Abstract
Streptococcus mutans is a recognized cariogenic bacterium and a major producer of biofilm matrix. The presence of Candida albicans in dental plaque with S. mutans enhances the virulence leading to the onset of rampant caries which is similar to early childhood caries (ECC). The purpose of this study was to explore the effect of Lactobacillus plantarum CCFM8724 (CCFM8724) on the treatment and prevention of dental caries induced by S. mutans and C. albicans in vivo. Rats were divided into 6 groups: the control group and model group, 2 treatment groups, and 2 prevention groups (0.02% chlorhexidine or CCFM8724). The fluctuation of microbial colonization and the change of bacteria flora in rat oral cavity after sowing of L. plantarum CCFM8724 were investigated by colony-forming units (CFU) and microflora analysis. The caries of rats were assessed by microcomputed tomography (micro-CT) and Keyes scoring method. The results showed that L. plantarum CCFM8724 in both the treatment and prevention groups could significantly decrease the population of S. mutans and C. albicans in the rats' oral cavity (p < 0.001), the mineral loss of enamel (p < 0.05), and the scores of caries (p < 0.05). Besides, L. plantarum CCFM8724 exhibited better effects than chlorhexidine. Hence, L. plantarum CCFM8724 was proved to be a potential oral probiotic on caries treatment and prevention in vivo and it may have the prospect of application in dental caries (especially ECC) prevention products.
1. Introduction
Dental caries is one of the most prevalent oral bacterial infectious diseases that afflict children and adults worldwide. It can lead to the irreversible tooth demineralization, which is mainly associated with acidic biofilm formation and sugar intake [1]. Early childhood caries (ECC) is a unique form of rampant caries developed in babies and toddlers, especially preschool children. ECC can result in rapid and aggressive destruction of primary teeth which not only causes painful pulpal but also impacts on the development of permanent dentition and systemic health [2, 3]. Traditional treatment for deciduous caries is extremely costly and time consuming. Hence, ECC has been a major challenge in public health until today.
Streptococcus mutans has been recognized as the main cariogenic bacteria for its strong acidogenic and aciduric capacities [4]. And it is also the major producer of biofilm matrix, providing acid milieus within which caries-associated organisms thrive [5]. Intriguingly, recent studies have found that, in addition to S. mutans, fungus such as Candida albicans was detected frequently in high numbers in plaque biofilms from toddlers with caries [6, 7]. Moreover, the presence of C. albicans in mixed-species biofilms induces S. mutans glucosyltransferase (GTF) expression, which converts dietary sucrose into extracellular polysaccharides (EPS) and build more cariogenic biofilms [8]. Rather, the synergistic alliance of them amplifies the biofilm virulence which led to rampant caries similar to ECC in an animal model [9].
Common mechanical measures for dental caries control, although effective, have been limited by the preference of children and the elderly. It is known that chlorhexidine (CHX) has a bactericidal effect on S. mutans and fungicidal effect on C. albicans [10]. While the limitation of antimicrobial drugs is that once the intervention with CHX stops, the same pathogens will repopulate in oral niches and result in recurrence of tooth decay. To avoid this, lower dental plaque virulence with ecological methods are being increasingly preferred over broad-spectrum antimicrobials for long-term caries control [11]. In this regard, it is essential to develop an alternative product which can disrupt the cariogenic virulence and promote oral microecological balance with no adverse effects.
The FAO/WHO (Food and Agriculture Organization of the United Nations, World Health Organization) define probiotics as live microorganisms that play a beneficial role in the health of the host by taking appropriate amounts. In the past 10 years, probiotics have achieved a lot in preventing or treating gastrointestinal infections and diseases such as acute diarrhea, ulcerative colitis, Crohn's disease, and pouchitis [12, 13]. Currently, with the increasing acceptance of probiotics, more and more attention has been paid to probiotic usage in other areas such as dental caries prevention, periodontitis relieving, and regulation of oral microecological balance [14]. And several clinical trials have demonstrated that the administration of Lactobacillus genus including L. rhamnosus, L. reuteri, and L. paracasei decreased the lesion of caries in children [15–17]. However, the number of studies on the interaction of Lactobacillus with cariogenic bacteria and fungi and the effects of the probiotic L. plantarum on ECC caries model in vivo is limited, which prompted us to address these knowledge gaps.
In our previous study, L. plantarum CCFM8724 isolated from healthy human feces exhibited a considerable effect on inhibiting S. mutans and C. albicans dual biofilm formation in vitro [18]. In this study, we examined the changes of S. mutans and C. albicans colonization and caries development in the rat oral cavity by plate culture counting, 16S rDNA sequencing, micro-CT, and caries scoring to evaluate the effect of L. plantarum CCFM8724 on the prevention or treatment of caries in vivo. These results will provide enlightenment and proof on probiotics preventing and treating rampant caries induced by S. mutans and C. albicans.
2. Methods
2.1. Ethics Statement
This research methodology was conducted in accordance with institutional ethical standards. The study on animals was approved by “Jiangsu Institute of Parasitic Diseases, Animal Care and Use Committee” (IACUC-JIPD-2019030). All experiments were in accordance with the guidelines of the China Ministry of Science and Technology Guide for the Care and Use of Laboratory Animals.
