient and graft survival and graft function and less acute rejection in patients with a low BMI. Moreover, there were more surgical complications in obese patients. Seventy-five to 80% of kidney transplant recipients present at least one cardiovascular risk factor [9]. Despite transplantation decreases the risk of death and cardiovascular events in relation to dialysis patients, cardiovascular events are still the leading cause of death in kidney recipients, with an annual risk of 3.5 – 5%, 50-fold higher than the general population [10].
There are some groups that have studied the outcomes of KT in obese patients, but post-transplant outcomes in relation to changes in BMI during the follow-up have not previously been described. The main objective of this study was to analyse the effect of basal obesity and BMI changes during the follow-up on long-term graft and patient survival and graft function.
MATERIALS AND METHODS
After obtaining the approval of the Institutional Review Board, we used data from the Catalan Renal Registry (RMRC). This is a mandatory population-based registry covering 7.5 million people that collects information on all patients with ESRD requiring RRT in Catalonia. At the time of starting RRT and at every switch of treatment throughout RRT, a registration form is completed. Every year an update has to be carried out and sent to the RMRC through the finalization of RRT, death of the patient or lost to follow-up. In Catalonia, there are no standardized exclusion criteria with regards to obesity and KT.
Between 1990 and 2011, patients who lived in Catalonia and received a first single kidney transplant from a deceased or living donor were considered for the analysis. Patients were followed until death, lost to follow-up or 31 December 2015. The median follow-up time was 9.3 years, with a maximum of 25 years. The BMI was classified in the following four groups: underweight (BMI <18.5), normal weight (BMI ≥18.5–<25), pre-obese (BMI ≥25–<30) and obese (BMI ≥30). The annual change in post-transplantation BMI was calculated over the patient's follow-up (until December 2015). The donor variables that are collected include sex, age, cause of death and presence of hepatitis C virus. The described variables of the recipient are age, sex, primary renal disease, maximum anti-human leucocyte antibodies (HLAs), dialysis time before transplantation and immunosuppression treatment during the first 6 weeks. Transplant variables such as cold ischaemia time and HLA mismatches (A, B and DR) between donor and recipient were also considered.
Comparisons between groups of patients with and without obesity at the time of transplantation were performed by chi-square test for categorical data and analysis of variance for continuous data (P < 0.05 was considered significant). Baseline characteristics of the study cohort were expressed as number and proportion or mean ± standard deviation (SD). To evaluate graft function and change in weight, data were only available for those kidney grafts that survived until the first follow-up at 31 December. To evaluate the change in weight, we calculated the percent change between basal weight and weight during the follow-up for each year and then the mean of the different periods: [(weight at follow-up basal weight)/(basal weight)]*100. The statistical approach to calculate the adjusted model was done using a generalized estimating equation, which is used to estimate the parameters of a generalized linear model with a possible unknown correlation between outcomes.
We assessed kidney graft survival, defined as the period from transplant date until graft loss or patient death, whichever came first. Cumulative incidence function (CIF) were used to calculate the unadjusted incidence of graft survival (1-CIF) and patient survival during transplantation, considering patient death with functioning graft and graft loss as competing events, respectively. We also calculated the adjusted risk of patient death with a functioning graft and the adjusted risk of graft loss by means of competing risks regression, considering graft failure and patient death with a functioning graft as a competing event, respectively. The model was calculated and adjusted for BMI at the moment of KT, loss of weight between transplantation and overall follow-up, age and gender of the recipient, age of the donor, pre-transplantation dialysis time, type of KT, presence of diabetes mellitus, cardiovascular comorbidities (ischaemic heart disease, cardiac failure, cardiac conduction disorders, cerebrovascular disease or peripheral vascular disease), period of time for transplantation (1990–2000 and 2001–11) and treatment with tacrolimus. The final model was chosen using the Akaike information criterion, which assumes that the lower the values, the better the value of the model.
Statistical analyses were performed using Stata software version 13 (StataCorp, College Station, TX, USA).
RESULTS
We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines to report this observational study. In this population-based study, we included adult recipients living in Catalonia who had received a first deceased (n = 5415) or living donor kidney transplant (n = 568) between January 1990 and December 2011 (n = 5983) whose weight and height at transplantation were known (n = 5607 patients).
Characteristics of included recipients
Of the 5607 patients, 194 (3.5%) were underweight, 2904 (51.8%) normal weight, 1900 (33.9%) pre-obese and 609 (10.9%) were obese. Basal characteristics of donors and recipients were compared between the obese and non-obese groups (Table 1). We found some differences between groups: more obese recipients were transplanted during the 2001–11 period compared with the previous decade (P < 0.001), obese patients have more comorbidities (P < 0.001) and there were younger donors (P < 0.001) and recipients (P < 0.001) in the non-obese group. When we analysed the percentage of weight change during the follow-up, we found a weight gain in the majority of patients of all groups with a low percentage of patients changing the basal BMI category to obese (Table 2 and Supplementary data, Table S1). However, there were differences between groups, with small changes in weight in the obese group, a moderate increase in normal and pre-obese patients and a large increase in weight in the underweight group, especially during the first 2 years. Afterwards, the trend of weight change remained stable in all groups (Supplementary data, Figure S1 and Table S2).
