Abstract
TAM receptors (Tyro3, Axl, and Mer) are receptor tyrosine kinases (RTKs) that are expressed by multiple immune cells including natural killer (NK) cells. Although RTKs typically enhance cellular functions, TAM receptor ligation blocks NK cell activation. The mechanisms by which RTKs block NK cell signaling downstream of activating receptors are unknown. In this report, we demonstrate that TAM receptors attenuate NK cell responses via the activity of E3 ubiquitin ligase Cbl-b. Specifically, we show that Tyro3, Axl, and Mer phosphorylate Cbl-b, and Tyro3 ligation activates Cbl-b by phosphorylating tyrosine residues 133 and 363. Ligation of TAM receptors by their ligand Gas6 suppresses activating receptor-stimulated NK cell functions such as IFN-γ production and degranulation, in a TAM receptor kinase- and Cbl-b-dependent manner. Moreover, Gas6 ligation induces the degradation of LAT1, a transmembrane adaptor protein required for NK cell activating receptor signaling, in WT but not in Cbl-b knock-out NK cells. Together, these results suggest that TAM receptors may attenuate NK cell function by phosphorylating Cbl-b, which in turn dampens NK cell activation signaling by promoting the degradation of LAT1. Our data therefore support a mechanism by which RTKs attenuate, rather than stimulate, signaling pathways via the activation of ubiquitin ligases.
Keywords: NK cells, signal transduction, cellular activation, TAM receptors, inhibitory receptors
Introduction
The TAM receptor family is a group of receptor tyrosine kinases (RTKs) that includes Tyro3, Axl, and Mer [1]. TAM receptors have a highly conserved cytoplasmic kinase domain that is activated upon binding with their ligands Gas6 or Protein S [2], [3]. TAM activation leads to their autophosphorylation and the phosphorylation of tyrosine residues of multiple downstream intracellular signaling molecules [1]. In hematopoietic cells, TAM receptors are primarily expressed by cells of the innate immune system.
One important function of TAM receptors is to clear apoptotic bodies. The phosphatidyl serine residues of the surface membrane of apoptotic bodies bind to Gas6, serving as a means for recognition and subsequent phagocytosis by TAM receptor-expressing macrophages [4]. Thus, Tyro3, Axl, and Mer triple knock-out (KO) display a defect in clearing apoptotic bodies, which causes a lupus-like spontaneous autoimmune disease [5]. In addition, TAM receptor ligation blocks pro-inflammatory cytokine release by macrophages and dendritic cells (DCs) [5], [6], [7].
In addition to macrophages and DCs, TAM receptors are also expressed by NK cells [8]. NK cells are innate lymphocytes that play an important role in killing cancerous and virally infected cells by direct cytotoxicity and through production of inflammatory cytokines such as IFN-γ [9]. Interestingly, the expression of TAM receptors is necessary for proper NK cell development and functional maturation [8]. In mature NK cells, TAM receptors attenuate NK cell function. Accordingly, the inhibition of TAM receptor signaling prevents metastasis in a mouse tumor model in an NK cell-dependent manner [10].
RTKs are typically associated with stimulation, rather than inhibition, of activating receptor-mediated signaling pathways. For example, ligand binding to epidermal growth factor receptor (EGFR) stimulates the ERK, MAPK and AKT pathways, which leads to cell growth and proliferation [11]. However, TAM receptors are unique within the RTK family in that they are able to both promote and inhibit signal transduction pathways.
The mechanism by which TAM receptors inhibit innate immune cells has only been well described in DCs, where the TAM receptor Axl is induced upon Toll-like receptor (TLR) activation by foreign pathogens. Axl associates with the type I interferon receptor, which activates the JAK/STAT pathway and drives the transcription of the suppressor of cytokine signaling (SOCS1 and 3) genes [7], [12], which are ubiquitin ligases that inhibit cytokine receptor and TLR-mediated signaling.
Cbl-b, another E3 ubiquitin ligase, was also found to be associated with TAM receptors in an in vitro proteomics assay [10]. Cbl-b ubiquitinates TAM receptors, causing decreased surface expression, likely through lysosomal degradation [10]. Although this interaction appeared to be important for the TAM receptor function in NK cells, the mechanism by which TAM receptors attenuate NK cell function is still unclear.
In this report, we find that while TAM receptors are targets for Cbl-b-mediated ubiquitination, Cbl-b is also a phosphorylation target for TAM receptors. Our data suggest that TAM receptors may attenuate NK cell function by phosphorylating Cbl-b, promoting the degradation of LAT1, a key protein necessary for activating receptor-mediated signaling in NK cells [13].
