Abstract
There has been interest in the function of adult neurogenesis since its discovery, by Joseph Altman, nearly 60 years ago. While controversy curtailed follow up studies, in the 1990s a second wave of research validated many of Altman’s original claims and revealed that factors such as stress and environmental stimulation altered the production of new neurons in the hippocampus. However, only with the advent of tools for manipulating neurogenesis did it become possible to perform causal tests of the function of newborn neurons. Here, we identify approximately 100 studies in which adult neurogenesis was manipulated to study its function. A majority of these studies demonstrate functions for adult neurogenesis in classic hippocampal behaviors such as context learning and spatial memory, as well as emotional behaviors related to stress, anxiety and depression. However, a closer look reveals a number of other, arguably understudied, functions in decision making, temporal association memory, and addiction. In this special issue, we present 16 new studies and review articles that continue to address and clarify the function of adult neurogenesis in behaviors as diverse as memory formation and consolidation, pattern separation and discrimination behaviors, addiction, and attention. Reviews of stem cell dynamics and regenerative properties provide insights into the mechanisms by which neurogenesis may be controlled to offset age- and disease-related brain injury. Finally, translation-oriented reviews identify next steps for minimizing the gap between discoveries made in animals and applications for human health. The articles in this issue synthesize and extend what we have learned in the last half century of functional neurogenesis research and identify themes that will define its future.
The discovery that neurons are added to the adult brain has generated tremendous excitement in recent decades, but, ultimately, neurogenesis is only important to the extent that it contributes to brain function and behavior. This fact was not lost on the pioneers of neurogenesis: when Joseph Altman discovered adult neurogenesis over 50 years ago, he was already speculating on its functional significance. In a study that quantified the protracted addition of hippocampal neurons, and found that they bind 3H-testosterone, he proposed that neurogenesis may be involved in late onset behaviors such as those related to sexual processes [1]. While modern approaches allow for causal tests of function, much of what we know about neurogenesis function stems from pioneering work that inferred function through quantification of changes in neuronal birth and survival in response to various manipulations, similar to Altman’s studies. Here, we review studies that, over the years, have led to our current understanding of the function of adult neurogenesis and highlight a collection of studies in this special issue that continue in this tradition.
Following the groundbreaking work of Altman, neurogenesis research was essentially “on hold” until the 1990s, when advances in immunohistochemistry and microscopy appeared, and at which point there was growing acceptance for the broader notion of neuroplasticity [2,3]. Early work showed that the cell proliferation in the adult dentate gyrus is suppressed by stress and stress hormones [4,5], and that newborn neurons express both glucocorticoid and mineralocorticoid receptors [6], thereby providing compelling early support for a neurogenic role in stress-related behaviors. Soon after, it was reported that survival of adult-born neurons is increased by enriched environment [7] and hippocampal-dependent forms of learning [8], suggesting that immature neurons may be rescued proportional to cognitive demands. Around that time it was also reported that exercise upregulates cell proliferation, increases dentate gyrus LTP, and improves spatial learning, thereby providing intriguing support for a possible role for neurogenesis in hippocampal circuit function and behavior [9,10]. A comprehensive review of the physiological and anatomical features of neurogenesis-associated circuitry that support the memory functions of the hippocampus can be found in the review article by Christian et al, in this issue [11].
The first direct test of a behavioral function for neurogenesis came in 2001, when Shors et al. found that inhibiting cell proliferation impaired rats’ ability to learn a conditioned trace eyeblink response [12]. A wave of additional studies soon followed, using chemical, radiological, transgenic and optogenetic approaches to modulate neurogenesis and assess behavioral changes. Figure 1 displays the trajectory of 96 such studies over time. The collection of studies was manually adapted from a recent survey [13] and is intended to be comprehensive (provided as supplementary material). Inevitably, some studies may have been missed but perhaps some insights can still be gleaned. For example, in 2008 there is an approximate tripling in the number of such studies per year, which coincides with the first use of transgenic animals where neurogenesis could be inducibly arrested in adulthood [14,15]. By 2018–19, 67% of studies (14/21) used transgenic models, highlighting the role these models have played in enabling more widespread investigation of the behavioral function of adult neurogenesis.