Table 1.
Characteristics of included recipients depending on BMI group
| Characteristics | Obese (n = 609) | Pre-obese (n = 1900) | Normal weight (n = 2904) | Underweight (n = 194) | P-value |
|---|---|---|---|---|---|
| BMI (mean ± SD) | 33.2 ± 3.3 | 27.1 ± 1.4 | 22.3 ± 1.7 | 17.3 ± 1.0 | |
| Recipient variables | |||||
| Primary renal disease | |||||
| Glomerular | 144 (23.6) | 494 (26.0) | 847 (29.2) | 62 (32) | <0.001 |
| Polycystic | 76 (12.5) | 306 (16.1) | 476 (16.4) | 19 (9.8) | – |
| Interstitial | 77 (12.6) | 239 (12.6) | 434 (14.9) | 29 (14.9) | – |
| Vascular | 92 (15.1) | 239 (12.6) | 294 (10.1) | 17 (8.8) | – |
| Diabetes | 84 (13.8) | 143 (7.5) | 139 (4.8) | 7 (3.6) | – |
| Others | 25 (4.1) | 133 (7.0) | 251 (8.6) | 29 (14.9) | – |
| Unknown | 111 (18.2) | 346 (18.2) | 463 (15.9) | 31 (16.0) | – |
| Sex | |||||
| Male | 333 (54.7) | 1274 (67.1) | 1851 (63.7) | 65 (33.5) | <0.001 |
| Age (years) | |||||
| <45 | 130 (21.3) | 397 (20.9) | 1123 (38.7) | 121 (62.4) | <0.001 |
| 45–54 | 160 (26.3) | 501 (26.4) | 679 (23.4) | 30 (15.5) | – |
| 55–64 | 196 (32.2) | 593 (31.2) | 700 (24.1) | 32 (16.5) | – |
| ≥65 | 123 (20.2) | 409 (21.6) | 402 (13.8) | 11 (5.7) | – |
| Morbidity | |||||
| Any cardiovascular morbiditya | 144 (27.7) | 353 (22.5) | 392 (16.8) | 23 (15.2) | <0.001 |
| Diabetes mellitus | 221 (36.7) | 408 (21.7) | 399 (13.8) | 18 (9.3) | <0.001 |
| Hypertension | 352 (79.1) | 865 (71.7) | 115 (63.0) | 64 (56.1) | <0.001 |
| Maximum CDC (%) | |||||
| 0–10 | 503 (83.4) | 1612 (85.1) | 2399 (83.1) | 157 (81.3) | 0.307 |
| 11–50 | 74 (12.3) | 221 (11.7) | 364 (12.6) | 30 (15.5) | – |
| >50 | 26 (4.3) | 61 (3.2) | 124 (4.3) | 6 (3.1) | – |
| Dialysis time before KT (years) | |||||
| ≤1 | 156 (25.6) | 431 (22.7) | 718 (24.7) | 47 (24.2) | 0.610 |
| 1–2 | 150 (24.6) | 511 (26.9) | 727 (25.0) | 50 (25.8) | – |
| >2 | 303 (49.8) | 958 (50.4) | 1459 (50.2) | 97 (50.0) | – |
| Donor variables | |||||
| Donor type | |||||
| Deceased | 541 (88.8) | 1756 (92.4) | 2630 (90.6) | 173 (89.2) | 0.022 |
| Living donor | 68 (11.2) | 144 (7.6) | 274 (9.4) | 21 (10.8) | |
| Sex | |||||
| Male | 351 (57.9) | 1123 (59.5) | 1734 (60.1) | 116 (59.8) | 0.790 |
| Age (years) | |||||
| <45 | 173 (28.5) | 576 (30.5) | 1152 (40.0) | 104 (54.2) | <0.001 |
| 45–54 | 147 (24.2) | 394 (20.9) | 651 (22.6) | 39 (20.3) | – |
| 55–64 | 156 (25.7) | 492 (26.1) | 615 (21.3) | 28 (14.6) | – |
| ≥65 | 131 (21.6) | 425 (22.5) | 465 (16.1) | 21 (10.9) | – |
| Hepatitis C virus positive | 8 (1.6) | 23 (1.5) | 46 (2.0) | 6 (3.9) | 0.160 |
| Transplant procedure | |||||
| Period | |||||
| 1990–2000 | 167 (27.4) | 719 (37.8) | 1245 (42.9) | 83 (42.8) | <0.001 |
| 2001–11 | 442 (72.6) | 1181 (62.2) | 1659 (57.1) | 111 (57.2) | – |
| Cold ischaemia time (h) | |||||
| 0–18 | 328 (59.7) | 959 (57.7) | 1526 (60.5) | 101 (62.0) | 0.392 |
| 19–24 | 171 (31.1 | 529 (31.8) | 739 (29.3) | 42 (25.8) | – |
| >24 | 50 (9.1) | 174 (10.5) | 257 (10.2) | 20 (12.3) | – |
| Immunosuppression treatment during the first 6 weeks | |||||
| Tacrolimus | 314 (51.5) | 876 (48.9) | 1336 (48.3) | 94 (50.5) | 0.051 |