Results
Gas6 inhibits IFN-γ production and degranulation by WT NK cells
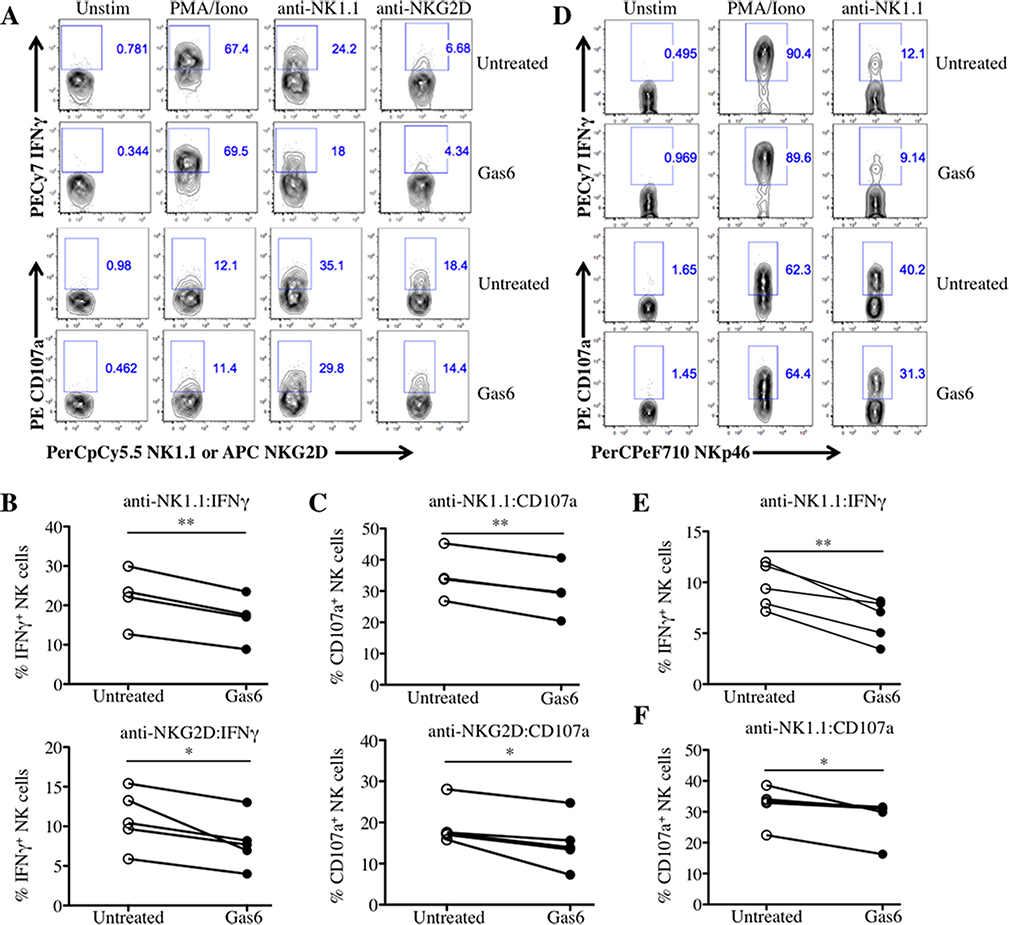
To examine the effect of TAM receptor-mediated signaling on NK cell activation, we stimulated IL-2-expanded NK cells (Lymphokine Activated Killer cells, LAKs) by ligating the activating receptors NK1.1 and NKG2D in the presence or absence of the TAM receptor ligand Gas6, and measured the production of IFN-γ and cell-surface CD107a expression (a surrogate for degranulation). The addition of Gas6 significantly decreased the proportion of IFN-γ+ and CD107a+ anti-NK1.1- or anti-NKG2D-stimulated LAKs (Fig.1A–C). To test whether a similar effect was observed in NK cells removed freshly ex vivo, NK cells from WT mice pre-treated with the viral mimetic PolyI:C were stimulated in the presence or absence of Gas6. Similar to LAKs, a decreased fraction of IFN-γ+ and CD107a+ NK cells was detected in freshly isolated NK cells treated with Gas6 and stimulated with anti-NK1.1 (Fig.1D–F). These data demonstrate that TAM receptor ligation negatively regulates NK cell activation.
Fig 1. Gas6 inhibits IFNγ production and degranulation by WT NK cells stimulated through activating receptors.
LAKs were serum/IL-2-starved for 2 hours, pre-treated with or without Gas6 for 2 hours, and left unstimulated (Unstim) or stimulated with PMA/Iono, anti-NK1.1, or anti-NKG2D for 5 hours and analyzed by flow cytometry using the strategy illustrated in Supporting Information (Figure S1A). (A) Representative flow cytometric plots of IFN-γ+ and CD107a+ LAK cells are shown. (B) The fraction of IFN-γ-expressing and (C) CD107a-expressing LAKs from 4 independent experiments are shown (n = 4 mice for anti-NK1.1 and n=5 mice for anti-NKG2D). (D) Splenoctyes from Poly I:C-treated mice were pre-treated with or without Gas6 for 2 hours and left unstimulated (Unstim) or stimulated with PMA/Iono or anti-NK1.1 for 5 hours and analyzed by flow cytometry using the strategy illustrated in Supporting Information (Figure S1B). Representative flow cytometric plots of IFN-γ+ and CD107a+ NK cells are shown. The fraction of (E) IFN-γ-expressing and (F) CD107a–expressing splenocytes of 4 independent experiments are shown (n =4–5 mice). *p<0.05 and **p<0.01 by paired Student t test, respectively; ns, not significant.
