Abstract
BACKGROUND
There is increasing interest in transplanting patients with hepatocellular carcinoma (HCC) with tumors greater than 5 cm (Milan criteria).
AIM
To investigate possible prognostically-useful factors for liver transplantation in HCC patients with large tumors.
METHODS
In this clinical study, 50 patients with HCC who were transplanted at our Liver Transplant Center between April 2006 and August 2019 and had tumors greater than 6 cm maximum diameter were retrospectively analyzed. Their survival and full clinical characteristics were examined, with respect to serum alpha-fetoprotein (AFP) and gamma glutamyl transpeptidase (GGT) levels. Kaplan-Meier survival estimates were used to determine overall survival and disease-free survival in these patients. The inclusion criterion was evidence of HCC. Exclusion criteria were the presence of macroscopic portal vein thrombosis or metastasis and a follow-up period of less than 90 d.
RESULTS
Using receiver operating characteristic curve (ROC) analysis, cutoff values of AFP 200 ng/mL and GGT 104 IU/L were identified and used in this study. Significantly longer overall survival (OS) and disease-free-survival (DFS) were found in patients who had lower values of either parameter, compared with higher values. Even greater differences in survival were found when the 2 parameters were combined. Two tumor size bands were identified, in searching for the limits of this approach with larger tumors, namely 6-10 cm and > 10 cm. Combination parameters in the 6-10 cm band reflected 5-year OS of 76.2% in patients with low AFP plus low GGT vs 0% for all other groups. Patients with tumors greater than 10 cm, did not have low AFP plus low GGT. The most consistent clinical correlates for longer survival were degree of tumor differentiation and absence of microscopic portal venous invasion.
CONCLUSION
Serum levels of AFP and GGT, both alone and combined, represent a simple prognostic identifier in patients with large HCCs undergoing liver transplant-ation.
Keywords: Hepatic malignancy, Advanced, Gamma glutamyl transpeptidase, Living donor, Beyond, Extended
Core Tip: Excellent long-term survival was found after liver transplantation in patients with hepatocellular carcinoma with a maximum tumor diameter greater than 6 cm and less than 10 cm, in whom serum alpha-fetoprotein was lower than 200 ng/mL and gamma glutamyl transpeptidase was lower than 104 IU/L.
INTRODUCTION
Rationale
The Milan criteria are still the most widely used criteria for liver transplantation in hepatocellular carcinoma (HCC). More recently, similar survival results to those with the Milan criteria were obtained following liver transplantation in patients with tumors exceeding the Milan criteria, and the need to expand the criteria has emerged. However, the degree of expansion that is compatible with prolonged survival is still a matter of debate.
Alpha-fetoprotein and gamma glutamyl transpeptidase as biomarkers for HCC
Alpha-fetoprotein (AFP) was initially reported as a cancer-related oncofetal antigen (fetal albumin) in several tumor types[1,2], but it has become an especially useful tool in evaluating and following treatment responses in patients with HCC. However, there is no general agreement on its usefulness as a surveillance tool, partly because it is not elevated in a significant proportion of patients with small HCCs[3]. Conversely, large HCCs can also be associated with low AFP values[4]. Furthermore, patients with unresectable HCC without elevated AFP levels, represent a variable but large patient subset, in the range of 30%-50%[5,6]. Nevertheless, AFP values have also been shown to be an important prognosticator in liver transplantation for HCC and have been included as a selection criterion in several recent reports[7-9]. Gamma glutamyl transpeptidase (GGT) has been found to be a useful marker for HCC, especially for small tumors or for those HCC patients with low AFP levels[4,5,10-12]. There is also a hepatoma-specific isozyme, GGT-II[10-13].
Malatya criteria for liver transplantation in HCC
We previously published the “Malatya criteria” as an expansion of the Milan criteria for liver transplantation in patients with HCC[14]. The Malatya criteria consist of HCCs with a maximum dominant tumor diameter (MTD) ≤ 6 cm, AFP ≤ 200 ng/mL, GGT ≤ 104 IU/L, and well/moderate tumor differentiation. We showed that there were still patients with long-term survival in the group who were even beyond the Malatya criteria, including HCCs with a MTD larger than 6 cm.
Objectives
In the current study, we focused on patients with HCCs larger than 6 cm who were beyond the Malatya criteria and studied the usefulness of serum AFP and GGT levels, both alone and in combination, with respect to survival after liver transplantation.
MATERIALS AND METHODS
Patients
This analysis involved 50 patients with HCC who were transplanted by live donation at our Liver Transplant Center between April 2006 and August 2019 and who had both survival data, baseline AFP and GGT laboratory data and a baseline MTD > 6 cm. As our practice concerns only live donor transplantation, we have no waiting list dropout.
Study design
Prospectively recorded data were analyzed retrospectively. Patients who had HCC on explanted liver pathology and survived more than 90 d post-transplant were included in this study. Patients with macroscopic portal vein invasion and metastases were excluded. We previously found, by multivariate analysis of a large HCC cohort, that an MTD of 6 cm had a high and significant Hazard Ratio for survival[14]. Therefore we chose patients with a MTD greater than 6 cm (n = 50) for this study, as we previously found[14] that transplanting patients with HCCs up to 6 cm resulted in similar survival to the Milan criteria[14], as has been shown by others[15]. This study was approved by Inonu University Institutional Review Board (Approval No. 2018/1-9) and registered in ClinicalTrials.gov (ID: NCT04412161).
