Abstract
American Indians and Alaska Natives experience higher lung cancer mortality than other races in Minnesota. We compared rates of standard-of-care genetic mutation testing in this group compared to non-Native controls. No significant difference was identified; further resources should therefore be focused on other potential contributing factors, such as commercial tobacco use and other social determinants of health.
Background:
American Indians and Alaska Natives (AI/AN) continue to experience extreme lung cancer health disparities. The state of Minnesota is home to over 70,000 AI/AN, and this population has a 2-fold increase in lung cancer mortality compared to other races within Minnesota. Genetic mutation testing in lung cancer is now a standard of high-quality lung cancer care, and EGFR mutation testing has been recommended for all adenocarcinoma lung cases, regardless of smoking status. However, genetic testing is a controversial topic for some AI/AN.
Patients and Methods:
We performed a multisite retrospective chart review funded by the Minnesota Precision Medicine Grand Challenge as a demonstration project to examine lung cancer health disparities in AI/AN. We sought to measure epidemiology of lung cancer among AI receiving diagnosis or treatment in Minnesota cancer referral centers as well as rate of EGFR testing. The primary outcome was the rate of EGFR mutational analysis testing among cases and controls with nonsquamous, non—small-cell lung cancer. We secured collaborations with 5 health care systems covering a diverse geographic and demographic population.
Results:
We identified 200 cases and 164 matched controls from these sites. Controls were matched on histology, smoking status, sex, and age. In both groups, about one third of subjects with adenocarcinoma received genetic mutation testing.
Conclusion:
There was no significant difference in mutation testing in AI compared to non-AI controls at large health care systems in Minnesota. These data indicate that other factors are likely contributing to the higher mortality in this group.
Keywords: Guideline adherence, Health disparities, Informatics, Lung cancer mutation, NSCLC
Introduction
Worldwide, lung cancer is the deadliest cancer.1 Tobacco use is responsible for 85% of lung cancers,2 with the remainder attributed to other etiologic factors. In the United States, lung cancer survival is improving.3 However, not all groups are experiencing improved survival. American Indians and Alaska Natives (AI/AN) continue to experience lung cancer health disparities. In the state of Minnesota, part of the Indian Health Service Great Lakes (Bemidji Area) region, AI/AN die of lung cancer more than twice the rate of non-Hispanic whites.4,5
Understanding this disparity is complex. Each tribe and regional group of AI/AN may differ in cultural norms and other health-promoting behaviors. Further, genetic variations may also contribute to disparities. For example, some AI/AN demonstrate rapid nicotine metabolism, which may increase risk of nicotine addiction and difficulty in quitting commercial tobacco use6; Finally, lack of access to high-quality cancer care also contributes to worse cancer outcomes.7,8 Furthermore, collecting and interpreting data on AI/AN is challenging as a result of small relative population sizes, the fragmented nature of the health care system, undercounting as a result of the use of “other” racial categories, and aggregating individuals from over 573 federally recognized tribes into one group. As a result, the estimated effects of AI/AN health disparities on overall cancer outcomes is not currently known.
Advances in lung cancer care have modestly improved survival in recent years.9 The most revolutionary advance in the treatment of lung cancer has been the discovery of genetic mutations that drive lung cancer and therapies targeted to those mutations. Lung cancer treatment has evolved from one-size-fits-all chemotherapy to targeted therapies that act on tumor-specific somatic genetic mutations. The National Comprehensive Cancer Network and other organizations10 strongly recommend mutational testing for all patients with adenocarcinoma lung cancer. Hence, genetic mutation testing in lung cancer is now a standard of high-quality lung cancer care and is routinely performed on cancer biopsy specimens.
Precision medicine, through testing for somatic mutations and targeting therapy to such mutations, has shown dramatic impact on lung cancer outcomes. Precision medicine has the potential to narrow or widen lung cancer disparities, depending on implementation and access. Of the tumor genetic mutations shown to vary by race, little is known on differences in AI/AN.11 The extent to which the AI/AN population receive testing for somatic cancer mutations is unknown as are the clinical consequences of this testing (or lack thereof).
Patients and Methods
We performed a multisite retrospective review of lung cancer mutational testing in Minnesota. The pilot was funded by the University of Minnesota and was exempted from review by the institutional review board. We invited potential collaborators and data analysts from large health systems in the state of Minnesota to participate. We secured collaborations with 5 tertiary referral health care systems covering a diverse geographic and demographic population; none were Indian Health Service, tribe affiliated, or Urban Indian Centers.