TAM receptor Tyro3 phosphorylates tyrosine residues 133 and 363 of Cbl-b
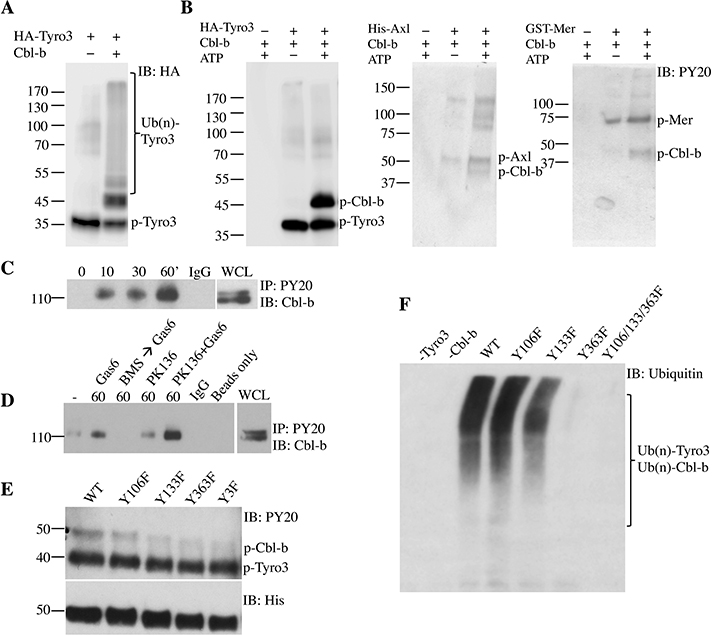
It has been previously shown that TAM receptors are direct targets of the ubiquitin ligase Cbl-b and that these interactions are important for TAM receptors to attenuate NK cell function [10]. To further examine the interaction between TAM receptors and Cbl-b, we generated recombinant phosphorylated Tyro3 and a truncated Cbl-b composed of the tyrosine kinase binding and RING finger domains (TKB+RF). As previously reported [10], the addition of Cbl-b to purified Tyro3 induced the ubiquitination of Tyro3 (Fig. 2A). While Cbl-b typically needs to be pre-activated by Lck-mediated phosphorylation to carry out its ubiquitinating function [14], we unexpectedly found that Cbl-b was able to ubiquitinate Tyro3 in the absence of Lck. Given that Tyro3 is a kinase, this raised the possibility that Tyro3 might be phosphorylating and activating Cbl-b. Indeed, phosphorylated Cbl-b was detected when Cbl-b was mixed with both Tyro3 and ATP (Fig. 2B). These data suggest that not only was Tyro3 a ubiquitination target of Cbl-b, but also that Cbl-b was a phosphorylation target of Tyro3. Furthermore, recombinant Axl and Mer were also able to phosphorylate Cbl-b (Fig. 2B).
Fig 2. TAM receptor Tyro3 phosphorylates tyrosine residues 133 and 363 of Cbl-b.
(A) HA-tagged Tyro3 was incubated with truncated Cbl-b (TKB+RF), E1, E2, ubiquitin, and ATP in vitro for 1 hour and immunoblotted with anti-HA antibody. (B) Truncated Cbl-b (TKB+RF) was incubated with or without recombinant Tyro3, Axl, or Mer and with or without ATP in vitro and immunoblotted with anti-phosphotyrosine antibody. (C) LAKs were IL-2-starved for 18 hours and serum-starved for 4 hours followed by stimulation with Gas6 for various time points or (D) followed by stimulation with or without Gas6, BMS 777607 with Gas6, or anti-NK1.1 (PK136) with or without Gas6 for 60 minutes. Lysates were de-ubiquitinated with DUB USp2core, followed by immunoprecipitation with anti-phosphotyrosine and immunoblotting with anti-Cbl-b antibodies. (E) 10XHis-tagged Y→F Cbl-b point mutants were incubated with Tyro3 and ATP, followed by immunoblotting with anti-phosphotyrosine antibody. Y3F refers to Y106/133/363F triple mutant. (F) 10XHis-tagged Y→F Cbl-b point mutants were incubated with Tyro3, E1, E2, ubiquitin, and ATP for 1 hour and immunoblotted with anti-ubiquitin antibody. One representative of three independent experiments is shown.
To test whether Cbl-b is phosphorylated upon TAM receptor ligation in NK cells, we next examined Cbl-b phosphorylation in LAKs treated with Gas6. Consistent with our purified protein assay results, the treatment of LAKs with Gas6 induced the phosphorylation of Cbl-b (Fig. 2C). To ensure that the phosphorylation was TAM kinase-dependent, we pre-treated LAKs with the pan-TAM kinase inhibitor BMS777607 for 30 minutes prior to stimulation with Gas6; this resulted in the reduction of Cbl-b phosphorylation to baseline levels (Fig. 2D), suggesting that the induction of Cbl-b phosphorylation by Gas6 was TAM kinase-dependent. The ligation of activating receptors such as Ly49D and CD16 can also lead to phosphorylation of the Cbl-b homologue c-Cbl [15], likely as a feedback mechanism to attenuate NK cell overactivation. To test whether Gas6 has a cumulative effect in enhancing Cbl-b phosphorylation with an activating receptor stimulus, we stimulated LAKs with anti-NK1.1 with or without Gas6. Stimulation of LAKs with anti-NK1.1 antibody PK136 led to minimal phosphorylation of Cbl-b, which was synergistically enhanced by Gas6 (Fig. 2D). These data collectively suggest that TAM receptors directly phosphorylate Cbl-b.
Tyrosines 106, 133, and 363 have been previously shown to be required for the ligase activity of Cbl-b [14], [16], [17]. Phosphorylation of Tyr363 opens Cbl-b from its auto-inhibitory confirmation, allowing E2 and substrates to bind to Cbl-b [16]. To determine which of the tyrosines of Cbl-b could potentially be phosphorylated by Tyro3, we examined Tyro3-dependent phosphorylation of Cbl-b mutants harboring single Y→F mutations at residues Tyr106, Tyr133, and Tyr363, and a triple mutation of all tyrosines (Y106/133/363F). Following co-incubation of Y133F, Y363F, and Y106/133/363F mutants with Tyro3, we found a decrease in phospho-Cbl-b, in comparison to WT Cbl-b (Fig. 2E). These data suggest that Tyr133 and Tyr363 are the primary tyrosine residues that are phosphorylated by Tyro3.
