Abstract
Sarcopenia is characterised by progressive and extensive skeletal muscle degeneration and is associated with functional decline. Sarcopenia has primary and secondary aetiology, arising as a result of the ageing process or through chronic cytokine-mediated inflammation (associated with health conditions including cancer), respectively. Diagnosis of sarcopenia is dependent upon detection of reduced skeletal muscle strength, mass and performance. A combination of non-radiological and radiological methods can be used to assess each of these in turn to accurately diagnose sarcopenia. Sarcopenia is known to adversely affect outcomes of patients with various forms of cancer. Early identification of sarcopenia is imperative in improving patient care and overall prognosis. Various interventions, such as resistance exercise, nutritional support, and amino acid and vitamin supplementation have shown promise in the management of sarcopenia. However, further insight into novel interventions and indeed, assessment of the benefits of management of sarcopenia in terms of survival, are required to better support cancer patients.
Keywords: Cancer, Sarcopenia, Radiotherapy, Chemotherapy, Prognosis, Biomarker
Introduction
In oncology, age is considered as a major factor in clinical decision making. Older patients tend to receive less intense treatment due to presence of multiple co-morbidities, declining organ function, cognitive impairment, lack of social support, and clinician decision to avoid adverse effects of treatment. Considering chronological age alone may disadvantage some patients who might be otherwise “fit”.
A multitude of pathophysiological changes to the musculoskeletal and neurological systems can be attributed to the ageing process. Conditions common in older patients, such as osteoporosis and Parkinson’s Disease, lead to a gradual decline in functional status through increased frailty, falls and fractures. Also common in patients over 65 is a gradual reduction in muscle mass. From 50 years of age, a 1% reduction in muscle mass per annum can be observed [1]. Accordingly, in 65–70 year-olds, severe muscle loss (defined as appendicular skeletal muscle mass less than two standard deviations below young, healthy controls) has a prevalence of approximately 13–24%; whereas in over 80 year-olds, this rises to approximately 50% [2], [3].
In addition to various factors such as age, performance status, stage of the disease, co-morbidities and frailty score to decide the treatment and predict the prognosis, sarcopenia could be another useful parameter that can be incorporated in our clinical decision making. In this review, we aim to define sarcopenia, understand the methods of evaluation, recognise the impact and explore potential options in management.
Sarcopenia involves loss of skeletal muscle form and function
One condition involving reductions in skeletal muscle mass is sarcopenia; first termed by Rosenberg in 1989; sarcopenia translates literally as “loss of flesh” [4]. Since this initial, fairly rudimentary definition, characterisation of the condition has evolved from being considered merely a loss of muscle. Nowadays, sarcopenia is known as a geriatric syndrome, characterised by muscle failure; that is, decreased muscle mass, accompanied by a loss of muscle function and performance levels. Poor performance level alone is a predictive factor for mobility limitation, hospitalisation and mortality in the elderly population [5]. The European Working Group on Sarcopenia in Older People 2 (EWGSOP2) defines sarcopenia as a “progressive and generalised skeletal muscle disorder that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability and mortality” [6], [7]. Reduced muscle strength, low muscle quality and low physical performance are all recognised as diagnostic criteria by the EWGSOP2 and sarcopenia is considered severe if all three criteria are satisfied [7] (Table 1). The Asian Working Group for Sarcopenia (ASWG) defines this condition as “age-related loss of skeletal muscle mass plus loss of muscle strength and/or reduced physical performance” with population specific cut offs for measurements [8]. Similarly, the Foundation for the National Institutes of Health defined muscle mass and muscle strength using a definite cut off [9]. Among the many definitions and criteria available, EWGSOP2 is widely accepted.
Table 1.
Simplified diagnosis of sarcopenia according to the EWGSOP2 [7].
| Sarcopenia criteria satisfied | Probability of sarcopenia diagnosis |
|---|---|
| Reduced muscle strength. | Probable sarcopenia. |
| Reduced muscle strength and low muscle quality/quantity. | Definite sarcopenia. |
| Reduced muscle strength, low muscle quality, low physical performance. | Severe sarcopenia. |
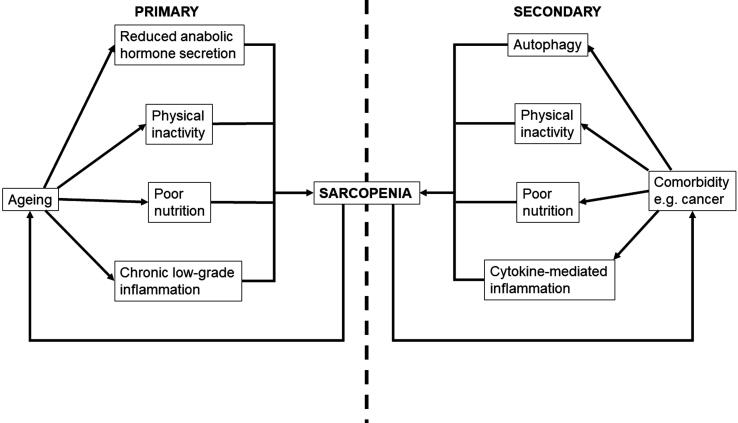
As alluded to by the EWGSOP2 definitions, sarcopenia is often associated with frailty and reduced performance status, with a predisposition towards falls and fractures [3], [10]. Sarcopenia also has a bidirectional relationship with age and comorbidity. Primary sarcopenia is attributable to age in the absence of any underlying comorbidity and develops as a result of the ageing process. Secondary sarcopenia, on the other hand, arises as a result of a chronic inflammatory state (caused by diseases such as organ failure and cancer). Both primary and secondary sarcopenia are of importance as they adversely affect patients’ functional status and can worsen prognosis of causative diseases [6], [11].
Inflammatory processes mediate loss of skeletal muscle
Secondary sarcopenia arises, at least in part, through systemic cytokine-mediated inflammation [12]. This shifts the body into a pro-catabolic state, wherein protein degradation outweighs protein synthesis [13], [14]. This, ultimately, leads to mobilisation and degradation of protein, as part of gluconeogenesis, and net loss of skeletal muscle tissue. Muscle loss is exacerbated further by components of ageing (and chronic diseases), such as physical inactivity, poor nutrition and reduced secretion of anabolic hormones. These factors attenuate generation of new skeletal muscle cells and facilitate neuromuscular degeneration. Consequently, a gradual and generalised loss of skeletal muscle may be observed throughout the course of advanced organ failure, chronic inflammatory diseases, endocrine diseases and cancer. These diseases typically have a higher incidence in older patients, compounding the issue [15].
