The ability to perceive motion is a fundamental property of the visual system, and one of its most basic aspects is the ability to discriminate moving objects from motionless ones, for example, motion detection. Velocity thresholds represent the minimum rate of displacement over time unit that an animal is able to perceive as movement, any slower motion being not discriminable from a still object. Although such topic has grabbed the attention of scientists already at the beginning of the 20th century, the interest has waned in time, and velocity thresholds have thus far been assessed in only a handful of species. Thresholds for pigeons Columba livia, rats Rattus norvegicus, and cats Felis catus fall in an approximate range of 1–10 deg/s. (Since perceived linear speed depends on the distance between the moving stimulus and the observer, angular speed [i.e., angle of the observer’s field of view/unit time], which is distance invariant, is the preferred unit in the speed perception literature) (Hawley and Munn 1933; Pasternak and Merigan 1980; Martinoya et al. 1983). Thresholds <1 deg/s are only reported for nonhuman primates (Carpenter and Carpenter 1958), falling to under 0.1 deg/s for adult humans (Snowden and Kavanagh 2006).
Dogs’ visual processing received growing interest in recent years (Byosiere et al. 2018). Within the area of motion perception, the topic that has received more attention is certainly the perception of biological motion (Delanoeije et al. 2020 and references therein). Fewer studies have explored more basic aspects of dogs’ motion perception. Very early work suggests that dogs could recognize moving objects from a distance of 800 to 900 m, but could recognize stationary objects only at a distance of 500–600 m, which would imply a particular aptness at detecting moving entities by dogs. More recently, however, dogs’ ability to detect motion as a function of the stimulus coherence has been explored (Kanizsár et al. 2017). The latter represents the proportion of local constituents in a visual scene, which moves with the same direction and speed. Dogs were found to require an average of 42% of coherence to discriminate a random-dot display from 1 with a coherence of 0%. This indicates a poor ability to detect coherent motion, especially when compared with humans’ 5% coherence threshold. Moreover, although such ability can be improved through experience, thresholds in dogs are higher than those reported for humans (Kanizsár et al. 2018). Therefore, the notion that dogs could be particularly apt to perceive motion was not supported, at least in terms of coherence threshold. To our knowledge, no one has explored what are the perceptual limits of dogs in terms of motion velocity thresholds. The aim of this study was therefore to determine the minimum velocity that dogs can perceive as motion.
To this aim, we employed a modified method of limits, whereby dogs (N = 6) were first trained to discriminate a static random-dot display from 1 where dots moved at a clearly perceivable velocity. Once dogs reached a discrimination accuracy of at least 90% over 6 consecutive 20-presentation sessions, subjects underwent a series of descending and ascending assessment, where the speed of dots in the discriminandum random-dot display was systematically varied. Methodological details can be found in the Supplementary Materials.
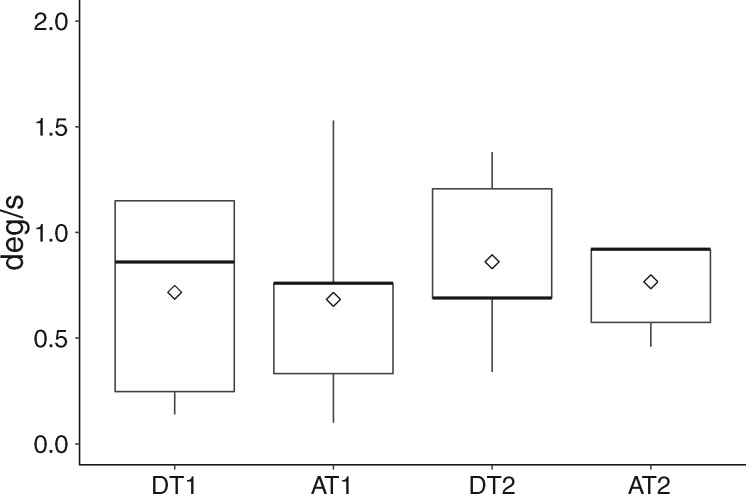
All the 6 enrolled dogs reached the learning criterion in the training phase. The mean number of sessions to reach such criterion was 23.3 ± 11.3, ranging from 13 to 42. The mean value and the distribution of thresholds obtained in each of the 4 assessments are presented in Figure 1. Individual dogs’ thresholds are presented in the Supplementary Material. The mean individual velocity threshold ranged between 0.26 deg/s and 1.24 deg/s. The overall mean velocity threshold in our sample was of 0.76 ± 0.38 deg/s. Mean velocity thresholds were not correlated with the length of the training (Pearson correlation, r = −0.67, df = 4, P = 1.44). Analysis of Pearson’s correlations revealed a correlation between the first and second descending assessments (r = 0.75, P = 0.083), between the first and second ascending assessments (r = 0.832, P = 0.048), as well as between the average descending and the average ascending thresholds (r = 0.878, P = 0.021). Finally, there was no significant within-subject difference between thresholds obtained in the 4 assessments (repeated-measures ANOVA, F = 0.66, P = 0.59).