Post-transplant follow up and management
Management was the same as in our previous study[14]. Standard triple immunosuppression (steroid, mycophenolate mofetil and tacrolimus) were administered for the first month, then mammalian target of rapamycin inhibitor (everolimus) was combined with low dose tacrolimus for maintenance immunosuppression. None of the 50 patients received adjuvant HCC therapy after transplantation, unless their tumor recurred. In patients with recurrence, resectability was assessed and systemic sorafenib therapy was administered.
Statistical analysis
The clinical parameters and tumor characteristics of the 50 patients were analyzed according to the AFP cutoff 200 ng/mL and GGT cutoff 104 IU/L, as we previously found on multivariate analysis that an AFP cutoff of 200 ng/mL and a GGT cutoff of 104 IU/L were highly statistically significant for survival[14]. Categorical (qualitative) variables were expressed as count and percentage. Comparisons of groups were made by Pearson chi-square test, continuity corrected chi-square test and Fisher's exact tests for categorical variables. Categorized variables were compared using univariate analysis methods (Pearson chi-square test, continuity corrected chi-square test, Fisher's exact test). Continuous variables were compared using the Mann-Whitney U test. Kaplan-Meier survival estimate was used to determine overall survival (OS) and disease-free survival (DFS) of the patients. The follow-up period was defined as the interval between liver transplantation and the date of the last outpatient department visit for living patients or the date of death. Time to disease recurrence was defined as the interval between liver transplantation and the date a lesion that appeared to be a tumor was detected by biochemical (AFP) and radiological examination and/or a lesion diagnosed as HCC.
Statistical tests were considered significant when the corresponding P value was less than 5%. All statistical analyses were performed using IBM SPSS Statistics for Windows version 25.0 (New York, United States).
RESULTS
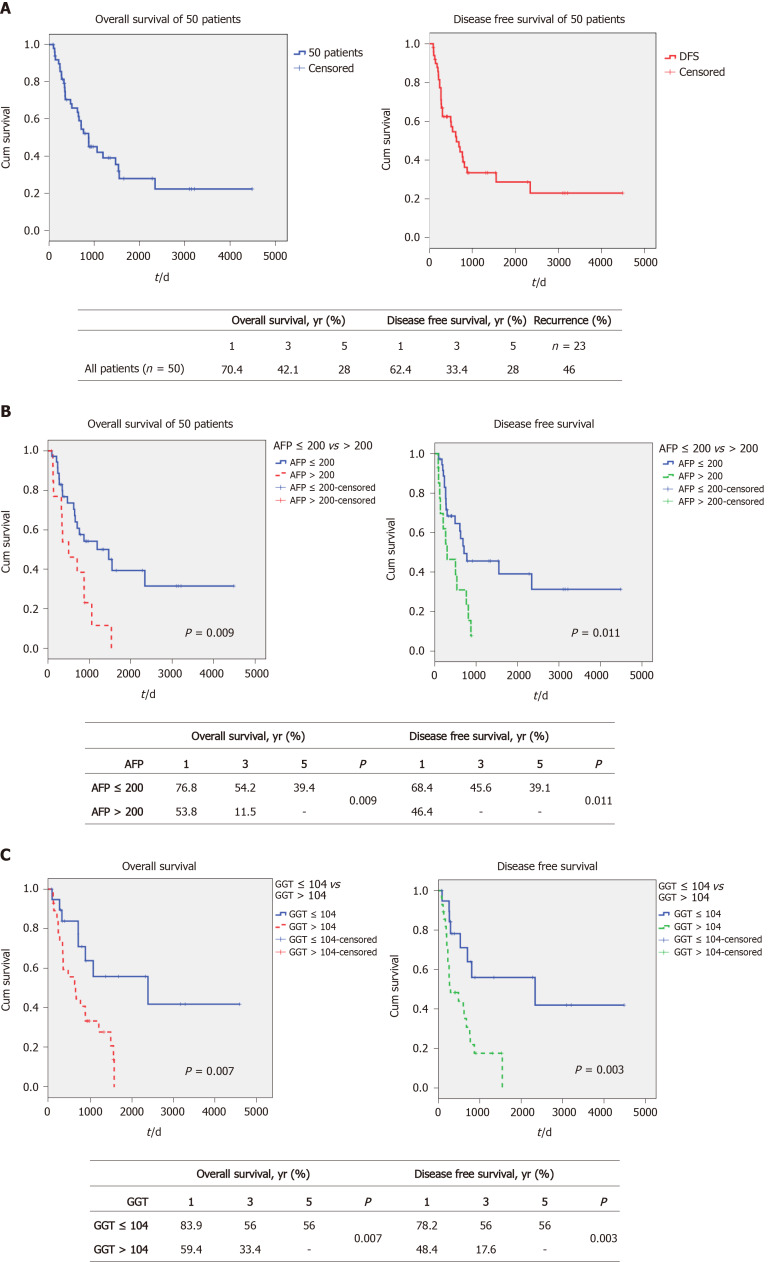
The demographics of all 50 patients are shown in Table 1 and 2. The 1-, 3- and 5-year OS and DFS of these advanced HCC patients were 70.4%, 42.1% and 28% vs 62.4%, 33.4% and 28%, respectively (Figure 1A). None of the 50 patients received adjuvant HCC therapy after transplantation, unless their tumor recurred. The post-transplant recurrence rate was 46% (n = 23). None of the patients with post-transplant recurrences were suitable for further surgery, and all of these patients received systemic HCC therapy. We previously found that both AFP and GGT serum levels were significantly associated with survival post-liver transplant in a large cohort[14]. In this analysis, we expanded on that analysis, to determine if this approach could yield useful prognostic information in patients with large tumors. We initially examined the effect of these 2 parameters when used singly, to the total cohort (n = 50 for AFP values, n = 48 for GGT values) of large HCCs > 6.0 cm. Figure 1B shows the results of examining this cohort according to AFP levels. We found that when AFP was dichotomized according to greater than or less than 200 ng/mL, 2 statistically different survival curves were found [preliminary data with a variety of cutoffs found that this AFP level provided optimal separation between the 2 survival groups, using receiver operating characteristic curve (ROC) analysis]. The 1-, 