2.2. Strains and Inoculum Preparation
L. plantarum CCFM8724 was isolated from healthy human feces and inoculated in MRS broth (Difco™, Detroit, MI, USA) under anaerobic conditions at 37°C. S. mutans ATCC 25175 was purchased from the China Common Microbial Species Preservation and Management Center (CGMCC, Beijing, China) and cultured in Tryptic Soy Broth (TSB, Difco™, Detroit, MI, USA) under anaerobic conditions at 37°C. C. albicans SJ was isolated from human carious dentin, which was grown in Yeast Extract Peptone Dextrose Medium (YPD, Difco™, Detroit, MI, USA) under aerobic conditions at 37°C. All strains were frozen in 30% (v/v) glycerol broth at -80°C and routinely streaked on corresponding agar plates. The plates were cultured at 37°C for 48 h and at least three times consecutively using 2% (v/v) inoculum in corresponding broth at 37°C for 18 h before use.
Prior to use in animal experiments, the microbial cultures were centrifuged at 3000 g for 10 min and washed twice with sterile saline solution. The bacteria and yeast were then centrifuged and resuspended in saline solution and diluted to a suspension of 1 × 109 living cells (CCFM8724 and S. mutans) or 106 (C. albicans) by colony counting.
2.3. Animals and General Procedures
Animal experimental design is shown in Figure 1. Female SPF Wistar rats (21 days old) were purchased from Charles River Laboratories (Beijing, China). All of the rats were divided randomly into 6 groups, comprising of 2 treatment groups (T1-CHX and T2-CCFM8724), 2 prevention groups (P1- CHX and P2-CCFM8724), and the caries-free and caries model groups. The rats in the caries-free group were given normal diet and distilled water during the whole experiment. Other groups were offered a cariogenic diet 2000 (obtained from Nantong Trophy Feed Technology Co., Ltd.) and water containing 5% sucrose ad libitum. The oral flora of rats was suppressed by ampicillin (0.5 μg/mL) and streptomycin (200 μg/mL) for 3 days before the experiment.
Figure 1.
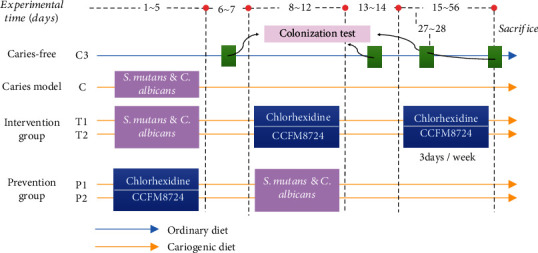
Animal experimental design.
The caries model group was infected with S. mutans and C. albicans for 5 consecutive days from the first day of the experiment. The specific manipulation was to saturate the sterile cotton sticks with suspension of the two cariogenic microorganisms separately and then coat the suspension onto the rat oral cavity for 15 s per quadrant, as described by Beiraghi et al. [19]. Diet and water were forbidden for half an hour after tooth coating to ensure the colonization of microorganisms [20]. The establishment of tested strains was verified on day 6 and 7. The treatment groups were then applied with 0.02% CHX or CCFM8724 from day 8 to 12 and day 15 to 56. After verifying the successful colonization of lactobacilli, CHX or Lactobacillus was given three times a week until the end of the experiment (from day 15 to 56).
To test the prevention effect of Lactobacillus on caries, CHX or CCFM8724 was firstly applied to coat the teeth on day 1-5. After the establishment of lactobacilli, S. mutans and C. albicans were used for another 5 consecutive days. No treatment was done in the prevention groups for the next 6 weeks to the end of the experiment. The whole experiment period was 8 weeks. The rats were weighed daily, and their weight gains were calculated.
2.4. Oral Microbial Count
Four samples (day 6, 13, 27, and 56) were taken during the whole experiment. Specific sampling method was scraping the surface of rat dentin with sterile cotton stick back and forth three to four times. S. mutans was counted on Mitis Salivarius Agar (Difco, No. 229810, BD Diagnostic Systems) with 200 IU/L bacitracin (MSB) [21], and C. albicans was plated on BIGGY Agar (BBL, No. 211027, BD Diagnostic Systems) [22], while MRS supplemented with 12 μg/mL vancomycin was used to enumerate lactobacilli [23].
2.5. DNA Extraction, PCR, and 16S rDNA Sequencing
Cotton swabs sampled from the rat oral cavity on day 56 before sacrificed were stored at -80°C before examination. According to the instructions, FastDNA Spin Kit for feces (MP Biomedical, United States) was used to extract microbial genome DNA. The V3-V4 region of the 16S rDNA gene was amplified by PCR. After cutting from the 1.5% agarose gel, the product was purified by a QIAquick Gel Extraction Kit (Qiagen, Germany) and quantified by a Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies, United States). Libraries were established by a TruSeq DNA LT Sample Preparation Kit (Illumina, United States) and were sequenced for 500 + 7 cycles on Illumina MiSeq using a MiSeq Reagent Kit. At last, the sequence data of 16S rDNA were analyzed by QIIME pipeline as described previously [24].
2.6. Caries Scoring
On day 56, the rats were anesthetized and decapitated. The soft tissues on the teeth and jaws were peeled off with a scalpel, and the residual debris in the sutures was washed by ultrasonic cleaning for 20 minutes. The maxillary and mandibular molars were soaked in 10% polyformaldehyde for 24 hours [25, 26], then washed and dried. All the specimens were immersed in a 0.4% ammonium purpurate staining solution for 12 h and hemisectioned in the mesiodistal direction with an ultrathin carborundum disk (0.2 mm in thickness). The caries of rat molars were observed under a stereomicroscope (Leica CLS 100X, Wetzlar, Germany) and scored according to the method reported by Keyes [27].