The primary outcomes were incidence of genetic mutational testing in AI with adenocarcinoma and incidence of targeted therapy use in AI receiving a diagnosis or treatment in Minnesota cancer centers. We also sought to describe lung cancer histopathology and the frequency of known lung cancer driver mutations in Minnesota AI: EGFR, ALK, MET, ROS-1, PIK3CA, BRAF, HER2, and KRAS.
Participant Selection and Matching
Deidentified cases were requested from each institution meeting the following inclusion criteria: self-identified AI/AN in the electronic health record, lung cancer diagnosis after 2010, and age > 18 years at the time of diagnosis. Lung cancer diagnoses were identified using the International Classification of Diseases, 9th Revision (ICD-9), before October 2015 and the International Classification of Diseases, 10th Revision (ICD-10) after October 2015.
Controls were requested on these inclusion criteria, self-identified non-AI/AN, lung cancer diagnosis after 2010, and age > 18 years at the time of diagnosis. Matched controls were requested from each center at a ratio of 1:1 on the basis of tumor histology, smoking status, sex, age, and date of diagnosis. From one center, we received a large number of controls, so to reduce the influence of this center on the analysis, the controls were matched 1:1 using the “nearest” neighbor propensity score method, using tumor histology, smoking status, sex, age, and date of diagnosis as matching factors. This was completed with the “MatchIt” package in R (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/).
Data Collection and Analysis
Each health system collected data by manual chart review or through computerized data extraction from a clinical data warehouse. One site utilized an existing lung cancer registry. Guidelines for data collection and documentation were provided for standardization across sites in the absence of a common data model across sites. Data were fully deidentified at each site, then submitted to the University of Minnesota Clinical and Translational Science Institute Biomedical Informatics team for analysis. Data were harmonized and integrated into a single data set. Statistical analysis was performed by the Biostatistics Core of the Masonic Cancer Center at the University of Minnesota.
Age is summarized with the mean and standard deviation, and cases and controls were compared by a 2-sample t test. All categorical measures are summarized with frequencies and percentages, and were compared by the Fisher exact test. P < .05 was considered statistically significant. All analyses were done in R 3.5.1. Histology responses were reviewed by investigators (A.B., R.K., P.Y.) to confirm and classify lung cancer. Eight cases and 23 controls were excluded as a result of lack of histopathology result or non—lung cancer histology (eg, lymphoma, sarcoma).
Occurrence of testing and results for the genetic mutations of interest (EGFR, ALK, MET, ROS-1, PIK3CA, BRAF, HER2, KRAS) were recorded. In addition, the use of any clinically available targeted therapies was recorded.
Results
Data for 200 cases and 164 controls were collected. Cases and controls had equal proportions of men and women. Most were current or former cigarette smokers. Lung cancer histology was mostly non—small-cell lung cancer (NSCLC), specifically adenocarcinoma (Table 1). One site (site 4) did not submit controls, so the 36 cases from that site were excluded from outcome analyses. Another site (site 2) did submit cases; however, some controls were diagnosed before 2010. These were included to give a more representative description of detected mutations.
Table 1.
Participant Characteristics
| Characteristic | Case (N = 164) | Control (N = 164) | P |
|---|---|---|---|
| Site | |||
| 1 | 75 (45.7) | 75 (45.7) | |
| 2 | 45 (27.4) | 45 (27.4) | |
| 3 | 33 (20.1) | 33 (20.1) | |
| 5 | 11 (6.7) | 11 (6.7) | |
| Female sex | 78 (47.6) | 79 (48.2) | .999 |
| Age (y), mean ± standard deviation | 65.1 ± 9.6 | 65.5 ± 9.3 | .724 |
| Year of Diagnosis | |||
| <2010 | 0 | 39 (23.8) | <.001 |
| 2010–2013 | 70 (42.7) | 48 (29.3) | |
| 2013+ | 94 (57.3) | 77 (47.0) | |
| Smoking Status | |||
| Current | 97 (59.1) | 103 (62.8) | .862 |
| Former | 57 (34.8) | 50 (30.5) | |
| Never | 9 (5.5) | 10 (6.1) | |
| Not asked | 1 (0.6) | 1 (0.6) | |
| Histology | .863 | ||
| Small cell | 18 (11.0) | 20 (12.2) | |
| NSCLC | 146 (89.0) | 144 (87.8) | |
| Adenocarcinoma | 84 (51.2) | 75 (45.7) | |
| Squamous-cell carcinoma | 35 (21.3) | 47 (28.7) | |
| NOS | 27 (16.5) | 22 (13.4) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: NOS = not otherwise specified; NSCLC = non–small-cell lung cancer.