It is also worth noting that patients may enter a vicious cycle, wherein concomitant progression of sarcopenia and chronic/degenerative disease leads to reduced prognosis (Fig. 1). As skeletal muscle is a key regulator of metabolic and inflammatory pathways, the development of sarcopenia facilitates a loss of metabolic/inflammatory regulation and subsequent promotion of a generalised inflammatory state [12], [15]. This leads to accelerated progression of existing health conditions. Research has demonstrated the prognostic nature of sarcopenia in terms of survival in multiple health conditions that are commonplace in the elderly, including cancer [12], [16], [17], [18], [19]. Sarcopenic patients also have poorer tolerance to cancer therapies, with greater incidence of complications such as infection, chemotherapy toxicity and perioperative problems, which makes cancer more difficult to treat [13], [20]. Whilst sarcopenia acts as a key driver in the progression of chronic disease, symptoms of chronic disease, such as physical inactivity and loss of appetite further exacerbate sarcopenia, further accelerating the index pathology and reducing prognosis. Fig. 2.
Fig. 1.
Pathophysiology of primary and secondary sarcopenia.
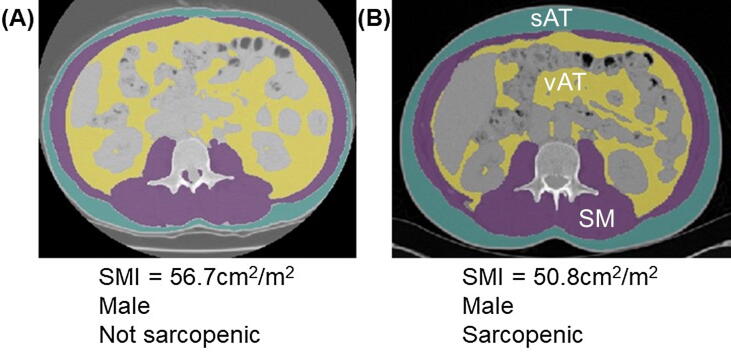
Fig. 2.
Segmentations of computed tomography scans at the level of the third lumbar vertebra (L3) in (A) a non-sarcopenic male patient and (B) a sarcopenic male patient. Blue segmentations indicate subcutaneous adipose tissue (sAT), purple segmentations indicate skeletal muscle (SM; paraspinal muscles adjacent to L3 and muscles of the abdominal wall); yellow segmentations indicate visceral adipose tissue [vAT] surrounding the abdominal viscera; and grey/blank segmentations indicate the abdominal organs (including the small and large bowel, liver and kidneys). Segmentation was processed using machine learning software [27]. Clearly, there is little visible difference between some non-sarcopenic and sarcopenic patients, highlighting the importance of segmentation and calculation of SMI values. Abbreviations: SMI, skeletal muscle index.
Sarcopenia can exist in isolation or as a component of cancer cachexia
Another multifactorial condition that is similar to sarcopenia, cancer cachexia, is characterised by ongoing loss of skeletal muscle that may or may not be accompanied by loss of adipose tissue, asthenia and anaemia [21]. Cancer cachexia is caused by a complex interplay between anorexia, increased protein catabolism, systemic inflammation and increased resting state energy expenditure. There is a considerable overlap in the pathophysiology of development of cachexia and sarcopenia. The inflammatory response is more pronounced in cachexia compared to sarcopenia. The key differentiating feature of cachexia is the recognition of hyper catabolic state and a negative protein energy imbalance which is difficult to reverse.
The cachexia spectrum recognises three stages- pre-cachexia, cachexia and refractory cachexia [22]. Pre-cachexia would involve a weight loss ≤ 5%, anorexia and metabolic change. Diagnosis of cachexia requires satisfaction of one or more of the following criteria: weight loss > 5% of total body weight over 6 months; body mass index (BMI) < 20 kg/m2 and weight loss > 2% of total body weight; or appendicular skeletal muscle index consistent with a diagnosis for sarcopenia and weight loss > 2% total body weight. Finally, the refractory stage is defined by active catabolism, non-responsive to treatment and poor functional status.
Whilst cancer cachexia is characterised by loss of total body weight, sarcopenia is specific to loss of lean muscle mass. As such, whilst cancer and sarcopenia may co-exist, it is possible that sarcopenia may present insidiously through concurrent loss of skeletal muscle mass, but with retention of a consistent total body weight.
Sarcopenic obesity represents a poor prognosis in cancer patients
Sarcopenia may be difficult to detect in some individuals, particularly those with sarcopenic obesity. These patients typically have low muscle mass, indicative of sarcopenia, but also a BMI greater than 30 kg/m2 (consistent with obesity) [23], [24]. In oncology, sarcopenia is known to increase the risk of complications from both malignancy and treatments, however sarcopenic obesity is often overlooked/misunderstood. Sarcopenia may develop due to increased metabolic demand (from the malignancy) leading to subsequent proteolysis (through processes such as inflammation and gluconeogenesis). In addition, lifestyle factors that are commonplace in cancer patients, such as physical inactivity and malnutrition can attenuate muscle growth and facilitate adipose tissue gain, leading to the development of sarcopenic obesity [15], [23], [25]. In cancer patients, this is associated with poor prognosis relative to non-sarcopenic, obese patients. For example, in patients with solid gastrointestinal or respiratory tumours, sarcopenic obesity leads to reduced functional status and survival, and increased chemotherapy toxicity [23].
Various tests are employed in the assessment of sarcopenia
Assessment of muscle mass and diagnosis of sarcopenia is achieved through consideration of diagnostic criteria, as outlined by the EWGSOP2. The prevalence of sarcopenia in different cancer types and stages is not well defined, which may explain a lack of universal diagnostic methods/thresholds. Nevertheless, various analytical methods can be used to infer muscle strength, volume/mass and performance, as summarised in Table 2 [7].
Table 2.
