Abstract
Objective:
To assess whether children with autoimmune cytopenias prior to or at diagnosis of systemic lupus erythematosus (cSLE), differ phenotypically from other cSLE patients; and have a lower risk and severity of lupus nephritis (LN) as observed in prior adult studies. To assess the effect of prior immune therapy for autoimmune cytopenias on 2-year risk of LN.
Methods:
This was a retrospective cohort study of incident cSLE cases. We included patients aged less than 17 years at diagnosis. We excluded patients with LN at cSLE diagnosis. Our follow-up period was 2 years. We defined autoimmune cytopenias as either autoimmune hemolytic anemia, immune thrombocytopenia or Evan’s syndrome.
Results:
Forty-three (33%) of the 130 patients had autoimmune cytopenias before or at cSLE diagnosis. Those with autoimmune cytopenias had significantly more neuropsychiatric symptoms and higher mean ESR but less arthritis, malar rash and myositis versus those without autoimmune cytopenias. They had lower 2-year incidence proportion of LN compared to other cSLE patients (7% vs 15%). Of the 16 patients who developed LN, those with autoimmune cytopenias had mostly class V (2 of 3 patients) versus mostly class III and IV in those without autoimmune cytopenias (6 of 12 patients). None of the 13 patients pre-treated for autoimmune cytopenias developed LN.
Conclusion:
Patients with autoimmune cytopenias before or at cSLE diagnosis have intriguing differences from other cSLE patients. They may represent a unique sub-type of cSLE patients and should be further explored.
Keywords: Systemic Lupus Erythematosus, Pediatrics, Nephritis, Anemia, Thrombocytopenia
INTRODUCTION
Autoimmune cytopenias including autoimmune hemolytic anemia (AIHA), idiopathic thrombocytopenic purpura (ITP) and Evans syndrome (ES) may precede or be present at the time of diagnosis of childhood-onset systemic lupus erythematosus (cSLE) 1–4. Surprisingly, studies suggest that adult patients with SLE and coexisting autoimmune cytopenias have lower rates of lupus nephritis (LN) compared to those patients without autoimmune cytopenias and may therefore represent a unique sub-population 5–7. This observation is unexplained and has not been well-studied in children.
Some patients with idiopathic autoimmune cytopenia, undergo immunosuppressive or immunomodulatory therapy similar to cSLE treatment. Mouse models suggest that early immune modifying therapy can impact SLE phenotype by preventing development of endothelial dysfunction and reducing progression of nephritis 8, 9. Despite advances in therapeutics, outcomes for LN, especially in cSLE, are still sub-optimal 2, 10, 11. It is unclear if coexisting autoimmune cytopenia is associated with a better clinical outcome in cSLE 12–16.
We conducted a retrospective cohort study comparing cSLE patients with preceding or co-existing autoimmune cytopenias at diagnosis to other cSLE patients. We hypothesized that patients with autoimmune cytopenias will have decreased 2-year risk and severity of LN compared to those without autoimmune cytopenia. We further hypothesized that receiving treatment for autoimmune cytopenia prior to cSLE diagnosis will decrease the 2-year risk of LN. Finally, as our study was conducted on one of the largest single-center cohorts of diverse cSLE patients in the United States, we performed a descriptive analysis of our population of pediatric patients without LN at cSLE diagnosis and assessed whether patient characteristics at diagnosis were associated with the 2-year risk of LN.
MATERIALS AND METHODS
Study design and setting
This was a retrospective cohort study of incident cSLE patients at the Emory Children’s Center/ Children’s Healthcare of Atlanta pediatric rheumatology service over a 16-year period. Approval of the study protocol with waiver of informed consent was obtained from the Children’s Healthcare of Atlanta Institutional Review Board (#16–114).
Characteristics of Study Population
We extracted patient data from electronic medical records and paper charts with ICD-9 or 10 codes corresponding to a diagnosis of SLE between January 1, 2000 and June 30, 2016. We included patients who were diagnosed at age less than 17 years and who met at least 4 of the 11 American College of Rheumatology (ACR) and/or at least 4 of the 17 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for SLE. For the SLICC criteria, this included at least 1 clinical and 1 immunologic criteria 17, 18. We excluded patients with a pre-existing diagnosis of cSLE who transferred care to our center and those with LN at time of cSLE diagnosis. Our follow-up period was 2 years from time of cSLE diagnosis.
Measurements
We defined time of cSLE diagnosis (baseline) as time of initial evaluation for cSLE by a pediatric rheumatologist at our institution. Variables defined at diagnosis included data at initial evaluation up to 1-month post cSLE diagnosis. Autoimmune cytopenia referred to AIHA, thrombocytopenia and/or ES. We defined the presence of autoimmune cytopenia as a preceding diagnosis of a primary autoimmune cytopenia and/or the presence of an autoimmune cytopenia at cSLE diagnosis and up to 1-month post cSLE diagnosis. We defined AIHA as hemoglobin ≤10 g/dl and positive direct Coombs. We defined ITP as thrombocytopenia <100,000/mm3 and ES as concurrent or sequential AIHA and ITP.