| Cyclosporine | 177 (30.8) | 682 (38.1) | 1122 (40.7) | 79 (42.5) | <0.001 |
| Mycophenolate | 449 (78.1) | 1257 (70.1) | 1857 (67.2) | 117 (62.9) | <0.001 |
| Basiliximab/daclizumab | 218 (38.2) | 549 (30.8) | 687 (25.1) | 41 (22.3) | <0.001 |
| Number of matches between donor and recipient (HLA-A, HLA-B and HLA-DR) | |||||
| 0 | 44 (7.3) | 118 (6.2) | 147 (5.1) | 14 (7.3) | 0.098 |
| 1 | 135 (22.4) | 385 (20.3) | 653 (22.6) | 34 (17.6) | – |
| 2 | 210 (34.9) | 689 (36.4) | 985 (34.1) | 67 (34.7) | – |
| 3 | 160 (26.6) | 508 (26.8) | 847 (29.3) | 60 (31.1) | – |
| ≥4 | 53 (8.8) | 194 (10.2) | 255 (8.8) | 18 (9.3) | – |
Values presented as n (%).
Ischaemic heart disease, cardiomyopathy, cardiac conduction disorders, cerebrovascular disease or vascular disease. CDC, classic complement-dependent cytotoxicity crossmatch technique.
Table 2.
Results of kidney transplantation depending on BMI group
| Results | Obese (n = 609) | Pre-obese (n = 1900) | Normal weight (n = 2904) | Underweight (n = 194) | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Delayed graft function, n (%) | |||||||||
| Yes | 229 (40.4) | 568 (32.4) | 750 (28.3) | 30 (17.6) | <0.001 | ||||
| No | 338 (59.6) | 1183 (67.6) | 1898 (71.7) | 140 (82.4) | – | ||||
| Cause of graft loss during the first year, n (%) | |||||||||
| Acute rejection | 12 (22.2) | 30 (22.7) | 39 (24.8) | 7 (35) | n/a | ||||
| Chronic allograft nephropathy/rejection | 4 (7.4) | 13 (9.8) | 16 (10.2) | 2 (10) | |||||
| Complications | 34 (63.0) | 70 (53.0) | 86 (54.8) | 8 (40) | |||||
| Renal primary disease recurrence | 1 (1.8) | 1 (0.8) | 4 (2.5) | 1 (5) | |||||
| De novo glomerulonephritis | 0 (0) | 0 (0) | 1 (0.6) | 0 (0) | |||||
| Unknown | 3 (5.6) | 18 (13.6) | 11 (7.0) | 2 (10) | |||||
|
| |||||||||
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | – | |
|
| |||||||||
| CKD-EPI | |||||||||
| First follow-up | 474 | 43.4 ± 19.1 | 1558 | 45.9 ± 18.5 | 2389 | 49.5 ± 19.5 | 157 | 54.8 ± 24.2 | <0.001 |
| Second follow-up | 449 | 47.4 ± 17.7 | 1487 | 47.4 ± 17.5 | 2339 | 51.1 ± 18.4 | 148 | 53.6 ± 20.2 | <0.001 |
| Third follow-up | 452 | 47.6 ± 18.6 | 1491 | 47.1 ± 17.6 | 2360 | 50.6 ± 18.8 | 146 | 53.7 ± 22.3 | <0.001 |
| Fourth follow-up | 449 | 47.3 ± 19.2 | 1444 | 48.0 ± 18.1 | 2304 | 50.5 ± 19.5 | 141 | 54.0 ± 22.7 | <0.001 |
| Fifth follow-up | 398 | 47.8 ± 18.4 | 1347 | 48.0 ± 18.6 | 2171 | 50.3 ± 19.5 | 130 | 54.5 ± 24.5 | <0.001 |
| % of weight change (from basal weight) | |||||||||
| First follow-up | 474 | −0.85 ± 8.6 | 1558 | 0.73 ± 7.6 | 2389 | 3.61 ± 8.4 | 157 | 7.04 ± 10.6 | <0.001 |
| Second follow-up | 449 | 0.17 ± 10.7 | 1487 | 2.99 ± 9.5 | 2339 | 6.73 ± 10.2 | 148 | 11.44 ± 12.2 | <0.001 |
| Third follow-up | 452 | 0.23 ± 10.9 | 1491 | 2.87 ± 10.0 | 2360 | 6.70 ± 10.9 | 146 | 12.39 ± 13.7 | <0.001 |
| Fourth follow-up | 449 | 1.22 ± 11.1 | 1444 | 3.42 ± 10.3 | 2304 | 7.52 ± 11.2 | 141 | 12.56 ± 13.0 | <0.001 |
| Fifth follow-up | 398 | 0.68 ± 11.2 | 1347 | 2.92 ± 10.3 | 2171 | 7.28 ± 11.0 | 130 | 11.85 ± 13.7 | <0.001 |
Yearly follow-ups.