Assessment methods for sarcopenia.
| Tool | Cut off | Comments |
|---|---|---|
| Screening for sarcopenia | ||
| SARC-F questionnaire [42] | Score of ≥ 4 | Self-administered scale with highest Total score = 10. Rapid tool for screening |
| Ishii screening tool [43] | Higher scores associated with increased probability of sarcopenia. | Individual scores for age, grip strength and calf circumference give a sum total score out of 135. |
| Assessing muscle strength | ||
| Grip strength [44] | <27 kg for men <16 kg for women |
Measured using handheld dynamometer. Good correlation with other muscle compartments. |
| Chair stand test [5] | >15 s for five rises. | Rise as quickly as possible five times, with arms folded. Mobility issue is a limiting factor. |
| Assessing muscle mass/volume | ||
| DEXA [45] | <7.0 kg/m2 for men <5.5 kg/m2 for women |
Measure appendicular skeletal mass (ASM) or total body skeletal muscle mass (SMM). These are adjusted for height, weight or BMI. |
| Bio-impedance analysis [46] | 8.87 kg/m2 for men 6.42 kg/m2 for women |
Measures total body fat and lean mass. Inexpensive and reproducible Used in ambulant and bed ridden patients. |
| CT imaging [22] | <55 cm2/m2 for men <39 cm2/m2 for women |
Muscle mass is measured at 3rd lumbar vertebra and normalised to height. |
| Physical Performance tests | ||
| SPPB [39] | ≤8 | Set of tests evaluating gait speed, balance and chair stand test. Maximum score = 12 |
| 400 m (Walk 20 m laps) [41] | Non completion or > 6minutes | Gait abnormalities, physical disability and cognitive impairment make it difficult to perform these tasks. |
| Gait speed (Over a 4 m course) [40] | <0.8 m/s |
Abbreviations: Dual energy X-ray absorptiometry; SPPB, short physical performance battery.
To measure muscle strength, clinical assessment methods such as handgrip strength and chair-stand testing can be used. These methods offer inexpensive, easy-to-measure means of assessing muscle function. Hand grip strength is a quantitative variable measured using a calibrated handheld dynamometer. The threshold values for low muscle strength are <27 kg for men and <16 kg for women [5], [26]. The chair stand test is another simple measurement that can be easily implemented in the clinical environment. The subject must rise from a chair, without support, five times in less than 15 seconds. Although both these methods are time efficient and convenient for clinical use, physical disability limits their use [5].
Muscle mass can be assessed using a range of non-radiological and radiological methods. One such non-radiological method is bio-impedance analysis, which is a measure of the insulative properties of tissues in response to an electrical stimulus. This can be converted to readings of fat mass and fat-free mass of the patient. To attain estimates of skeletal muscle mass, segmental bioimpedance analysis (such as, of the upper and lower limbs) is conducted. The accuracy of bioimpedance analysis, however, is limited by variance in the volume of intracellular fluid. As such, radiological methods are preferred in the assessment of muscle mass [27].
Dual energy X-ray absorptiometry (DEXA) measures attenuation of X-rays at two separate frequencies to ascertain indices of body composition (such as total body mass, skeletal muscle mass, total body fat percentage). These are typically normalised against BMI/height, yielding useful barometers of body composition, such as appendicular lean mass to height/BMI ratio [28]. Analysis of dual energy X-ray absorptiometry at the level of the mid-thigh can be used to calculate SMA and SMI of lower limb skeletal muscle. Sex-specific appendicular SMI thresholds (of < 7.26 kg/m2 in males and < 5.45 kg/m2 in females) can then be applied to define sarcopenia [7], [22]. Whilst DEXA represents a precise means of assessing body composition and skeletal muscle mass, it is limited by its expense, additional radiation exposure and an inability to operate on conventional X-ray machines.
Muscle mass can also be assessed using slices taken from computed tomography (CT) scans, at the level of the third lumbar vertebra (L3). Using CT slices, cross-sectional area of skeletal muscle (SMA; including psoas, quadratus lumborum, erector spinae muscles, transversus abdominis, internal and external obliques and rectus abdominis) can be calculated [29]. This involves segmentation of skeletal muscle, which can be achieved through manual contouring; or automated segmentation through use of machine learning software [30], [31], [32]. Similar to BMI, SMA can be adjusted to the square of the patient’s height yielding a skeletal muscle index (SMI/cm2/m2) [23]. Sex-specific threshold values can then be applied to SMI readings in order to diagnose sarcopenia. European consensus definitions of sarcopenia are an SMI < 55 cm2/m2 in males and < 39 cm2/m2 in females [22].
Assessment of SMI at L3 is the current gold standard for inferring total skeletal muscle mass using CT (and therefore, diagnosing sarcopenia) (Fig. 1). However, this is reliant upon imaging of the abdomen being available. Unfortunately, abdominal imaging is not routinely performed in many patients who are at risk of developing sarcopenia. In particular, patients with head and neck cancer are at increased risk of malnutrition (and therefore, sarcopenia) relative to other malignancies [33]. Clearly, there is greater need for assessment of skeletal muscle mass in those at greater risk of developing sarcopenia. In this light, numerous studies have investigated whether SMA/SMI at vertebral levels other than L3 (and different muscles/muscle groups) can be used in the assessment of sarcopenia [34], [35], [36].
In patients with head and neck cancer, a strong positive correlation has been observed between SMA at the third cervical vertebra (C3; calculated using sternocleidomastoid and the paravertebral muscles) and SMA at L3 [34]. Similarly, in patients treated with transcatheter aortic valve replacement, SMI at the seventh and twelfth thoracic vertebrae (T7 and T12 respectively) were both associated with SMI at L3. Interestingly, SMI at T12 and L3 were both associated with length of stay in hospital, suggesting sarcopenia has a prognostic role in these patients [35]. Moreover, in patients with small-cell lung cancer, SMI taken at L1 was positively associated with SMI at L3; however, SMI taken specifically from pectoralis major at the level of the aortic arch (T4) was not associated with SMI at L3 [36]. Altogether, this suggests that valid assessment of skeletal muscle mass can be achieved through measurement of SMI at multiple vertebral levels and should probably encompass all skeletal muscle at that given level, rather than delineating individual muscles.
Following segmentation, skeletal muscle density and adipose infiltration can be measured, giving indication of skeletal muscle mass and quality. Skeletal muscle density is reported as a CT number, measured in Hounsfield Units (HU), and characterises the percentage difference in density relative to water (which has a CT number of 0 HU). Skeletal muscle typically occupies a CT number of approximately 50–60 HU [37], [38]. The lower the CT number, the less dense and therefore, the more adipose infiltration is present. Whilst there is potential for applications in this regard, muscle density readings are limited by error margins of up to 15%, owing to the instability of CT images, generated by artefact and deviation of water values from 0 HU [37].