Demographic, clinical and laboratory data were obtained at baseline. We used age greater than 9 years as a proxy for puberty for both males and females. We defined positive ANA as titers ≥ 1:40 17, 18. We included the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) as a measure of overall cSLE activity 19. Neuropsychiatric symptoms were classified using ACR nomenclature and case definitions 20. Renal parameters (urinalysis, urine microscopy, urine protein to creatinine ratio (UPCr) and estimated glomerular filtration rate (eGFR)) were extracted at baseline and at LN diagnosis. UPCr and eGFR were analyzed as continuous variables. UPCr > 0.5mg/mg was sub-classified as a dichotomous outcome with ≥ 2mg/mg as nephrotic range proteinuria and < 2 mg/mg as sub-nephrotic proteinuria. eGFR was calculated using the modified Schwartz formula which is a validated measure for patients aged 2 to 18 years 21. eGFR < 90mL/min/1.73m2 was indicative of decreased renal function 22.
We defined LN as the presence of persistent UPCr > 0.5mg/mg, ≥ 3+ proteinuria and/or a renal biopsy demonstrating LN. Time of LN diagnosis was categorized as within the first 6 months from baseline, between > 6 months to ≤ 12 months from baseline or > 12 months to ≤ 24 months from baseline. LN classification was based on the 2003 International Society of Nephrology/Renal Pathology Society criteria for renal biopsy 23. We sub-classified the six broad classes into mild (Class I or II only) and severe renal disease (Class III, IV, V, VI or any combination of Class V with other classes). The use of immune-directed therapy prior to time of cSLE diagnosis was analyzed as a dichotomous variable for rituximab, cyclophosphamide, intravenous immunoglobulin (IVIG) and corticosteroids.
Statistical Analyses
We carried out descriptive statistics for all variables of interest including demographic, clinical, and laboratory variables. We summarized continuous variables as means and standard deviations and/or medians and interquartile ranges. We calculated counts and percentages for categorical variables. Differences in continuous variables were tested using two sample t-tests or Wilcoxon rank sum test for non-normal measures. Differences in categorical variables were tested using chi-square tests or Fisher’s exact test for expected counts less than 5. All analyses were performed in SAS v 9.4 (Cary, NC). We used a statistical significance level of p < 0.05.
Association of autoimmune cytopenia at baseline and 2-year LN risk
Among all cSLE patients, we compared characteristics between patients with autoimmune cytopenia at baseline to other cSLE patients. Univariate and multivariable logistic regression models were developed with the outcome of LN to identify the independent and adjusted association between the presence of autoimmune cytopenias and LN. Variables with univariate associations with incident LN showing p-values < 0.2 as well as our covariates of interests (age at cSLE diagnosis, sex, ethnicity, race, the presence of anti-dsDNA, and prior use of immune-directed therapy) were included as candidate predictors in the initial model. Forward selection strategies were used to produce the final model using an a priori list of confounders.
Sensitivity Analysis
We planned a sensitivity analysis a priori to re-analyze our data excluding those patients with less than 2 years of follow-up to assess if the observed association of autoimmune cytopenia and 2-year LN risk still held.
RESULTS
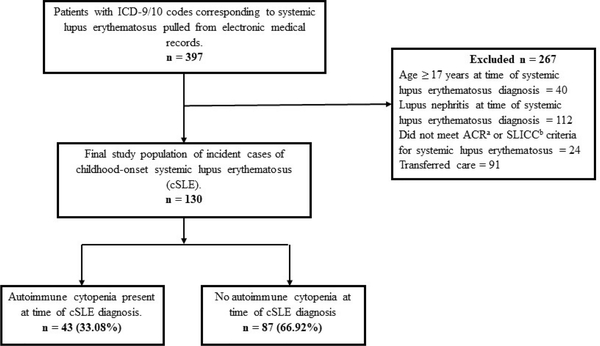
We identified 397 patients with ICD-9 or ICD-10 codes corresponding to SLE between January 1, 2000 and June 30, 2016 from our medical records. Our final study population had 130 incident cases of cSLE without LN at baseline (Figure 1).
Figure 1:
Flow diagram showing selection of study population
Abbreviations: aACR = American College of Rheumatology: bSLICC = Systemic Lupus International Collaborating Criteria for Systemic Lupus Erythematosus
Demographic, clinical and laboratory characteristics of final study population at baseline
Our final population was predominantly female. Table 1 summarizes the demographic, clinical and laboratory characteristics of 130 incident cSLE patients. We had a predominantly black population. There were also 23 (19%) non-Hispanic white, 7 (6%) Asian, 10 (8%) Hispanic and 1 (1%) mixed race/native American patients.
Table 1.