3- and 5-year OS was found to be 76.8%, 54.2% and 39.4% for the lower AFP cohort and 53.8%, 11.5% and 0% for the higher AFP cohort, P = 0.009 (see box, Figure 1B). Similar results were found when the same approach was used for DFS (Figure 1B). Survival was then examined according to serum GGT levels (using a cutoff of 104 IU/L according to the ROC). We also found a statistically significant difference between the 2 GGT groups, with a 1-, 3- and 5-year OS of 83.9%, 56% and 56%, respectively, for the lower GGT group and 59.4%, 33.4% and 0%, respectively, for the higher GGT group, P = 0.007 (see box, Figure 1C). Similarly, DFS was also found to be significantly different between the 2 GGT groups. In order to explain these survival differences, we compared the main clinical parameters of the 2 sets of AFP groups (n = 50) and the 2 sets of GGT groups (n = 48), examining mainly liver function and tumor characteristics (Table 3). We found that AFP levels were lower in the low GGT group, compared to the high GGT group, possibly reflecting differences in tumor aggressiveness, as expected. However, the converse was not true, as the low and high AFP groups showed non-significant changes in GGT values. Similarly, the percent of well differentiated tumors was 55% in the low and 15% in the high GGT group, P = 0.011. Microvascular invasion was also significantly different between the 2 GGT groups, being 55% in the low GGT group vs 82% in the high GGT group, P = 0.04. When we compared the 2 AFP groups, we found that neither tumor differentiation nor microvascular invasion were significantly different between the 2 AFP groups (Table 3). Serum AST and total bilirubin levels were similar across all groups.
Table 1.
Demographics of 50 patients with maximum dominant tumor diameter > 6 cm
|
Parameter
|
Median
|
Min-Max
|
| Age (yr) | 56 | 8-72 |
| MELD score | 10 | 6-28 |
| AFP (ng/mL) | 24.5 | 0.8-12863 |
| Platelets (103/uL) | 112 | 16-528 |
| NLR | 2.75 | 0.3-13.3 |
| PLR | 97.1 | 25.7-302.9 |
| Albumin (g/dL) | 3 | 1.9-4.2 |
| Total bilirubin (mg/dL) | 1.58 | 0.23-13.9 |
| GGT (IU/L) | 111.5 | 17-1396 |
| AST (IU/L) | 76.5 | 19-566 |
| ALT (IU/L) | 50 | 10-657 |
| MTD (cm) | 8.5 | 6.4-24 |
| Number of nodules | 2 | 1-20 |
AFP: Alpha-fetoprotein; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; GGT: Gamma glutamyl transpeptidase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; MTD: Maximum dominant tumor diameter.
Table 2.
Demographics of 50 patients with maximum dominant tumor diameter > 6 cm
|
Parameter
|
n
|
%
|
| Gender, male | 41 | 82 |
| Child-Pugh score | ||
| A | 19 | 38 |
| B | 26 | 52 |
| C | 5 | 10 |
| Etiology | ||
| Viral hepatitis | 40 | 80 |
| Cryptogenic | 7 | 14 |
| Fibrolamellar-HCC | 1 | 2 |
| Budd-Chiari | 1 | 2 |
| Ethanol | 1 | 2 |
| Differentiation | ||
| Well | 16 | 32 |
| Moderate | 26 | 52 |
| Poor | 8 | 16 |
| Microvascular invasion negative | 15 | 30 |
| Microvascular invasion positive | 35 | 70 |
| TACE/TARE/RFA (+) | 8 | 16 |
HCC: Hepatocellular carcinoma; TACE: Transarterial chemoembolization; TARE: Transarterial radioembolization; RFA: Radiofrequency ablation.
Figure 1.
Survival of patients according to alpha-fetoprotein and gamma glutamyl transpeptidase alone. A: Overall and disease-free survival of 50 patients; B: Overall and disease-free survival according to alpha-fetoprotein (AFP) ≤ 200 ng/mL vs AFP > 200 ng/mL; C: Overall and disease-free survival according to gamma glutamyl transpeptidase (GGT) ≤ 104 IU/L vs GGT > 104 IU/L.
