Abstract
Bariatric and metabolic surgery (BMS) is the most effective treatment for obesity, type 2 diabetes (T2D) and comorbidities, including nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). The beneficial effects of BMS are beyond the primary goal of gastric restriction and nutrients malabsorption. Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are the two most commonly performed procedures of BMS. Both surgeries lead to physiological changes in gastrointestinal tract; subsequently alter bile acids pool and composition, gut microbial activities, gut hormones and circulating exosome; and ultimately contribute to the improved glycemic control, insulin sensitivity, lipid metabolism, energy expenditure, as well as weight loss. The mechanisms underlying the benefits of BMS likely involve the bile acids signaling pathway mediated mainly by nuclear farnesoid X receptor (FXR) and the membrane Takeda G protein-coupled receptor (TGR5), bile acids-gut microbiota interaction, and exosomes. In this review, we focus on recent advances in potential mechanisms and aim to learn novel insights into the molecular mechanisms underlying metabolic disorders.
Keywords: Bariatric and metabolic surgery, bile acids, FXR, TGR5, NAFLD, T2D
FXR and TGR5 differentially contribute to the metabolic improvement associated with RYGB and VSG.
1. Introduction
The global prevalence of obesity (Body mass index, BMI ≥30 kg/m2) was estimated to be 13% of adult population [1]. In the United States, 39.6% of adults were obese according to NHANES data from 2015–2016 [2]. The epidemic of obesity has led to a parallel increase in the prevalence of type 2 diabetes (T2D) and non-alcoholic fatty liver disease (NAFLD) [3, 4]. NAFLD is present in up to 75% of patients with overweight and in 90–95% of patients with grade 3 obesity [5]. Bariatric and metabolic surgery (BMS) has proven to be an effective and durable therapy for grade 3 obesity (BMI ≥40 kg/m2) as well as for patients with BMIs between 35–39.9 kg/m2 with poor glycemic control. The criteria for surgery have been expanded to include some patients with a BMI ≤ 35 kg/m2 [6, 7].
Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are the two most commonly performed procedures in BMS, and they comprise 76% of currently performed procedures [8]. Work from a recent study involving 1,156 patients with grade 3 obesity showed that total body weight loss achieved by RYGB was 35%, 28% and 26.9% at 2, 6 and 12 years post-procedure, respectively. Moreover, T2D was resolved in 75%, 62% and 51% of patients at 2, 6 and 12 years, respectively [7]. In addition, a recent meta-analysis demonstrated the efficacy of BMS in the treatment of NAFLD, as shown by a biopsy-confirmed resolution of steatosis, inflammation, ballooning degeneration, and fibrosis in 66%, 50%, 76%, and 40% of patients, respectively [9]. Moreover, both RYGB and VSG have similar effects on the attenuation of NAFLD regardless the potential different mechanisms [10, 11]. Importantly, BMS also resulted in new or worsened NAFLD in 12% of patients [9]. Currently, BMS is not recommended by American Association for the Study of Liver Diseases (AASLD) to specifically treat nonalcoholic steatohepatitis (NASH) due to the safety issue [12]; but it may be considered an option for obese patients (BMI ≥35 kg/m2) with one or more severe obesity-related complications (ORCs) remediable by weight loss, including NAFLD and NASH [8, 12].
Nonetheless, the mechanisms underlying the benefits of BMS are of great importance for understanding the pathogenesis of metabolic diseases. Although the primary goal of BMS was designed for gastric restriction and malabsorption to produce weight loss, a growing body of evidence indicates that the beneficial effects of BMS (improvement of hyperglycemia, insulin sensitivity, and hyperlipidemia as well as steatosis) are beyond weight loss [13–15]. Thus, understanding the underlying mechanisms is of significant importance. In this review, we focus on the recent advances in BMS, including altered physiology, mechanistic studies involving bile acid and bile acid receptors, gut microbiota, gut hormones, and exosome.
2. Physiology
VSG is now the most commonly performed procedure of BMS, and it reduces gastric volume by approximately 70–80% through removal of a large portion of stomach along the greater curvature (Figure 1) [16, 17]. By doing so, VSG removes ghrelin-producing cells in the stomach resulting in decreased circulating ghrelin, accelerated gastric emptying, and increased secretion of the intestinal hormones, glucagon-like polypeptide 1 (GLP-1) and peptide YY (PYY) [18]. A recent study compared several procedures with different gastric volume reductions with standard VSG in rats. They found that gastric volume was negatively correlated to gastric emptying rate, glucose, and GLP-1 response. Therefore, significant gastric volume reduction is required to achieve the goal of metabolic improvement [19].
Figure. 1. Vertical sleeve gastrectomy (VSG).