Cancer patients undergo multiple evaluations with CT scans during their treatment. In a radiation oncology setting, the planning CT scan is a good source to calculate the sarcopenia indices. Although segmentation at L3 vertebral level is considered the standard for evaluation, alternate vertebral levels may be chosen as described previously. This evaluation can be carried out during the planning process to identify individuals with sarcopenia.
Muscle performance tests evaluate whole body function in terms of mobility and transfer. Short Physical Performance Battery (SPPB), gait speed and 400 m walk testing are the most commonly used methods. Assessment tools such as the (SPPB) are useful in combining surrogates for musculoskeletal performance that impact quality of life. The SPPB produces an aggregate score between 0 and 12, based on balance, gait speed and chair stand tests, of which a score ≤ 8 is diagnostic for low performance [39]. Gait speed is a straightforward measurement of time taken to walk 4 meter distance with a specified cut off at < 0.8 m/s [40]. Since it is a very short distance, impediment in gait initiation phase could impact the evaluation. The 400 m walk test allows testing of both physical function and endurance. The task is to complete 20 laps of 20 m distance in the least amount of time [41]. All the performance tests can be conducted without the need for additional equipment. Cognitive impairment, physical disability, and gait and balance disorders preclude its utility.
In terms of the diagnostic algorithm, muscle strength testing should be performed first, followed by assessment of mass/volume and then performance. Fulfilment of all three criteria is indicative of severe sarcopenia, whereas one or two of these criteria indicates probable and definite sarcopenia respectively [7].
Sarcopenia has profound impact on cancer treatment and outcomes
Sarcopenic is increasingly recognised as a poor prognostic factor in cancer treatment and outcomes. There is a growing body of evidence suggesting that sarcopenic patients are at higher risk of developing immediate post-operative complications and prolonged hospital stays after cancer resection [47], [48], [49]. Chemoradiotherapy tolerance in sarcopenia patients is poor due to increased prevalence of side effects and treatment interruption. This can be explained by the variation in pharmacokinetics of drugs when using body surface area for dose calculation. Patients with poor lean body mass have a low volume of distribution of drugs and are relatively overdosed [50]. In addition, pro-inflammatory states can suppress hepatic cytochrome enzymes leading to increased systemic exposure of cytotoxic drugs [51]. The prevalence of sarcopenia in different types of cancer and stages is not well defined in the literature. Perhaps this could be due to lack of universal definition and diagnostic models.
In general, sarcopenic patients with various forms of cancer have reduced survival relative to those without sarcopenia [52]. This difference is seen in both non metastatic patients receiving curative treatment and in metastatic patients. Various groups have studied the effect of sarcopenia in surgical, chemotherapy and radiotherapy cohorts. Radiotherapy plays a vital role in curative treatments of head and neck cancers, upper GI cancers and pelvic malignancies such as cervix and bladder. Here we highlight a few studies evaluating sarcopenia in patients undergoing radiotherapy.
Sarcopenia in head and neck cancer patients
Radiotherapy, with or without chemotherapy, is a standard treatment for head and neck squamous cell cancer (HNSCC) and is associated with significant side effects such as mucositis, dysgeusia, dysphagia, odynophagia, nausea and vomiting. Treatment toxicity leads to dietary insufficiency and subsequent malnutrition. As a result, HNSCC patients may lose approximately 6–12% of their body weight during the treatment course [53]. Other factors related to weight loss include: advanced disease stage, use of concurrent chemotherapy and higher BMI [54]. In locally advanced disease, higher treatment volume and radiation dose augments damage to normal tissues, increasing the likelihood and severity of side effects.
Ganju et al. (2019) showed that sarcopenia reduces chemoradiotherapy compliance and increases chemotherapy toxicity in patients with locally advanced HNSCC [55]. 58% of the 246 patients investigated had pre-treatment sarcopenia and approximately 20% of these sarcopenic patients had treatment interruptions lasting more than one week. In addition, 45% of sarcopenia patients experienced dose-limiting chemotherapy toxicities. Moreover, overall survival (OS) at 3 years was poor relative to non-sarcopenic patients (65.3% versus 79.9%). A similar study demonstrated an approximately threefold increased risk of developing dose-limiting toxicity in sarcopenic patients treated with chemoradiotherapy (CRT) [56].
Grossberg et al. (2016) showed that pre- and post-treatment sarcopenia is associated with worse OS in a cohort of 190 HNSCC patients, treated with CRT [57]. A significant reduction in OS, from 75% to 62%, was observed in sarcopenic patients, relative to non-sarcopenic patients. Post-treatment sarcopenia was also associated with a reduction in OS, relative to non-sarcopenic patients. A similar drop in OS (from 86% to 64%) was observed in patients experiencing post treatment skeletal muscle depletion. Post-treatment sarcopenia was much higher in prevalence than pre-treatment sarcopenia (65.8% versus 35.3%). Moreover, generalised weight loss was not associated with significant changes to patient outcomes, thus emphasising the importance of measuring SMI specifically, rather than simply total body weight. A similar study reported a two-fold increase in the prevalence of sarcopenia after treatment relative to before treatment [58]. Furthermore, a threefold increase in risk of recurrence and death was also observed among sarcopenic patients.
Sarcopenia in upper gastrointestinal (UGI) cancer patients
Cancers arising from oesophagus, stomach, liver and pancreas carry a limited prognosis. This relates to their late stage presentation and high predilection for local and distant spread. Poor nutritional status is also implicated in the poor prognosis of patients with UGI cancers. Patients with UGI cancer typically present with dysphagia, weight loss, loss of appetite, nausea, vomiting, haematemesis and deranged liver function. These factors facilitate nutritional insufficiency, which can lead to the development of sarcopenia. Furthermore, treatment of UGI cancers involves major resections of the organ, chemotherapy and radiation, all of which precipitate alterations in body composition.
A sub-study from a Swedish phase 3 trial evaluating trimodality treatment with a biological agent in oesophageal cancer reported higher incidence of CRT-related toxicity in the sarcopenia group [59]. The incidence of toxicity ≥ grade 3 was 83.3% versus 54.4% in non-sarcopenic patients. Moreover, the prevalence of sarcopenia increased from 29.5% before treatment to 63.9% during neoadjuvant CRT. However, no significant difference in survival was observed between sarcopenic and non-sarcopenic patients. A retrospective analysis of patients undergoing similar treatment also demonstrated increased side effects of neoadjuvant CRT without significant impact on survival [60].
