Abstract
Aim.
Children surviving cardiac arrest are at high risk of neurological morbidity and mortality; however, there is a lack of validated prognostic biomarkers. We aimed to evaluate brain magnetic resonance imaging (MRI) and spectroscopy (MRS) as predictors of death and disability. Secondly, we evaluated whether MRI/S by randomized group.
Methods.
This single center study analyzed clinically indicated brain MRI/S data from children enrolled in a randomized controlled trial of 24 versus 72 hours of hypothermia following cardiac arrest. Two pediatric radiologists scored conventional MRIs. Lactate and N-acetyl-aspartate (NAA) concentrations (mmol/kg) were determined from spectra acquired from the basal ganglia, thalamus, parietal white matter and parietooccipital gray matter. Mortality and neurological outcomes (favorable = Pediatric Cerebral Performance Category [PCPC] 1, 2, 3 or increase < 2) were assessed at hospital discharge. Non-parametric tests were used to test for associations between MRI/S biomarkers and outcome and randomized group.
Results.
23 children with (median [interquartile range]) age of 1.5 (0.3–4.0) years. Ten (44%) had favorable outcome. There were more T2 brain lesions in the lentiform nuclei in children with unfavorable 12 (92%) versus favorable 3 (33%) outcome, p=.007. Increased lactate and decreased NAA concentrations in the parietooccipital gray matter and decreased NAA in the parietal white matter were associated with unfavorable outcome (p’s<.05). There were no differences for any biomarker by randomized group.
Conclusion.
Regional cerebral and metabolic MRI/S biomarkers are predictive of neurological outcomes at hospital discharge in pediatric cardiac arrest and should undergo validation testing in a large sample.
Keywords: pediatric, cardiac arrest, prognostication, magnetic resonance imaging, outcome, magnetic resonance spectroscopy
INTRODUCTION
Morbidity and mortality following pediatric cardiac arrest is chiefly due to global hypoxia-ischemia and reperfusion injury as part of the post-cardiac arrest syndrome1,2. Surviving children are at high-risk of long-term neurological morbidity adversely affecting child and family function3–7. Early, accurate prognostication can help clinicians and families understand the breadth of the child’s brain injury to aid in decision-making and planning for approach to subsequent care including surgery for technological support (e.g., tracheostomy), rehabilitation, and withdrawal of support depending on the family’s goals.
Brain magnetic resonance imaging (MRI) is increasingly used as standard of care for diagnostic, therapeutic, and prognostic purposes in pediatric neurocritical care8. Small observational studies show that conventional (T1, T2, and diffusion-weighted [DWI]) MRI can identify regions selectively vulnerable to hypoxic-ischemic injury and shows promise to assist in informing accurate prognostication9–13. Similarly, MR Spectroscopy (MRS), an advanced MRI technique, which is used to determine regional brain metabolite concentrations such as including N-acetyl-aspartate (NAA) and lactate, can assist in outcome prognostication14,15.
Brain MRI and MRS studies in children with cardiac arrest are often limited by evaluation of a single brain region, heterogeneity in the timing of imaging, and the use of a non-quantitative analytic approach. The objective of this study was to prospectively determine the predictive utility of brain MRI biomarkers and MRS metabolites, NAA and lactate, from multiple brain regions at a single-center in the setting of a phase I/II randomized, controlled trial (RCT) of therapeutic hypothermia. The primary objective was to evaluate MRI/S biomarkers as predictors of outcomes at hospital discharge. The secondary objective was to compare MRI/S biomarkers across randomized treatment groups. We hypothesized that regional alterations in both conventional MRI signal (basal ganglia lesions) and metabolism (low NAA and high lactate) would be predictive of poor 6-month outcomes.
PATIENTS AND METHODS
Study Design.