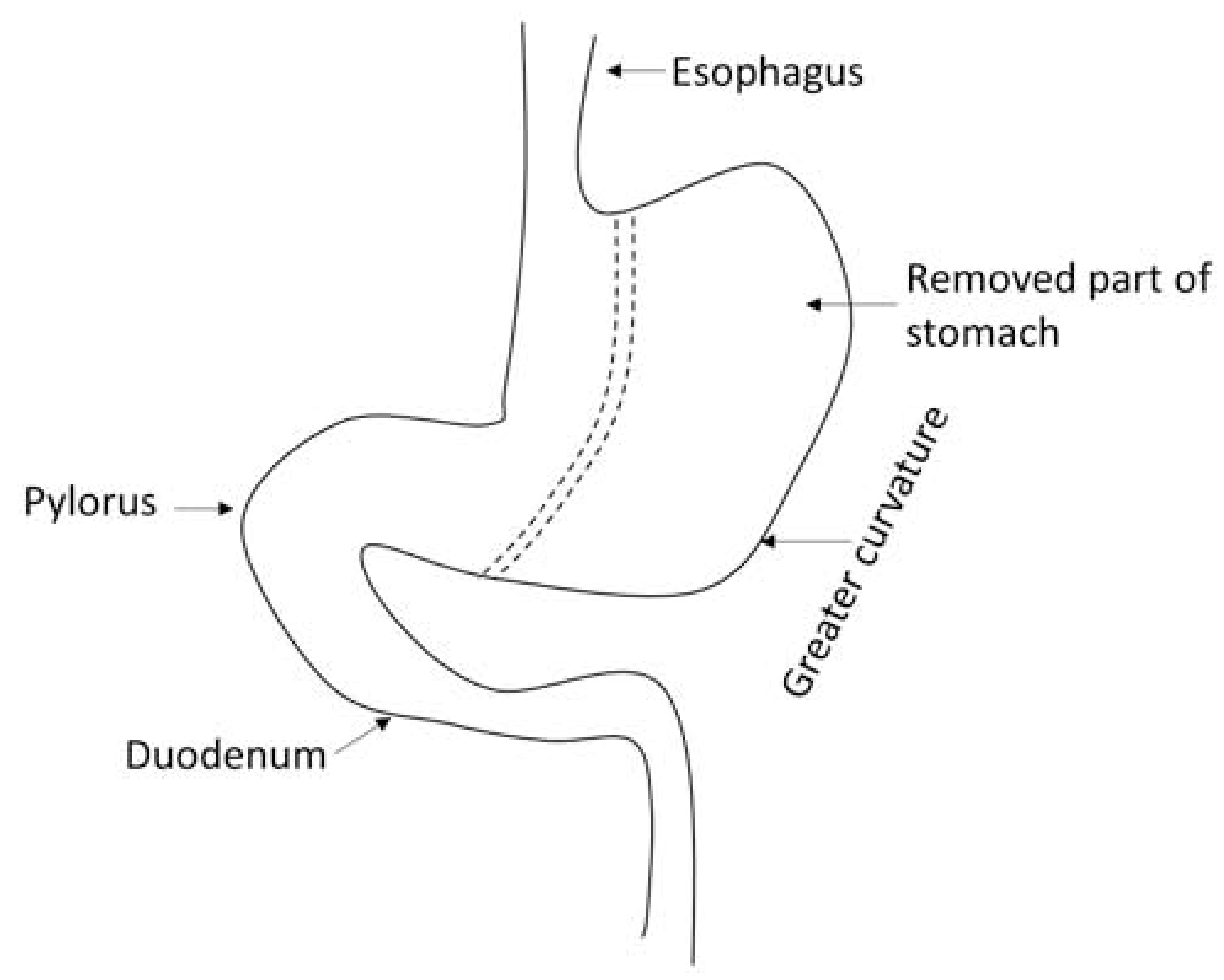
Schematic diagram of VSG. VSG creates a tube-like stomach with the majority (approximately 70–80%) of stomach is removed along the greater curvature. The dotted line denote where the excision is made (in between).
RYGB is the combination of gastric reduction with intestinal rearrangement, including generation of a small gastric pouch and bypass of the stomach and upper gastrointestinal tract, leading to accelerated nutrients flow to the middle jejunum (Figure 2) [16, 17]. While both RYGB and VSG lead to metabolic improvement, they change gut physiology in different ways. A recent clinical trial showed that RYGB and VSG differentially altered nutrient absorption and gut hormone secretion after 12 months. RYGB was associated with accelerated postprandial absorption of glucose and amino acids compared to controls, as shown by stable isotope tracers. However, altered amino acid absorption was not observed in patients after VSG. Moreover, gut hormones secretion rates, such as GLP-1, PYY and cholecystokinin (CCK), were enhanced after RYGB compared to VSG, highlighting the potentially different mechanisms underlying the metabolic benefits of these two procedures [20].
Figure. 2. Roux-en-Y Gastric Bypass (RYGB).