Delayed graft function
Delayed graft function (DGF) was defined as the need for dialysis in first week, excluding the first 24 h. A greater incidence of DGF was found in the cohort of obese recipients (40.4), compared with 17.6% in underweight (n = 30), 28.3% in normal weight (n = 750) and 32.4% in pre-obese (n = 568) (Table 2).
Graft function
To evaluate graft function, we only had data for those kidney grafts that survived at least until the first post-transplantation year (n = 5262). During the first 5 years of follow-up, all the included population except the underweight group tended to improve eGFR [Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula]. In the underweight patients, we found an initial decline in eGFR, but afterwards they maintained better graft function during the follow-up compared with other groups (P < 0.001). On the other hand, obese patients had the worst eGFR during the follow-up (P < 0.001) (Supplementary data, Figure S1 and Table S2).
In obese patients, we did not find any beneficial effect of weight change or after adjusting for other covariables (Table 3). In this cohort of recipients, factors that improved the value of eGFR were male sex or age <45 years, receiving a kidney from a donor <65 years of age and immunosuppression with tacrolimus. On the other hand, those obese patients who developed DGF had worse eGFR in the multivariate analysis [mean difference −5.90 mL/min/1.73 m2 (range−8.66 to −3.14), P < 0.05] (Table 3).
Table 3.
Multivariate model evaluating the change in the mean value of the CKD-EPI equation (mL/min/1.73 m2) depending on the change in BMI in obese patients
| Variables | Change in mean value (range) of CKD-EPI (mL/min/1.73 m2) |
|---|---|
| Percentage of weight change | |
| Gain/loss <1% | Reference |
| Loss 10–1% | 1.21 (−0.68–3.10) |
| Loss >10% | 0.63 (−1.87–3.13) |
| Gain >1% | −0.86 (−2.51–0.79) |
| Sex | |
| Male | Reference |
| Female | −3.39 (−6.04 to −0.73)* |
| Donor age (years) | |
| <45 | Reference |
| 45–54 | −13.22 (−16.83 to −9.60)* |
| 55–64 | −16.04 (−20.02 to −12.05)* |
| ≥65 | −18.60 (−23.01 to −14.2)* |
| Recipient age (years) | |
| <45 | Reference |
| 45–54 | 0.09 (−3.71–3.89) |
| 55–64 | −0.85 (−4.60–2.91) |
| 65–69 | −5.26 (−10.08 to −0.44)* |
| ≥70 | −5.70 (−11.99–0.59) |
| Period | |
| 1990–2000 | Reference |
| 2001–11 | 4.12 (0.84–7.40)* |
| DGF | |
| No | Reference |
| Yes | −5.90 (−8.66 to −3.14)* |
| Tacrolimus | |
| No | Reference |
| Yes | 5.00 (1.96–8.04)* |
P < 0.05.
Graft survival
Short-term graft survival was worse in underweight (89.8%) and obese patients (91.1%) compared with pre-obese (93%) or normal weight patients (94.6%) (P = 0.043) (Supplementary data, Figure S2). The adjusted subhazard ratio (SHR) showed a statistically significant increased risk of graft loss in the underweight [SHR 2.28 (95% CI 1.27–4.12); P < 0.001] and obese group [SHR 1.67 (95% CI 1.23–2.28); P < 0.001] compared with the normal group. Presenting with DGF significantly increased the risk of graft loss [SHR 2.91 (95% CI 2.32–3.64); P < 0.001] (Table 4).
Table 4.