2.7. Micro-CT Analysis
All mandibles were imaged using a microcomputed tomography (micro-CT) system (Quantum GX2; PerkinElmer, Hopkinton, MA, USA). An acquisition setting was used for scanning all the samples: 90 KV, 88 μA; field of view: 18 μm; acquisition time: 4 min; camera mode: high resolution. Each sample was rotated 360°, and all images were imported into Analyze 12.0 software (AnalyzeDirect, Overland Park, KS, USA) to reconstruct three-dimensional images of the mandibles, respectively. And a fixed threshold of 5,200 Hounsfield units was used to separate enamel from the whole mandible [28]. Mineral density (MD) of the enamel was calculated after calibration with hydroxyapatite standards of appropriate density.
2.8. Statistical Analysis
SPSS Statistics 25.0 (SPSS, Inc., Chicago, IL, USA) was used for the analysis. Graphpad Prism 8.0 and Origin 8.5 were used to map and analyze the data. The differences between the mean values of the groups were analyzed by one-way analysis of variance using Duncan's multiple range tests. Data expressed as mean ± standard error of the mean (s.d, n = 8).
3. Results
3.1. Microorganism Colonization of the Rat Oral Cavity
During the animal experiment, all animals appeared to be in good physical condition and no significant differences in weight gain were observed among the groups (p > 0.05). The colonization of S. mutans and C. albicans were 5.6 and 4.1 log (CFU/mL) in the first sampling, respectively (Figures 2(a) and 2(b)). After 5 consecutive days of Lactobacillus intervention, the population of S. mutans in T2 groups decreased from 5.4 to 4.0 log (CFU/mL) (p < 0.001) and remained at 4.1 log (CFU/mL) until the end of the experiment. CHX (T1) also inhibited the growth of S. mutans throughout the experiment, which reduced the population of S. mutans to 4.5 log (CFU/mL) (p < 0.001) and stabilized at this level.
Figure 2.
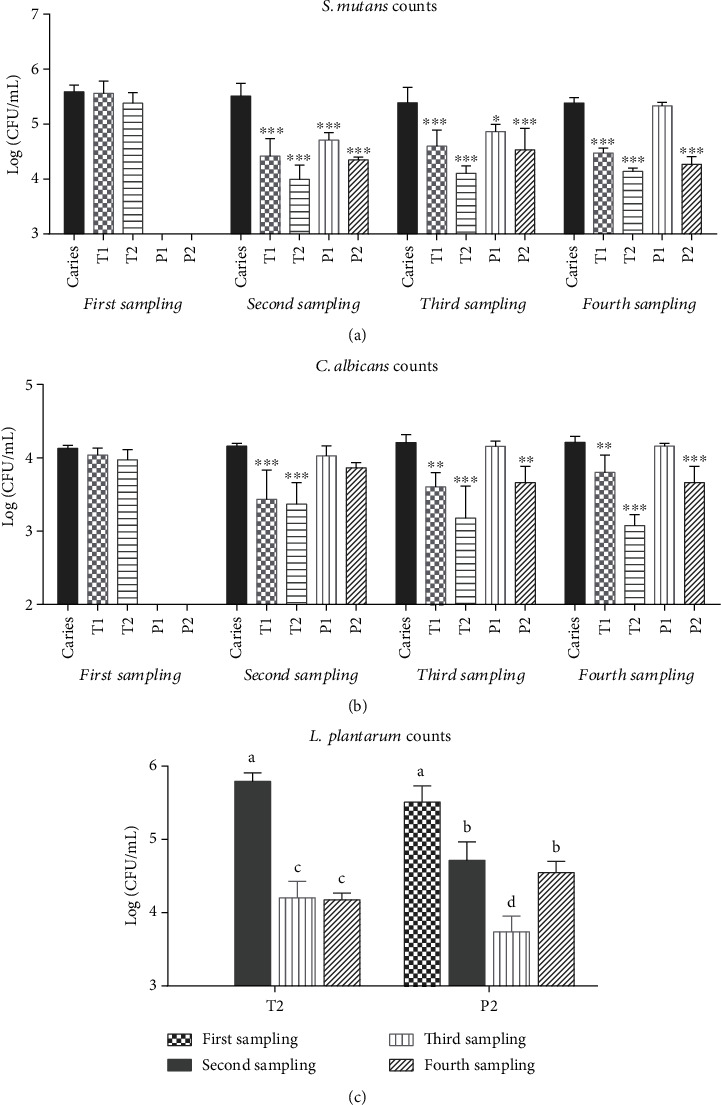
Microorganism count (log CFU/mL) recovered from rat oral swabs for 4 times. (a) Counting results of S. mutans. (b) Counting results of C. albicans. Values are significantly different from the caries model group in each sampling at ∗p < 0.05, ∗∗p < 0.01, or ∗∗∗p < 0.001 (a, b). (c) Counting results of L. plantarum. Groups with dissimilar letters differ, p < 0.05.
After administered 0.02% CHX or CCFM8724 on day 1-5, the colonization of S. mutans in the prevention groups (P1 and P2) was significantly lower than that in the caries model group (p < 0.001). However, once CHX was stopped, the number of S. mutans continued rising, from 4.7 log (CFU/mL) (second sampling) to 5.3 log (CFU/mL) (fourth sampling), which was close to the number observed in the caries model group (p > 0.05), while the population of S. mutans in the oral cavity (P2) remained at a low level (4.3 log (CFU/mL), p < 0.001) until the end of the experiment after CCFM8724 administration was stopped.
A similar phenomenon was observed on the change of C. albicans (Figure 2(b)). The effect of CHX treatment (T1) on C. albicans tended to decrease with time. Moreover, CHX cannot prevent the colonization and growth of C. albicans (P1, p > 0.05), while CCFM8724 intervention exhibited a significant inhibitory effect on C. albicans (p < 0.001) during the experiment. Interestingly, the CCFM8724 prevention group (P2) did not immediately affect C. albicans after the colonization of Lactobacillus but reduced the number of C. albicans in the third (p < 0.05) and fourth (p < 0.001) sampling.