Demographic, clinical and laboratory characteristics of incident patients with childhood-onset systemic lupus erythematosus from January 1, 2000 to June 30, 2016.
| All cSLE (n=130) N (%) | cSLE with AC (n=43 ) N (%) | cSLE without AC (n=87) N (%) | P-value | Missing | |
|---|---|---|---|---|---|
| Demographics | |||||
| Sex | |||||
| Female | 107 (82) | 35/43 (81) | 72/87 (83) | 0.848 | |
| Race | 0.620 | 8 | |||
| Black | 81 (66) | 26/41 (63) | 55/81 (68) | ||
| Other | 41 (34) | 15/41 (37) | 26/81 (32) | ||
| Age in years at cSLE diagnosis (mean (SD))c | 12 (3) | 13 (3) | 12 (3) | 0.321 | |
| Clinical features | |||||
| Fever | 54 (42) | 21/42 (50) | 33/87 (38) | 0.193 | 1 |
| Malar rash | 47 (36) | 10/42 (24) | 37/87 (43) | 0.038S | 1 |
| Photosensitivity | 47 (36) | 13/42 (33) | 34/87 (39) | 0.369 | 1 |
| Discoid lupusa | 3 (2) | 1/42 (2) | 2/87 (2) | 1.000 | 1 |
| Vasculitic rash | 33 (26) | 13/42 (31) | 20/87 (23) | 0.331 | 1 |
| Raynaudsa | 14 (11) | 5/42 (12) | 9/87 (10) | 0.770 | 1 |
| Oral ulcers | 31 (24) | 9/42 (21) | 22/87 (25) | 0.631 | 1 |
| Nasal ulcersa | 4 (3) | 2/42 (5) | 2/87 (2) | 0.596 | 1 |
| Alopecia | 21 (16) | 4/42 (10) | 17/87 (20) | 0.149 | 1 |
| Arthritis | 64 (50) | 13/42 (31) | 51/86 (60) | 0.003S | 2 |
| Angioedemaa | 8 (6) | 2/41 (5) | 6/86 (7) | 1.000 | 3 |
| Pleural effusion | 26 (31) | 12/32 (38) | 14/53 (26) | 0.283 | 45 |
| Pericardial effusion | 17 (32) | 7/19 (37) | 10/34 (29) | 0.578 | 77 |
| Neuropsychiatric symptomsa | 8 (6) | 6/43 (14) | 2/87 (2) | 0.016S | |
| Myositis* | 20 (29) | 2/20 (10) | 18/49 (37) | 0.026S | 61 |
| Laboratory features | |||||
| Positive ANA ≥ 1:40 | 130 (100) | 43 (100) | 87 (100) | ||
| Positive anti-dsDNA ≥ 1:10 | 88 (70) | 31/43 (72) | 57/83 (69) | 0.692 | 4 |
| Positive anti-RNP* | 72 (58) | 24/43 (56) | 48/81 (59) | 0.711 | 6 |
| Positive anti-Smith* | 71 (57) | 22/43 (51) | 49/81 (61) | 0.318 | 6 |
| Positive anti-SSA* | 56 (45) | 20/43 (47) | 36/81 (44) | 0.826 | 6 |
| Positive anti-SSB* | 20 (16) | 10/43 (23) | 10/80 (13) | 0.123 | 7 |
| Leukopenia ≤ 4,000/uL | 59 (46) | 21/43 (49) | 38/85 (45) | 0.658 | 2 |
| Lymphopenia ≤1,500/uL | 81 (66) | 29/42 (69) | 52/81 (64) | 0.591 | 7 |
| Neutropenia ≤1,500/uL | 32 (27) | 14/42 (33) | 18/79 (23) | 0.210 | 9 |
| Low C3 complement* | 63 (53) | 25/41 (61) | 38/79 (48) | 0.180 | 10 |
| Low C4 complement* | 78 (65) | 29/41 (71) | 49/79 (62) | 0.343 | 10 |
| ESR in mm/hr (mean(SD)) | 63 (41) | 80 (45) | 55 (36) | 0.003S | 19 |
| eGFR < 90mL/min/1.73m2 | 32 (29) | 14/39 (36) | 18/73 (25) | 0.210 | 18 |
| SLEDAI-2k (median(IQR,range) | 9 (7 – 12) | 9 (6 – 11) | 10 (7 – 12) | 0.655 | 11 |
| Prior Treatment | |||||
| Corticosteroidsa | 10 (8) | 10/43 (23) | 0/87 (0) | <0.001S | |
| Cyclophosphamide | 0 (0) | 0/43 (0) | 0/87 (0) | ||
| IVIGa | 7 (5) | 7/43 (16) | 0/87 (0) | <0.001S | |
| Rituximaba | 2 (2) | 2/43 (5) | 0/87 (0) | 0.108 | |
| 2 year risk of lupus nephritis after cSLE diagnosis | 16 (12) | 3/43 (7) | 13/87 (15) | 0.190 | |
| Follow-up period in years (mean(SD)) | 4.03 (2) | 3.96 (2) | 4.16 (2) | 0.641 |
Abbreviations: AC = Autoimmune cytopenias; cSLE = childhood-onset systemic lupus erythematosus; dsDNA = double-stranded DNA; ESR = Erythrocyte sedimentation rate; eGFR = Estimated glomerular filtration rate; IVIG = intravenous immunoglobulin; RNP = ribonucleoprotein; SLEDAI-2k = Systemic Lupus Erythematosus Disease Activity Index 2000; SS = Sjogren syndrome-related antigen
= significant with p value < 0.05
By laboratory reference range, Comparisons were by chi square test except otherwise stated
Fisher’s exact test
Wilcoxon rank sum test
Student’s t-test
The 43 patients with autoimmune cytopenia included 13 patients who were pretreated for autoimmune cytopenia (8 for ITP, 4 for AIHA and 1 with ES).