To test whether phosphorylation of Cbl-b at Tyr133 and/or Tyr363 by Tyro3 is important for its ubiquitin ligase function, we examined the ubiquitination of the Cbl-b mutants and Tyro3. Tyr106, Tyr133, and Tyr363 have been previously shown to be required for the auto-ubiquitination of Cbl-b [17]. Ubiquitinated Tyro3 and Cbl-b bands were detected when Tyro3 is mixed with WT Cbl-b (Fig. 2F). These ubiquitination bands were slightly decreased in the Y133F mutant and almost undetectable in the Y363F and triple mutants. These results show that Tyro3 regulates the function of Cbl-b by phosphorylating Tyr363 of Cbl-b.
TAM receptors attenuate NK cell function by phosphorylating Cbl-b, which promotes LAT1 degradation
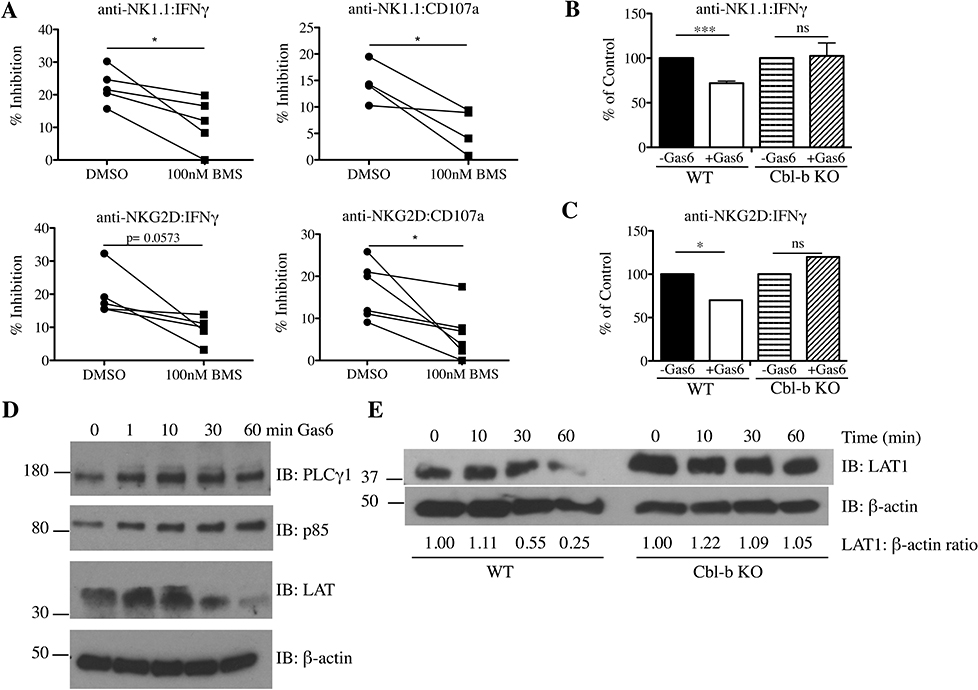
Cbl-b negatively regulates NK cell function by ubiquitinating key signaling molecules and targeting them for re-localization or degradation [13]. Thus, we hypothesized that TAM receptors might inhibit NK cell function by activating Cbl-b through its phosphorylation. To test this possibility, we first examined whether the kinase activity of TAM receptors was necessary for the suppression of NK cell activation. Cells were pretreated with the pan-TAM kinase inhibitor BMS777607 for 2 hours prior to stimulation with plate-bound anti-NK1.1 or anti-NKG2D in the presence of Gas6. The suppressive effect of Gas6 on LAK IFN-γ production and degranulation was partially reversed by BMS777607, suggesting that Gas6 may regulate NK cells at least in part by the kinase activity of TAM receptors (Fig. 3A, Supplemental Fig. 2).
Fig 3. TAM receptors attenuate NK cell function by phosphorylating Cbl-b, which promotes LAT1 degradation.
(A) LAKs were treated with media (0.05% DMSO) or BMS 777607 (100 nM) for 2 hours in IL-2/serum-free conditions, followed by treatment with or without Gas6 for another 2 hours prior to stimulation with anti-NK1.1 or NKG2D for 5 hours. Data is shown as mean % inhibition {(Untreated - Gas6-treated)/Untreated]*100} ± SEM of 4 independent experiments (n = 4–5 mice for anti-NK1.1 and n=5–6 mice for anti-NKG2D). P values were determined by paired Student t test. (B) WT and Cbl-b KO LAKs were serum/IL-2-starved for 2 hours, followed by Gas6 treatment for 2 hours, and stimulated with anti-NK1.1 or (C) anti-NKG2D antibodies for 24 hours. IFNγ content in the supernatants was measured by ELISA. Data are represented as % of control + SEM of n=4 mice for anti-NK1.1 and n=3 mice for anti-NKG2D. *p< 0.05 and ***p <0.001 by paired Student t test. ns, not significant. (D) LAKs were serum and IL-2-starved for 4 hours followed by Gas6 stimulation for various time points. Cell lysates were immunoblotted with anti-PLCγ1, anti-p85, anti-LAT1, and β-actin antibodies. (E) LAKs from WT and Cbl-b KO mice were serum and IL-2 starved for 4 hours followed by Gas6 stimulation for various time points. Cell lysates were immunoblotted with anti-LAT1 and β-actin antibodies. The relative ratio of LAT1 to β-actin for each time point, normalized to the 0 timepoint for each genotype, is shown below the blots. One representative of three independent experiments is shown.