Risk factors for graft loss
| Variables | Graft loss |
|||
|---|---|---|---|---|
| At 1 year |
Long term |
|||
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Obesity | ||||
| Normal weight | Reference | |||
| Underweight | 1.95 (1.23–3.11)* | 2.28 (1.27–4.12)* | 1.39 (1.09–1.78)* | 1.39 (1.05–1.84)* |
| Pre-obese | 1.30 (1.03–1.63)* | 1.11 (0.83–1.48) | 0.97 (0.87–1.08) | 0.93 (0.82–1.05) |
| Obese | 1.67 (1.23–2.28)* | 1.59 (1.11–2.26)* | 1.22 (1.04–1.44)* | 1.29 (1.08–1.54)* |
| Period | ||||
| 1990–2000 | Reference | – | – | – |
| 2001–11 | 0.75 (0.62–0.91)* | 1.41 (1.04–1.91)* | 0.67 (0.61–0.74)* | 0.96 (0.84–1.08) |
| Recipient age (years) | ||||
| <45 | Reference | – | – | – |
| 45–54 | 1.08 (0.81–1.42) | 0.81 (0.54–1.19) | 0.84 (0.75–0.95)* | 0.69 (0.59–0.79)* |
| 55–64 | 1.19 (0.92–1.55) | 0.90 (0.60–1.34) | 0.80 (0.71–0.90)* | 0.58 (0.49–0.68)* |
| ≥65 | 1.56 (1.18–2.07)* | 1.09 (0.69–1.73) | 0.79 (0.68–0.92)* | 0.49 (0.39–0.60)* |
| Maximum CDC (%) | ||||
| 0–10 | Reference | – | – | – |
| 11–50 | 1.62 (1.24–2.11)* | 1.55 (1.10–2.18)* | 1.31 (1.14–1.49)* | 1.26 (1.08–1.47)* |
| >50 | 2.52 (1.77–3.60)* | 3.02 (1.94–4.72)* | 1.69 (1.37–2.09)* | 1.71 (1.35–2.16)* |
| Tacrolimus | ||||
| No | Reference | – | – | – |
| Yes | 0.60 (0.48–0.75)* | 0.63 (0.48–0.85)* | 0.58 (0.52–0.64)* | 0.63 (0.55–0.71)* |
| Donor type | ||||
| Deceased | Reference | – | – | – |
| Living | 0.39 (0.24–0.64)* | 0.75 (0.39–1.45) | 0.53 (0.41–0.66)* | 0.62 (0.46–0.84)* |
| Donor age (years) | ||||
| <45 | Reference | – | – | – |
| 45–54 | 1.25 (0.95–1.64) | 1.37 (0.95–1.98) | 1.17 (1.03–1.32)* | 1.45 (1.25–1.68)* |
| 55–64 | 1.21 (0.92–1.59) | 1.38 (0.93–2.07) | 1.27 (1.12–1.43)* | 1.82 (1.55–2.13)* |
| ≥65 | 1.58 (1.20–2.07)* | 1.81 (1.16–2.81)* | 1.37 (1.20–1.57)* | 2.45 (2.01–2.98)* |
| DGF | ||||
| No | Reference | – | – | – |
| Yes | 2.91 (2.32–3.64)* | 2.83 (2.18–3.69)* | 1.57 (1.41–1.74)* | 1.47 (1.31–1.65)* |
P < 0. 05.
CDC, classic complement-dependent cytotoxicity crossmatch technique.
We found 363 graft losses during the first year. The majority of them (54.5%) were due to vascular thrombosis not related to immunological rejection, graft infection or primary non-function (Table 2).
The median graft survival time was 8.6 years, with a maximum value of 25 years. Until the end of follow-up, taking into account death as a competing risk, graft survival was also worse in obese (68%) and underweight (62.7%) patients compared with normal weight (69.5%) and pre-obese (71.2%) patients (P = 0.003) (Supplementary data, Figure S2). When the basal weight category was adjusted to other factors, obesity still increased graft loss [SHR 1.29 (95% CI 1.08–1.54); P < 0.05] (Table 4).
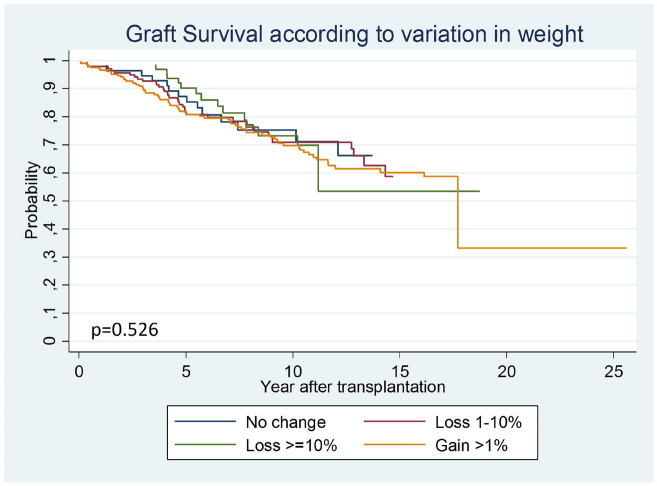
In obese recipients, when we evaluated the effect of post-transplant weight change on graft survival, we did not find any benefit of losing weight (P = 0.526). When we performed the multivariate model we found no benefit [1–10% loss: SHR 1.30 (95% CI 0.66–2.57), if >10% loss: SHR 0.98 (95% CI 0.44–2.18)], nor worse outcomes if weight increased [SHR 1.68 (95% CI 0.91–3.12)] (Figure 1, Supplementary data, Table S2).
FIGURE 1.
Comparison of graft survival curves according to variations in weight in obese patients. In the obese patients, weight change did not modify graft survival.