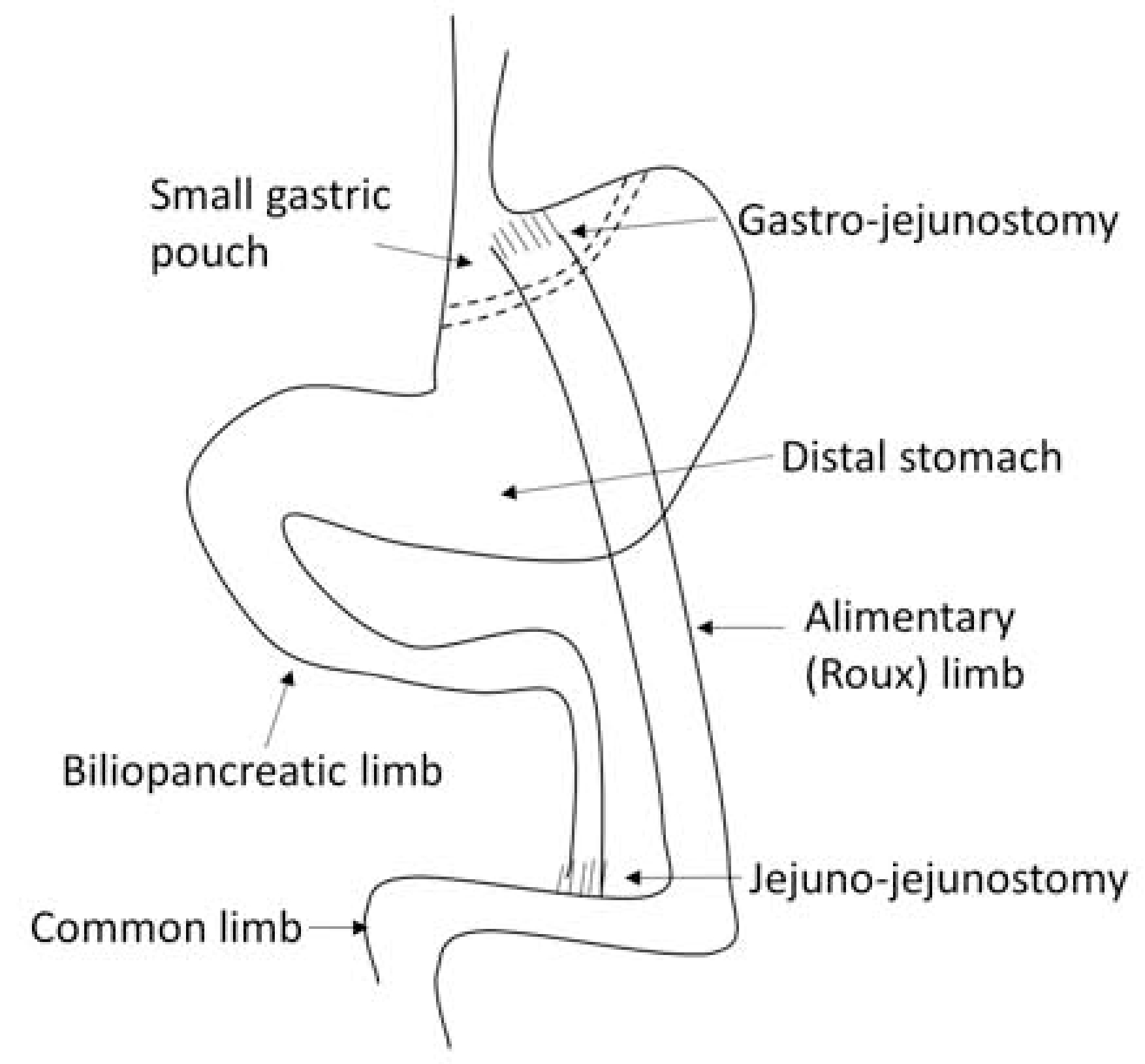
Schematic diagram of RYGB. The stomach is divided into a small gastric pouch and a distal stomach along the dotted lines. The jejunum is transected and the distal part is connected to the gastric pouch through a gastro-jejunostomy, which creates a Roux limb or alimentary limb, as indicated. The continuity of the gastrointestinal tract is re-established by connecting biliopancreatic limb to the jejunum through a jejunojejunostomy. The small intestine distal to the jejuno-jejunostomy is called common limb. RYGB leads ingested food to bypass the distal stomach, duodenum and proximal jejunum, and rapidly go through the small gastric pouch and flow into the jejunum. Therefore, nutrients are present in the Roux limb without bile, whereas bile and pancreatic secretions are present in the biliopancreatic limb, but no nutrients. Nutrients are mixed with bile and pancreatic secretions in the common limb.
3. Bile acid and bile acid receptors
Increased systemic bile acid levels and altered bile acid composition were observed after both RYGB and VSG, particularly after RYGB [21, 22]. In addition to the role as a surfactant, bile acids act as signaling molecules for a number of nuclear receptors and plasma membrane receptors, including farnesoid X receptor (FXR), pregnane X receptor (PXR), constitutive androstane receptor (CAR), vitamin D receptor (VDR) and the membrane Takeda G protein-coupled receptor (TGR5, also known as GPBAR1) [23]. Among them, FXR and TGR5 are the most studied in BMS research. Ryan et al. found that VSG-induced weight loss and improvement of glucose tolerance were significantly blunted or abolished in Fxr knockout mice compared to wild type mice fed with high-fat diet, suggesting that FXR plays an essential role in the metabolic benefits of VSG [24]. Of note, the metabolic effects of FXR signaling are diet- [25, 26] and tissue-dependent [27–29]. While Fxr null mice develop a deleterious metabolic phenotype on chow diet [25], they display improved glucose tolerance when fed with a high-fat diet [26]. Although global activation of FXR is beneficial in improving metabolic disorders [27, 30–32], the role of intestinal FXR signaling is mixed [28, 29, 33]. To understand intestinal FXR signaling in the benefits of BMS, intestine-specific Fxr null mice were fed with a high-fat diet for twelve weeks before and after bile diversion surgery, which diverts bile flow from the gallbladder to the ileum without gastric reduction and had similar effects as RYGB. As a result, bile diversion-induced weight loss and glucose tolerance improvement were abolished in intestine-specific Fxr null mice fed with a high-fat diet, but not in Tgr5 knockout mice fed the same diet [34], suggesting that the beneficial effects of BMS are mediated by intestinal FXR signaling. However, TGR5 is required for the metabolic improvement and GLP-1 secretion produced by VSG [35], indicating distinct mechanisms might be involved in RYGB and VSG. This concept was supported by clinical studies [20, 36]. In addition, the glucoregulatory effects of bile diversion surgery were abrogated by either GLP-1 receptor (GLP-1R) antagonist or by the bile acids sequestrant, cholestyramine, as well as in Glp-1r knockout mice [34], indicating the essential role of GLP-1 in glucose homeostasis. To determine the role of FXR and TGR5 in GLP-1 secretion, mice were treated with a FXR agonist (obeticholic acid, OCA), a TGR5 agonist (INT-777), or a dual agonist of FXR and TGR5 (INT-767). Glucose-induced GLP-1 secretion was markedly increased by all three agonists, with the dual agonist being the strongest stimulator. Conversely, serum GLP-1 levels were significantly reduced in both Fxr and Tgr5 knockout mice. Moreover, INT-767 stimulated GLP-1 secretion was observed in Tgr5 knockout mice, but not in the Fxr knockout mice, suggesting that FXR is required for the GLP-1 secretion. Further, a FXR-response element was identified on the Tgr5 gene promoter, suggesting FXR is upstream of TGR5 [37]. In another study, the intestine-restricted FXR agonist, fexaramine (FEX), markedly increased glucose-induced GLP-1 secretion in wild type mice, but not in Fxr or Tgr5 knockout mice, suggesting both FXR and TGR5 are required in bile acid-stimulated GLP-1 secretion [38]. Collectively, intestinal FXR and GLP-1 signaling pathways are key players in the beneficial role of BMS.