Figure 1: Studies that have manipulated neurogenesis to study behavioral functions of adult-born neurons.
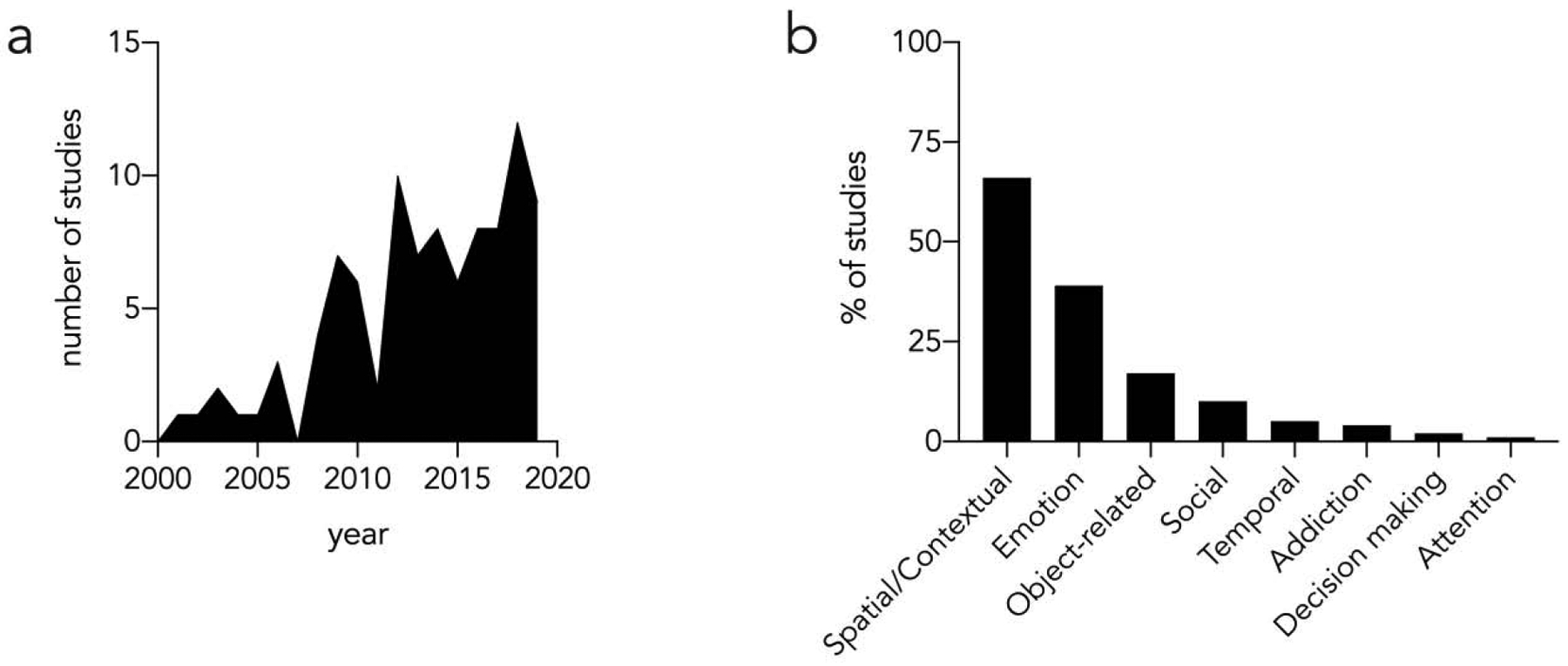
a) The number of functional behavior studies per year, from 2001 to 2019. b) The proportion of studies that have examined various types of behavior. Since many studies employed multiple tests, the sum of all the categories is greater than 100%.
In addition to plotting the temporal trajectory, one can also examine which types of behaviors have been investigated. Not surprisingly, 66% of studies have examined classic hippocampal functions in memory, using well-established tests such as contextual fear conditioning and spatial learning and memory in navigational tasks. Notably, not all studies have found behavioral requirements for neurogenesis and only over time have themes emerged from apparent discrepancies. For example, numerous studies have reported both that neurogenesis is, and is not, required for contextual fear conditioning. A meta-analysis of these data found no consistent effect but noted a high degree of variability in the reported effects across studies [16], suggesting that the role of neurogenesis may only be apparent in certain conditions. Indeed, one study that carefully manipulated the duration of training and the number of footshocks that mice were exposed to found that neurogenesis was required for context learning only when there was a brief opportunity to form a context-footshock association [17]. The duration of neurogenesis ablation [15,18] also matters (see [19] for review).