Table 3.
Clinical characteristics of 50 hepatocellular carcinoma patients, maximum dominant tumor diameter > 6 cm, according to serum gamma glutamyl transpeptidase or alpha-fetoprotein levels
| Parameters | Low GGT ≤ 104, n = 20, median (min-max) | High GGT > 104, n = 28, median (min-max) | P value | Low AFP ≤ 200, n = 36, median (min-max) | High AFP > 200, n = 14, median (min-max) | P value |
| AFP (ng/mL) | 20.1 (0.8-6388) | 45.7 (1.9-12863) | 0.818 | |||
| GGT (IU/L) | 114 (17-1396) | 106 (29-393) | 0.862 | |||
| AST (IU/L) | 76.5 (19-566) | 71 (25-313) | 0.544 | 81 (19-566) | 76 (28-245) | 0.596 |
| Total Bilirubin (mg/dl) | 1.7 (0.23-13.9) | 1.7 (0.38-13.9) | 0.660 | 1.78 (0.23-13.9) | 1.13 (0.38-11.7) | 0.270 |
| MTD (cm) | 8.5 (6.5-22) | 8.5 (6.4-24) | 0.992 | 8.5 (6.4-24) | 8.5 (6.5-22) | 0.896 |
| Microvascular invasion, n (%) | 0.04a | 0.360 | ||||
| Negative | 9 (45) | 5 (18) | 11 (31) | 4 (29) | ||
| Positive | 11 (55) | 23 (82) | 25 (69) | 10 (71) | ||
| Differentiation, n (%) | ||||||
| Well | 11 (55) | 4 (15) | 0.011a | 13 (36) | 3 (21) | 0.324 |
| Moderate | 7 (35) | 18 (64) | 19 (53) | 7 (50) | ||
| Poor | 2 (10) | 6 (21) | 4 (11) | 4 (29) | ||
| Number of nodules | ||||||
| 1 | 10 (50) | 12 (43) | 0.845 | 16 (44) | 6 (43) | 1.000 |
| > 1 | 10 (50) | 16 (57) | 20 (56) | 8 (57) |
P < 0.05.
AFP: Alpha-fetoprotein; GGT: Gamma glutamyl transpeptidase; AST: Aspartate aminotransferase; MTD: Maximum dominant tumor diameter.
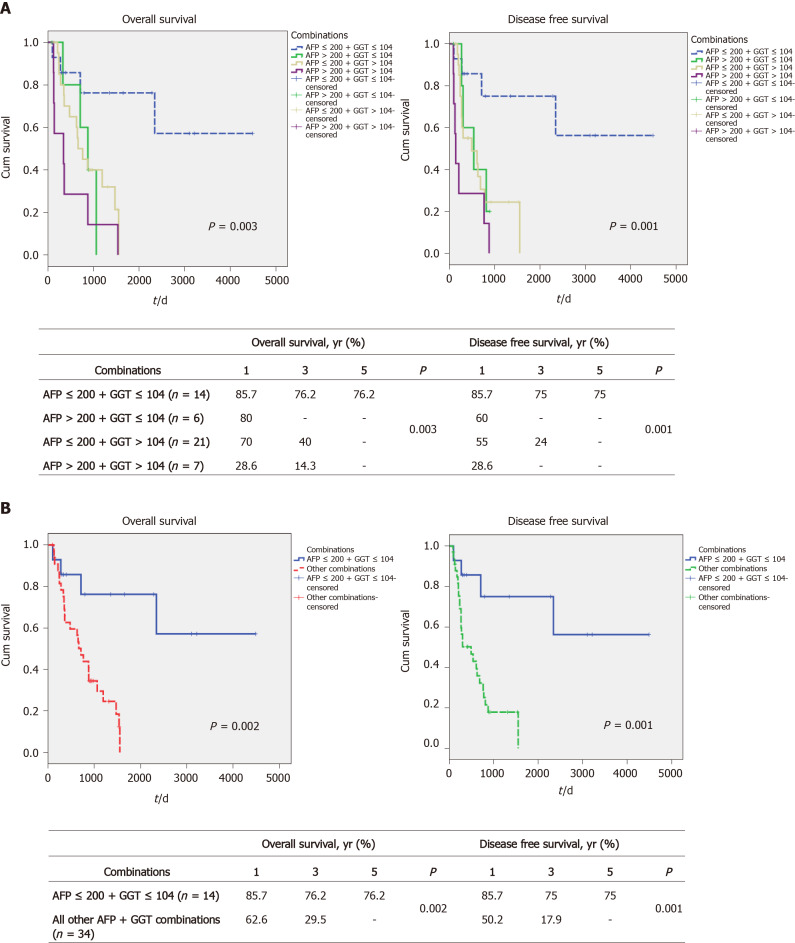
We next examined whether combinations of the 2 markers, AFP and GGT, might identify subsets or result in better survival discrimination. We found that the combination of low AFP plus low GGT levels (low AFP/GGT) was associated with the best survival, with 1-, 3- and 5-year OS being 85.7%, 76.2% and 76.2%, respectively (Figure 2A, left). By contrast, all the other 3 combinations (high AFP plus low GGT, low AFP plus high GGT, high AFP plus high GGT) were similar to each other and all had poor survival (none of them had 5-year OS, see box, Figure 2A, left). Similar differences between the 4 groups were found for DFS (Figure 2A, right). As a result of these findings, the rest of the analysis was performed by comparing the low AFP/GGT group with all the other 3 groups combined. Taking this approach, we found similar large and statistically significant survival differences. The 1-, 3- and 5-year OS for the low AFP/GGT group was 85.7%, 76.2% and 76.2%, respectively, vs 62.6%, 29.5% and 0%, respectively, for the other groups combined, P = 0.002 (Figure 2B, left) and DFS differences (Figure 2B, right). The clinical characteristics of the 2 combination parameter groups were then examined (Table 4). The main differences found were in the percent of poorly differentiated tumors, 14% for the low AFP/GGT group vs 24% for the combination high parameter groups, P = 0.020, and in the percent of microvascular invasion, being 50% in the low AFP/GGT group vs 79% in the combination high parameter groups, P = 0.04.