The relationship between sarcopenia and survival in patients treated with radical CRT for oesophageal cancer has also been characterised [61]. Interestingly, no significant differences were observed with respect to OS or disease control in patients with pre-treatment sarcopenia. However, patients developing sarcopenia after CRT had poorer survival relative to the rest of the cohort (median OS 45 versus 74 months). It appears that CRT, either used as neoadjuvant or radical treatment, increases the risk of nutritional insufficiency, possibly reducing treatment tolerance. Specifically, post-treatment complications (as well as male gender) were associated with development of post-treatment sarcopenia.
Sarcopenia in preoperative gastric cancer patients varies greatly, with a prevalence ranging from 7-70% [62]. Postoperative radiotherapy to the gastric bed may be considered in selected locally advanced gastric cancers. This involves irradiation of a significant volume of the small bowel and pancreas, increasing the risks of treatment side effects. A study evaluating postoperative radiation to the gastric bed reported incidences of sarcopenia as 29.4% (45/153), 27.3% (35/128) and 37.0% (37/100) prior to CRT, 6 months and 12 monthsafter treatment respectively [63]. Increased radiation dose to the pancreas saw a two-fold increase in sarcopenia prevalence at 12 months (V46 ≥ 57%). As such, functional decline of the exocrine and endocrine pancreas could prevent post-treatment recovery, further contributing to muscle loss.
Sarcopenia in pelvic cancer patients
The prevalence of sarcopenia is often underestimated in cancers of the pelvic organs. In locally advanced cervical cancer, the prevalence of sarcopenia ranges from 33-51% [64]. One study found that a third of patients with cervical cancer met diagnostic criteria for sarcopenia criteria despite not being clinically malnourished. Following treatment, 69% of patients were clinically malnourished and 58% were sarcopenic [65]. This was attributed to decreased calorie intake and early satiety. It was also noted that many women intentionally cut down on food intake to avoid gastrointestinal symptoms. Patients experiencing > 10% reduction in SMI had higher early recurrence of disease and reduced survival following CRT. In addition, sarcopenia was found to predict severe haematological toxicity (≥grade 3). By contrast, another study failed to identify associations between pre-treatment sarcopenia and survival [66].
With respect to bladder cancer, Psutka et al (2014) found that, in a cohort of 205 bladder cancer patients treated with radical cystectomy, sarcopenia was associated with worse cancer-specific survival (CSS) and OS [67]. Approximately 68% of patients had sarcopenia and these patients tended to be older (median age 72 versus 67.5 years) than non-sarcopenic patients. Specifically, sarcopenic patients had significantly lower 5-year CSS (49% vs 72%) and OS (39% vs 70%) relative to non-sarcopenic patients. In addition, Fukushima et al. (2015) demonstrated that in patients with inoperable bladder cancer, concurrent sarcopenia was associated with very poor OS (11 versus 31 months in non-sarcopenic patients) [18]. Stangl-Kremser et al. (2017) reported a sarcopenia prevalence of 72% in a series of 68 patients undergoing CRT [68]. By contrast to surgical studies, whilst the prevalence of sarcopenia was high, no significant associations between sarcopenia and survival could be made. This could be due to the high median age (82 years) and mortality due to other causes.
Management of sarcopenia in cancer patients
Due to the prognostic significance of sarcopenia in cancer patients, early identification of sarcopenia and appropriate intervention is clearly imperative. For decades, inference of prognosis has been solely based upon performance status (PS; measured as per the World Health Organisation [WHO] and Karnofsky PS scales). PS can be used to separate patients into strata, according to their ability to carry out activities of daily living and their functional status. Whilst PS scales allow quantitative assessment of patients’ performance, PS scales are highly subjective and are reliant upon sound clinical judgement. Other prognostic factors, such as total body weight and BMI can also be used to predict prognosis in cancer patients. As explained earlier, total body weight and BMI are not truly reflective of changes in body composition, increasing the risk of false negative sarcopenia diagnoses. As such, supplementation of PS, total body weight and BMI, with additional prognostic factors, such as sarcopenia, could aid estimation of prognosis and clinical decision making.
Exercise and resistance training are currently used to treat age-associated sarcopenia [69], [70], [71]. Similar structured physical activities have been evaluated in cancer patients during treatment. In a cohort of HNSCC, progressive resistance exercises for 12 week periods improved the lean body mass by 4.2% [72]. Progressive resistance exercises aim to strengthen large muscle groups of the body. This involves working the muscles against resistance using free weights, machines or elastic bands. Typically, these exercises are done in repetitions in multiple sessions. The resistance is progressively increased over a period based on the strength gained in the muscle groups. Benefits of structured resistance training programmes include reduction in cancer related fatigue, decreased anxiety, weight gain and BMI correction, as well as improved functional status [73], [74]. That said, exercise therapy may not be suitable in few patients due to poor adherence, advanced stage disease, fatigue due to cancer and side effects of treatment limiting functional capacity. As such, evaluation of patients followed by a supervised and structured training programme is the ideal approach.
The hypermetabolic state in cancer patients increases the energy expenditure. As a compensatory mechanism, lipolysis and proteolysis in the body reserves accelerates leading to a negative energy balance. A study evaluating amino acid supplements like glutamine, L-arginine and b-hydroxy b-methyl butyrate (HMB) has shown an increase in lean body weight of about a kilogram at the end of 24 weeks compared to the control group [75]. Leucine as a standalone therapy (or combined with other supplements) appears to promote an anabolic response and protein synthesis [76]. Various other supplements, such as the omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as Vitamin D and Vitamin C supplements have shown varied response [77]. The studies evaluating nutritional intervention have heterogeneous groups of patients and the end points differ between them, making the evidence equivocal. Clearly then, randomised, double-blinded data is required to evaluate the efficacy of nutritional supplementation in the treatment of sarcopenia. Nutritional counselling and feeding procedures such as nasogastric or percutaneous endogastric feeding have proven beneficial to reducing sarcopenia prevalence in patients with head and neck cancer. Nutritional prophylaxis and early intervention are known to reduce weight loss during treatment and improve treatment tolerance [78]. Importantly, nutritional support should be timed effectively to maintain or increase muscle mass. In order to reverse sarcopenia, nutritional support must be provided in the initial disease stages, as opposed to terminal disease, when the life expectancy is less than 3 months [79].