One theme that has emerged is that neurogenesis appears to be particularly important for learning when there is greater possibility for interference between memories. This work has been inspired by theoretical predictions that a core function of the dentate gyrus is pattern separation, whereby incoming sensory signals are decorrelated in order to minimize representational overlap between experiences [20,21]. Indeed, neurogenesis functions in context fear conditioning are particularly salient in discriminative paradigms, where footshock is paired with only one of two similar-appearing contexts [22–26]. Consistent with a discrimination function, neurogenesis-deficient animals are often capable of initial learning in spatial tasks but have difficulty performing a spatial reversal or discriminating nearby locations or cues [27–35].
Two studies in this special issue provide additional support for a neurogenic role in spatial discrimination behaviors. First, van Dijk et al. use the Intellicage to behaviorally phenotype five species of rodents in their home cage environment, finding that spatial reversal learning correlates with levels of neurogenesis in the septal hippocampus [36]. Remarkably, this correlation was observed across both domesticated and wild species, providing an important validation of previous findings from rather artificial laboratory testing experiments. Second, Yu et al. tested transgenic neurogenesis-deficient rats in a water maze paradigm where the escape platform alternated between 2 possible locations on each trial [37]. Whereas intact rats developed an efficient sequential strategy for targeting the 2 locations, neurogenesis-deficient rats vacillated in their choice behavior and were generally inefficient, consistent with a role for neurogenesis in guiding adaptive behavioral responses in conditions that require discrimination of related stimuli.
What are the cellular- and circuit-level mechanisms by which neurogenesis contributes to memory formation? While the enhanced plasticity and excitability of newborn neurons is often cited as providing physiological support for a role in memory, it has remained unclear how these properties promote the formation of precise memories that enable discrimination. Here, Lodge and Bischofberger provide a detailed review of the physiology and offer a proposal for how excitable immature neurons with weak but plastic synapses might provide the sparse code that is needed for precise memories [38]. Also in the current issue, Tuncdemir et al. offer a complementary perspective where they emphasize a diversity of circuit mechanisms by which neurogenesis might promote distributed representations of spatial, object-related, and temporal information in the hippocampus [39]. Indeed, blocking neurogenesis in mice can elevate overall hippocampal activity, which may reduce sparse coding and promote memory interference. In a test of this hypothesis, Cahill et al., in this issue, found that blocking neurogenesis did not alter activity of neonatally-born dentate gyrus neurons in rats [40]. However, treatment with exercise and memantine, which are well-known pro-neurogenic manipulations, greatly suppressed neural activity in the dentate gyrus. The asymmetrical effects of decreasing and increasing neurogenesis show that neurogenesis rates do not have a simple, direct effect on neural activity.
Memory is dynamic and can transform or be forgotten in the days, weeks or years after encoding. In contrast to the large body of work that has investigated neurogenesis functions in learning and memory over shorter intervals, there is relatively little study of neurogenesis functions in long-term processes [41–43]. In this issue, Terranova et al. review previous evidence that has implicated neurogenesis in long-term memory retention and systems consolidation [44]. By highlighting approaches for precisely manipulating neuronal populations, they identify strategies for addressing key mechanistic questions in the future. Tran et al. address the question of how neurogenesis contributes to another dynamic aspect of memory: forgetting [45]. By generating an artificial neural network model of the trisynaptic circuit, and using biologically plausible rates of neurogenesis, they propose that the excitability and efferent connectivity of newborn neurons play particularly important roles in the forgetting of previously-acquired memories. They also report that, by promoting forgetting, neurogenesis also improves learning that conflicts with previously-acquired memories, thereby providing additional insights into the mechanisms by which neurogenesis contributes to the types of reversal learning described above.