Figure 2.
Survival of patients according to alpha-fetoprotein and gamma glutamyl transpeptidase combinations. A: Overall and disease-free survival according to alpha-fetoprotein (AFP) and gamma glutamyl transpeptidase (GGT) combinations (low AFP + low GGT, high AFP + low GGT, low AFP + high GGT and high AFP + high GGT); B: Overall and disease-free survival according to low AFP + low GGT vs all other AFP + GGT combinations.
Table 4.
Clinical characteristics of combination low alpha-fetoprotein/gamma glutamyl transpeptidase patients vs all other combinations
| Parameters | Low AFP ≤ 200, low GGT ≤ 104, n = 14 | Other combos, n = 34 | P value |
| AFP (ng/mL) | |||
| Median | 7.35 | 132.5 | 0.009b |
| mean ± SD | 21.6 ± 32.4 | 758.5 ± 2397.2 | |
| GGT (IU/L) | |||
| Median | 63 | 140.5 | < 0.001c |
| mean ± SD | 59.6 ± 31.6 | 208.9 ± 232.4 | |
| Total Bilirubin (mg/dL) | |||
| Median | 2 | 1.4 | 0.511 |
| mean ± SD | 3.2 ± 3.8 | 2.5 ± 3.0 | |
| AST (IU/L) | |||
| Median | 68.5 | 77.5 | 0.892 |
| mean ± SD | 126.2 ± 145.9 | 93.2 ± 66.4 | |
| MTD (cm) | |||
| Median | 8.75 | 8.25 | 0.317 |
| mean ± SD | 8.66 ± 1.18 | 9.9 ± 4.5 | |
| Number of nodules, n (%) | |||
| 1 | 8 (57) | 14 (41) | 0.490 |
| > 1 | 6 (43) | 20 (59) | |
| Microvascular invasion, n (%) | |||
| Negative | 7 (50) | 7 (21) | 0.041a |
| Positive | 7 (50) | 27 (79) | |
| Differentiation, n (%) | |||
| Well | 7 (50) | 7 (20) | 0.020a |
| Moderate | 5 (36) | 19 (56) | |
| Poor | 2 (14) | 8 (24) |
P < 0.05.
P < 0.01.
P < 0.001.
AFP: Alpha-fetoprotein; GGT: Gamma glutamyl transpeptidase; AST: Aspartate aminotransferase; MTD: Maximum dominant tumor diameter; SD: Standard deviation.
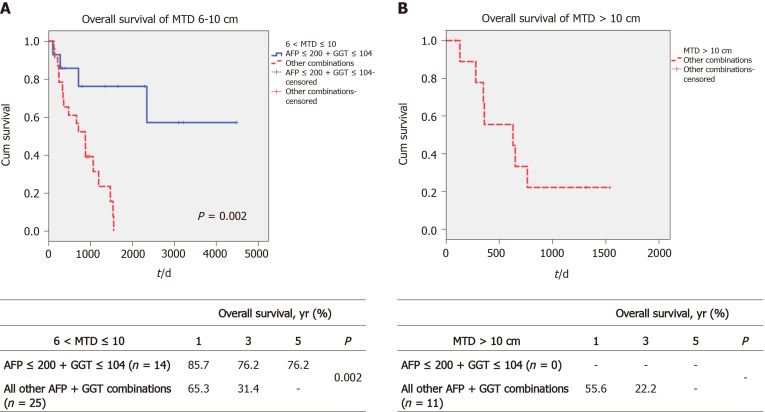
We then examined whether we could identify an upper limit of tumor size that was associated with prolonged post-transplant survival. The total cohort was divided into MTD groups of 6 < MTD ≤ 10 cm and greater than 10 cm. We then dichotomized each MTD band according to low AFP/GGT parameters vs all 3 others combined, as in Figure 3. We found a large survival difference between the low AFP/GGT group vs the other parameter groups, for the 6 < MTD ≤ 10 cm MTD band. The 1-, 3- and 5-year OS was 85.7%, 76.2% and 76.2%, respectively, P = 0.002 (Figure 3A). Finally, we examined the > 10 cm MTD group. We did not find patients with MTD > 10 cm who also had low serum AFP plus low serum GGT levels. However, there were 11 patients in the other combination parameter group and their 1, 3 and 5-year OS was 55.6%, 22.2% and 0%, respectively (Figure 3B).