Figure 1.
Boxplot showing the mean (diamond shape) and distribution (min, 1st, 2nd [bold line], 3rd quartile, and max) of velocity thresholds of each dog in descending assessment 1 (DT1), ascending assessment 1 (AT1), descending assessment 2 (DT2), ascending assessment 2 (AT2).
The findings of this study set dogs’ threshold at lower values than that of other nonprimate species, including pigeons, rats, and cats. Average thresholds comparable with those found in dogs are reported for chimpanzees, whereas thresholds reported for adult humans are clearly lower. Thus, dogs seem to be better at detecting slow motion than other nonprimate species, and possibly close to that of nonhuman primates.
Before exploring biological reasons for the difference between dogs and other species, some methodological aspects should be considered. First, in the current experiment, random-dot displays were used. However, different stimuli may lead to different results. For example, in cats, a threshold of 6.4 deg/s was obtained using rotating crosses and of 1.3 deg/s using random-dot displays (Pasternak and Merigan 1980). Another methodologically relevant aspect is the angle at which stimuli were presented, relative to the animal’s eyes. Evidence from humans and pigeons indicates that different retinal regions have different sensitivity to motion (Martinoya et al. 1983). Therefore, optimal viewing angles for motion detection are shifted laterally for species with eyes placed laterally on the head, as the case of pigeons, and frontally for those with more forward-oriented eyes, as humans or cats. As regards the present experiment, stimuli were presented on 2 monitors, each of which fell slightly off-center in the dogs’ field of view, thus matching the relatively lateral placement of eyes on dogs’ heads. In addition, dogs’ head movement was not constrained, allowing subject to rotate their head to their liking. In this sense, it can be assumed that thresholds were obtained in the best conditions for detection—at least for what concerns viewing angle. Finally, methods based on psychometric estimation in most cases define thresholds as the value of the physical entity (in this case velocity), which results in an estimated detection accuracy of 75%. In the method of limits, thresholds represent a hypothetical accuracy of 50% and should be compared with corresponding accuracies, not with the common 75% thresholds, obtained in studies relying on psychometric estimations.
Even after taking into account appropriate corrections due to methodological differences, dogs’ thresholds are still lower than those of pigeons and rats. Interestingly, a negative relationship seems to exist between detection thresholds for motion velocity and motion coherence, that is, the degree of consistency in terms of direction and speed of movement among local elements composing a visual scene. Coherent motion detection thresholds are substantially lower in pigeons and rats, than in dogs (Kanizsár et al. 2018), while the opposite holds for velocity thresholds, as discussed above. It is possible that the trade-off between velocity and coherence detection abilities reflects a specialization of the visual system linked to the species’ ecology. For instance, while the diet of all such species includes live animals, pigeons’ and rats’ preys are relatively smaller than those of dogs. Thus, pigeons’ and rats’ visual system may have evolved to favor the detection of relatively smaller moving items (i.e., patches of motion with lower coherence within the visual field), but at the expense of velocity. Vice versa, dogs’ visual system seems tuned to the detection of relatively larger objects but allowing the detection of slower movements.