Comparison of final study population at baseline by autoimmune cytopenia status
As shown in Table 1, there was no significant difference in sex, race or mean age at baseline comparing those with autoimmune cytopenia to those without. When we compared differences in serologic markers, there was no meaningful difference in the frequency of positive anti-dsDNA antibodies between those with autoimmune cytopenia and those without. Patients with autoimmune cytopenia had a higher frequency of elevated erythrocyte sedimentation rate (45% versus 36% p=0.003). There were no statistically significant differences in eGFR, SLEDAI-2K or low C3 and C4 complements.
Comparison of final study population at baseline by race
When we compared black patients versus patients of other races, females were still the predominant sex in both groups and there was no statistically significant difference in mean age at baseline. Black patients had fewer oral ulcers (16% versus 34% p=0.025) but higher frequency of positive anti-RNP (67% versus 39% p=0.003), and anti-Smith (70% versus 33% p= <0.001) antibodies.
Comparison of final study population at baseline by age and by sex
In our study, 15 of 129 children were aged less than 9 years. Mean age of patients less than 9 years at baseline was 7 (SD 1) and 13 (SD 2) in patients 9 years or older. The younger patients had a higher frequency of fever than the older patients (73% versus 38% p=0.009). There were no statistically significant differences in eGFR or SLEDAI-2K. There were also no statistically significant differences in race, age, clinical or laboratory features comparing females to males.
Association of autoimmune cytopenia at baseline and 2-year LN risk
The 2-year incidence proportion of LN was 12% in our study population. As shown in Table 1, the 2-year risk of LN was lower in patients with autoimmune cytopenia (7%) compared to those without autoimmune cytopenias (15%), but this difference was not statistically significant.
As shown in Table 2, in our univariate analysis, examining the association of patient characteristics with incident LN, the odds of developing LN in patients with autoimmune cytopenia at baseline were 0.43 lower than the odds of developing LN in those without autoimmune cytopenia at baseline ( 95% CI 0.12, 1.60, p=0.204). The odds of developing LN in patients with low C3 at baseline were 3.81 times higher than the odds of developing LN in those without low C3 at baseline (95% CI 1.01, 14.42, p=0.049).
Table 2:
Univariate logistic regression analysis examining the association of patient characteristics with incident lupus nephritis (in the 130 cSLE patients without lupus nephritis at baseline).
| Characteristics | Odds ratio | 95% CI for Odds ratio | P-value |
|---|---|---|---|
| Presence of autoimmune cytopenia at cSLE diagnosis | 0.43 | 0.12, 1.60 | 0.204 |
| Age at cSLE diagnosis (in years) | 0.99 | 0.83, 1.18 | 0.888 |
| Sex (female versus male) | 0.92 | 0.24, 3.54 | 0.906 |
| Race (Black versus other) | 2.20 | 0.59, 8.29 | 0.243 |
| Arthritis | 0.56 | 0.19, 1.64 | 0.290 |
| Myositis* | 3.07 | 0.85, 11.07 | 0.086 |
| Positive anti-double-stranded DNA* | 1.84 | 0.49, 6.94 | 0.367 |
| Positive anti-ribonucleoprotein* | 1.52 | 0.49, 4.73 | 0.474 |
| Positive anti-Smith* | 2.25 | 0.67, 7.49 | 0.188 |
| Positive anti-SSA* | 1.98 | 0.66, 5.95 | 0.744 |
| Positive anti-SSB* | 0.77 | 0.16, 3.71 | 0.224 |
| low C3 complement at cSLE diagnosis* | 3.81 | 1.01, 14.42 | 0.049S |
| eGFR < 90mL/min/1.73m2 at cSLE diagnosis | 2.92 | 0.93, 9.15 | 0.066 |
| SLEDAI-2K at cSLE diagnosis (per 1 point in score) | 0.98 | 0.86, 1.11 | 0.740 |
Abbreviations: cSLE = childhood-onset systemic lupus erythematosus; ESR = Erythrocyte sedimentation rate; eGFR = Estimated glomerular filtration rate; SLEDAI-2k = Systemic Lupus Erythematosus Disease Activity Index 2000; SS = Sjogren syndrome-related antigen
= significant with p value < 0.05
By laboratory reference range
Autoimmune cytopenia at baseline and low C3 at baseline were included in our final multivariable logistic regression model. We lost 8% of patients when we used complete case analysis following list-wise deletion. After adjusting for the presence of autoimmune cytopenia at baseline, the odds of developing LN in low C3 patients at baseline were 4.24 times higher than the odds of developing LN in those without low C3 at baseline (95% CI 1.10, 16.34, p= 0.036).