Figure 3.
Survival of patients according to maximum dominant tumor diameter size and alpha-fetoprotein-gamma glutamyl transpeptidase combinations. A: Overall survival in the maximum tumor diameter band 6-10 cm according to low alpha-fetoprotein (AFP) + low gamma glutamyl transpeptidase (GGT) vs all other combinations; B: Overall survival in the maximum tumor diameter band > 10 cm according to low AFP + low GGT vs all other AFP + GGT combinations.
DISCUSSION
Tumor size has always been an important aspect for liver transplant selection in HCC patients. For many years, the Milan criteria using a maximum size of 5 cm has been the gold standard. More recently, several groups have proposed that equally good results (70% or more 5-year survival) could be obtained in patients with slightly larger tumors[15,16]. Indeed, one group (extended Toronto) claimed that there was no size limit[15]. We recently found that we can obtain equally good survival results to the Milan criteria, but in patients with a tumor diameter up to 6 cm[14]. In the current analysis of patients with HCCs larger than 6 cm, we analyzed factors that we considered might be prognostically important and focused on the 2 HCC tumor markers, serum AFP and GGT levels.
Our main findings were that serum AFP or GGT levels alone, were significantly associated with survival (Figure 1B and C), likely due to their relationship to tumor differentiation or microscopic invasion (Table 3). We obtained AFP values in all 50 patients and GGT values in 48 of these patients. We then combined any and all of the 4 possible 2-parameter combinations (AFP-/GGT-, AFP+/GGT+, AFP-/GGT+, AFP+/GGT-). We found that patients with AFP-/GGT- (low AFP/GGT) tumors survived significantly longer than any of the other 2-parameter combinations, and these other combination groups showed similar survival rates. We therefore combined all 3 of the 2-parameter combination groups with poor survival (not the AFP-/GGT- group). On comparing low AFP/GGT (AFP-/GGT-) with the 3 other groups combined, a significant separation of the survival curves was obtained (Figure 2B). Both GGT alone, as well as the AFP/GGT combinations, both predicted microscopic invasion and tumor differentiation, unlike AFP alone (Table 3 and 4). We also investigated GGT and microscopic portal vein invasion (microPVT) in 270 liver transplant patients[17] and found a significant relationship between AFP levels and microPVT and an almost statistically significant relationship between high GGT levels and microPVT (P = 0.053). However, for survival in microPVT patients, GGT levels were statistically significant but not AFP levels. The association between survival and AFP levels is thus weak, but strong and significant for GGT levels.
In this study, the AFP cutoff used was 200 ng/mL, as per our previous studies[14]. However, specifically in this study, we obtained the same results when we applied the AFP cutoff of 150 or 100 or 50 separately. Therefore, based on our previous studies, we used the 200 ng/mL AFP cutoff.
The other main finding related to our attempt to define the upper limits of tumor size that might benefit post-transplant prolonged survival was that patients in the 2-parameter combination group of low AFP/GGT had improved survival compared with all other 2-parameter AFP/GGT combinations for HCCs in the tumor size band of 6-10 cm. However, in the > 10 cm band, there were no patients in the good prognosis low AFP/GGT (AFP-/GGT-) group. There is general agreement that patient prognosis worsens with increased tumor size, but the exact mechanisms are unexplained. Possibly there is an increase in the number of tumor cells undergoing vascular invasion per unit size of tumor, and thus larger tumors have a larger total number of invading HCC cells. Equally possible, however, is that beyond a certain small tumor size (as with tumor angiogenesis), there is a change in HCC biology with more aggressive characteristics, that would explain the higher mortality associated with larger tumors. However, the small sample size limited the availability of patients in all parameter combinations. In the HCC size band of 6-10 cm, a significant increase in survival was found in the good prognosis low GGT/AFP groups, P = 0.002 (Figure 3A) compared to the other groups.
There are several outstanding questions concerning these results. Although AFP and GGT are well-described HCC biomarkers, they appear to reflect different HCC properties (Table 3). In this respect, GGT appeared to be a better discriminator of HCC aggressiveness. We combined AFP and GGT, but found only a small increase in predicted survival. Nevertheless, the Milan criteria have been the most useful guidelines for successful outcomes after liver transplant for HCC for 24 years. Recent similar success, but with larger tumors have been observed[15,17]. Another consideration is that 5 cm seemed to be a limitation for 70% 5-year survival, as in the Milan criteria. Do the tumors change with increasing size and if so, how? Or does 5 cm mark a watershed in HCC biology, with > 5 cm tumors exhibiting more aggressive features? A partial answer is that there is an increase in portal vein invasion and in AFP levels with an increase in HCC size[18]. This may explain the high (70%) incidence of PVT positivity in these larger tumors, as shown in Table 1 and 2. We did not find a relationship between AFP levels and tumor differentiation, possibly because we were dealing with larger tumors, or because of the relatively small sample size. Our findings point to serum GGT levels as having both prognostic and tumor discrimination power. Why that should be so, is not yet clear. However, as an HCC biomarker[10-13], it likely reflects certain HCC aggressiveness properties, although an action in its own right cannot be excluded.