Interventions aimed at controlling symptoms such as nausea and vomiting may encourage enteral feeding. Exogenous hormone therapies such as low dose corticosteroids, progesterone and testosterone supplements exhibit modest advantage in terms of increasing weight gain and appetite [80], [81]. However, the benefits of such therapies must be considered in the context of their risks and side effects.
In addition, non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, celecoxib and indomethacin have been studied with the aim of suppressing systemic inflammation, thereby interrupting weight loss and cachexia [82]. Moreover, a synthetic neuropeptide mimicking the action of ghrelin has been shown to improve lean muscle mass in cancer patients [83]. However, this had no functional improvements. Another drug, enobosarm is a selective androgen receptor modulator which has shown lean body mass gain in lung cancer patients [84]. Further novel therapies (Bimagrumab and follistatin) targeting the myostatin pathway are being explored. Overall, pharmacotherapy for sarcopenia is still evolving.
Conclusion
Sarcopenia is a widely prevalent problem among cancer patients, but often it is not recognised in the clinical setting. Screening patients for sarcopenia is relatively simple and it can be incorporated along with performance status. The multiple CT images obtained during a treatment provide us opportunity to objectively quantify muscle loss at various time points. By integrating sarcopenia assessment in treatment algorithms, patients can be risk categorised for developing treatment complications and necessary steps can be taken to mitigate them. Along with PS evaluation, a rapid screening tool in the clinic would help in identifying at risk individuals. This can be followed up with a more formal evaluation using a physical test or by calculating the sarcopenia indices using CT scans. These assessments further help in predicting outcomes and can serve as a useful biomarker. Timely nutritional intervention and exercise therapy has shown moderate benefit in overcoming the complications associated with muscle depletion. Moving forward, more research exploring other management options and supportive therapies is necessary.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This work was supported by Cancer Research UK RadNet Manchester [C1994/A28701] and Cancer Research UK via funding to the Cancer Research Manchester Centre [C147/A25254].
Professor Ananya Choudhury and Professor Peter Hoskin are supported by NIHR Manchester Biomedical Research Centre.
References
- 1.Larsson L., Degens H., Li M., Salviati L., Lee Y.I., Thompson W. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol Rev. 2019;99:427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim T.N., Choi K.M. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20:1–10. doi: 10.11005/jbm.2013.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner R.N., Koehler K.M., Gallagher D., Romero L., Heymsfield S.B., Ross R.R. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg I.H. Summary comments. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- 5.Cesari M., Kritchevsky S.B., Newman A.B., Simonsick E.M., Harris T.B., Penninx B.W. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57:251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L.-K., Woo J., Assantachai P., Auyeung T.-W., Chou M.-Y., Iijima K. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft A.J., Kiesswetter E., Drey M., Sieber C.C. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017;29:43–48. doi: 10.1007/s40520-016-0709-0. [DOI] [PubMed] [Google Scholar]
- 11.Santilli V., Bernetti A., Mangone M., Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 12.Roubenoff R., Parise H., Payette H.A., Abad L.W., D’Agostino R., Jacques P.F. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima H., Koga F. Impact of sarcopenia in the management of urological cancer patients. Expert Rev Anticancer Ther. 2017;17:455–466. doi: 10.1080/14737140.2017.1301209. [DOI] [PubMed] [Google Scholar]
- 14.Sandri M. Autophagy in skeletal muscle. FEBS Lett. 2010;584:1411–1416. doi: 10.1016/j.febslet.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 15.Dhillon R.J.S., Hasni S. Pathogenesis and Management of Sarcopenia. Clin Geriatr Med. 2017;33:17–26. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vashi P.G., Gorsuch K., Wan L., Hill D., Block C., Gupta D. Sarcopenia supersedes subjective global assessment as a predictor of survival in colorectal cancer. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0218761. e0218761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chargi N., Bril S.I., Emmelot-Vonk M.H., de Bree R. Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur Arch Otorhinolaryngol. 2019;276:1475–1486. doi: 10.1007/s00405-019-05361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukushima H., Yokoyama M., Nakanishi Y., Tobisu K.-I., Koga F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0115895. e0115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villaseñor A., Ballard-Barbash R., Baumgartner K., Baumgartner R., Bernstein L., McTiernan A. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6:398–406. doi: 10.1007/s11764-012-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazemi-Bajestani S.M.R., Mazurak V.C., Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol. 2016;54:2–10. doi: 10.1016/j.semcdb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Dhanapal R., Saraswathi T., Govind R.N. Cancer cachexia. J Oral Maxillofac Pathol. 2011;15:257–260. doi: 10.4103/0973-029X.86670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 23.Prado C.M.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 24.Lee D.-C., Shook R.P., Drenowatz C., Blair S.N. Physical activity and sarcopenic obesity: definition, assessment, prevalence and mechanism. Future Sci OA. 2016;2 doi: 10.4155/fsoa-2016-0028. FSO127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batsis J.A., Villareal D.T. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–537. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodds R.M., Syddall H.E., Cooper R., Benzeval M., Deary I.J., Dennison E.M. Grip strength across the life course: normative data from twelve British studies. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0113637. e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil S.F., Mohktar M.S., Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors. 2014;14:10895–10928. doi: 10.3390/s140610895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd J.A., Ng B.K., Sommer M.J., Heymsfield S.B. Body composition by DXA. Bone. 2017;104:101–105. doi: 10.1016/j.bone.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zopfs D., Theurich S., Große Hokamp N., Knuever J., Gerecht L., Borggrefe J. Single-slice CT measurements allow for accurate assessment of sarcopenia and body composition. Eur Radiol. 2020;30:1701–1708. doi: 10.1007/s00330-019-06526-9. [DOI] [PubMed] [Google Scholar]