Association of autoimmune cytopenia at baseline and LN severity
Table 3 summarizes the characteristics of the 16 patients who developed lupus nephritis within the 2-year follow-up period. At baseline, 3 of these 16 patients had autoimmune cytopenia. When we compared these patients to those without autoimmune cytopenia at baseline, we found no statistically significant difference in age, baseline eGFR or SLEDAI-2K (all p> 0.05). We also found no statistically significant difference in eGFR or UPCr at the time of LN diagnosis (all p> 0.05). Two of the 3 patients with autoimmune cytopenias had isolated class V LN; those without autoimmune cytopenia who had only 1 case of isolated class V LN.
Table 3.
Comparing characteristics of patients who developed lupus nephritis within the first 2 years of diagnosis by baseline autoimmune cytopenia status.
| Characteristics | All cSLE* | cSLE with AC | cSLE without AC* |
|---|---|---|---|
| (n = 16) | (n = 3) | (n =13) | |
| N (%) | N (%) | N (%) | |
| LN by classa | |||
| II | 3/15 (20) | 0/3 (0) | 3/12 (25) |
| III | 2/15 (13) | 0/3 (0) | 2/12 (17) |
| IV | 5/15 (33) | 1/3 (33) | 4/12 (33) |
| V | 3/15 (20) | 2/3 (67) | 1/12 (8) |
| IV/V | 2/15 (13) | 0/3 (0) | 2/12 (17) |
| LN severitya | |||
| Mild i.e. Class I and II only | 3/15 (20) | 0/3 (0) | 3/12 (25) |
| Renal characteristics at time of LN diagnosis | |||
| eGFR < 90mL/min/1.73m2a | 6/15 (40) | 1/3 (33) | 5/12 (42) |
| UPCr ≥2 mg/mga | 10/16 (63) | 3/3 (100) | 7/13 (54) |
| RBC casts at time of LN diagnosisa | 1/16 (6) | 0/13 (0) | 1/13 (8) |
| Hematuria >5RBC/hpfa | 12/16 (75) | 1/3 (33) | 11/13 (85) |
| Interval of LN from time of cSLE diagnosis in months | |||
| >1 to ≤ 6 | 6/16 (38) | 1/3 (33) | 5/13 (39) |
| > 6 to ≤ 12 | 3/16 (19) | 0/3 (0) | 3/13 (23) |
| > 12 to ≤ 24 | 7/16 (44) | 2/3 (67) | 5/13 (39) |
Abbreviations: AC = Autoimmune cytopenia; cSLE = Childhood-onset systemic lupus erythematosus; eGFR= Estimated glomerular filtration rate; LN= Lupus nephritis; UPCr = Urine protein to creatinine ratio
Fisher’s exact test
Missing 1 patient without kidney biopsy
Association of immune-directed treatment for autoimmune cytopenia prior to cSLE diagnosis and 2-year LN risk
Table 1 summarizes the prior immune-directed treatment received. Mean interval from rituximab administration to cSLE diagnosis was 6 months (SD 5.30). Median interval from IVIG administration to cSLE diagnosis was 4 months (IQR 51). Corticosteroids were mostly administered with rituximab or IVIG. None of the 13 patients who had prior immune-directed treatment for autoimmune cytopenia developed LN. Of the 30 patients who had autoimmune cytopenia but did not receive prior immune-directed treatment, 3 (10%) developed LN. Of the 87 other cSLE patients without autoimmune cytopenia at baseline, 13 (15%) developed LN. However, the difference in risk of LN among these three groups of patients was not statistically significant (p = 0.41).
Sensitivity analysis
When we re-analyzed our data excluding those patients with less than 2-year follow-up, the 2-year risk of LN was 8% in patients with autoimmune cytopenia compared to 15% in those without autoimmune cytopenia (p=0.036).
DISCUSSION
Our primary objective was to compare cSLE patients with autoimmune cytopenias to those without autoimmune cytopenias. Overall, our study found clinically relevant differences to support prior adult studies that SLE patients with autoimmune cytopenias may be a distinct sub-population from other patients with SLE 5–7.
We examined the first 2 years after cSLE diagnosis because this is when LN is more likely to develop. The relatively lower 2-year risk of LN we found in cSLE patients with autoimmune cytopenias is similar to earlier reports from adult studies that showed lower incidence and prevalence of LN in this subset of patients. We did not find that the presence of anti-dsDNA, or other commonly tested serologic markers, at the time of cSLE diagnosis were associated with LN risk. While 29% of our study population had low eGFRs at cSLE diagnosis, and low C3 at cSLE diagnosis was associated with an increased 2-year risk of LN, we did not find differences in eGFR or low C3 between patients with autoimmune cytopenias and those without autoimmune cytopenias to suggest that these factors are associated with their different LN risk. Similarly, we did not find a difference in SLEDAI-2K index or low C4 at cSLE diagnosis between the two subgroups to explain the difference in LN risk. However, we noted that cSLE patients with autoimmune cytopenias had more neuropsychiatric disease but less arthritis, malar rash and myositis than those without autoimmune cytopenias. Erythrocyte sedimentation rate (ESR) is a non-specific marker of ongoing systemic inflammation and was higher in the patients with autoimmune cytopenias compared to the other patients. It is known that ESR may be elevated in the presence of anemia which likely contributed to this observation here since at the time of cSLE diagnosis, the SLEDAI-2K, which is a measure of overall disease activity, was similar between the two groups.