According to the counts of L. plantarum (Figure 2(c)), cariogenic bacterial and fungi did not affect the colonization of Lactobacillus (P2 and T2, p > 0.05), which ranged from 5.5 to 5.8 log (CFU/mL) in both two groups. The population of L. plantarum in the CCFM8724 prevention group (P2) experienced significant fluctuations in the second, third, and fourth sampling, from 4.7 (CFU/mL) to 3.7 log (CFU/mL), and back to 4.5 log (CFU/mL) (p < 0.05), while that in the CCFM8724 treatment group decreased from 5.8 log (CFU/mL) (second sampling) to 4.2 log (CFU/mL) (third sampling) and then stabilized to 4.2 log (CFU/mL) (fourth sampling).
3.2. Microflora Analysis
Since the counts of S. mutans, C. albicans, and Lactobacillus shifted in different groups during the experiment, we also revealed the alterations of bacterial abundance and proportion among different groups by Illumina 16S rDNA gene sequencing. Compared with the caries-free group, the application of 0.02% CHX or CCFM8724 revealed major changes in relative abundance in the oral microbiome of rats (Figure 3(a)). The notable difference in Streptococcus was found between the caries-free group (CF, 3.65%) and the caries model group (C, 22.5%) (p < 0.05). Treatment with CCFM8724 significantly decreased the abundance of Streptococcus when compared with the caries model group (p < 0.05), although there was no difference observed between the T1 (7.24%) and T2 (6.01%) groups (Figure 3(b)). The same degree of decrease was obtained in the two prevention groups (P1, 17.24%, P2, 9.43%, p < 0.05). Relative abundance of Lactobacillus remained lowest in the caries-free group (CF, 0.22%), caries model group (C, 0.43%), and CHX treatment group (T1, 0.22%). No significant difference was observed among these three groups. Treatment with CCFM8724 can significantly increase the relative abundance of Lactobacillus, as shown in Figure 3(b), while the Lactobacillus abundance in the CCFM8724 prevention group (P2) was more than 1%, which is the highest in all groups (1.5%, p < 0.05).
Figure 3.
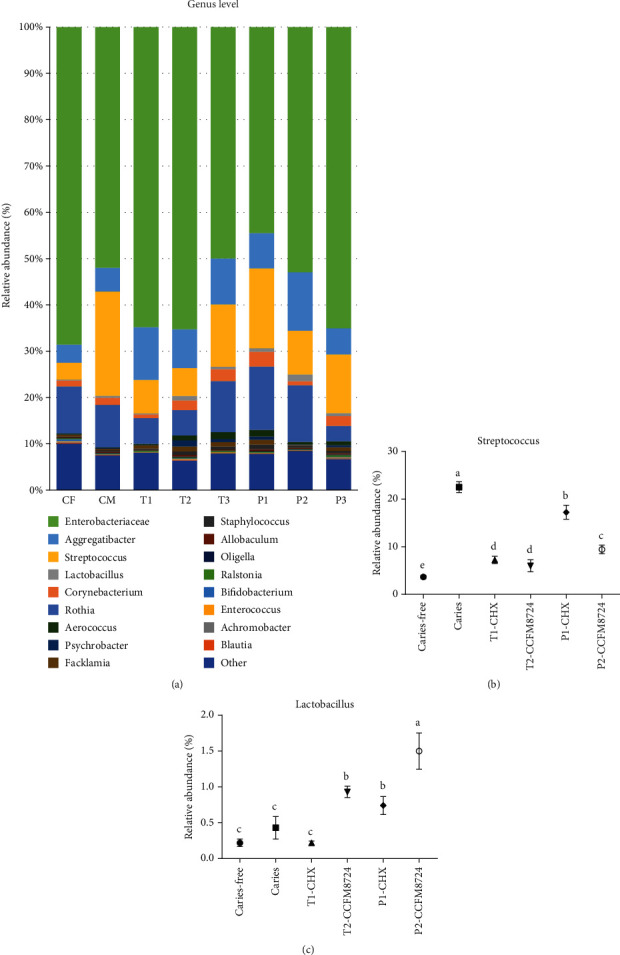
Changes in the level of genus in the oral cavity of rats in different groups. (a) Relative abundance of main genus > 0.1% in different groups. (b) Changes of the abundance of Streptococcus genus in different groups. Groups with dissimilar letters differ, p < 0.05. (c) Changes of the abundance of Lactobacillus genus in different groups. Groups with dissimilar letters differ, p < 0.05.
3.3. Micro-CT Analysis
To enhance the visibility of caries site on rat molars, 3D reconstructions of mandible molars were performed by micro-CT. Enamel and dentin were stripped according to the density threshold (Hounsfield units). Meanwhile, the corresponding sagittal slice of the same molar was taken for comparative observation (Figure 4). Compared with the sagittal slice image between the caries model group and caries-free group, it is obvious that the enamel (green) of sulcal or adjacent areas were not continuous if caries occurred.
Figure 4.
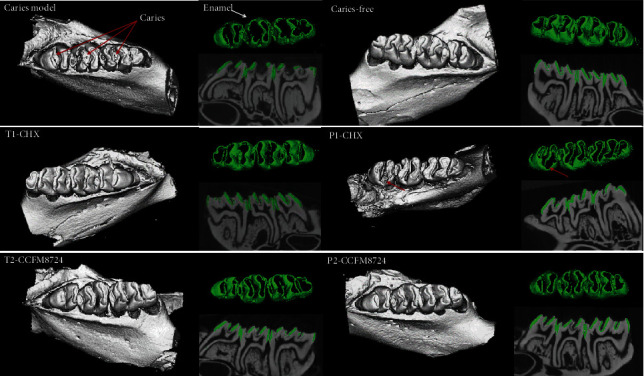
3D micro-CT image of mandibular molars, separated enamel (green), and corresponding 2D scale sagittal slice of the same molar (enamel is green) in each group. Red arrows: caries lesion site.