TGR5 is a transmembrane G-protein coupled bile acid receptor [39, 40]. Recent studies revealed that TGR5 is required for the beneficial role of VSG in the improvement of glucose control, weight loss, hepatic steatosis, and energy expenditure in a diet-induced obesity mouse model [35, 41]. Investigators found the mRNA expression of Tgr5 and its target gene, proglucagon, in the ileum were significantly upregulated after VSG in wild type mice, but not in Tgr5 knockout mice, indicating activated TGR5 signaling by VSG. They further demonstrated that glucose clearance was significantly enhanced after VSG compared to sham-operation in wild type mice in response to an oral glucose load, and this was associated with increased GLP-1 and insulin secretion. However, these effects were blunted in Tgr5 knockout mice, suggesting that TGR5 is required in the regulation of GLP-1 and insulin secretion following VSG [35]. Primary bile acids are synthesized in the hepatocytes and secreted to the intestine where they are deconjugated to secondary bile acids by the gut microbiota. Therefore, bile acids can modulate the abundance and the composition of gut microbiota. Conversely, gut microbiota can modulate bile acids composition via microbial enzyme activities [42]. However, the relative abundance of gut microbiota did not differ between wild type and Tgr5 KO mice after VSG; therefore, it is questionable as to whether VSG-induced bile acids alterations were attributable to gut microbiota [35, 41]. How bile acid levels and composition are altered by BMS remains elusive. While primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), are FXR agonists [42], tauro-β muricholic acid (TβMCA) (in mice) is a FXR antagonist [43]. Secondary bile acids, lithocholic acid (LCA) and deoxycholic acid (DCA), are potent ligands for TGR5 [42]. Research in mice showed that serum total and unconjugated, as well as taurine-conjugated, bile acids were increased by VSG, whereas fecal taurine-conjugated bile acids, including TβMCA, were decreased in a high-fat diet induced obesity mouse model [35]. However, it is unclear whether and how decreased fecal TβMCA and/or altered bile acids composition contribute to the metabolic improvement by BMS.
Although both FXR and TGR5 are essential in the beneficial effects of BMS, their roles in the regulation of lipid and glucose metabolism are divergent. To understand the differential roles of FXR and TGR5 in the regulation of lipid and glucose homeostasis, bile acid sequestrants were administered to either Fxr or Tgr5 null mice. The results revealed that the beneficial role of bile acid sequestrants on cholesterol and triglyceride metabolism is mediated by FXR [44, 45], whereas the improvement on glycemia control is dependent on TGR5/GLP-1 [45]. An FXR mutation led to improved glucose tolerance and adipose tissue insulin sensitivity, but aggravated hepatic steatosis, and had no effect on hepatic insulin sensitivity in either genetic or diet-induced murine model of obesity [26]. Conversely, CA, a natural ligand of FXR, protected against hepatic steatosis and attenuated hypertriglyceridemia in KK-Ay mice, characterized with hyperglycemia, hyperlipidemia and hepatic steatosis [46]. In support of this concept, a FXR agonist, OCA, showed beneficial effects on NASH patients [30, 32, 47].
Several studies examined the role of TGR5 on the development of diet-induced hepatic steatosis. The results from different groups uniformly showed that Tgr5 knockout mice developed comparable hepatic steatosis compared to wild type mice in response to a high-fat or a high-fat/high-fructose diet [35, 41, 48], suggesting TGR5 is not required in diet-induced hepatic steatosis. However, debate exists regarding the role of TGR5 in mediating the benefits of VSG on hepatic steatosis. While Ding et al.'s study showed that TGR5 is required for the beneficial role of VSG in reducing liver fat accumulation [35], McGavigan et al.'s results did not [41]. The discrepancy likely relates to the age of mice, dietary fat proportion and the duration of the experiment [35, 41]. TGR5 is expressed in a variety of tissues and cells, including liver sinusoidal endothelial cells [49], adipose tissue, skeletal muscle [50], ileum and colon enteroendocrine cells [40]. Future studies with tissue- or cell-specific Tgr5 knockout animals will lead to a better understanding of the role of TGR5 in metabolic diseases.
4. Gut Hormones
Increased gut hormone secretion after BMS contributes to appetite and glycemic control, including proximal intestine derived hormones, such as CCK and glucose-dependent insulinotropic polypeptide (GIP), and distal intestinal hormones, such as GLP-1, PYY and neurotensin (NT). Yet, the specific role of a particular macronutrient in stimulating gut hormones secretion after BMS is unclear. A recent study showed that distal, but not proximal gut hormones, were significantly increased in RYGB patients compared to controls in response to dietary long-chain fatty acids (LCFAs) [51].