Patient survival
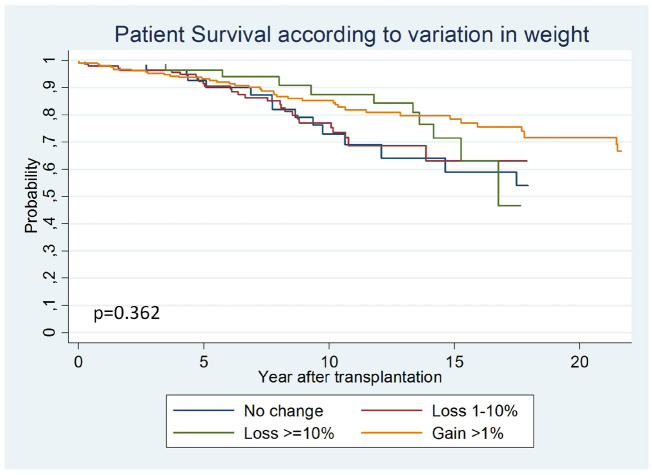
Patient survival considering the entire cohort of patients was worse in the pre-obese group (P < 0.001) (Supplementary data, Figure S3 and Table S5). When the hazard ratio (HR) was adjusted for other variables in a multivariate analysis, this effect disappeared (Table 5). When we evaluated the effect of weight change on obese patient mortality in a multivariate analysis, changes in weight had no impact on patient mortality [1–10% loss: SHR 1.79 (95% CI 0.79–4.06), >10% loss: SHR 1.24 (95% CI 0.48–3.16) and weight gain >1%: SHR 1.18 (95% CI 0.54–2.54)] (Figure 2 and Supplementary data, Table S3).
Table 5.
Risk factors for patient mortality
| Patient mortality |
||
|---|---|---|
| Variables | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
| Obesity | ||
| Normal weight | Reference | |
| Underweight | 0.71 (0.49–1.02) | 1.29 (0.84–1.96) |
| Pre-obese | 1.29 (1.14–1.46)* | 0.90 (0.78–1.03) |
| Obese | 1.20 (0.99–1.46) | 0.89 (0.71–1.1) |
| Recipient sex | ||
| Male | Reference | – |
| Female | 0.81 (0.72–0.9)* | 0.72 (0.63–0.83)* |
| Recipient age (years) | ||
| <45 | Reference | – |
| 45–54 | 3.08 (2.49–3.8)* | 2.87 (2.17–3.79)* |
| 55–64 | 6.18 (5.08–7.51)* | 6.05 (4.67–7.83)* |
| 65–69 | 9.53 (7.7–11.81)* | 9.59 (7.25–12.67)* |
| ≥70 | 11.47 (9.1–14.45)* | 13.11 (9.83–17.48)* |
| Any cardiovascular morbidity* | ||
| No | Reference | – |
| Yes | 1.96 (1.71–2.24)* | 1.28 (1.11–1.48)* |
| Dialysis time before KT (years) | ||
| ≤1 | Reference | |
| 1–2 | 1.67 (1.41–1.98)* | 1.34 (1.11–1.63)* |
| >2 | 2.00 (1.72–2.33)* | 1.43 (1.2–1.72)* |
P < 0. 05.
FIGURE 2.
Comparison of patient survival curves according to variations in weight in obese patients. In the obese patients, survival was not different depending on the weight change category.
DISCUSSION
Although transplantation is the best option for RRT in terms of patient survival [5], one of the main limitations of access to waiting lists is overweight. The main objective of this study was to analyse the effect of basal obesity and BMI changes during the follow-up and their relation with graft function and graft and patient survival.
In a prospective cohort of the French Renal Epidemiology and Information Network Registry [11], the authors did a multivariate analysis (evaluating also age, diabetes, congestive heart failure, cancer, albuminaemia level and type of dialysis) and they found that patients with a BMI ≥31 kg/m2 at the start of dialysis were less likely to receive a kidney transplant and this probability increased as the weight decreased [HR 1.09 (95% CI 1.06–1.10)]. There are four major KT guidelines regarding this cardiovascular risk factor. Both the UK Renal Association [7] and Kidney Health Australia– Caring for Australasians with Renal Impairment) [12] state that although obesity is not a formal contraindication of KT, the benefits of KT are doubtful in individuals with a BMI ≥40. European Renal Best Practice guidelines [13] only recommend weight loss in obese patients. The Kidney Disease: Improving Global Outcomes guidelines [14] state that weight reduction before the surgical procedure did not provide as much beneficial effect as could be expected in general population, so they did not make any specific recommendation about the topic. There are many studies that have evaluated the outcomes in KT related to weight, but this is the first one that evaluates post-transplant outcomes in obese patients in relation to long-term changes in BMI during the follow-up.
In our Catalan cohort, we found 10.9% of patients were obese before KT. This prevalence is quite different from other registries; for example, the United Network for Organ Sharing data showed a prevalence of 30% [15] and a group published data from the Australia and New Zealand renal registry estimating 24% of patients were obese [16].