To evaluate the results of micro-CT, the mean enamel volume (Figure 5(a)) and density (Figure 5(b)) of molars were analyzed in each group. Morphometric volume analysis revealed that the enamel volume in the CCFM8724 prevention group (P2) was closest to that of the caries-free group, followed by the CCFM8724 intervention group (T2). There was no significant difference in enamel volume between the CHX prevention group (P1) and intervention group (T1), but the enamel volume in both two groups was significantly larger than that of the caries model group (p < 0.05). The enamel density showed a similar result to those of enamel volume, but there was no difference in enamel density between the CHX prophylaxis group (P1) and dental caries model group (p > 0.05).
Figure 5.
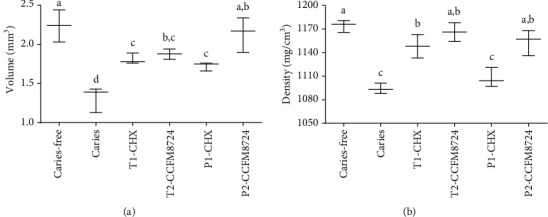
(a) Volume of enamel of mandibular molars. (b) MD of enamel of mandibular molars. Groups with dissimilar letters differ, p < 0.05.
3.4. Caries Scoring
Under stereoscopic microscopy, various caries lesions were observed in all of the dyed molars except the caries-free group. The caries scores (Table 1) indicated that the lesion level E (enamel caries) of smooth surface or sulcal surface was considerably decreased in all the four groups, except that the CHX prevention group did not exhibit a significant effect on sulcal caries. According to the scores of different severity, the CCFM8724 prevention group showed the best effect, in which smooth surface and fissures did not appear with extensive caries (Dm, 3/4 of the dentin affected). And the total score (E) of this group was the lowest. The order of anticaries effect is as follows: the Lactobacillus treatment group, CHX treatment group, and lastly, CHX prevention group.
Table 1.
Effect of different groups on caries development (incidence and severity) in rats.
| Group | Smooth surface | Sulcal surface | ||||
|---|---|---|---|---|---|---|
| Total | Severity | Total | Severity | |||
| E | Ds | Dm | E | Ds | Dm | |
| Caries model | 13.2 ± 5.2 | 8.3 ± 0.8 | 5.7 ± 0.5 | 23.7 ± 5.2 | 18.8 ± 4.1 | 9.3 ± 2.4 |
| T1-CHX | 8.6 ± 2.6∗ | 5.0 ± 1.1∗ | 2.1 ± 0.6∗ | 21.3 ± 6.8∗ | 12.9 ± 3.5∗ | 7.1 ± 1.4 |
| T2-CCFM8724 | 6.6 ± 3.8∗ | 4.7 ± 1.1∗ | 2.0 ± 1.4∗ | 19.6 ± 4.0∗ | 6.6 ± 2.7∗ | 2.4 ± 0.8∗ |
| P1-CHX | 7.9 ± 5.6∗ | 6.5 ± 2.8 | 4.5 ± 2.1 | 21.8 ± 6.2 | 16.3 ± 4.9 | 6.9 ± 1.9 |
| P2-CCFM8724 | 6.5 ± 2.4∗ | 1.9 ± 0.1∗ | — | 15.4 ± 7.3∗ | 3.1 ± 3.0∗ | — |
Data are expressed as mean ± standard error of the mean (n = 8). E: enamel caries; Ds: dentin exposed; Dm: 3/4 of the dentin affected. ∗p < 0.05 when compared with the caries model group.
4. Discussion
In view of the increasing incidence and prevalence of dental cavity and its detrimental effects on oral health, novel strategies are required for its prevention and control. In recent years, the application of probiotics to prevent dental caries has become more and more common. Krzyściak et al. proved that L. salivarius HM6 could inhibit S. mutans and C. albicans dual biofilm formation and reduce the pathogenic species in vitro [29]. Although lactobacilli themselves could produce organic acid, it can be concluded that the overall effect of lactobacilli on caries prevention seems favorable when probiotics candidates are carefully selected [30]. And other studies have shown that Lactobacillus could attenuate the growth of S. mutans [31] or C. albicans [32] in human mouth. However, little is known whether Lactobacillus could exhibit effect on the mutually reinforcing alliances of S. mutans and C. albicans that coexist in the mouth.
In our study, CCFM8724 showed a considerable inhibitory effect on S. mutans and C. albicans during the intervention which lasted less than 2 months, three times a week (Figures 2(a), 2(b), and 3(b)). The prevention group in which CCFM8724 colonized firstly succeeded in controlling the proliferation of S. mutans but did not affect the colonization of C. albicans at first (Figures 2(a) and 2(b)). Although C. albicans decreased significantly and stabilized at a certain level in the later stage, this may be related to the protective effect of S. mutans on C. albicans [33, 34]. The colonization of Lactobacillus is also the key to its function. From Figure 2(c), it can be seen that the colonization of CCFM8724 was a fluctuating process, which may be the result of competitive adherence with pathogenic bacteria in the mouth [35]. Meanwhile, Lactobacillus could maintain a healthy oral environment by producing antimicrobial substances including organic acids, hydrogen peroxide, bacteriolytic enzymes, bacteriocins, and biosurfactants to inhibit the growth of pathogenic bacteria [36, 37]. However, the oral cavity is a nutrient fluctuating environment, whether CCFM8724 could maintain the balance of the oral environment for a longer time should be investigated further.