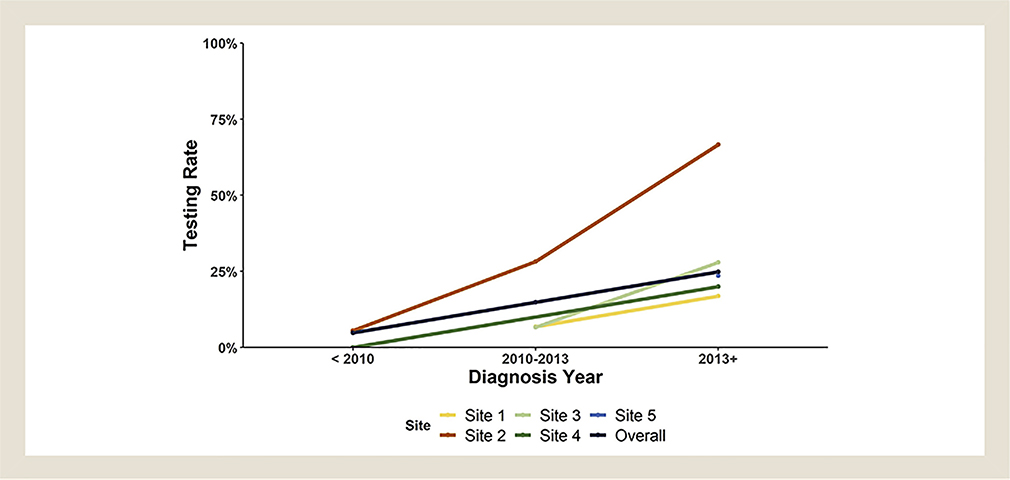
Overall, cases of adenocarcinoma or NSCLC not otherwise specified received testing 35% of the time, while 22% of controls were tested (Table 2). More cases had an EGFR mutation detected, while more controls had KRAS mutation detected. Seven patients were prescribed targeted therapy, most of whom were cases. Missing data were minimal, with only one missing mutation test result. The Fisher test for within-site differences in rates of testing among cases and controls showed a lower rate of testing in controls at site 2, but we did not identify between-site difference for testing rates (Table 3). Testing rates increased over time in all groups (Figure 1). One interesting finding was that 2 of 3 cases tested for KRAS had the mutation, while none of the 5 controls tested did.
Table 2.
Any Mutation Test Rates for Subjects With Adenocarcinoma or Non–Small-Cell Lung Cancer, Not Otherwise Specified
| Site | Case | Control | P |
|---|---|---|---|
| 1 | 10 (27.0) | 9 (24.3) | .999 |
| 2 | 19 (42.2) | 4 (13.3) | .010 |
| 3 | 6 (28.6) | 7 (21.8) | .999 |
| 5 | 4 (50.0) | 2 (25.0) | .608 |
| Total | 39 (35.1) | 22 (22.7) | .067 |
| P | .357 | .453 |
Data are presented as n (%) unless otherwise indicated.
Table 3.
Specific Mutation Test Rates and Results for Subjects With Adenocarcinoma or Non–Small-Cell Lung Cancer, Not Otherwise Specified
| Mutation | Cases (N = 111) | Controls (N = 97) | P |
|---|---|---|---|
| EGFR | |||
| Tested | 35 (31.5) | 17 (17.5) | .025 |
| Mutation detected | 2 (5.7) | 4 (23.5) | .140 |
| ALK | |||
| Tested | 17 (15.3) | 12 (12.4) | .556 |
| Mutation detected | 1 (5.9) | 0 | .469 |
| ROS1 | |||
| Tested | 2 (1.8) | 5 (5.1) | .255 |
| Mutation detected | 0 | 1 (20) | .495 |
| KRAS | |||
| Tested | 3 (2.7) | 5 (5.1) | .477 |
| Mutation detected | 2 (66.7) | 0 | .035 |
Data are presented as n (%) unless otherwise indicated.
Figure 1.