Fibroblast growth factor 19 (FGF19) has been proposed as a potential therapeutic target from the beneficial effects of BMS. FGF19 (in humans and its mouse ortholog FGF15) is a target gene of FXR. Once FXR is activated by bile acids in the ileum, FGF15/19 is released from the enterocytes of the small intestine and enters the liver via the portal vein, where FGF15/19 binds to FGF receptor 4 (FGFR4), and consequently suppresses cholesterol 7α-hydroxylase (CYP7A1) expression and inhibits bile acids synthesis. Not only does FGF15/19 promote glycogen synthesis and reduce gluconeogenesis, it also decreases hepatic triglycerides. While circulating FGF19 was decreased in NAFLD patients, it was increased after BMS, indicating that FGF19 may be a mediator for the beneficial effects of BMS [52].
5. Gut Microbiota
Gut microbiota dysbiosis is increasingly recognized as an important mechanism leading to obesity and the metabolic syndrome. BMS-induced weight loss is associated with alterations of the gut microbiome characterized by increased microbial gene richness (MGR) [53], Gammaproteobacteria [54–56], Akkermansia muciniphila [55, 57, 58] and a decreased ratio of Firmicutes to Bacteroidetes [54, 59, 60]. However, the alterations of the gut microbiota are likely independent of calorie restriction [55, 61]. Fecal microbiota transplantation (FMT) from RYGB-treated mice to non-operated germ-free mice resulted in weight loss and reduced fat mass in recipient mice compared to the sham-operated recipient mice [55], suggesting that the beneficial role of BMS in improving metabolic diseases is mediated, at least partially, by gut microbiota. Consistent with this, germ-free mice that received fecal microbiota from patients 9-years post RYGB or VSG exhibited significantly less body fat accumulation (43% and 26%) in 2 weeks compared to mice that received microbiota from severely obese patients. Moreover, mice colonized with RYGB microbiota gained more lean body mass, while the body weight gain and food intake did not differ compared to controls. In addition, mice with RYGB microbiota displayed a lower respiratory quotient (RQ, ratio of CO2 produced and O2 consumed), suggesting increased lipid oxidation and decreased carbohydrate oxidation [62]. This study further validated that the long-term beneficial effects of BMS are through functional gut microbiota. However, only modest effects were achieved in the patients with the metabolic syndrome who received gut microbiota from donor-RYGB patients compared to those who received gut microbiota from metabolic syndrome donors. The FMT recipients of RYGB gut microbiota exhibited a trend toward faster intestinal transit time, altered fecal bile acids profile and decreased adipose tissue C-C motif chemokine ligand 2 (CCL2) mRNA as well as decreased plasma CCL2 levels. The discrepancy between human and mice studies is likely due to the greater variation of external environment and individual gut microbiota composition in humans [63]. Although the role of gut microbiota in the beneficial effects of BMS has been established, the underlying mechanisms remain largely unknown. Despite significantly improved metabolic effects and weight loss, MGR was only restored in a small portion of patients undergoing BMS and remained low [53]. Moreover, Proteobacteria are considered potentially pro-inflammatory bacteria [64] and a hallmark of gut microbiota dysbiosis [65], which is a signature of gut microbiota alteration after BMS. Therefore, future research is necessary to understand how the altered gut microbial activity following BMS improves host metabolism.
6. Exosomes
Adipose tissue is a major source of circulating exosome microRNAs (miRNAs). Adipose tissue-derived circulating exosome miRNAs regulate gene expression in the liver [66]. It has been demonstrated that adipose tissue-derived exosomes activate peripheral monocytes and subsequently release inflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which contribute to the pathogenesis of insulin resistance [67]. Therefore, it is plausible that adipose tissue-derived exosomes are a potential mediator of beneficial effects after BMS. In fact, a growing body of evidence demonstrated that distinct alterations of circulating miRNAs occurred after BMS [68–71]. A recent study showed that BMS-responsive miRNAs were found from circulating adipocyte-derived exosomes identified by fatty acid binding protein 4 (FABP4), a specific marker for adipocytes. Further, they showed that the insulin signaling pathway was a target of 10 miRNAs and that the changes in levels of these miRNAs correlated to the improved insulin signaling after BMS [72]. In line with this, markedly reduced serum C-reactive protein (CRP), TNF-α and IL-6 were observed after BMS, suggesting improved systemic inflammation [57, 73]. However, the link between the altered miRNA profiles and improved systemic inflammation as well as other metabolic phenotypes remains to be established concerning the beneficial effects of BMS. Future studies identifying specific circulating miRNAs as biomarkers for the prediction of successful BMS would be of great interest.
7. Glucose Metabolism
The changed anatomy of GI tract due to RYGB leads the undigested nutrients going directly to the Roux limb (Figure 2), which reprograms intestinal glucose metabolism associated with the hypertrophy of Roux limb and renders the intestine as a major site for glucose disposal, consequently leading to improved glucose tolerance. This effect is mediated by the upregulation of glucose transporter-1 which leads to increased glucose uptake from circulation and concomitant increased glycolysis. Not only were these findings demonstrated in a rat model [74], but they were also verified in human studies [36]. Paradoxically, Baud’s study showed that glucose uptake from the alimentary Roux limb was decreased after RYGB in minipigs, owing to deprived bile in the Roux limb. Addition of bile to the Roux limb restored glucose uptake. Mechanically, bile diversion results in the concomitant diversion of salt which contributes a functional defect in sodium glucose cotransporter 1 (SGLT1), consequently reducing glucose absorption [75]. Although both RYGB and VSG improve glycemia, the mechanisms underlying the two approaches are different. While RYGB increases intestinal glucose disposal, VSG decreases alimentary glucose absorption without intestinal hypertrophy, and is associated with increased density of GLP-1 secreting cells [36]. A human study showed that gastric bypass surgery was superior to VSG for the remission of type 2 diabetes [76]. To understand the mechanisms underlying VSG-induced improvement in glucose homeostasis, Harris et al. evaluated tissue-specific glucose uptake using 18-FDG PET/CT in a mouse model. They found VSG resulted in a significant increase in glucose uptake by visceral adipose tissue, which was associated with the upregulation of transcripts involved in energy metabolism, suggesting increased glucose utilization in adipose tissue after VSG [77]. However, these results were from a non-obese mouse model and need to be validated in obese mouse models as well as in humans.