The oral microbiome analysis of the rats in different tested groups was a novel aspect to supplementarily certificate the CFU counting results. The genus level composition of all groups seemed no notable differences, and the major genus detected throughout the study were Enterobacteriaceae, Aggregatibacter, Streptococcus, and Lactobacillus as displayed in Figure 3(a). Meanwhile, the relative abundance of S. mutans in the caries model (22.5%) is in agreement to a recent report by Garcia et al. [38], in which the caries model was established by S. mutans alone. The diversity of oral microbiota in the caries model seemed to decrease, which may be attributed to the colonization of Candida. Haukioja et al. also found that increased Candida was related to reduced diversity of salivary microbiota [39]. Furthermore, the relative abundance of the Lactobacillus and Streptococcus in T2 and P2 (Figure 3(b)) groups was negatively correlated, which was consistent with the counting results in Figure 2. Intriguingly, Aggregatibacter actinomycetemcomitans has been reported to produce a signaling molecule called autoinducer-2 (AI-2) which could regulate the C. albicans biofilm formation [40]. Combined with the relative abundance changes of Aggregatibacter in the model group and other test groups, there may be a negative correlation between Aggregatibacter and C. albicans. More explorations should be taken to demonstrate this in further study. After all, C. albicans can affect the composition of bacteria in the oral cavity and its status in the oral ecosystem is no longer neglectable [41]. However, the microbiome of oral fungi in caries model needs to be further explored by gene sequencing.
Microtomography (micro-CT) is a modified version of medical computed tomography, which can capture images from multiple angles and produce nondestructive visualization of dental structures in three dimensions [42]. As a tool to provide high-resolution images as well as both qualitative and quantitative analyses of the tooth, microtomography is the gold standard for caries detection and evaluation in vitro, which gained increasing popularity in use for dental research [43]. Hence, mciro-CT has been recommended as a reliable method to evaluate the volume and MD of dental hard tissue, especially to focus on the density of the hard tissues [42, 44]. It is well known that caries occur with demineralization of hard tissues, and enamel is the initial site of dental caries. Therefore, the enamel which separated from the dentin is often used to analyze the caries severity in most studies [45–47]. In this study, the enamel volume and MD of one side of the mandible were calculated. The larger the volume of the residual enamel indicated the stronger the anticaries effect of the tested group. And the higher MD value proved the less loss of minerals or the stronger remineralization effect. And the result showed that the enamel caries severity increased in the order of P2, T2, T1, and P1, which provided evidence of the notable effect of Lactobacillus, while little effect of CHX prevention.
Despite these considerable advantages of micro-CT, the high cost and long scanning time are the disadvantages. Therefore, the Keyes scoring method is still widely used for analyzing the primary caries in animal models along with the supplementary testimony of micro-CT. Klinke et al. [22] revealed that the combination of S. mutans and C. albicans could lead to more serious pit and fissure caries than smooth caries, which is similar to the results of the model group (Table 1). No significant difference of smooth surface caries was observed in caries scores or images among four tested groups. What really widened the score gap was the relief of pit and fissure caries. Except that the CHX intervention group had no significant effect on occlusal caries, other groups showed a notable effect on smooth and fissure caries. The lowest total score (level E) was the CCFM8724 prevention group, which indicated that Lactobacillus exhibited better anticaries effect when colonized firstly in the oral cavity than later treatment. Probiotics can be promising candidates for novel anticariogenic substances owning to their essential ability of oral colonization and competition with oral pathogens for adhesion sites [35, 48].
In this study, both CCFM8724 treatment and prevention significantly attenuated the growth of S. mutans and C. albicans in the rat oral cavity, while CCFM8724 showed potential ability to colonize the oral cavity which is also the key to its function. In addition, the significant difference of caries lesions between the model group and lactobacillus-associated groups were verified via micro-CT and Keyes scoring method, demonstrating the anticaries properties of CCFM8724 in vivo. Further confirmation by clinical studies, would confirm the oral probiotic nature of CCFM8724 in alleviating dually infected caries.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFD0400600), the National Natural Science Foundation of China (Nos. 31820103010, 31530056), National First-class Discipline Program of Food Science and Technology (JUFSTR20180102), and Collaborative innovation center of food safety and quality control in Jiangsu Province.
Contributor Information
Zhenmin Liu, Email: liuzhenmin@brightdairy.com.
Wei Chen, Email: chenwei66@jiangnan.edu.cn.
Data Availability
Data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interests.