Prior reports showed that adult patients with autoimmune cytopenias had less severe renal involvement 5, 7. Our study showed that of the 16 cSLE patients who developed lupus nephritis, those with autoimmune cytopenias at cSLE diagnosis had mostly isolated Class V, while those without autoimmune cytopenias, had mostly Class III and IV LN. While our inference is limited by the small number of patients who developed LN, this observation should be further explored as Class III and IV LN are associated with a higher risk of progression to end-stage renal disease. More so, there is evidence to suggest that pathologic mechanism in Class III and IV LN may differ from pure Class V LN 24.
We examined whether some of the decreased risk of LN in autoimmune cytopenia could be attributed to prior immune-directed treatment. Interestingly, our study showed that none of the 13 cSLE patients with autoimmune cytopenia who received immune-directed treatment prior to cSLE diagnosis developed LN. In comparison, the 2-year risks of LN for those with autoimmune cytopenia and cSLE who did not receive pre-treatment and for cSLE patients without any autoimmune cytopenia were 10% and 15%, respectively. The absence of LN in cSLE patients with autoimmune cytopenia who were pre-treated, suggests that early treatment may play a role in preventing development of LN and warrants further investigation.
Our results add to the growing discussion on phenotypic heterogeneity in cSLE 25. While larger studies are needed, we raise important considerations regarding how disease drivers in cSLE patients with autoimmune cytopenias may differ from those without autoimmune cytopenias, and the impact of such mechanism on LN. Our focus on red blood cell and platelet cytopenias is relevant as both of these cellular subtypes are actively involved in the systemic inflammation that occurs in cSLE 26–31. Recent blood transcriptome analyses of cSLE patients found a strong correlation between neutrophil signature and the presence of LN 24. Similarly, another gene expression study of adult and pediatric SLE patients found neutrophil-driven clusters to be associated with an increased risk of proliferative LN 32. However, the association of autoimmune cytopenias with these molecular stratifications, and particularly the risk of LN, has been under explored.
In our study, we were uniquely able to describe a population of 130 patients without LN at cSLE diagnosis. Two-thirds (66 %) of our patients were black. Our population had a mean age at cSLE diagnosis of 12 years, which is similar previous reports 33, 34. Our female: male ratio of 4.65:1 was similar to reports from the large cSLE cohort studies from France and Toronto 34, 35. Also, arthritis, fever and malar rash were the most commonly occurring clinical features. Nasal ulcers and discoid rash were uncommon. Black patients had statistically significant higher frequency of positive anti-RNP and anti-Smith antibodies, as has been previously reported 36–39. However, we did not find higher positive anti-SSA/SSB antibodies as reported by others 33. We did not find many differences in cSLE manifestation by age, though younger children had more fever. It is thought that factors such as younger age and age-related physiologic changes in the kidney may independently contribute to increased risk of LN. However, there was no difference in the 2-year risk of LN or decreased eGFR at cSLE diagnosis in our study when we compared the two age groups. We also found no statistically significant sex differences in clinical and laboratory features or 2-year risk of LN. Interestingly, 29% of our patients presented with some renal insufficiency though they did not have persistent proteinuria to suggest LN. This renal insufficiency did not differ by autoimmune cytopenia status, age at cSLE diagnosis, race or sex.
A major strength of our study is that it was conducted on a predominantly black pediatric lupus population. We were able to examine age, sex and racial/ethnic differences and we approached renal outcomes in cSLE from a hematologic perspective. To the best of our knowledge, we are the first to examine the association of pre-treatment with immune-directed therapy for autoimmune cytopenia on renal outcomes within the first 2 years of cSLE diagnosis.
Our study has some limitations. While it was a relatively large single-center study on cSLE, we had a low 2-year incidence proportion of LN and so our study was underpowered to detect statistically significant differences in risk and severity of LN and prior therapy effects. Also, it was a retrospective study and we were limited to using the available data. Our study spanned a 16-year period during which there were differences in physician practice in obtaining laboratory and imaging data to evaluate for cSLE. A single center study may limit generalizability. However, there are no other pediatric rheumatology or nephrology practices in the Atlanta metropolitan statistical area and thus most cSLE patients are seen at our practice. Therefore, we have a representative sample population. Our center does not routinely perform activity or chronicity scoring of kidney biopsies which would have added more information about LN severity. Finally, we had a short follow-up period of 2 years, but more than 80% of LN develop within the first 2 years of cSLE diagnosis 2.
In conclusion, our findings indicate that patients with autoimmune cytopenia before or at cSLE diagnoses have statistically significant and clinically relevant differences from other cSLE patients. Our study highlights the need for further studies to understand the inherent and external factors contributing to these differences, and understand the impact of prior exposure to immune suppressing therapy on cSLE phenotype.