- 30.Green A., Cipriano C., Osorio E.V., Weaver J., Van Herk M., McWilliam A. PO-0960 Automated sarcopenia assessment and its predictive power in lung cancer radiotherapy patients. Radiother Oncol. 2019;133:S521. [Google Scholar]
- 31.Dabiri S., Popuri K., Cespedes Feliciano E.M., Caan B.J., Baracos V.E., Beg M.F. Muscle segmentation in axial computed tomography (CT) images at the lumbar (L3) and thoracic (T4) levels for body composition analysis. Comput Med Imaging Graph. 2019;75:47–55. doi: 10.1016/j.compmedimag.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnard R., Tan J., Roller B., Chiles C., Weaver A.A., Boutin R.D. Machine Learning for Automatic Paraspinous Muscle Area and Attenuation Measures on Low-Dose Chest CT Scans. Acad Radiol. 2019;26:1686–1694. doi: 10.1016/j.acra.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pressoir M., Desné S., Berchery D., Rossignol G., Poiree B., Meslier M. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. 2010;102:966–971. doi: 10.1038/sj.bjc.6605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swartz J.E., Pothen A.J., Wegner I., Smid E.J., Swart K.M.A., de Bree R. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016;62:28–33. doi: 10.1016/j.oraloncology.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Nemec U., Heidinger B., Sokas C., Chu L., Eisenberg R.L. Diagnosing Sarcopenia on Thoracic Computed Tomography: Quantitative Assessment of Skeletal Muscle Mass in Patients Undergoing Transcatheter Aortic Valve Replacement. Acad Radiol. 2017;24:1154–1161. doi: 10.1016/j.acra.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Kim E.Y., Kim Y.S., Park I., Ahn H.K., Cho E.K., Jeong Y.M. Evaluation of sarcopenia in small-cell lung cancer patients by routine chest CT. Support Care Cancer. 2016;24:4721–4726. doi: 10.1007/s00520-016-3321-0. [DOI] [PubMed] [Google Scholar]
- 37.Engelke K., Museyko O., Wang L., Laredo J.-D. Quantitative analysis of skeletal muscle by computed tomography imaging-State of the art. J Orthop Translat. 2018;15:91–103. doi: 10.1016/j.jot.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aubrey J., Esfandiari N., Baracos V.E., Buteau F.A., Frenette J., Putman C.T. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavasini R., Guralnik J., Brown J.C., di Bari M., Cesari M., Landi F. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14:215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Sousa M.A., Venegas-Sanabria L.C., Chavarro-Carvajal D.A., Cano-Gutierrez C.A., Izquierdo M., Correa-Bautista J.E. Gait speed as a mediator of the effect of sarcopenia on dependency in activities of daily living. J Cachexia Sarcopenia Muscle. 2019;10:1009–1015. doi: 10.1002/jcsm.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H., Ambrosius W.T., Murphy T.E., Fielding R., Pahor M., Santanasto A. Imputation of Gait Speed for Noncompleters in the 400-Meter Walk: Application to the Lifestyle Interventions for Elders Study. J Am Geriatr Soc. 2017;65:2566–2571. doi: 10.1111/jgs.15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malmstrom T.K., Miller D.K., Simonsick E.M., Ferrucci L., Morley J.E. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7:28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishii S., Tanaka T., Shibasaki K., Ouchi Y., Kikutani T., Higashiguchi T. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014;14(Suppl 1):93–101. doi: 10.1111/ggi.12197. [DOI] [PubMed] [Google Scholar]
- 44.Roberts H.C., Denison H.J., Martin H.J., Patel H.P., Syddall H., Cooper C. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 45.Buckinx F., Landi F., Cesari M., Fielding R.A., Visser M., Engelke K. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. 2018;9:269–278. doi: 10.1002/jcsm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chien M.-Y., Huang T.-Y., Wu Y.-T. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56:1710–1715. doi: 10.1111/j.1532-5415.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 47.Gruber E.S., Jomrich G., Tamandl D., Gnant M., Schindl M., Sahora K. Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0215915. e0215915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simonsen C., de Heer P., Bjerre E.D., Suetta C., Hojman P., Pedersen B.K. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann Surg. 2018;268:58–69. doi: 10.1097/SLA.0000000000002679. [DOI] [PubMed] [Google Scholar]
- 49.Rutten I.J.G., Ubachs J., Kruitwagen R.F.P.M., van Dijk D.P.J., Beets-Tan R.G.H., Massuger L.F.A.G. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol. 2017;43:717–724. doi: 10.1016/j.ejso.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Prado C.M.M., Lima I.S.F., Baracos V.E., Bies R.R., McCargar L.J., Reiman T. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 2011;67:93–101. doi: 10.1007/s00280-010-1288-y. [DOI] [PubMed] [Google Scholar]
- 51.Sharma R., Kacevska M., London R., Clarke S.J., Liddle C., Robertson G. Downregulation of drug transport and metabolism in mice bearing extra-hepatic malignancies. Br J Cancer. 2008;98:91–97. doi: 10.1038/sj.bjc.6604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shachar S.S., Williams G.R., Muss H.B., Nishijima T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 53.Trotti A., Bellm L.A., Epstein J.B., Frame D., Fuchs H.J., Gwede C.K. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 54.Zhao J.-Z., Zheng H., Li L.-Y., Zhang L.-Y., Zhao Y., Jiang N. Predictors for Weight Loss in Head and Neck Cancer Patients Undergoing Radiotherapy: A Systematic Review. Cancer Nurs. 2015;38:E37–E45. doi: 10.1097/NCC.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 55.Ganju R.G., Morse R., Hoover A., TenNapel M., Lominska C.E. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol. 2019;137:117–124. doi: 10.1016/j.radonc.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 56.Wendrich A.W., Swartz J.E., Bril S.I., Wegner I., de Graeff A., Smid E.J. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017;71:26–33. doi: 10.1016/j.oraloncology.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Grossberg A.J., Chamchod S., Fuller C.D., Mohamed A.S.R., Heukelom J., Eichelberger H. Association of Body Composition With Survival and Locoregional Control of Radiotherapy-Treated Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016;2:782–789. doi: 10.1001/jamaoncol.2015.