The differences between dogs’ and cats’ thresholds are less striking than with other species. Nevertheless, the current results suggest that dogs may be slightly better than cats in detecting slow motion. Again, these differences may hold a functional relevance for the species’ behavioral ecology. Both species use a variety of sensory cues for successful predation, among which vision plays a considerable role (Fitzgerald and Turner 2000; Coppinger and Coppinger 2001). Cats, however, are ambush predators, which hunt the prey from their close surrounding and therefore rely on vision for short distances. In dogs, predatory behavior seems to be triggered by the distant prey movement. Since perceived velocity is negatively correlated with the distance between the observer and the moving object, detection of distant preys also requires the ability to detect slow motion. Therefore, the relatively lower velocity detection threshold in dogs compared to another carnivore species, such as the cat, might have evolved as an adaption to spot relatively distant preys.
The study employed a limited number of adult subjects and did not explore aspects that are believed to or known to affect motion perception abilities. For instance, as only adult animals were employed, no conclusion can be drawn from our data about the developmental trajectory of dogs’ ability to detect slow motion in the first weeks or months of life, an aspect which received some attention in both primates and humans. On the contrary, ontogeny of a perceptual process does not just depend on developmental age, but also on experience. It is possible that lower thresholds were to be found in dogs that underwent specific training to detect moving stimuli—as could be the case, for instance, of dogs used for sight hunting. At the same time, the existence of dog breeds specifically selected for sight-hunting would represent an ideal condition to investigate genetic contributions to motion detection. Moreover, the diversity of dogs in terms of head morphology provides a good opportunity to investigate differences due to eye positioning, in terms of both absolute thresholds and relative sensitivity to movement of different sectors of the visual field. Exploration of the role of age, experience, and breed on dogs’ motion detection abilities has important theoretical and practical implications and will be tackled in future studies.
Experimental Ethics
The current experiment was carried out in accordance with the national and European legislation regarding the involvement of animals in scientific research.
Funding
This study was supported through funds of the University of Padua to the Department of Comparative Biomedicine and Food Science (PhD grant awarded to O.K., research scholarship awarded to C.G.), of the Italian Ministry of Education, University and Research (MIUR, Progetto Dipartimenti di Eccellenza, DM 11/05/2017 n. 262) to the Department of General Psychology, and of Fondazione CARIPARO (PhD grant awarded to M.L.).
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
Conflict of Interest statement
The authors decleare no conflict of interest.
Supplementary Material
References
- Byosiere SE, Chouinard PA, Howell TJ, Bennett PC, 2018. What do dogs Canis familiaris see? A review of vision in dogs and implications for cognition research. Psychon Bull Rev 25:1798–1813. [DOI] [PubMed] [Google Scholar]
- Carpenter B, Carpenter JT, 1958. The perception of movement by young chimpanzees and human children. J Comp Physiol Psychol 51:782–784. [DOI] [PubMed] [Google Scholar]
- Coppinger R, Coppinger L, 2001. Dogs: A Startling New Understanding of Canine Origin, Behavior & Evolution. New York: Simon and Schuster. [Google Scholar]
- Delanoeije J, Gerencsér L, Miklósi Á, 2020. Do dogs mind the dots? Investigating domestic dogs’ Canis familiaris preferential looking at human-shaped point-light figures. Ethology 126:637–650. [Google Scholar]
- Fitzgerald BM, Turner DC, 2000. Hunting behavior of domestic cats and their impact on prey population In: Turner DC, Bateson P, editors. The Domestic Cat: The Biology of Its Behavior. Cambridge: Cambridge University Press; 152–175. [Google Scholar]
- Hawley JM, Munn NL, 1933. Visual discrimination of movement by white rats. J Comp Psychol 16:137–142. [Google Scholar]
- Kanizsár O, Mongillo P, Battaglini L, Campana G, Marinelli L, 2017. Dogs are not better than humans at detecting coherent motion. Sci Rep 7:11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanizsár O, Mongillo P, Battaglini L, Campana G, Lõoke M et al. , 2018. The effect of experience and of dots’ density and duration on the detection of coherent motion in dogs. Anim Cogn 21:651–660. [DOI] [PubMed] [Google Scholar]
- Martinoya C, Rivaud S, Bloch S, 1983. Comparing frontal and lateral viewing in the pigeon. II. Velocity thresholds for movement discrimination. Behav Brain Res 8:375–385. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Merigan WH, 1980. Movement detection by cats: invariance with direction and target configuration. J Comp Physiol Psychol 94:943–952. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Kavanagh E, 2006. Motion perception in the ageing visual system: minimum motion, motion coherence, and speed discrimination thresholds. Perception 35:9–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.