Rates of Mutational Testing by Site Over Time
Discussion
This project was designed as a secondary research use of electronic health records with data sharing to aggregate a sample of Minnesota AI/AN with lung cancer from different health systems. AI/AN comprise 1.88% of Minnesota’s population,5 so each large health system has a small number of AI/AN patients with lung cancer. The study is limited by its retrospective nature, as well as potential bias due to reliance on self-report of race.12 However, difficulty studying heterogeneous groups of marginalized persons with justifiable mistrust of researchers limits prospective study design. AI/AN are underrepresented in clinical trials.13 As such, we believe creative methods like this pragmatic and deidentified approach to examining the glaring disparities observed in lung cancer outcomes between whites and AI/AN are necessary steps toward understanding tumor genetic mutations in AI with lung cancer and its effects on outcomes.14
The rate of genetic testing was higher in cases than controls, though this may be attributable to inclusion of controls diagnosed before 2010. Sensitivity analysis was performed to exclude subjects diagnosed before 2010, resulting in a higher rate of testing for both cases and controls at that site. Cases found to have a mutation were not less likely to receive targeted therapy.
Lung cancer histology in this study of Minnesota AI/AN was similarly distributed to the general United States population (primarily adenocarcinoma), in contrast to the squamous-cell predominance shown in a large review of Surveillance, Epidemiology, and End Results program data.15 That study was published in 2010 and reported a larger proportion of NSCLC not otherwise specified, which may account for the difference. As expected, testing rates increased over time (Figure 1), consistent with the 2013 practice guideline calling for testing in all adenocarcinoma.16 In this study, the proportion of current, former, and never smokers does not match that of the general population with lung cancer. This is consistent with the known higher rate of commercial tobacco use in Minnesota AI/AN compared to whites.17 Commercial tobacco use stems from colonialization of traditional practices in some tribes. As Carol Hernandez (Anishinaabe), an AI/AN tobacco program advocate, notes,
In the past, Minnesota tribes used noncommercial tobacco for ceremonial use. Some tribes used kinnikinnick (“that which is mixed”) with red willow bark often mixed with plants such as bearberry. Others used asemaa, which is a plant in the Nicotiana rustica family. Today, Nicotiana tabacum (commercial tobacco) is used by many American Indians as a substitute for the Nicotiana rustica.18
Although the small numbers do not permit us to draw firm conclusions, the difference in KRAS frequency between cases and controls is intriguing, especially considering that KRAS mutation is associated with poor prognosis in lung cancer and has no targeted therapy yet available.19 KRAS mutations are typically seen in tumors from patients who smoke, and only 5% to 10% of KRAS-mutant lung cancers are reported in never or light smokers. Conversely, EGFR mutation detection rates appear lower in cases, which represents a lack of opportunity for treatment with this class of targeted therapy. Further studies are warranted to determine if this mutation discrepancy might at least in part underlie differences in lung cancer mortality.
Our study found a shockingly low rate of targeted therapy prescription for detected mutations. This may be the result of missing data, but we cannot ascertain that from this study. Alternative explanations include cost of targeted therapy and underinsurance.
In the AI/AN community, some justifiably doubt the intents of the mainstream medical system, and genetic testing in particular is a controversial topic. Controversy over genetic testing is due primarily to concerns about genetic lineage and its impact on cultural identity as well as tribal membership.12 However, testing for somatic cancer mutation is limited to the genes known to affect lung cancer and is considered theragnostic—that is, standardized personalized testing used to guide therapy. Approval for such a test would not typically be sought from a patient because it is now a routine clinical test. Thus, lack of consent or suspicion should not lower the rate of testing AI/AN for target mutations. Our findings confirm this assumption.
Conclusion
This study provided evidence that despite disparities in access to care among AI/AN,20 among those receiving tertiary cancer care, disparities in testing for somatic gene mutations and targeted treatments are unlikely. Thus, efforts to improve the disparities seen in lung cancer mortality in this and similar populations will be more effective by focusing on improving differences in commercial tobacco use and other disparities in care, including access to early cancer detection.21,22 Additionally, our data show that there is a need for increased standard-of-care genetic testing in all patients with nonsquamous NSCLC. Finally, further investigation into lung cancer mutation rates in AI, particularly KRAS, might uncover reasons for mortality disparities.
Supplementary Material
Clinical Practice Points.
AI/AN experience higher lung cancer mortality than other races, but the cause of this disparity is unknown.
We compared rates of standard-of-care EGFR mutation testing in Minnesota AI/AN with lung cancer compared to non-AI/AN controls.