We have previously found that GGT values represent a useful discriminant for HCC tumor aggressiveness in a large percentage of untransplantable HCC patients who have low AFP values[19].
We also analyzed our current database in terms of OS according to this new model for internal validation and compared our new model with our previously defined Malatya criteria according to survival. According to this new model, 1-, 5- and 10-year OS were 89.7%, 77.6%, and 69.9%, respectively, and were similar to the Malatya criteria (1-, 5-, 10-year OS of 90.1%, 79.7% and 72.8%, respectively, P = 0.753).
As a result of the previously published Malatya criteria, we expanded the Milan criteria by an additional 27% of patients[14]. With this new model of expanded Malatya criteria, we were able to increase the patients who were eligible for liver transplantation by 42.7% beyond the number who were eligible by Milan criteria alone. Thus, OS of 77.6% at 5 years was achieved with transplantation in 65 HCC patients who were beyond the Milan criteria.
The limitations of this study are the small sample size, although it is not small for transplantation in patients with large tumors and its retrospective design. The results of this study should be confirmed in a prospective trial.
CONCLUSION
In conclusion, the current study shows that in the setting of liver transplantation, GGT and AFP both independently and together allow discrimination of better or worse survival in HCC patients with large tumors who undergo liver transplant.
ARTICLE HIGHLIGHTS
Research background
For many years, liver transplantation for hepatocellular carcinoma (HCC) has been performed according to the Milan criteria for patient selection, using a maximum dominant tumor diameter (MTD) of 5 cm and resulting in a 5-year survival of 75%.
Research motivation
It has recently become apparent, that an expansion of the Milan criteria may be possible for patients with a slightly larger MTD than 5 cm to be transplanted and achieve similar good survival. However, the extent by which MTD can be increased has not been clearly identified.
Research objectives
To identify subsets of HCC patients with larger tumors, who also have longer survival after liver transplant, despite having an MTD > 5 cm.
Research methods
We retrospectively evaluated a prospectively accrued database of 50 patients who had HCCs > MTD of 5 cm and were treated by living donor liver transplantation.
Research results
We found that HCC patients with a MTD 6-10 cm could be stratified according to the presence of low or high levels of serum alpha-fetoprotein (AFP) or gamma glutamyl transpeptidase (GGT), or the 2 serum markers in combination. The results showed that patients with a combination of low AFP plus low GGT had excellent long-term survival. Patients who had either low AFP alone or low GGT alone had intermediate survival and patients with both high AFP plus high GGT had the worst survival.
Research conclusions
Measurement of serum AFP and GGT levels permits the identification of HCC patients with tumors greater than the Milan criteria size who can have excellent post-liver transplant survival.
Research perspectives
Future research should be directed towards the identification of new HCC biomarkers that will correlate with tumor biology and will help refine the selection of HCC patients with large tumors who can also benefit from treatment with liver transplantation. This will increase the number of HCC patients who can then benefit from increased survival following liver transplantation.
Footnotes
Institutional review board statement: This study was approved by Inonu University Institutional Review Board (Approval No. 2018/1-9).
Clinical trial registration statement: This study is registered in ClinicalTrials.gov. The registration identification number is NCT04412161.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
CONSORT 2010 statement: The authors have read the CONSORT 2010 statement, and the manuscript was prepared and revised according to the CONSORT 2010 statement.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Society for Organ Transplantation, No. 40102; and International Liver Transplantation Society, No. 17667.
Peer-review started: July 24, 2020
First decision: October 21, 2020
Article in press: November 13, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ji G, Zheng H S-Editor: Gao CC L-Editor: Webster JR P-Editor: Li JH
Contributor Information
Volkan Ince, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey. volkanince@outlook.com.
Brian I Carr, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Harika Gozukara Bag, Department of Biostatistics, Inonu University, School of Medicine, Malatya 44280, Turkey.