There is a relationship between obesity and an increase in DGF. This incremental risk could be explained because of the more complex surgical procedure, which consequently brings a longer duration of cold ischaemia time [17]. Lafranca et al. [8] published a recent meta-analysis including 30 studies (15 262 recipients) that showed an overall risk ratio of 1.52 (95% CI 1.35–1.72; P < 0.001). There are two concomitant publications, Hill et al. [4] and Nicoletto et al. [18], that found similar results. In a newly published study, the authors showed the beneficial effects of laparoscopic sleeve gastrectomy before KT in patients that met the criteria for bariatric surgery (BMI ≥40kg/m2 or ≥35 kg/m2 with two or more obesity-related conditions) [19]. They found less DGF and better eGFR in those patients who underwent gastrectomy before KT compared with a matched control group. This finding is similar in our data, with the highest incidence of DGF in the obese group (40.4%), remaining statistically significant after multivariate analysis. Apart from surgical factors, the higher metabolic demand in obese patients could also contribute to the higher incidence of DGF in this cohort.
The only published study that shows data about GFR is one by Moreira et al. [20]. It concludes that pre-transplant overweight and obese patients present significantly lower GFR at 5 years. In our study, when data were analysed for subgroups, we found that obesity was related with worse eGFR and that weight changes in the obese group did not modify eGFR. This information has not been previously reported. Our eGFR data were analysed based on the CKD-EPI equation that is based on serum creatinine, which is related with muscle mass and is also a contributor to BMI. It has to be considered equation that underweight patients have lower muscle mass and therefore better eGFR measured by the CKD-EPI equation. In support of this interference, there were no differences in graft survival between groups. DGF impacted negatively on long-term eGFR in obese patients. On the other hand, underweight recipients that remained with a functioning graft had a better eGFR during the follow-up compared with the other groups. This effect could be explained because obese recipients have higher rates of hypertension, post-transplant diabetes and hyperfiltration due to an unpaired ratio between donor nephron mass and recipient BMI that cannot be changed later with a weight loss.
In our study, when we analysed all the included population, we found increased first-year graft loss in underweight and obese patients, which can be explained by a worse basal clinical situation to receive a kidney transplant. In the case of obese recipients, this increased weight brings greater surgical difficulties and more complications related to it. We also evaluated change in weight of obese patients and show that these weight changes did not change graft survival. These results have been adjusted by risk factors such as sex of the recipient, type of donor, age of the donor and recipient, time in dialysis before transplantation, DGF or use of tacrolimus. Those short-term, obesity-related complications may jeopardize the possible long-term benefits of losing weight. The idea of worse short- and long-term graft survival in patients with higher BMI is well established, but the finding of the absence of a beneficial effect of losing weight has never been published before. This short-term effect could be explained because of more surgical complications, wound infections and a higher risk of DGF.
KT brings higher patient survival compared with haemodialysis in all patients, including obese patients. There are three recent meta-analyses that evaluated this outcome post-KT and show different results in patient survival [8, 18]. These differences could be explained because survival was evaluated at different time points (1, 2, 3 or 5 years of follow-up) or because the selection of the included studies is different. Perhaps the year of publication may also explain these differences; as Nicoletto et al. [18] have already described, they found better survival in obese patients in reports published after 2003. Similar to graft survival, patient survival can be negatively affected by cardiovascular risk factors. All the published studies except one used BMI data from the pre-transplant period to classify the patients [3]. That study takes into account weight changes and post-transplant BMI. They described that both pre-transplant and especially 1-year post-transplantation obesity brings a higher risk for mortality and graft failure and that weight gain 1 year after transplantation brings a higher risk of death and graft failure independent of pre-transplant BMI. In our experience, after considering all confounding risk factors, the weight change during follow-up did not have any effect on patient survival.
Our study has several strengths and limitations. In this study, we report long-term follow-up, with a median of 8.6 years and a maximum of 25 years, and it is the only study with baseline and follow-up data for BMI variations. The main limitation is the retrospective nature of the routine data obtained through the Catalonian Renal Registry. Although multiple confounding factors were considered, there may be unmeasured residual confounders not collected by the registry that could also have contributed to the study findings.
In conclusion, in this large retrospective cohort study with long-term follow-up, we show worse renal function and short-term graft survival outcomes in obese patients. The possible long-term benefits of losing weight for graft survival and graft function may be jeopardized by short-term obesity-related complications, particularly DGF. The implication for practice of these findings is that it is necessary to focus on losing weight before KT and not after.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all the staff working at the RMRC and all the health professionals involved in data management in the nephrology and KT units in Catalonia. The Follow-up Committee of the RMRC includes the following members: Dr A. Martínez Castelao, Hospital Universitari de Bellvitge; Dr M. Troya, Hospital de Badalona Germans Trias i Pujol; Dr A. Cases Hospital Clínic i Provincial de Barcelona; Dr J. Calabia, Hospital de Girona Dr. Josep Trueta; Dr H. Cao, Hospital del Mar; Dr A. Segarra, Hospital de Lleida Arnau de Vilanova; Dr A. Martínez Vea, Hospital de Tarragona Joan XXIII; Dr S. Gil-Vernet, Comissió Assessora de Trasplantament Renal i Pancreàtic; Dr J.M. Díaz, Fundació Puigvert; Dr E. Espinel, Hospital General Vall d’Hebron; Dr E. Lara, Hospital Maternoinfantil Vall d’Hebron; E. Arcos, J. Comas i J. Tort, Registre de Malalts Renals de Catalunya, Organització Catalana de Trasplantaments.