After spatial and contextual memory, emotional behaviors are the second-most common type of behavior examined in functional studies of neurogenesis. Interest in emotional functions of neurogenesis stems from the early work on stress, described above, and sharply increased following early findings that neurogenesis is upregulated by antidepressant drugs [46] and is required for their behavioral effects [47]. Since that time, many subsequent studies have focused on neurogenesis functions in behaviors related to anxiety, depression, and the response to stress [48]. While social and emotional behaviors are separated from each other in Figure 1, studies showing neurogenesis modulates social interaction have typically done so in the context of stress, depression, and social dominance situations that are relevant for understanding the role of neurogenesis in emotional disorders [49–52].
Whereas antidepressant drugs increase neurogenesis, drugs of abuse have typically been associated with decreased production of new neurons [53], and several studies have reported that neurogenesis regulates addiction behavior in animal models [54–56]. Nonetheless, the role of neurogenesis in addiction is massively understudied relative to its roles in memory and emotional behaviors. In this issue, Bulin et al. [57] therefore sought to address the role of morphine self-administration on new neuron survival, given seemingly contradictory evidence that morphine reduces cell proliferation [58] but increases neuronal production [59]. In finding no effects of morphine on measures of neuronal proliferation, survival, or maturation they systematically review the important role that dose, timing, and other methodological factors may play in morphine-dependent regulation of neurogenesis. Given that similar complexities are observed in the regulation of neurogenesis by learning [60], and have taken years to untangle, these results are perhaps not surprising, and they highlight the need for future work in this relatively understudied area.
A final look at the behavioral categories in Figure 1 highlights a number of additional behaviors that have received relatively little attention but perhaps warrant additional investigation. A handful of studies have investigated neurogenesis functions in object-related memory, which may be worth pursuing given the preferential connectivity of new neurons with the lateral perforant path [61], which relays ventral stream object-related information to the hippocampus [62]. Despite the initial discovery that new neurons are important for trace eyeblink conditioning [12], there are only a few other investigations of the role of neurogenesis in temporal association memory [63–65]. Additionally, while neurogenesis may contribute to complex spatial behavior, there are few, but nonetheless positive, reports of neurogenesis involvement in nonspatial forms of decision making and reward seeking [66,67]. Lastly, in this issue, Weeden et al. [68] provide novel support for a role for neurogenesis in shifting attention. Given the ubiquitous nature of attentional regulation, this raises new questions of how neurogenesis functions in learning may be influenced by other functions in cognition.
The early work of Altman demonstrated the profound reduction in neurogenesis that occurs with age. In this issue, Kirschen and Ge review the aging literature that has amassed since Altman’s time and address key questions about how neurogenesis might impact hippocampal function later in life when cell addition rates have slowed [69]. On a related note, Lazutkin et al. highlight key gaps in our understanding of stem cell dynamics that, once clarified, may lead to the development of tools for promoting the long-term maintenance and activity of neural precursors throughout aging [70]. Strategies for enhancing neurogenesis are not only relevant for aging but, as discussed by Williamson and Drew in this issue, may also be valuable for disorders where neurons are lost, such as stroke [71]. However, as these authors point out, only a relatively small proportion of the newborn cells that migrate toward the site of a stroke differentiate into neurons. The putative beneficial effects of proliferation may thus be mediated by non-neuronal newborn cells. Moreover, neurogenesis in the damaged brain is not always beneficial. As discussed by Bielefeld et al. in this issue, disorders such as epilepsy and traumatic brain injury may be characterized by dysregulated activation of stem cells and abnormal integration of new neurons into hippocampal circuits, which may ultimately worsen the situation [72]. Thus, additional research is needed to learn how to both promote and restrain neurogenesis depending on the condition.
While the vast majority of research on adult neurogenesis is performed in animal models, findings need to be applied to the human condition to be truly relevant [13]. In this issue, Millon and Shors highlight a translational project for promoting mental health that is directly inspired by preclinical evidence that rodent neurogenesis is promoted by both exercise and specific cognitive tasks [73]. They review recent reports that applying this mental and physical training regimen to humans reduces anxiety and depressive symptoms, providing an important bridge between animal and human research on activity, plasticity, and mental health. Given the complex relationship between neurogenesis and behavior, it may not be surprising that there is still much to learn about how new neurons might contribute to human hippocampal function. This issue was in the spotlight following recent reports that came to opposite conclusions about whether neurogenesis even occurs in the adult human hippocampus [74–78]. In this issue, Lucassen et al [reference to be added once published] provides a detailed review of these studies and outlines key questions and methodologies for resolving the issue.