Next, we determined whether Cbl-b was functionally required for TAMs to inhibit NK cells. Cbl-b KO and WT LAKs were stimulated with plate-bound anti-NK1.1 or anti-NKG2D antibodies in the presence or absence of Gas6, and the supernatants were assayed for IFN-γ by ELISA at 24 hours. Consistent with our flow cytometric data, the addition of Gas6 to WT LAKs significantly inhibited IFN-γ release. However, Gas6 treatment displayed no inhibitory effect towards anti-NK1.1 or anti-NKG2D-stimulated Cbl-b KO LAKs (Fig.3B–C, Supplemental Fig. 3). Together, these data suggest that the phosphorylation of Cbl-b is required for the Gas6/TAM pathway to inhibit pro-inflammatory cytokine production by NK cells.
To gain insight into how the TAM/Cbl-b pathway may be regulating NK cell signaling, we assessed for degradation of PLCγ1, the p85 subunit of PI3K, and LAT1- key signaling molecules downstream of NK cell activating receptors and known targets for ubiquitination and degradation by Cbl-b [13]. Upon Gas6 ligation, only LAT1 levels were decreased over time in WT LAKs (Fig. 3D), whereas Cbl-b KO LAKs were unaffected (Fig. 3E). Together, these data support a novel mechanism whereby TAM receptors negatively regulate NK cell function by phosphorylating Tyr363 of Cbl-b, which in turn dampens NK cell activation signaling by promoting the degradation of positive signaling molecules such as LAT1 (Fig. 4).
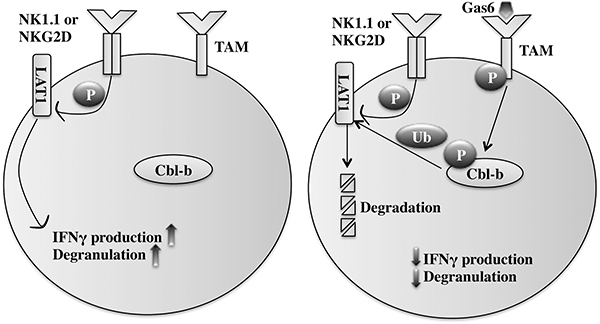
Fig 4. Gas6/TAM signaling inhibits NK cell activation through Cbl-b.
Gas6 binds and activates TAM receptors, which directly phosphorylates Cbl-b. Phosphorylated Cbl-b, in turn, ubiquitinates and targets LAT1 for degradation. NK1.1/NKG2D signaling is attenuated by decreased LAT1 protein, which is required for signal transduction downstream of NK cell activating receptors.
Discussion
TAM receptors are RTKs that inhibit the pro-inflammatory function of a variety of cell types. While it has been shown that TAM receptors attenuate DC function through the transcriptional induction of SOCS proteins [7], the mechanism by which TAM receptors attenuate activating receptor-stimulated NK cell function is unknown. In this report, we provide data that supports a mechanism by which TAM receptors attenuate pro-inflammatory responses via post-translational modification of the E3 ubiquitin ligase Cbl-b. By activating Cbl-b through its phosphorylation, TAM receptor ligation can inhibit NK cell function potentially by inducing the degradation of key molecules necessary for activating receptor signaling, such as LAT1.
RTKs are typically associated with stimulation rather than inhibition of activating receptor-mediated signaling pathways [11]. Accordingly, the tyrosine kinase activity of TAM receptors activates Erk, Akt, and NFκB – key signaling pathways required for NK cell cytokine production [1]. Thus, it is puzzling that TAM receptors attenuate, rather than augment, NK cell function. Our data reveal the mechanism by which RTKs inhibit activating receptor stimulation in NK cells. Namely, by phosphorylating and activating Cbl-b, TAM receptors can selectively suppress signaling through activating receptors by targeting LAT1 for degradation.
Cbl-b has been shown to negatively regulate T cells, macrophages, and more recently NK cells [18]. In T cells, Cbl-b has been shown to ubiquitinate not only the T cell receptor, but also downstream signaling molecules such as p85, PLCγ, and PLC-θ – which are all required for T cell effector function [19]. In NK cells, Cbl-b has also been shown to ubiquitinate key signaling molecules including the transmembrane adaptor protein LAT1 [13]; LAT1 is part of the multi-molecular signaling complex that forms downstream of activating receptors and is required for signaling and cytolytic activity [20],[21].
Along with LAT1, we also assessed the stability of other key signaling molecules such as PLCγ1 and the p85 subunit of PI3K after Gas6 stimulation. Although only LAT1 degraded after 60 minutes of Gas6 stimulation, lack of degradation of these proteins does not preclude them from being involved in TAM/Cbl-b-mediated inhibition of NK cell activation. Ubiquitination can also affect localization of the protein within the cell, block posttranslational modifications, or can even alter binding to other proteins [22],[23]. Therefore, although these proteins are not degraded upon Gas6 stimulation, Cbl-b may still be regulating NK cell responses by changing their localization. In addition, although our results show that Gas6-mediated LAT1 degradation is mainly controlled by Cbl-b, other additional ubiquitin ligases can be involved LAT1 degradation.