Authors' Contributions
Qiuxiang Zhang contributed to the conception, design, and data analysis of the study and critically revised the manuscript. Sujia Qin contributed to the conception, design, data acquisition, analysis, and interpretation of the study and drafted and critically revised the manuscript. Xianyin Xu contributed to the study conception and critically revised the manuscript. Jianxin Zhao contributed to the study conception and critically revised the manuscript. Hao Zhang contributed to the study conception and critically revised the manuscript. Zhenmin Liu contributed to the study conception and critically revised the manuscript. Wei Chen contributed to the study conception and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
References
- 1.Pitts N. B., Zero D. T., Marsh P. D., et al. Dental caries. Nature Reviews Disease Primers. 2017;3(1, article 17030) doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 2.Anil S., Anand P. S. Early childhood caries: prevalence, risk factors, and prevention. Frontiers in Pediatrics. 2017;5:p. 157. doi: 10.3389/fped.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen K., Gao S., Duangthip D., Lo E., Chu C. Managing early childhood caries for young children in China. Healthcare. 2018;6(1):p. 11. doi: 10.3390/healthcare6010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simón-Soro A., Mira A. Solving the etiology of dental caries. Trends in Microbiology. 2015;23(2):76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Klein M. I., Falsetta M. L., Xiao J., Bowen W. H., Koo H. The Role of Extracellular Polysaccharides Matrix in Virulent Oral Biofilms. Norfolk, UK: Caister Academic Press; 2013. [Google Scholar]
- 6.Raja M., Hannan A., Ali K. Association of oral candidal carriage with dental caries in children. Caries Research. 2010;44:p. 272. doi: 10.1159/000314675. [DOI] [PubMed] [Google Scholar]
- 7.Yang X. Q., Zhang Q., Lu L. Y., Yang R., Liu Y., Zou J. Genotypic distribution of Candida albicans in dental biofilm of Chinese children associated with severe early childhood caries. Archives of Oral Biology. 2012;57:1048–1053. doi: 10.1016/j.archoralbio.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Gregoire S., Xiao J., Silva B., et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Applied and Environmental Microbiology. 2011;77:6357–6367. doi: 10.1128/AEM.05203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falsetta M. L., Klein M. I., Colonne P. M., et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infection and Immunity. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shino B., Peedikayil F. C., Jaiprakash S. R., Ahmed Bijapur G., Kottayi S., Jose D. Comparison of antimicrobial activity of chlorhexidine, coconut oil, probiotics, and ketoconazole on Candida albicans isolated in children with early childhood caries: an in vitro study. Scientifica. 2016;2016:5. doi: 10.1155/2016/7061587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philip N., Suneja B., Walsh L. J. Ecological approaches to dental caries prevention: paradigm shift or shibboleth? Caries Research. 2018;52:153–165. doi: 10.1159/000484985. [DOI] [PubMed] [Google Scholar]
- 12.Jun S., Zhi-Xiang Z., Ai-Ping M. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn's disease, and pouchitis: meta-analysis of randomized controlled trials. Inflammatory Bowel Diseases. 2014;20:21–35. doi: 10.1097/01.MIB.0000437495.30052.be. [DOI] [PubMed] [Google Scholar]
- 13.Pooneh S., Shekoufeh N., Mohammad A. A meta-analysis and systematic review on the effect of probiotics in acute diarrhea. Inflammation & Allergy - Drug Targets. 2012;11(1):3–14. doi: 10.2174/187152812798889394. [DOI] [PubMed] [Google Scholar]
- 14.Gruner D., Paris S., Schwendicke F. Probiotics for managing caries and periodontitis: systematic review and meta-analysis. Journal of Dentistry. 2016;48:16–25. doi: 10.1016/j.jdent.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Marigo A., Marega C., Zannetti R., Morini G., Ferrara G. Oral administration of Lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age. Caries Research. 2014;48:111–117. doi: 10.1159/000354412. [DOI] [PubMed] [Google Scholar]
- 16.Näse L., Hatakka K., Savilahti E., et al. Effect of long–term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Research. 2001;35:412–420. doi: 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- 17.Pahumunto N., Piwat S., Chankanka O., Akkarachaneeyakorn N., Rangsitsathian K., Teanpaisan R. Reducing mutans streptococci and caries development by Lactobacillus paracasei SD1 in preschool children: a randomized placebo-controlled trial. Acta Odontologica Scandinavica. 2018;1 doi: 10.1080/00016357.2018.1453083. [DOI] [PubMed] [Google Scholar]
- 18.Sujia Q., Wanqing X., Qiuxiang Z., Jianxin Z., Hao Z., Wei C. Inhibitory effect of Lactobacillus plantarum CCFM8724 on caries- causing dual biofilms. Food and Fermentation Industries. 2020;46:127–132. [Google Scholar]
- 19.Beiraghi S., Rosen S., Beck F. The effect of stannous and sodium fluoride on coronal caries, root caries and bone loss in rice rats. Archives of Oral Biology. 1990;35:79–80. doi: 10.1016/0003-9969(90)90120-y. [DOI] [PubMed] [Google Scholar]
- 20.Wang R., Zhao P., Zhu B., Li J. Inhibitive effect of extracts of Galla chinesis on caries development in rats. Sichuan da xue xue bao Yi xue ban= Journal of Sichuan University Medical Science Edition. 2008;39:474–477. [PubMed] [Google Scholar]
- 21.Quishida C. C. C., Mima E. G. D. O., Jorge J. H., Vergani C. E., Bagnato V. S., Pavarina A. C. Photodynamic inactivation of a multispecies biofilm using curcumin and LED light. Lasers in Medical Science. 2016;31:997–1009. doi: 10.1007/s10103-016-1942-7. [DOI] [PubMed] [Google Scholar]
- 22.Klinke T., Guggenheim B., Klimm W., Thurnheer T. Dental caries in rats associated with Candida albicans. Caries Research. 2011;45:100–106. doi: 10.1159/000324809. [DOI] [PubMed] [Google Scholar]
- 23.Montella R., Malfa P., Giuliano A., Brustia G., Coïsson J. D., Arlorio M. Vaginal adhesion of Lactobacillus plantarum P17630 after probiotic food supplement oral administration: a preliminary in vivo study. Nutrafoods. 2013;12:35–42. [Google Scholar]