So, how far have we come in the half century since Altman’s discovery? As the papers in this issue illustrate, with the establishment of increasingly precise methods for identifying, quantifying and manipulating neurogenesis in rodents we have amassed a great deal of evidence that neurogenesis occurs in many, if not all, mammalian species and that it can, in principle, modulate a variety of hippocampus-dependent processes. But other important questions remain largely unanswered. The cellular and circuit mechanisms through which the putative functions of neurogenesis are implemented remain largely the domain of theory. We still know very little how adult-born neurons behave in vivo; with respect to this question, this special issue is unfortunately representative of the larger scientific literature, which contains only a vanishingly few studies in which adult-born neurons were recorded in vivo [79]. Finally, after two decades of research supporting the existence of adult neurogenesis in different mammalian species, it is perhaps ironic that we should now be enmeshed in a debate about whether adult neurogenesis occurs in humans. We hope that the work in this issue serves to reinforce the view that we should not let questions about the extent of adult neurogenesis once again impede our progress toward understanding the phenomenon and how it might be harnessed to improve human health.
Supplementary Material
Acknowledgements
The authors’ research is supported by the Canadian Institutes of Health Research (JSS), the Michael Smith Foundation for Health Research (JSS), and the National Institutes of Health (MRD). The authors also thank Sue Rim Baek for assistance with generating the list of functional studies of adult neurogenesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Altman J, Das GD, Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats, J Comp Neurol. 124 (1965) 319–335. [DOI] [PubMed] [Google Scholar]
- [2].Gross CG, Neurogenesis in the adult brain: death of a dogma, Nat Rev Neurosci. 1 (2000) 67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- [3].Altman J, The Discovery of Adult Mammalian Neurogenesis, in: Neurogenesis in the Adult Brain I, Springer, Tokyo, Tokyo, 2011: pp. 3–46. doi: 10.1007/978-4-431-53933-9_1. [DOI] [Google Scholar]
- [4].Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS, Adrenal hormones suppress cell division in the adult rat dentate gyrus, J Neurosci. 12 (1992) 3642–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E, Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation, J Neurosci. 17 (1997) 2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cameron HA, Woolley CS, Gould E, Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus, Brain Res. 611 (1993) 342–346. [DOI] [PubMed] [Google Scholar]
- [7].Kempermann G, Kuhn HG, Gage FH, More hippocampal neurons in adult mice living in an enriched environment, Nature. 386 (1997) 493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- [8].Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ, Learning enhances adult neurogenesis in the hippocampal formation, Nat Neurosci. 2 (1999) 260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- [9].van Praag H, Christie BR, Sejnowski TJ, Gage FH, Running enhances neurogenesis, learning, and long-term potentiation in mice, Proc Natl Acad Sci USA. 96 (1999) 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van Praag H, Kempermann G, Gage FH, Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus, Nat Neurosci. 2 (1999) 266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- [11].Christian KM, Ming G-L, Song H, Adult neurogenesis and the dentate gyrus: Predicting function from form, Behav Brain Res. 379 (2020) 112346. doi: 10.1016/j.bbr.2019.112346. [DOI] [PubMed] [Google Scholar]
- [12].Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E, Neurogenesis in the adult is involved in the formation of trace memories, Nature. 410 (2001) 372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- [13].Snyder JS, Recalibrating the Relevance of Adult Neurogenesis, Trends Neurosci. (2019) 1–15. doi: 10.1016/j.tins.2018.12.001. [DOI] [PubMed] [Google Scholar]