Our investigation was initially prompted by a previous report showing that TAM receptors are ubiquitinated by Cbl-b [10]. In this study, Gas6 treatment of NK cells led to a decrease in surface expression of TAM receptors in a Cbl-b-dependent manner. This ubiquitin-dependent endocytosis of TAM receptors was proposed to be necessary for the induction of its inhibitory signal transduction pathway. Consistent with these data, we also found that Tyro3 is ubiquitinated by Cbl-b. However, while it is possible that Cbl-b-mediated endocytosis of TAM receptors is critical for the inhibitory function of TAM receptors, it remained unclear as to how this endocytosis could subsequently inhibit NK cell functions. Instead, our data favor a model in which the inhibitory function of TAM receptors is carried out by Cbl-b-mediated ubiquitination of activating receptor-associated signaling molecules. In this model, the downregulation of TAM receptors by Cbl-b-mediated ubiquitination could play a role in feedback attenuation of TAM receptor signaling. In support of this type of negative feedback, another RTK, epidermal growth factor receptor (EGFR), has been shown to be ubiquitinated by a homolog of Cbl-b, c-Cbl [24].
Despite differences in the molecules involved, there are similar mechanisms by which TAM receptors inhibit cytokine production in DCs; in these cells, Axl ligation up-regulates the transcription of the ubiquitin ligases SOCS1 and SOCS3, which block TLR-mediated (MyD88 and TRIF) and cytokine-mediated (JAK/STAT) signaling [7]. While the increased expression of SOCS1 and SOCS3 does not affect activating receptor-mediated activation in NK cells, TAM receptors post-transcriptionally activate Cbl-b, which downregulates components of activating receptor-mediated but not TLR- or cytokine-mediated signaling. Thus, TAM receptors may (through two independent mechanisms) induce ubiquitin ligase-mediated attenuation of signaling through TLRs, cytokines, and activating receptors, to provide broad range control of signaling pathways in innate cells of the immune system.
Materials and Methods
Mice
Cbl-b KO mice were generated and generously provided by Richard Hodes (National Cancer Institute, Bethesda, MD). SJL/Thy1.1 were created by breeding B6.SJL and B6.PL1 mice. B6.SJL, B6.PL1, and C57BL/6 mice were purchased from The Jackson Laboratory. Mice were sacrificed and analyzed between 8 and 12 weeks of age. Mice were housed in pathogen-free conditions and treated in strict compliance with the Institutional Animal Care and Use Committee regulations at the University of Pennsylvania.
Reagents, Flow cytometry, antibodies, and data analysis
Recombinant murine Gas6 was purchased from R&D systems (Minneapolis, MN). Recombinant Axl and Mer were purchased from SignalChem (Richmond, BC, Canada). TAM inhibitor BMS 777607 was purchased from Santa Cruz Biotechnology (Dallas, TX). Antibodies for NK cell stimulation and phenotyping were purchased from BD Pharmingen (San Diego, CA), BioLegend (San Diego, CA), eBioscience (San Diego, CA), Bio X Cell (West Lebanon, NH), and Molecular Probes, Invitrogen (Carlsbad, CA). Western blot antibodies were purchased from Cell Signaling Technology (Danvers, MA), Santa Cruz Biotechnology (Dallas, TX), or Sigma Aldrich (St. Louis, MO). All chemicals are from Sigma Aldrich (St. Louis, MO), unless otherwise specified.
For flow cytometric analysis, single-cell suspensions were stained with fluorochrome-labeled antibodies in PBS for 30 minutes at 4 °C. Fc receptors were blocked with anti-CD16/32 antibodies (BD Pharmingen). Lymphocytes were identified by their scatter properties (FSC-A x SSC-A plot), followed by exclusion of doublets by gating on FSC-H x FSC-W and SSC-H x SSC-W as per guidelines set by Cossarizza, et al. and illustrated in Supporting Information (Figure S1A) [25]. LAKs were identified by fluorochrome-labeled antibodies against CD4, CD8, NK1.1, and NKG2D (BD Pharmingen), including Live/Dead (Invitrogen). Data were acquired using a multicolor flow cytometer FACSCanto equipped with FACSDiva software (BD Biosciences). Data analysis was performed using FlowJo (BD Biosciences). Statistical analysis was performed using Prism (GraphPad, San Diego, CA) computer software.
NK cell stimulation assays
Splenocytes from 10–12 week old WT or Cbl-b KO mice were stained with anti-DX5-biotin antibody, enriched using anti-biotin MACS beads (Miltenyi Biotec), and expanded in LAK media [MEMα, 10 mM HEPES, 10% FBS, 1% penicillin/streptomycin, 1% L-glutamine, 1 × 10–5 M 2-mercaptoethanol, and 1000 U/ml human IL-2 (PeproTech)] for at least 5 days to create LAKs. After 5–6 days of expansion, ∼80–90% of the LAKs in culture were NK cells (CD4–CD8–NK1.1+).
For NK cell activation, freshly isolated splenocytes (untreated or from Poly I:C-injected mice, 200 μg per mouse, injected i.p. 16 hours before harvesting) or LAKs were rested (deprived of IL-2 and serum) for 2 hours with 450 ng/mL Gas6 and then cultured with PE-labeled anti-CD107a (0.4 μg/ml), IL-2 (1000 U/ml), and brefeldin A (10 μg/ml) for 5 hours in tissue culture plates that were pre-coated with antibodies against NK cell–activating receptors NK1.1 (PK136) or NKG2D (HMG2A) plated at 30 μg/ml overnight at 4 °C. Unstimulated and PMA/Ionomycin controls shown in the figures did not contain exogenous hIL-2. The cells were then stained with cell surface antibodies, fixed and permeabilized (BD Biosciences), intracellularly stained with anti- IFN-γ antibody, and analyzed by flow cytometry. In experiments involving the TAM inhibitor BMS 777607, splenocytes were preincubated with 100 nM of the inhibitor for 2 hours before stimulation, and the inhibitor was maintained throughout the assay. To measure IFN-γ release in the supernatant, LAKs were deprived of serum and IL-2 for 2 hours and simultaneously pre-treated with 450 ng/mL murine Gas6 at 37 °C. Cell free supernatant was collected 24 hours later after stimulation with anti-NK1.1 and anti-NKG2D, and IFN-γ content was measured by ELISA (BioLegend). All experiments were performed in serum-deprived conditions during the stimulation to avoid contamination of our cultures with Gas6, which is present in FBS. When using LAK cells, the cells were starved of IL-2 for 2–18 hours prior to stimulation, since long term culture in IL-2 led to high baseline levels of Cbl-b phosphorylation (data not shown).