FUNDING
N.M. is supported by a grant of the Spanish Nephrologist Society. N.M., M.Q., I.R., N.L., E.M., O.B. and J.M.C. have funding from ISCIII-FEDER RD16/0009/0003.
AUTHORS’ CONTRIBUTIONS
N.M. analysed and interpreted the data and drafted the manuscript. E.A. and J.C. acquired the data, analysed data, drafted the manuscript and revised it critically. M.Q., I.R., N.L., A.C., M.M., A.M., E.M., O.B. and J.T drafted the manuscript. J.M.C. designed the study, interpreted data and drafted the manuscript and revised it critically.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.World Health Organization Obesity. 2015. http://www.who.int/gho/ncd/risk_factors/obesity_text/en/ (2 July 2017, date last accessed)
- 2. Krauss RM, Winston M, Fletcher BJ et al. Obesity: impact on cardiovascular disease. Circulation 1998; 98: 1472–1476 [PubMed] [Google Scholar]
- 3. Hoogeveen EK, Aalten J, Rothman KJ et al. Re: effect of obesity on the outcome of kidney transplantation: a 20-year follow-up. Transplantation 2011; 91: 869–874 [DOI] [PubMed] [Google Scholar]
- 4. Hill CJ, Courtney AE, Cardwell CR et al. Recipient obesity and outcomes after kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant 2015; 30: 1403–1411 [DOI] [PubMed] [Google Scholar]
- 5. Gill JS, Lan J, Dong J et al. The survival benefit of kidney transplantation in obese patients. Am J Transplant 2013; 13: 2083–2090 [DOI] [PubMed] [Google Scholar]
- 6. Lanton C, Ao T, Ruess DAC et al. Impact of renal transplantation on survival in end-stage renal disease patients with elevated body mass index. Kidney Int 2003; 63: 647–653 [DOI] [PubMed] [Google Scholar]
- 7. Dudley C, Harden P. Renal association clinical practice guideline on the assessment of the potential kidney transplant recipient. Nephron Clin Pract 2011; 118: 209–224 [DOI] [PubMed] [Google Scholar]
- 8. Lafranca JA, IJermans JN, Betjes MG et al. Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med 2015; 13: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation 2006; 82: 603–611 [DOI] [PubMed] [Google Scholar]
- 10. Ducloux D, Bourrinet E, Motte G et al. Antiphospholipid antibodies as a risk factor for atherosclerotic events in renal transplant recipients. Kidney Int 2003; 64: 1065–1070 [DOI] [PubMed] [Google Scholar]
- 11. Hannedouche T, Lassalle M, Massy A. et al. Obesity and access to kidney transplantation in patients starting dialysis: a prospective cohort study. PLoS One 2017; 12: e0176616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campbell S, Pilmore H, Gracey D et al. KHA-CARI guideline: recipient assessment for transplantation. Nephrology (Carlton) 2013; 18: 455–462 [DOI] [PubMed] [Google Scholar]
- 13. Abramowicz D, Cochat P, Claas FHJ et al. European Renal Best Practice guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant 2015; 30: 1790–1797 [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(Suppl 3): S1–S155 [DOI] [PubMed] [Google Scholar]
- 15. Cannon RM, Jones CM, Hughes MG et al. The impact of recipient obesity on outcomes after renal transplantation. Ann Surg 2013; 257: 978–984 [DOI] [PubMed] [Google Scholar]
- 16. Zrim S, Furlong T, Grace BS et al. Body mass index and postoperative complications in kidney transplant recipients. Nephrology (Carlton) 2012; 17: 582–587 [DOI] [PubMed] [Google Scholar]
- 17. Daly PJA, Power RE, Healy DA et al. Delayed graft function: a dilemma in renal transplantation. BJU Int 2005; 96: 498–501 [DOI] [PubMed] [Google Scholar]
- 18. Nicoletto BB, Fonseca NKO, Manfro RC et al. Effects of obesity on kidney transplantation outcomes. Transplantation 2014; 98: 167–176 [DOI] [PubMed] [Google Scholar]
- 19. Kim Y, Jung AD, Dhar VK et al. Laparoscopic sleeve gastrectomy improves renal transplant candidacy and posttransplant outcomes in morbidly obese patients. Am J Transplant 2017; 18: 410–416 [DOI] [PubMed] [Google Scholar]
- 20. Moreira TR, Bassani T, de Souza G et al. Obesity in kidney transplant recipients: association with decline in glomerular filtration rate. Ren Fail 2013; 35: 1199–1203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.