8. Lipid Metabolism
Dyslipidemia is one of manifestations associated with obesity and the metabolic syndrome as shown by elevated blood total cholesterol, low-density lipoprotein (LDL), triglycerides and decreased high-density lipoprotein (HDL), all of which were reversed by BMS [70, 78–80]. However, the underlying mechanisms are not clear. A recent study showed that intestinal LDL receptor (LDLR) was significantly upregulated at both mRNA and protein levels in jejunal biopsies from patients after RYGB. This was accompanied by the upregulation of Niemann-Pick C1-like protein 1 (NPC1L1), acetyl-coenzyme A acetyltransferase 2 (ACAT2), sterol regulatory element-binding protein 2 (SREBP2), and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), implicating the enhanced intestinal cholesterol absorption, uptake, and synthesis. Consistent with this, mice with intestine-specific overexpression of LDLR displayed significantly decreased circulating total cholesterol and LDL cholesterol levels, as well as body weight, either on regular chow diet or high-fat, high-cholesterol (HFHC) diets. Moreover, increased fecal cholesterol as well as total lipids levels were observed when intestine-specific Ldlr overexpression mice were fed with HFHC diet, highlighting that the reprogrammed intestinal cholesterol metabolism might produce at least some of the beneficial effects of RYGB [81].
Conclusions
While RYGB and VSG are effective in the improvement of metabolic phenotypes, they likely involve different mechanisms. Tissue-specific roles of FXR and TGR5 in the mediation of the metabolic benefits of RYGB and VSG, as well as in the metabolic disorders, remain elusive. The benefits of BMS are transmissible through FMT in rodents. However, again, the underlying mechanisms are largely unknown. Circulating exosomes and miRNA profiles were substantially altered by BMS. The specific roles of miRNAs on the regulation of gene expression involved in metabolic signaling pathways are not clear. Future studies are warranted to decipher how BMS confers its benefits.
Acknowledgments
We thank Dr. Russell A. Prough and Marion McClain for careful reading of this manuscript. We thank Dr. Craig J. McClain for the support of this study. This study was supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under grant number P20GM113226 (Sub-Project: 7948) (M.S.), P20GM113226 (C.J.M), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) of the NIH under award number P50AA024337, U01AA026934, U01AA026936, U01AA026980, and R01AA023681(C.J.M.). Support was also provided by the Jewish Heritage Fund for Excellence Pilot Grant Program at the University of Louisville School of Medicine (M.S.) and T35ES014559 (R.A.P., C.J.M.); and the Veterans Administration 1I01BX002996 (C.J.M). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- BMS
bariatric and metabolic surgery
- RYGB
Roux-en-Y gastric bypass
- VSG
vertical sleeve gastrectomy
- FXR
farnesoid X receptor
- TGR5
Takeda G protein-coupled receptor
- NAFLD
nonalcoholic fatty liver disease
- BMI
body mass index
- T2DM
type 2 diabetes
- NASH
nonalcoholic steatohepatitis
- AASLD
American Association for the Study of Liver Diseases
- ORCs
obesity-related complications
- GLP-1
glucagon-like polypeptide 1
- PYY
peptide YY
- PXR
pregnane X receptor
- CAR
constitutive androstane receptor
- VDR
vitamin D receptor
- GPBAR1
G protein-coupled bile acid receptor 1
- GLP-1R
GLP-1 receptor
- OCA
obeticholic acid
- FEX
fexaramine
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- TβMCA
tauro-β muricholic acid
- LCA
lithocholic acid
- DCA
deoxycholic acid
- GIP
glucose-dependent insulinotropic polypeptide
- NT
neurotensin
- LCFAs
long-chain fatty acids
- FGF19
fibroblast growth factor 19
- FGFR4
FGF receptor 4
- CYP7A1
cholesterol 7α-hydroxylase
- MGR
microbial gene richness
- FMT
fecal microbiota transplantation
- CCL2
C-C motif chemokine ligand 2
- miRNAs
microRNAs
- TNF-α
tumor necrosis factor-α
- IL-6
interleukin-6
- FABP4
fatty acid binding protein 4
- CRP
C-reactive protein
- SGLT1
sodium glucose cotransporter 1
- 18FDG
18F-fluorodeoxyglucose
- PET
positron emission tomography
- CT
computed tomography
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- LDLR
LDL receptor
- NPC1L1
Niemann-Pick C1-like protein 1
- ACAT2
acetyl-coenzyme A acetyltransferase 2
- SREBP2
sterol regulatory element-binding protein 2
- HMGCR
3-hydroxy-3-methylglutaryl-coenzyme A reductase
- HFHC
high-fat, high-cholesterol diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- 1.Afshin A, et al. , Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med, 2017. 377(1): p. 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CM, et al. , Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. Jama, 2018. 319(16): p. 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y,Ley SH, and Hu FB, Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol, 2018. 14(2): p. 88–98. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, et al. , Epidemiology of chronic liver diseases in the USA in the past three decades. Gut, 2020. 69(3): p. 564–568. [DOI] [PubMed] [Google Scholar]
- 5.Cotter TG and Rinella M, Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology, 2020. 158(7): p. 1851–1864. [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE and Rubino F, Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia, 2018. 61(2): p. 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams TD, et al. , Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med, 2017. 377(12): p. 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mechanick JI, et al. , Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures - 2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity (Silver Spring), 2020. 28(4): p. O1–o58. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, et al. , Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol, 2019. 17(6): p. 1040–1060.e11. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin D, Chennakesavalu M, and Gangemi A, Systematic review and meta-analysis of Roux-en-Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg Obes Relat Dis, 2019. 15(12): p. 2123–2130. [DOI] [PubMed] [Google Scholar]