Western Blot and Immunoprecipitation
For immunoprecipitation of phosphotyrosine, LAKs were serum-starved for 4 hours and IL-2-starved for 18 hours, followed by incubation with the proteasome inhibitor MG132 (5 μM, SelleckChem) for 30 minutes and stimulation with 450 ng/mL recombinant murine Gas6 for various time points at 37 °C. In some experiments, cells were co-incubated with MG132 and the pan-TAM inhibitor BMS777607 (300 nM), 30 minutes prior to Gas6 stimulation or incubated with anti-NK1.1 antibody (30 μg/ml) with or without 450ng/mL Gas6 for 60 minutes. Protein was extracted with protein lysis buffer (Pierce Crosslink Magnetic IP/Co-IP Kit; Thermo Scientific) containing a cocktail of protease and phosphatase inhibitors (dichloroisocoumarin, benzamidine hydrochloride, sodium orthovanadate, sodium pyrophosphate, and sodium fluoride). Lysis was performed on ice for 5 minutes while vortexing followed by the addition of 10 μg deubiquitinating enzyme Usp2core (Life Sensors) for 1 hour at room temperature. 5 μg of PY20 antibody was prepared for immunoprecipitation using Pierce Crosslink Magnetic IP/Co-IP Kit. Crosslinked antibodies were incubated with lysates for 1 hour at room temperature. Samples were run on SDS-PAGE gels, transferred to nitrocellulose membranes, and blotted for Cbl-b.
For western blot lysates, LAKs were serum-starved for 4 hours and stimulated with 450 ng/mL recombinant murine Gas6 for various time points at 37 °C. Protein was extracted using 1% NP-40 lysis buffer containing a protease inhibitor cocktail (Roche) and phosphatase inhibitors. Samples were run on SDS-PAGE gels, transferred to nitrocellulose membranes, and blotted for the indicated protein. The band intensity of LAT1 and the loading control β-actin were measured by ImageJ and the ratio was normalized to unstimulated conditions.
Production and purification of proteins
Human Cbl-b TKB+RF (residues 38–429) and Tyro3 kinase catalytic domain (residues 495–810) were cloned into pET-24d(+)-based vectors for bacterial expression. Tyro3 isolated from E. coli was found to be in a phosphorylated state, possibly due to autophosphorylation. Cbl-b point mutations (Y106F, Y133F, and Y363F), in addition to a compound mutant (Y106/133/363F) were generated from plasmid pE-10xHis-HA-Cbl-b TKB+RF by site-directed mutagenesis. WT and mutant Cbl-b TKB+RF proteins were purified from IPTG-induced bacteria transformed with the various constructs. The cells were lysed and sonicated for 30 seconds x 3 cycles, loaded onto HIS-select spin columns (Sigma), and purified according to the manufacturer’s protocol. To remove the imidazole contained in the elution buffer prior to assays, buffer exchange was performed using NAP-5 Sephadex G-25 columns (GE Healthcare) using 20 mM Tris HCl pH 8.0. Proteins were stored in 150 mM NaCl, 10% glycerol, and 10mM DTT for stability. Protein concentration was measured using Pierce 600 nm kit (Thermo Scientific).
Phosphorylation and ubiquitination assays
ATP (0.2 mM), WT or mutant Cbl-b recombinant protein (500 nM), and p-Tyro3 (50 nM), p-Axl (100 nM), or p-Mer (100 nM) were added to a final volume of 50 μL in kinase assay buffer (50 mM Bicine pH8.0, 5 mM MgCl2, 0.05% Chaps, and 1mM beta-mercaptoethanol) and incubated at 25 °C for 1 hour. For ubiquitination assays, E1 (5 nM), E2 (100 nM), ATP (0.2 mM), ubiquitin (600 nM), p-Tyro3 (50 nM), and Cbl-b (50 nM) were incubated in kinase assay buffer for 1 hour at room temperature in a final volume of 20 μL. The E2 enzyme used in the assays was UBE2D3/UbcH5C (LifeSensors). The reactions were stopped with 4X sample buffer (NuPage) with 100 mM DTT and immunoblotted with anti-phosphotyrosine or with anti-ubiquitin.
Supplementary Material
Acknowledgements
We thank Dr. Richard Hodes for kindly providing the Cbl-b KO mice. We thank all current and former members of the Kambayashi lab for helpful discussions and Dr. Hamid Bassiri for review of this manuscript. We also thank Drs. Hamid Bassiri, Martha Jordan, Janis Burkhardt, and Warren Pear for helpful discussions. This work was supported by grants from the National Institutes of Health (T32CA009140 to L.C.; R01HL107589, R01HL111501, R01AI121250, R21AI135359, R43CA213582 to T.K.)