- [14].Dupret D, Revest J-M, Koehl M, Ichas F, De Giorgi F, Costet P, et al. , Spatial relational memory requires hippocampal adult neurogenesis, PLoS ONE. 3 (2008) e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, et al. , Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain, Nat Neurosci. 11 (2008) 1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- [16].Groves JO, Leslie I, Huang G-J, McHugh SB, Taylor A, Mott R, et al. , Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model, PLoS Genet. 9 (2013) e1003718. doi: 10.1371/journal.pgen.1003718.s002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Drew MR, Denny CA, Hen R, Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning, Behav Neurosci. 124 (2010) 446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR, 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning, Hippocampus. 22 (2012) 1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Drew MR, Huckleberry KA, Modulation of Aversive Memory by Adult Hippocampal Neurogenesis, Neurotherapeutics. 14 (2017) 646–661. doi: 10.1007/s13311-017-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rolls ET, A theory of hippocampal function in memory, Hippocampus. 6 (1996) 601–620. doi:. [DOI] [PubMed] [Google Scholar]
- [21].O’Reilly RC, McClelland JL, Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off, Hippocampus. 4 (1994) 661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- [22].Tronel S, Belnoue L, Grosjean N, Revest J-M, Piazza P-V, Koehl M, et al. , Adult-born neurons are necessary for extended contextual discrimination, Hippocampus. 22 (2012) 292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- [23].Niibori Y, Yu T-S, Epp JR, Akers KG, Josselyn SA, Frankland PW, Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region, Nat Comms. 3 (2012) 1253. doi: 10.1038/ncomms2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, et al. , Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation, Nature. 472 (2011) 466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kheirbek MA, Tannenholz L, Hen R, NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination, J Neurosci. 32 (2012) 8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, et al. , Young Dentate Granule Cells Mediate Pattern Separation, whereas Old Granule Cells Facilitate Pattern Completion, Cell. 149 (2012) 188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Luu P, Sill OC, Gao L, Becker S, Wojtowicz JM, Smith DM, The role of adult hippocampal neurogenesis in reducing interference, Behav Neurosci. 126 (2012) 381–391. doi: 10.1037/a0028252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Winocur G, Becker S, Luu P, Rosenzweig S, Wojtowicz JM, Adult hippocampal neurogenesis and memory interference, Behav Brain Res. 227 (2012) 464–469. doi: 10.1016/j.bbr.2011.05.032. [DOI] [PubMed] [Google Scholar]
- [29].Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, et al. , A functional role for adult hippocampal neurogenesis in spatial pattern separation, 325 (2009) 210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, et al. , Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories, Hippocampus. 24 (2014) 905–911. doi: 10.1002/hipo.22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW, Posttraining ablation of adult-generated neurons degrades previously acquired memories, J Neurosci. 31 (2011) 15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Epp JR, Silva Mera R, Köhler S, Josselyn SA, Frankland PW, Neurogenesis-mediated forgetting minimizes proactive interference, Nat Comms. 7 (2016) 10838. doi: 10.1038/ncomms10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Garthe A, Behr J, Kempermann G, Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies, PLoS ONE. 4 (2009) e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Burghardt NS, Park EH, Hen R, Fenton AA, Adult-born hippocampal neurons promote cognitive flexibility in mice, Hippocampus. 22 (2012) 1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Swan AA, Clutton JE, Chary PK, Cook SG, Liu GG, Drew MR, Characterization of the role of adult neurogenesis in touch-screen discrimination learning, Hippocampus. 24 (2014) 1581–1591. doi: 10.1002/hipo.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van Dijk RM, Wiget F, Wolfer DP, Slomianka L, Amrein I, Consistent within-group covariance of septal and temporal hippocampal neurogenesis with behavioral phenotypes for exploration and memory retention across wild and laboratory small rodents, Behav Brain Res. 372 (2019) 112034. doi: 10.1016/j.bbr.2019.112034. [DOI] [PubMed] [Google Scholar]
- [37].Qi Yu R, Cooke M, Seib DR, Zhao J, Snyder JS, Adult neurogenesis promotes efficient, nonspecific search strategies in a spatial alternation water maze task, Behav Brain Res. (2019) 112151. doi: 10.1016/j.bbr.2019.112151. [DOI] [PubMed] [Google Scholar]
- [38].Lodge M, Bischofberger J, Synaptic properties of newly generated granule cells support sparse coding in the adult hippocampus, Behav Brain Res. 372 (2019) 112036. doi: 10.1016/j.bbr.2019.112036. [DOI] [PubMed] [Google Scholar]