- 24.Wang L., Pan M., Li D., et al. Metagenomic insights into the effects of oligosaccharides on the microbial composition of cecal contents in constipated mice. Journal of Functional Foods. 2017;38:486–496. [Google Scholar]
- 25.Bowen W. H. Rodent model in caries research. Odontology. 2013;101:9–14. doi: 10.1007/s10266-012-0091-0. [DOI] [PubMed] [Google Scholar]
- 26.Stookey G., Warrick J., Miller L., Greene A. Animal caries models for evaluating fluoride dentifrices. Advances in Dental Research. 1995;9:198–207. doi: 10.1177/08959374950090030301. [DOI] [PubMed] [Google Scholar]
- 27.Keyes H. P. Dental caries in the molar teeth of rats: II. A method for diagnosing and scoring several types of lesions simultaneously. Journal of Dental Research. 1958;37:p. 1088. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]
- 28.Gasque K. C. S., Foster B. L., Kuss P., et al. Improvement of the skeletal and dental hypophosphatasia phenotype in Alpl?/? mice by administration of soluble (non-targeted) chimeric alkaline phosphatase. Bone. 2015;72:137–147. doi: 10.1016/j.bone.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krzyściak W., Kościelniak D., Papież M., et al. Effect of a lactobacillus salivarius probiotic on a double-species Streptococcus mutans and Candida albicans caries biofilm. Nutrients. 2017;9:p. 1242. doi: 10.3390/nu9111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamatova I., Meurman J. H. Probiotics: health benefits in the mouth. American Journal of Dentistry. 2009;22:329–338. [PubMed] [Google Scholar]
- 31.Pahumunto N., Sophatha B., Piwat S., Teanpaisan R. Increasing salivary IgA and reducing Streptococcus mutans by probiotic Lactobacillus paracasei SD1: a double-blind, randomized, controlled study. Journal of Dental Sciences. 2019;14 doi: 10.1016/j.jds.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishikawa K. H., Mayer M. P., Miyazima T. Y., et al. A multispecies probiotic reduces oral Candida colonization in denture wearers. Journal of Prosthodontics. 2015;24:194–199. doi: 10.1111/jopr.12198. [DOI] [PubMed] [Google Scholar]
- 33.Barbieri D. S. A. V., Vicente V. A., Fraiz F. C., Lavoranti O. J., Svidzinski T. I. E., Pinheiro R. L. Analysis of the in vitro adherence of Streptococcus mutans and Candida albicans. Brazilian Journal of Microbiology. 2007;38:624–631. [Google Scholar]
- 34.Morales D. K., Hogan D. A. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathogens. 2010;6, article e1000886 doi: 10.1371/journal.ppat.1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin T.-H., Lin C.-H., Pan T.-M. The implication of probiotics in the prevention of dental caries. Applied Microbiology and Biotechnology. 2018;102:577–586. doi: 10.1007/s00253-017-8664-z. [DOI] [PubMed] [Google Scholar]
- 36.Oelschlaeger T. A. Mechanisms of probiotic actions–a review. International Journal of Medical Microbiology. 2010;300:57–62. doi: 10.1016/j.ijmm.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Wannun P., Piwat S., Teanpaisan R. Purification, characterization, and optimum conditions of fermencin SD11, a bacteriocin produced by human orally Lactobacillus fermentum SD11. Applied Biochemistry and Biotechnology. 2016;179:572–582. doi: 10.1007/s12010-016-2014-y. [DOI] [PubMed] [Google Scholar]
- 38.Garcia S. S., Blackledge M. S., Michalek S., et al. Targeting of Streptococcus mutans biofilms by a novel small molecule prevents dental caries and preserves the oral microbiome. Journal of Dental Research. 2017;96:807–814. doi: 10.1177/0022034517698096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haukioja A., Loimaranta V., Tenovuo J. Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral Microbiology and Immunology. 2008;23:336–343. doi: 10.1111/j.1399-302X.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- 40.Bachtiar E. W., Bachtiar B. M., Jarosz L. M., et al. AI-2 of Aggregatibacter actinomycetemcomitans inhibits Candida albicans biofilm formation. Frontiers in Cellular and Infection Microbiology. 2014;4:p. 94. doi: 10.3389/fcimb.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janus M., Crielaard W., Volgenant C., Van der Veen M., Brandt B., Krom B. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. Journal of Oral Microbiology. 2017;9, article 1270613 doi: 10.1080/20002297.2016.1270613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swain M. V., Jing X. State of the art of micro-CT applications in dental research. International Journal of Oral Science. 2009;1:177–188. doi: 10.4248/IJOS09031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo E. C. M., Zhi Q. H., Itthagarun A. Comparing two quantitative methods for studying remineralization of artificial caries. Journal of Dentistry. 2010;38:352–359. doi: 10.1016/j.jdent.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Dong G., Dong Q., Liu Y., et al. High-resolution micro-CT scanning as an innovative tool for evaluating dental hard tissue development. Journal of Applied Clinical Medical Physics. 2014;15:335–344. doi: 10.1120/jacmp.v15i4.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chih-Ko Y., Harris S. E., Sumathy M., et al. Hyperglycemia and xerostomia are key determinants of tooth decay in type 1 diabetic mice. Laboratory Investigation. 2012;92:p. 868. doi: 10.1038/labinvest.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gantt D. G., Kappleman J., Ketcham R. A., Alder M. E., Deahl T. H. Three-dimensional reconstruction of enamel thickness and volume in humans and hominoids. European Journal of Oral Sciences. 2006;114:360–364. doi: 10.1111/j.1600-0722.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang T. T., Guo H. J., Liu X. J., Chu J. P., Zhou X. D. Galla chinensis compounds remineralize enamel caries lesions in a rat model. Caries Research. 2016;50:159–165. doi: 10.1159/000445036. [DOI] [PubMed] [Google Scholar]
- 48.Haukioja A. Probiotics and oral health. European Journal of Dentistry. 2010;4:p. 348. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to support the findings of this study are available from the corresponding author upon request.