No significant difference was identified; therefore, further study should be focused on other potential contributing factors.
Acknowledgment
This study was funded by the University of Minnesota.
Footnotes
Disclosure
The authors have stated that they have no conflict of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy WJ, Meza R, Jeon J, Moolgavkar SH. Chapter 6: Lung cancer in never smokers: epidemiology and risk prediction models. Risk Anal 2012; 32(suppl 1): S69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards TB, Henley SJ, Puckett MC, et al. Lung cancer survival in the United States by race and stage (2001–2009): findings from the Concord-2 study. Cancer 2017; 123(suppl 24):5079–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minnesota Department of Health; Minnesota Cancer Alliance; American Cancer Society. Minnesota cancer facts and figures, 2015. Available at https://www.health.state.mn.us/communities/environment/tracking/docs/cancerfandf.pdf. Accessed: November 1, 2019.
- 5.Great Lakes Inter-Tribal Epidemiology Center. American Indian and Alaska Native health in Michigan, Minnesota, and Wisconsin, 2016. Available at: http://www.glitc.org/forms/epi/profiles/chp-report-final-050117-web.pdf. Accessed: November 1, 2019.
- 6.Tanner JA, Henderson JA, Buchwald D, Howard BV, Nez Henderson P, Tyndale RF. Variation in cyp2a6 and nicotine metabolism among two American Indian tribal groups differing in smoking patterns and risk for tobacco-related cancer. Pharmacogenet Genomics 2017; 27:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soneji S, Tanner NT, Silvestri GA, Lathan CS, Black W. Racial and ethnic disparities in early-stage lung cancer survival. Chest 2017; 152:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non—small cell lung cancer among black and white Medicare beneficiaries, 2000–2003. J Natl Med Assoc 2011; 103:711–8. [DOI] [PubMed] [Google Scholar]
- 9.Xia W, Yu X, Mao Q, et al. Improvement of survival for non—small cell lung cancer over time. Onco Targets Ther 2017; 10:4295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 2018; 142:321–46. [DOI] [PubMed] [Google Scholar]
- 11.Schabath MB, Cress D, Munoz-Antonia T. Racial and ethnic differences in the epidemiology and genomics of lung cancer. Cancer Control 2016; 23: 338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haozous EA, Strickland CJ, Palacios JF, Solomon TG. Blood politics, ethnic identity, and racial misclassification among American Indians and Alaska Natives. J Environ Public Health 2014; 2014:321604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regnante JM, Richie NA, Fashoyin-Aje L, et al. US cancer centers of excellence strategies for increased inclusion of racial and ethnic minorities in clinical trials. J Oncol Pract 2019; 15:e289–99. [DOI] [PubMed] [Google Scholar]
- 14.Lohinai Z, Klikovits T, Moldvay J, et al. KRAS-mutation incidence and prognostic value are metastatic site-specific in lung adenocarcinoma: poor prognosis in patients with kras mutation and bone metastasis. Sci Rep 2017; 7: 39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fesinmeyer MD, Goulart B, Glough DK, Buchwald D, Ramsey SD. Lung cancer histology, stage, treatment, and survival in American Indians and Alaska Natives and whites. Cancer 2010; 116:4810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013; 8:823–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults—United States, 2005–2015. MMWR Morb Mortal Wkly Rep 2016; 65:1205–11. [DOI] [PubMed] [Google Scholar]
- 18.Boudreau G, Hernandez C, Hoffer D, et al. Why the world will never be tobacco-free: reframing “tobacco control” into a traditional tobacco movement. Am J Public Health 2016; 106:1188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrer I, Zugazagoitia J, Herbertz S, John W, Paz-Ares L, Schmid-Bindert G. KRAS-mutant non—small cell lung cancer: from biology to therapy. Lung Cancer 2018; 124:53–64. [DOI] [PubMed] [Google Scholar]
- 20.Marley TL. Ambiguous jurisdiction: governmental relationships that affect American Indian health care access. J Health Care Poor Underserved 2019; 30: 431–41. [DOI] [PubMed] [Google Scholar]
- 21.Charkhchi P, Kolenic GE, Carlos RC. Access to lung cancer screening services: preliminary analysis of geographic service distribution using the acr lung cancer screening registry. J Am Coll Radiol 2017; 14:1388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeliadt SB, Hoffman RM, Birkby G, et al. Challenges implementing lung cancer screening in federally qualified health centers. Am J Prev Med 2018; 54:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.