- [39].Tuncdemir SN, Lacefield CO, Hen R, Contributions of adult neurogenesis to dentate gyrus network activity and computations, Behav Brain Res. 374 (2019) 112112. doi: 10.1016/j.bbr.2019.112112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cahill SP, Martinovic A, Cole JD, Seib DR, Snyder JS, A combination of running and memantine increases neurogenesis and reduces activation of developmentally-born dentate granule neurons in rats, Behav Brain Res. 372 (2019) 112005. doi: 10.1016/j.bbr.2019.112005. [DOI] [PubMed] [Google Scholar]
- [41].Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM, A role for adult neurogenesis in spatial long-term memory, Neuroscience. 130 (2005) 843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- [42].Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, et al. , Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory SUPPL, Cell. (2009) 1–33. [DOI] [PubMed] [Google Scholar]
- [43].Ben Abdallah NMB, Filipkowski RK, Pruschy M, Jaholkowski P, Winkler J, Kaczmarek L, et al. , Impaired long-term memory retention: common denominator for acutely or genetically reduced hippocampal neurogenesis in adult mice, Behav Brain Res. 252 (2013) 275–286. doi: 10.1016/j.bbr.2013.05.034. [DOI] [PubMed] [Google Scholar]
- [44].Terranova JI, Ogawa SK, Kitamura T, Adult hippocampal neurogenesis for systems consolidation of memory, Behav Brain Res. 372 (2019) 112035. doi: 10.1016/j.bbr.2019.112035. [DOI] [PubMed] [Google Scholar]
- [45].Tran LM, Josselyn SA, Richards BA, Frankland PW, Forgetting at biologically realistic levels of neurogenesis in alarge-scale hippocampal model, Behav Brain Res. (2019). doi: 10.1016/j.bbr.2019.112180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Malberg JE, Eisch AJ, Nestler EJ, Duman RS, Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus, J Neurosci. 20 (2000) 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. , Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants, Science. 301 (2003) 805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- [48].Schoenfeld TJ, Cameron HA, Adult neurogenesis and mental illness, Neuropsychopharmacology. 40 (2015) 113–128. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M, Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress, Mol Psychiatry. 15 (2010) 1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, et al. , Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus, Nature. 559 (2018) 1–22. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Opendak M, Offit L, Monari P, Schoenfeld TJ, Sonti AN, Cameron HA, et al. , Lasting Adaptations in Social Behavior Produced by Social Disruption and Inhibition of Adult Neurogenesis, J Neurosci. 36 (2016) 7027–7038. doi: 10.1523/JNEUROSCI.4435-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, et al. , Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance, Proceedings of the National Academy of Sciences. 107 (2010) 4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mandyam CD, Koob GF, The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery, Trends Neurosci. 35 (2012) 250–260. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Noonan MA, Bulin SE, Fuller DC, Eisch AJ, Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction, J Neurosci. 30 (2010) 304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Galinato MH, Takashima Y, Fannon MJ, Quach LW, Morales Silva RJ, Mysore KK, et al. , Neurogenesis during abstinence is necessary for context-driven methamphetamine-related memory, J Neurosci. 38 (2018) 2011–17–14. doi: 10.1523/JNEUROSCI.2011-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Deroche-Gamonet V, Revest J-M, Fiancette J-F, Balado E, Koehl M, Grosjean N, et al. , Depleting adult dentate gyrus neurogenesis increases cocaine-seeking behavior, Mol Psychiatry. 229 (2018) 1. doi: 10.1038/s41380-018-0038-0. [DOI] [PubMed] [Google Scholar]