- 11.Cherla DV, et al. ,Impact of sleeve gastrectomy and Roux-en-Y gastric bypass on biopsy-proven non-alcoholic fatty liver disease. Surg Endosc, 2020. 34(5): p. 2266–2272. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani N, et al. , The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology, 2018. 67(1): p. 328–357. [DOI] [PubMed] [Google Scholar]
- 13.Miras AD and le Roux CW, Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol, 2013. 10(10): p. 575–84. [DOI] [PubMed] [Google Scholar]
- 14.Sinclair P, Brennan DJ, and le Roux CW, Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nat Rev Gastroenterol Hepatol, 2018. 15(10): p. 606–624. [DOI] [PubMed] [Google Scholar]
- 15.Chambers AP, et al. , Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology, 2011. 141(3): p. 950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Najim W, Docherty NG, and le Roux CW, Food Intake and Eating Behavior After Bariatric Surgery. Physiol Rev, 2018. 98(3): p. 1113–1141. [DOI] [PubMed] [Google Scholar]
- 17.Albaugh VL, et al. , Bile acids and bariatric surgery. Mol Aspects Med, 2017. 56: p. 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albaugh VL, et al. , Recent advances in metabolic and bariatric surgery. F1000Research, 2016. 5: p.F1000 Faculty Rev-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evers SS, et al. , The Unconventional Role for Gastric Volume in the Response to Bariatric Surgery for Both Weight Loss and Glucose Lowering. Ann Surg, 2020. 271(6): p. 1102–1109. [DOI] [PubMed] [Google Scholar]
- 20.Svane MS, et al. , Postprandial Nutrient Handling and Gastrointestinal Hormone Secretion After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology, 2019. 156(6): p. 1627–1641.e1. [DOI] [PubMed] [Google Scholar]
- 21.Nemati R, et al. , Increased Bile Acids and FGF19 After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Correlate with Improvement in Type 2 Diabetes in a Randomized Trial. Obes Surg, 2018. 28(9): p. 2672–2686. [DOI] [PubMed] [Google Scholar]
- 22.Flynn CR, Albaugh VL, and Abumrad NN, Metabolic Effects of Bile Acids: Potential Role in Bariatric Surgery. Cell Mol Gastroenterol Hepatol, 2019. 8(2): p. 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calkin AC and Tontonoz P, Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol, 2012. 13(4): p. 213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan KK, et al. , FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature, 2014. 509(7499): p. 183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinal CJ, et al. , Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell, 2000. 102(6): p. 731–44. [DOI] [PubMed] [Google Scholar]
- 26.Prawitt J, et al. , Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes, 2011. 60(7): p. 1861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. , Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A, 2006. 103(4): p. 1006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang C, et al. , Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest, 2015. 125(1): p. 386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang C, et al. , Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun, 2015. 6: p. 10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuschwander-Tetri BA,et al. , Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet, 2015. 385(9972): p. 956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe M, et al. , Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem, 2011. 286(30): p. 26913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Younossi ZM, et al. , Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet, 2019. 394(10215): p. 2184–2196. [DOI] [PubMed] [Google Scholar]
- 33.Fang S, et al. , Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med, 2015. 21(2): p. 159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albaugh VL, et al. , Role of Bile Acids and GLP-1 in Mediating the Metabolic Improvements of Bariatric Surgery. Gastroenterology, 2019. 156(4): p. 1041–1051.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding L, et al. , Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology, 2016. 64(3): p. 760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavin JB, et al. , Differences in Alimentary Glucose Absorption and Intestinal Disposal of Blood Glucose After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology, 2016. 150(2): p. 454–64.e9. [DOI] [PubMed] [Google Scholar]
- 37.Pathak P, et al. , Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem, 2017. 292(26): p. 11055–11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pathak P, et al. , Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology, 2018. 68(4): p. 1574–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maruyama T, et al. , Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun, 2002. 298(5): p. 714–9. [DOI] [PubMed] [Google Scholar]
- 40.Thomas C, et al. , TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab, 2009. 10(3): p. 167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGavigan AK, et al. , TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut, 2017. 66(2): p. 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahlström A, et al. , Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab, 2016. 24(1): p. 41–50. [DOI] [PubMed] [Google Scholar]
- 43.Sayin SI, et al. , Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab, 2013. 17(2): p. 225–35. [DOI] [PubMed] [Google Scholar]
- 44.Herrema H, et al. , Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology, 2010. 51(3): p. 806–16. [DOI] [PubMed] [Google Scholar]
- 45.Potthoff MJ, et al. , Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol, 2013. 304(4): p. G371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe M, et al. , Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest, 2004. 113(10): p. 1408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mudaliar S, et al. , Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology, 2013. 145(3): p. 574–82.e1. [DOI] [PubMed] [Google Scholar]