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung A.R., Roh J.-L., Kim J.S., Kim S.-B., Choi S.-H., Nam S.Y. Prognostic value of body composition on recurrence and survival of advanced-stage head and neck cancer. Eur J Cancer. 2019;116:98–106. doi: 10.1016/j.ejca.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Panje C.M., Höng L., Hayoz S., Baracos V.E., Herrmann E., Garcia Schüler H. Skeletal muscle mass correlates with increased toxicity during neoadjuvant radiochemotherapy in locally advanced esophageal cancer: A SAKK 75/08 substudy. Radiat Oncol. 2019;14:166. doi: 10.1186/s13014-019-1372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murimwa G.Z., Venkat P.S., Jin W., Leuthold S., Latifi K., Almhanna K. Impact of sarcopenia on outcomes of locally advanced esophageal cancer patients treated with neoadjuvant chemoradiation followed by surgery. J Gastrointest Oncol. 2017;8:808–815. doi: 10.21037/jgo.2017.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma D.W., Cho Y., Jeon M.-J., Kim J.-H., Lee I.J., Youn Y.H. Relationship Between Sarcopenia and Prognosis in Patient With Concurrent Chemo-Radiation Therapy for Esophageal Cancer. Front Oncol. 2019;9:366. doi: 10.3389/fonc.2019.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamarajah S.K., Bundred J., Tan B.H.L. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2019;22:10–22. doi: 10.1007/s10120-018-0882-2. [DOI] [PubMed] [Google Scholar]
- 63.Li Y., Wang W.-B., Jiang H.-G., Dai J., Xia L., Chen J. Predictive value of pancreatic dose-volume metrics on sarcopenia rate in gastric cancer patients treated with adjuvant chemoradiotherapy. Clin Nutr. 2019;38:1713–1720. doi: 10.1016/j.clnu.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 64.Chung E., Lee H.S., Cho E.-S., Park E.J., Baik S.H., Lee K.Y. Prognostic significance of sarcopenia and skeletal muscle mass change during preoperative chemoradiotherapy in locally advanced rectal cancer. Clin Nutr. 2020;39:820–828. doi: 10.1016/j.clnu.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Sánchez M., Castro-Eguiluz D., Luvián-Morales J., Jiménez-Lima R., Aguilar-Ponce J.L., Isla-Ortiz D. Deterioration of nutritional status of patients with locally advanced cervical cancer during treatment with concomitant chemoradiotherapy. J Hum Nutr Diet. 2019;32:480–491. doi: 10.1111/jhn.12649. [DOI] [PubMed] [Google Scholar]
- 66.Matsuoka H., Nakamura K., Matsubara Y., Ida N., Nishida T., Ogawa C. Sarcopenia Is Not a Prognostic Factor of Outcome in Patients With Cervical Cancer Undergoing Concurrent Chemoradiotherapy or Radiotherapy. Anticancer Res. 2019;39:933–939. doi: 10.21873/anticanres.13196. [DOI] [PubMed] [Google Scholar]
- 67.Psutka S.P., Carrasco A., Schmit G.D., Moynagh M.R., Boorjian S.A., Frank I. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer. 2014;120:2910–2918. doi: 10.1002/cncr.28798. [DOI] [PubMed] [Google Scholar]
- 68.Stangl-Kremser J., D’Andrea D., Vartolomei M., Abufaraj M., Goldner G., Baltzer P. Prognostic value of nutritional indices and body composition parameters including sarcopenia in patients treated with radiotherapy for urothelial carcinoma of the bladder. Urol Oncol. 2019;37:372–379. doi: 10.1016/j.urolonc.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Beaudart C., Dawson A., Shaw S.C., Harvey N.C., Kanis J.A., Binkley N. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28:1817–1833. doi: 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montero-Fernández N., Serra-Rexach J.A. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med. 2013;49:131–143. [PubMed] [Google Scholar]
- 71.Bullo V., Bergamin M., Gobbo S., Sieverdes J.C., Zaccaria M., Neunhaeuserer D. The effects of Pilates exercise training on physical fitness and wellbeing in the elderly: A systematic review for future exercise prescription. Prev Med. 2015;75:1–11. doi: 10.1016/j.ypmed.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Lønbro S., Dalgas U., Primdahl H., Johansen J., Nielsen J.L., Aagaard P. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy–results from the randomized DAHANCA 25B trial. Radiother Oncol. 2013;108:314–319. doi: 10.1016/j.radonc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Speck R.M., Courneya K.S., Mâsse L.C., Duval S., Schmitz K.H. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 74.Fong D.Y.T., Ho J.W.C., Hui B.P.H., Lee A.M., Macfarlane D.J., Leung S.S.K. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344 doi: 10.1136/bmj.e70. e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.May P.E., Barber A., D’Olimpio J.T., Hourihane A., Abumrad N.N. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–479. doi: 10.1016/s0002-9610(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 76.Antoun S., Raynard B. Muscle protein anabolism in advanced cancer patients: response to protein and amino acids support, and to physical activity. Ann Oncol. 2018:ii10–ii17. doi: 10.1093/annonc/mdx809. [DOI] [PubMed] [Google Scholar]
- 77.Dev R., Wong A., Hui D., Bruera E. The Evolving Approach to Management of Cancer Cachexia. Oncology. 2017;31:23–32. [PubMed] [Google Scholar]
- 78.Paccagnella A., Morello M., Da Mosto M.C., Baruffi C., Marcon M.L., Gava A. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer. 2010;18:837–845. doi: 10.1007/s00520-009-0717-0. [DOI] [PubMed] [Google Scholar]
- 79.Prado C.M., Sawyer M.B., Ghosh S., Lieffers J.R., Esfandiari N., Antoun S. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr. 2013;98:1012–1019. doi: 10.3945/ajcn.113.060228. [DOI] [PubMed] [Google Scholar]
- 80.Ruiz Garcia V., López-Briz E., Carbonell Sanchis R., Gonzalvez Perales J.L., Bort-Marti S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD004310.pub3. CD004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Willox J.C., Corr J., Shaw J., Richardson M., Calman K.C., Drennan M. Prednisolone as an appetite stimulant in patients with cancer. Br Med J. 1984;288:27. doi: 10.1136/bmj.288.6410.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reid J., Hughes C.M., Murray L.J., Parsons C., Cantwell M.M. Non-steroidal anti-inflammatory drugs for the treatment of cancer cachexia: a systematic review. Palliat Med. 2013;27:295–303. doi: 10.1177/0269216312441382. [DOI] [PubMed] [Google Scholar]
- 83.Temel J.S., Abernethy A.P., Currow D.C., Friend J., Duus E.M., Yan Y. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519–531. doi: 10.1016/S1470-2045(15)00558-6. [DOI] [PubMed] [Google Scholar]
- 84.Crawford J., Johnston M.A., Taylor R.P., Dalton J.T., Steiner M.S. Enobosarm and lean body mass in patients with non-small cell lung cancer. J Clin Orthod. 2014;32:9618. [Google Scholar]