- [57].Bulin SE, Simmons S, Richardson DR, Latchney SE, Deutsch HM, Yun S, et al. , Indices of dentate gyrus neurogenesis are unaffected immediately after or following withdrawal from morphine self-administration compared to saline self-administering control male rats, Behav Brain Res. (2019) 1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ, Opiates inhibit neurogenesis in the adult rat hippocampus, Proc Natl Acad Sci USA. 97 (2000) 7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang H, Jia M, Wang X-W, Ye C, Li Y, Wang N, et al. , Dentate gyrus μ-opioid receptor-mediated neurogenic processes are associated with alterations in morphine self-administration, Sci Rep. 9 (2019) 1471–11. doi: 10.1038/s41598-018-37083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shors TJ, Anderson ML, Curlik DM, Nokia MS, Use it or lose it: how neurogenesis keeps the brain fit for learning, Behav Brain Res. 227 (2012) 450–458. doi: 10.1016/j.bbr.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vivar C, Potter MC, Choi J, Lee J-Y, Stringer TP, Callaway EM, et al. , Monosynaptic inputs to new neurons in the dentate gyrus, Nat Comms. 3 (2012) 1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Knierim JJ, Neunuebel JP, Deshmukh SS, Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames, Philosophical Transactions of the Royal Society B: Biological Sciences. 369 (2014) 20130369. doi: 10.1098/rstb.2013.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E, Neurogenesis may relate to some but not all types of hippocampal-dependent learning, Hippocampus. 12 (2002) 578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Seo DO, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR, Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms, J Neurosci. 35 (2015) 11330–11345. doi: 10.1523/JNEUROSCI.0483-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Miller LN, Weiss C, Disterhoft JF, Genetic Ablation of Neural Progenitor Cells Impairs Acquisition of Trace Eyeblink Conditioning, eNeuro. 6 (2019) ENEURO.0251–19.2019. doi: 10.1523/ENEURO.0251-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Karlsson R-M, Wang AS, Sonti AN, Cameron HA, Adult neurogenesis affects motivation to obtain weak, but not strong, reward in operant tasks, Hippocampus. 28 (2018) 512–522. doi: 10.1002/hipo.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Seib DR, Espinueva D, Princz-Lebel O, Chahley E, Floresco SB, Snyder JS, Hippocampal neurogenesis promotes preference for future rewards, bioRxiv. (2018) 1–25. doi: 10.1101/399261. [DOI] [PubMed] [Google Scholar]
- [68].Weeden CSS, Mercurio JC, Cameron HA, A role for hippocampal adult neurogenesis in shifting attention toward novel stimuli, Behav Brain Res. (2019) 112152. doi: 10.1016/j.bbr.2019.112152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kirschen GW, Ge S, Young at heart: Insights into hippocampal neurogenesis in the aged brain, Behav Brain Res. 369 (2019) 111934. doi: 10.1016/j.bbr.2019.111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lazutkin A, Podgorny O, Enikolopov G, Modes of division and differentiation of neural stem cells, Behav Brain Res. 374 (2019) 112118. doi: 10.1016/j.bbr.2019.112118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Williamson MR, Jones TA, Drew MR, Functions of subventricular zone neural precursor cells in stroke recovery, Behav Brain Res. 376 (2019) 112209. doi: 10.1016/j.bbr.2019.112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bielefeld P, Durá I, Danielewicz J, Lucassen PJ, Baekelandt V, Abrous DN, et al. , Insult-induced aberrant hippocampal neurogenesis: Functional consequences and possible therapeutic strategies, Behav Brain Res. 372 (2019) 112032. doi: 10.1016/j.bbr.2019.112032. [DOI] [PubMed] [Google Scholar]
- [73].Millon EM, Shors TJ, Taking neurogenesis out of the lab and into the world with MAP Train My Brain™, Behav Brain Res. 376 (2019) 112154. doi: 10.1016/j.bbr.2019.112154. [DOI] [PubMed] [Google Scholar]
- [74].Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, et al. , Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults, Nature. 555 (2018) 377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cipriani S, Ferrer I, Aronica E, Kovács GG, Verney C, Nardelli J, et al. , Hippocampal Radial Glial Subtypes and Their Neurogenic Potential in Human Fetuses and Healthy and Alzheimer’s Disease Adults, Cerebral Cortex. 27 (2018) 16. doi: 10.1093/cercor/bhy096. [DOI] [PubMed] [Google Scholar]
- [76].Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT, Human adult neurogenesis across the ages: An immunohistochemical study, Neuropathol. Appl. Neurobiol 42 (2016) 621–638. doi: 10.1111/nan.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. , Human Hippocampal Neurogenesis Persists throughout Aging, Stem Cell. 22 (2018) 589–599.e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, et al. , Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease, Nature Publishing Group. 108 (2019) 621. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- [79].Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, et al. , Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding, Neuron. 90 (2016) 101–112. doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