- 48.Carino A, et al. , Agonism for the bile acid receptor GPBAR1 reverses liver and vascular damage in a mouse model of steatohepatitis. Faseb j, 2019. 33(2): p. 2809–2822. [DOI] [PubMed] [Google Scholar]
- 49.Keitel V, et al. , The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology, 2007. 45(3): p. 695–704. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe M, et al. , Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature, 2006. 439(7075): p. 484–9. [DOI] [PubMed] [Google Scholar]
- 51.Martinussen C, et al. , Intestinal sensing and handling of dietary lipids in gastric bypass-operated patients and matched controls. Am J Clin Nutr, 2020. 111(1): p. 28–41. [DOI] [PubMed] [Google Scholar]
- 52.Bozadjieva N, Heppner KM, and Seeley RJ, Targeting FXR and FGF19 to Treat Metabolic Diseases-Lessons Learned From Bariatric Surgery. Diabetes, 2018. 67(9): p. 1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aron-Wisnewsky J, et al. , Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut, 2019. 68(1): p. 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, et al. , Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A, 2009. 106(7): p. 2365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liou AP, et al. , Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med, 2013. 5(178): p. 178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao Y, et al. , Alterations of Gut Microbiota After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in Sprague-Dawley Rats. Obes Surg, 2017. 27(2): p. 295–302. [DOI] [PubMed] [Google Scholar]
- 57.Lu C, et al. , Alterations of Serum Uric Acid Level and Gut Microbiota After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in a Hyperuricemic Rat Model. Obes Surg, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmisano S, et al. , Changes in Gut Microbiota Composition after Bariatric Surgery: a New Balance to Decode. J Gastrointest Surg, 2019. [DOI] [PubMed] [Google Scholar]
- 59.Li JV, et al. , Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut, 2011. 60(9): p. 1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.AlAssal K, et al. , Gut Microbiota Profile of Obese Diabetic Women Submitted to Roux-en-Y Gastric Bypass and Its Association with Food Intake and Postoperative Diabetes Remission. Nutrients, 2020. 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong LC, et al. , Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr, 2013. 98(1): p. 16–24. [DOI] [PubMed] [Google Scholar]
- 62.Tremaroli V, et al. , Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab, 2015. 22(2): p. 228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Groot P, et al. , Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut, 2020. 69(3): p. 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Chatelier E, et al. , Richness of human gut microbiome correlates with metabolic markers. Nature, 2013. 500(7464): p. 541–6. [DOI] [PubMed] [Google Scholar]
- 65.Shin NR, Whon TW, and Bae JW, Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol, 2015. 33(9): p. 496–503. [DOI] [PubMed] [Google Scholar]
- 66.Thomou T, et al. , Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature, 2017. 542(7642): p. 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng ZB, et al. , Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes, 2009. 58(11): p. 2498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alkandari A, et al. , Improved physiology and metabolic flux after Roux-en-Y gastric bypass is associated with temporal changes in the circulating microRNAome: a longitudinal study in humans. BMC Obes, 2018. 5: p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atkin SL, et al. , Changes in Blood microRNA Expression and Early Metabolic Responsiveness 21 Days Following Bariatric Surgery. Front Endocrinol (Lausanne), 2018. 9: p. 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bae YU, et al. , Bariatric Surgery Alters microRNA Content of Circulating Exosomes in Patients with Obesity. Obesity (Silver Spring), 2019. 27(2): p. 264–271. [DOI] [PubMed] [Google Scholar]
- 71.Ortega FJ, et al. , Surgery-Induced Weight Loss Is Associated With the Downregulation of Genes Targeted by MicroRNAs in Adipose Tissue. J Clin Endocrinol Metab, 2015. 100(11): p. E1467–76. [DOI] [PubMed] [Google Scholar]
- 72.Hubal MJ, et al. ,Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring), 2017. 25(1): p. 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Askarpour M, et al. , Effect of Bariatric Surgery on Serum Inflammatory Factors of Obese Patients: a Systematic Review and Meta-Analysis. Obes Surg, 2019. 29(8): p. 2631–2647. [DOI] [PubMed] [Google Scholar]
- 74.Saeidi N, et al. , Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science, 2013. 341(6144): p. 406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baud G, et al. , Bile Diversion in Roux-en-Y Gastric Bypass Modulates Sodium-Dependent Glucose Intestinal Uptake. Cell Metab, 2016. 23(3): p. 547–53. [DOI] [PubMed] [Google Scholar]
- 76.Hofsø D, et al. , Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol, 2019. 7(12): p. 912–924. [DOI] [PubMed] [Google Scholar]
- 77.Harris DA, et al. , Sleeve Gastrectomy enhances glucose utilization and remodels adipose tissue independent of weight loss. Am J Physiol Endocrinol Metab, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mulla CM, et al. , Plasma FGF-19 Levels are Increased in Patients with Post-Bariatric Hypoglycemia. Obes Surg, 2019. 29(7): p. 2092–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahlin S, et al. , Bile acid changes after metabolic surgery are linked to improvement in insulin sensitivity. Br J Surg, 2019. 106(9): p. 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salman MA, et al. , Laparoscopic Sleeve Gastrectomy on the Horizon as a Promising Treatment Modality for NAFLD. Obes Surg, 2020. 30(1): p. 87–95. [DOI] [PubMed] [Google Scholar]
- 81.Meoli L, et al. , Intestine-Specific Overexpression of LDLR Enhances Cholesterol Excretion and Induces Metabolic Changes in Male Mice. Endocrinology, 2019. 160(4): p. 744–758. [DOI] [PMC free article] [PubMed] [Google Scholar]