Acknowledgments
Funding: Dr. Ogbu was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 and TL1TR002382. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Prahalad is supported in part by a grant from the Marcus Foundation Inc., Atlanta.
Footnotes
Conflicts of interest: None
DATA ACCESSIBILTY STATEMENT
The data analyzed for this study is available from the corresponding author on reasonable request.
REFERENCES
- 1.Gormezano NW, Kern D, Pereira OL, et al. Autoimmune hemolytic anemia in systemic lupus erythematosus at diagnosis: differences between pediatric and adult patients. Lupus 2017; 26: 426–430. 2016/11/09. DOI: 10.1177/0961203316676379. [DOI] [PubMed] [Google Scholar]
- 2.Hafeez F, Tarar AM and Saleem R. Lupus nephritis in children. Journal of the College of Physicians and Surgeons--Pakistan: JCPSP 2008; 18: 17–21. 2008/05/03. DOI: 01.2008/jcpsp.1721. [PubMed] [Google Scholar]
- 3.Hazzan R, Mukamel M, Yacobovich J, et al. Risk factors for future development of systemic lupus erythematosus in children with idiopathic thrombocytopenic purpura. Pediatric blood & cancer 2006; 47: 657–659. 2006/08/26. DOI: 10.1002/pbc.20970. [DOI] [PubMed] [Google Scholar]
- 4.Kokori SI, Ioannidis JP, Voulgarelis M, et al. Autoimmune hemolytic anemia in patients with systemic lupus erythematosus. The American journal of medicine 2000; 108: 198–204. 2000/03/21. [DOI] [PubMed] [Google Scholar]
- 5.Alger M, Alarcon-Segovia D and Rivero SJ. Hemolytic anemia and thrombocytopenic purpura: two related subsets of systemic lupus erythematosus. The Journal of rheumatology 1977; 4: 351–357. 1977/01/01. [PubMed] [Google Scholar]
- 6.Zhang L, Wu X, Wang L, et al. Clinical Features of Systemic Lupus Erythematosus Patients Complicated With Evans Syndrome: A Case-Control, Single Center Study. Medicine 2016; 95: e3279 2016/04/16. DOI: 10.1097/md.0000000000003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavalle C, Hurtado R, Quezada JJ, et al. Hemocytopenia as initial manifestation of systemic lupus erythematosus. Prognostic significance. Clinical rheumatology 1983; 2: 227–232. 1983/09/01. [DOI] [PubMed] [Google Scholar]
- 8.Bekar KW, Owen T, Dunn R, et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis and rheumatism 2010; 62: 2443–2457. 2010/05/28. DOI: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virdis A, Tani C, Duranti E, et al. Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Arthritis research & therapy 2015; 17: 277 2015/10/09. DOI: 10.1186/s13075-015-0790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vachvanichsanong P and McNeil E. Pediatric lupus nephritis: more options, more chances? Lupus 2013; 22: 545–553. 2013/05/01. DOI: 10.1177/0961203313485490. [DOI] [PubMed] [Google Scholar]
- 11.Wenderfer SE, Ruth NM and Brunner HI. Advances in the care of children with lupus nephritis. Pediatric research 2017; 81: 406–414. 2016/11/18. DOI: 10.1038/pr.2016.247. [DOI] [PubMed] [Google Scholar]
- 12.Aladjidi N, Fernandes H, Leblanc T, et al. Evans Syndrome in Children: Long-Term Outcome in a Prospective French National Observational Cohort. Frontiers in pediatrics 2015; 3: 79 2015/10/21. DOI: 10.3389/fped.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarr T, Derfalvi B, Gyori N, et al. Similarities and differences between pediatric and adult patients with systemic lupus erythematosus. Lupus 2015; 24: 796–803. 2014/12/18. DOI: 10.1177/0961203314563817. [DOI] [PubMed] [Google Scholar]
- 14.Nossent JC and Swaak AJ. Prevalence and significance of haematological abnormalities in patients with systemic lupus erythematosus. The Quarterly journal of medicine 1991; 80: 605–612. 1991/07/01. [PubMed] [Google Scholar]
- 15.Costallat GL, Appenzeller S and Costallat LT. Evans syndrome and systemic lupus erythematosus: clinical presentation and outcome. Joint, bone, spine : revue du rhumatisme 2012; 79: 362–364. 2011/09/29. DOI: 10.1016/j.jbspin.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Aleem A, Al Arfaj AS, khalil N, et al. Haematological abnormalities in systemic lupus erythematosus. Acta reumatologica portuguesa 2014; 39: 236–241. 2014/05/28. [PubMed] [Google Scholar]
- 17.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis and rheumatism 2012; 64: 2677–2686. 2012/05/04. DOI: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism 1982; 25: 1271–1277. 1982/11/01. [DOI] [PubMed] [Google Scholar]
- 19.Uribe AG, Vila LM, McGwin G Jr., et al. The Systemic Lupus Activity Measure-revised, the Mexican Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. The Journal of rheumatology 2004; 31: 1934–1940. 2004/10/07. [PubMed] [Google Scholar]