Abbreviations:
- TAM
Tyro3, Axl, Mer
- RTK
receptor tyrosine kinase
- NK
natural killer
- LAKs
lymphokine-activated killer cells
- Cbl-b
Casitas B- lineage lymphoma b
- IFN
γ interferon gamma
- LAT
linker for activation of T cells
- KO
knock-out
- DCs
dendritic cells
- EGFR
epidermal growth factor receptor
- SOCS
suppressor of cytokine signaling
- TLR
Toll-like receptor
- TKB
tyrosine kinase binding
- RF
ring finger
- ATP
adenosine triphosphate
- ELISA
enzyme-linked immunosorbent assay
- i.p.
intraperitoneal
- PMA
Phorbol 12-myristate 13-acetate
- PI3K
Phosphoinositide 3-kinase
- PLCγ1
Phospholipase C gamma 1
Footnotes
Conflict of interest
The authors declare no commercial or financial conflict of interest.
References
- 1.Linger R,M,A*, Keating, Earp H,S†, Graham DK. TAM Receptor Tyrosine Kinase: Biological Functions, Signaling, and Potential Theraputics Targeting in human Cancer. Adv. Cancer Res. 2008; 100:35–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manfioletti G, Brancolini C, Avanzi G, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol. Cell. Biol. 1993; 13:4976–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, Hunt RW, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature. 1995; 373:623–626. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006; 16:189–197. [DOI] [PubMed] [Google Scholar]
- 5.Lu Q, Lemke G. Homeostatic Regulation of the Immune System by Receptor Tyrosine Kinases of the Tyro 3 Family. Science (80-.). 2001; 293:306–311. [DOI] [PubMed] [Google Scholar]
- 6.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 1999; 162:3498–3503. [PubMed] [Google Scholar]
- 7.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MBA, Lemke G. TAM Receptors Are Pleiotropic Inhibitors of the Innate Immune Response. Cell. 2007; 131:1124–1136. [DOI] [PubMed] [Google Scholar]
- 8.Caraux A, Lu Q, Fernandez N, Riou S, Di Santo JP, Raulet DH, Lemke G, et al. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006; 7:747–754. [DOI] [PubMed] [Google Scholar]
- 9.Freund-brown J, Chirino L, Kambayashi T. Strategies to Enhance NK Cell Functio for the Treatment of Tumors and Infections. Crit. Rev. Immunol. 2018; 38:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, Jamieson AM, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014; 507:508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel). 2017; 9:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007; 7:454–465. [DOI] [PubMed] [Google Scholar]
- 13.Matalon O, Fried S, Ben-shmuel A, Pauker MH, Joseph N, Keizer D, Piterburg M, et al. Dephosphorylation of the adaptor LAT and phospholipase C – g by SHP-1 inhibits natural killer cell cytotoxicity. 2016; 9:1–16. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Qiao G, Tang J, Tang R, Guo H, Warwar S, Langdon WY, et al. Protein Tyrosine Phosphatase SHP-1 Modulates T Cell Responses by Controlling Cbl-b Degradation. J. Immunol. 2015; 195:4218–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McVicar DW, Taylor LS, Gosselin P, Willette-Brown J, Mikhael AI, Geahlen RL, Nakamura MC, et al. DAP12-mediated Signal Transduction in Natural Killer Cells. J. Biol. Chem. 1998; 273:32934–32942. [DOI] [PubMed] [Google Scholar]
- 16.Kobashigawa Y, Tomitaka a., Kumeta H, Noda NN, Yamaguchi M, Inagaki F. Autoinhibition and phosphorylation-induced activation mechanisms of human cancer and autoimmune disease-related E3 protein Cbl-b. Proc. Natl. Acad. Sci. 2011; 108:20579–20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dou H, Buetow L, Hock A, Sibbet GJ, Vousden KH, Huang DT. Structural basis for autoinhibition and phosphorylation-dependent activation of c-Cbl. Nat. Struct. Mol. Biol. 2012; 19:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz-Nicoladoni C, Wolf D, Sopper S. Modulation of Immune Cell Functions by the E3 Ligase Cbl-b. Front. Oncol. 2015; 5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz ML. Activation of T cells: Releasing the brakes by proteolytic elimination of Cbl-b. Sci. Signal. 2009; 2:2–5. [DOI] [PubMed] [Google Scholar]
- 20.May RM, Okumura M, Hsu CJ, Bassiri H, Yang E, Rak G, Mace EM, et al. Murine natural killer immunoreceptors use distinct proximal signaling complexes to direct cell function. Blood. 2013; 121:3135–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiesa S, Mingueneau M, Fuseri N, Malissen B, Raulet DH, Malissen M, Vivier E, et al. Multiplicity and plasticity of natural killer cell signaling pathways. Blood. 2006; 107:2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thien CBF, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem. J. 2005; 391:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang D, Wang HY, Fang N, Altman Y, Elly C, Liu YC. Cbl-b, a RING-type E3 Ubiquitin Ligase, Targets Phosphatidylinositol 3-Kinase for Ubiquitination in T Cells. J. Biol. Chem. 2001; 276:4872–4878. [DOI] [PubMed] [Google Scholar]
- 24.Ravid T, Heidinger JM, Gee P, Khan EM, Goldkorn T. c-Cbl-mediated ubiquitinylation is required for epidermal growth factor receptor exit from the early endosomes. J. Biol. Chem. 2004; 279:37153–37162. [DOI] [PubMed] [Google Scholar]
- 25.Cossarizza A, Chang HD, Radbruch A, Akdis M, Andrä I, Annunziato F, Bacher P, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 2017; 47:1584–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.