Veysel Ersan, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Sertac Usta, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Cemalettin Koc, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Fatih Gonultas, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Baris Kemal Sarici, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Serdar Karakas, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Koray Kutluturk, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Adil Baskiran, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Sezai Yilmaz, Department of General Surgery, Inonu University, Liver Transplantation Institute, Malatya 44280, Turkey.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Abelev GI. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295–358. doi: 10.1016/s0065-230x(08)60523-0. [DOI] [PubMed] [Google Scholar]
- 2.Elgort DA, Abelev GI, O'Conor GT. Dependence of the specificity of the serologic test for primary liver cancer in different areas of the world on sensitivity of the method used for detecting alpha-fetoprotein. Int J Cancer. 1972;10:331–337. doi: 10.1002/ijc.2910100214. [DOI] [PubMed] [Google Scholar]
- 3.Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–575. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 4.Carr BI, Pancoska P, Branch RA. Low alpha-fetoprotein hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1543–1549. doi: 10.1111/j.1440-1746.2010.06303.x. [DOI] [PubMed] [Google Scholar]
- 5.Carr BI, Guerra V, Giannini EG, Farinati F, Ciccarese F, Rapaccini GL, Di Marco M, Benvegnù L, Zoli M, Borzio F, Caturelli E, Chiaramonte M, Trevisani F. Low alpha-fetoprotein HCC and the role of GGTP. Int J Biol Markers. 2014;29:e395–e402. doi: 10.5301/jbm.5000092. [DOI] [PubMed] [Google Scholar]
- 6.Carr BI, Buch SC, Kondragunta V, Pancoska P, Branch RA. Tumor and liver determinants of prognosis in unresectable hepatocellular carcinoma: a case cohort study. J Gastroenterol Hepatol. 2008;23:1259–1266. doi: 10.1111/j.1440-1746.2008.05487.x. [DOI] [PubMed] [Google Scholar]
- 7.Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493–500. doi: 10.1001/jamaoncol.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmud N, John B, Taddei TH, Goldberg DS. Pre-transplant alpha-fetoprotein is associated with post-transplant hepatocellular carcinoma recurrence mortality. Clin Transplant. 2019;33:e13634. doi: 10.1111/ctr.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, Dharancy S, Gugenheim J, Bernard PH, Adam R, Radenne S, Muscari F, Conti F, Hardwigsen J, Pageaux GP, Chazouillères O, Salame E, Hilleret MN, Lebray P, Abergel A, Debette-Gratien M, Kluger MD, Mallat A, Azoulay D, Cherqui D Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012; 143: 986-94. :quiz e14–5. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 10.Xu K, Meng XY, Wu JW, Shen B, Shi YC, Wei Q. Diagnostic value of serum gamma-glutamyl transferase isoenzyme for hepatocellular carcinoma: a 10-year study. Am J Gastroenterol. 1992;87:991–995. [PubMed] [Google Scholar]
- 11.Yao D, Jiang D, Huang Z, Lu J, Tao Q, Yu Z, Meng X. Abnormal expression of hepatoma specific gamma-glutamyl transferase and alteration of gamma-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer. 2000;88:761–769. [PubMed] [Google Scholar]
- 12.Xu KC, Meng XY, Shi YC, Ge ZJ, Ye L, Yu ZJ, Yang DM. The diagnostic value of a hepatoma-specific band of serum gamma-glutamyl transferase. Int J Cancer. 1985;36:667–669. doi: 10.1002/ijc.2910360608. [DOI] [PubMed] [Google Scholar]
- 13.Fu SJ, Zhao Q, Ji F, Chen MG, Wu LW, Ren QQ, Guo ZY, He XS. Elevated Preoperative Serum Gamma-glutamyltranspeptidase Predicts Poor Prognosis for Hepatocellular Carcinoma after Liver Transplantation. Sci Rep. 2016;6:28835. doi: 10.1038/srep28835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ince V, Akbulut S, Otan E, Ersan V, Karakas S, Sahin TT, Carr BI, Baskiran A, Samdanci E, Bag HG, Koc C, Usta S, Ozdemir F, Barut B, Gonultas F, Sarici B, Kutluturk K, Dogan MS, Ozgor D, Dikilitas M, Harputluoglu M, Aladag M, Kutlu R, Varol I, Dirican A, Aydin C, Isik B, Ara C, Kayaalp C, Emre S, Yilmaz S. Liver Transplantation for Hepatocellular Carcinoma: Malatya Experience and Proposals for Expanded Criteria. J Gastrointest Cancer. 2020;51:998–1005. doi: 10.1007/s12029-020-00424-w. [DOI] [PubMed] [Google Scholar]
- 15.Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, Cleary SP, Lilly L, Cattral MS, Marquez M, Selzner M, Renner E, Selzner N, McGilvray ID, Greig PD, Grant DR. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology. 2016;64:2077–2088. doi: 10.1002/hep.28643. [DOI] [PubMed] [Google Scholar]
- 16.Carr BI, Ince V, Bag HG, Ersan V, Usta S, Yilmaz S. Microscopic vascular invasion by hepatocellular carcinoma in liver transplant patients. Clin Pract. 2020;17:1497–1505. [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–1732. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 18.Akkiz H, Carr BI, Kuran S, Karaoğullarından Ü, Üsküdar O, Tokmak S, Arslan B, Doran F, Balli HT, Ülkü A, Akçam TA, Bahçeci Hİ, Polat KY, Örmeci N, Şimşek H, Sonsuz A, Demir A, Altıntaş E, Demir M, Yalçın K, Ekinci N, Harmancı Özakyol A, Yücesoy M, Uygun A, Guerra V, Delik A, Tokat Y, Yilmaz S, Bektaş A, Kılıç M. Macroscopic Portal Vein Thrombosis in HCC Patients. Can J Gastroenterol Hepatol. 2018;2018:3120185. doi: 10.1155/2018/3120185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr BI, Akkiz H, Üsküdar O, Yalçın K, Guerra V, Kuran S, Karaoğullarından Ü, Altıntaş E, Özakyol A, Tokmak S, Ballı T, Yücesoy M, Bahçeci Hİ, Ülkü A, Akçam T, Polat KY, Ekinci N, Şimşek H, Örmeci N, Sonsuz A, Demir M, Kılıç M, Uygun A, Demir A, Delik A, Arslan B, Doran F, Yilmaz S, Tokat Y. HCC with low- and normal-serum alpha-fetoprotein levels. Clin Pract (Lond) 2018;15:453–464. doi: 10.4172/clinical-practice.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.