- 20.Liang MH, Corzillius M, Bae SC, et al. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis and rheumatism 1999; 42: 599–608. 1999/04/22. DOI: . [DOI] [PubMed] [Google Scholar]
- 21.Selistre L, De Souza V, Cochat P, et al. GFR estimation in adolescents and young adults. Journal of the American Society of Nephrology: JASN 2012; 23: 989–996. 2012/04/14. DOI: 10.1681/asn.2011070705. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–266. 2002/03/21. [PubMed] [Google Scholar]
- 23.Chow TK, Looi LM and Cheah PL. A comparison of 1995 WHO classification with 2003 ISN/RPS classification of lupus nephritis: a single centre observation. The Malaysian journal of pathology 2015; 37: 239–246. 2015/12/30. [PubMed] [Google Scholar]
- 24.Banchereau R, Hong S, Cantarel B, et al. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell 2016; 165: 551–565. 2016/04/05. DOI: 10.1016/j.cell.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alarcon-Riquelme ME. New Attempts to Define and Clarify Lupus. Current rheumatology reports 2019; 21: 11 2019/02/27. DOI: 10.1007/s11926-019-0810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alegretti AP, Mucenic T, Merzoni J, et al. Expression of CD55 and CD59 on peripheral blood cells from systemic lupus erythematosus (SLE) patients. Cell Immunol 2010; 265: 127–132. 2010/08/24. DOI: 10.1016/j.cellimm.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Brilland B, Scherlinger M, Khoryati L, et al. Platelets and IgE: Shaping the Innate Immune Response in Systemic Lupus Erythematosus. Clinical reviews in allergy & immunology 2019. 2019/06/30. DOI: 10.1007/s12016-019-08744-x. [DOI] [PubMed] [Google Scholar]
- 28.Linge P, Fortin PR, Lood C, et al. The non-haemostatic role of platelets in systemic lupus erythematosus. Nature reviews Rheumatology 2018; 14: 195–213. 2018/03/22. DOI: 10.1038/nrrheum.2018.38. [DOI] [PubMed] [Google Scholar]
- 29.Inada Y, Kamiyama M, Kanemitsu T, et al. Relationships between C3b receptor (CR1) activity of erythrocytes and positive Coombs’ tests. Annals of the rheumatic diseases 1986; 45: 367–372. 1986/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katyal M, Tiwari SC, Kumar A, et al. Association of complement receptor 1 (CR1, CD35, C3b/C4b receptor) density polymorphism with glomerulonephritis in Indian subjects. Molecular immunology 2004; 40: 1325–1332. 2004/04/10. DOI: 10.1016/j.molimm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Kavai M Immune complex clearance by complement receptor type 1 in SLE. Autoimmunity reviews 2008; 8: 160–164. 2008/07/08. DOI: 10.1016/j.autrev.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Toro-Dominguez D, Martorell-Marugan J, Goldman D, et al. Stratification of Systemic Lupus Erythematosus Patients Into Three Groups of Disease Activity Progression According to Longitudinal Gene Expression. Arthritis & rheumatology (Hoboken, NJ) 2018; 70: 2025–2035. 2018/06/26. DOI: 10.1002/art.40653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gedalia A, Molina JF, Molina J, et al. Childhood-onset systemic lupus erythematosus: a comparative study of African Americans and Latin Americans. Journal of the National Medical Association 1999; 91: 497–501. 1999/10/12. [PMC free article] [PubMed] [Google Scholar]
- 34.Bader-Meunier B, Armengaud JB, Haddad E, et al. Initial presentation of childhood-onset systemic lupus erythematosus: a French multicenter study. The Journal of pediatrics 2005; 146: 648–653. 2005/05/05. DOI: 10.1016/j.jpeds.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 35.Hiraki LT, Benseler SM, Tyrrell PN, et al. Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: a longitudinal study. The Journal of pediatrics 2008; 152: 550–556. 2008/03/19. DOI: 10.1016/j.jpeds.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Petri M, Perez-Gutthann S, Longenecker JC, et al. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. The American journal of medicine 1991; 91: 345–353. 1991/10/01. [DOI] [PubMed] [Google Scholar]
- 37.Arnett FC, Hamilton RG, Roebber MG, et al. Increased frequencies of Sm and nRNP autoantibodies in American blacks compared to whites with systemic lupus erythematosus. The Journal of rheumatology 1988; 15: 1773–1776. 1988/12/01. [PubMed] [Google Scholar]
- 38.Gulko PS, Reveille JD, Koopman WJ, et al. Survival impact of autoantibodies in systemic lupus erythematosus. The Journal of rheumatology 1994; 21: 224–228. 1994/02/01. [PubMed] [Google Scholar]
- 39.Barron KS, Silverman ED, Gonzales J, et al. Clinical, serologic, and immunogenetic studies in childhood-onset systemic lupus erythematosus. Arthritis and rheumatism 1993; 36: 348–354. 1993/03/01. [DOI] [PubMed] [Google Scholar]