This study reports results from a phase I/II RCT of hypothermia following resuscitation from pediatric cardiac arrest conducted between November 2009 and December 201316. Patients received either 24 or 72 hours of therapeutic hypothermia to a core body temperature of 33.5°C, which was maintained by use of a servo-controlled cooling blanket (Cincinnati Sub-Zero Blanketrol II-III, Monroe, NC). Brain imaging (MRI/S) was obtained after therapeutic hypothermia as part of routine clinical care. The University of Pittsburgh Institutional Review Board approved the RCT (NCT00797680) at the UPMC Children’s Hospital of Pittsburgh. Children were enrolled following parental/guardian informed consent and assent when applicable.
Enrollment of Patients.
Thirty-four children between the ages 1 week and 17 years who were admitted to the ICU with return of spontaneous circulation after in- or out-of-hospital CA were enrolled in the parent RCT16. Patients with a simultaneous acute neurological injury prior to the cardiac arrest event were excluded from the RCT (e.g., traumatic brain injury).
Data Collection.
Medical record review was used to collect patient demographics, disease characteristics, and patient outcomes. Under the parent RCT, Pediatric Cerebral Performance Category (PCPC) scores at baseline (pre-arrest), hospital discharge and 6-month follow-up were assigned by the lead author (EF), who was not blinded to treatment assignment. Neuroradiologists reading MRI scans were blinded to each other’s initial interpretation, and patient outcome and treatment assignment. PCPC score was assigned based on telephone, in-person interview, or chart review regarding the child’s neurological functioning prior to and at hospital discharge following the cardiac arrest event. A PCPC score of 4, 5, or 6 or increase > 1 was classified as an unfavorable outcome17.
MRI Acquisition and Analysis.
Standard care for brain MRI performed for clinical purposes after hypoxic-ischemic event includes brain MRS at our institution, and brain imaging was performed as part of clinical care as indicated by the clinical team. To undergo MRI, the child must have been clinically stable without the requirement for high-frequency oscillator ventilation, extracorporeal membrane oxygenation or cardiac pacemaker/pacing wires.
MRI data were acquired on a 1.5T or 3T GE Signa MR Scanner (GE Healthcare, Milwaukee, Wisconsin), by use of an 8-channel head coil. The MR imaging protocol included axial and coronal T2-weighted as well as axial, coronal, and sagittal T1-weighted imaging. Whole brain DWI was performed at b=1000 s/mm2 with 6 directions. In-plane resolution was 1.1 × 1.1 × 6.0 mm3 with no gap. Scan time was echo time/repetition time= 87.4/10000 ms.
Brain MRI images were analyzed by 2 pediatric neuroradiologists blinded to subject outcome. T1, T2, and DWI/apparent diffusion coefficient (ADC) images were analyzed for signal intensity abnormalities and scored using a system adapted from Christophe, et al13. Brain regions from both hemispheres, thalamus, basal ganglia, cerebellum, and brain stem were examined for evidence of hypoxic-ischemic injury. Inter-observer variability was described, disagreement resolved by consensus, and kappa statistic reported.
MRS.
MRS data were acquired using a single-voxel point-resolved spectroscopy (PRESS) sequence with an echo time of 35ms and repetition times of 1.5 sec (1.5 Tesla) and 2 sec (3 Tesla), and both the raw MRS data and water reference data were archived for data processing. MRS data were obtained from 4 regions of interest (ROI): 1) Basal ganglia; 2) Thalamus, 3) Parietal white matter (WM) and 4) Parietooccipital gray matter (GM) (Figure 1). These ROIs are standard and correspond to regions that are vulnerable to global hypoxic-ischemic injury. Basal ganglia and thalamus, which are highly interconnected with the cerebral cortex, have various important functional roles relevant to outcome whereas WM and GM ROIs include a prominent watershed region and the medial parietal cortex, which is associated with diseases of consciousness. Axial, sagittal, and coronal orientations were assessed for accurate voxel placement. Volumes of ROIs were typically 3–6 cm3 and acquisition times (128 averages) were 3–5 min per ROI.
Figure 1.
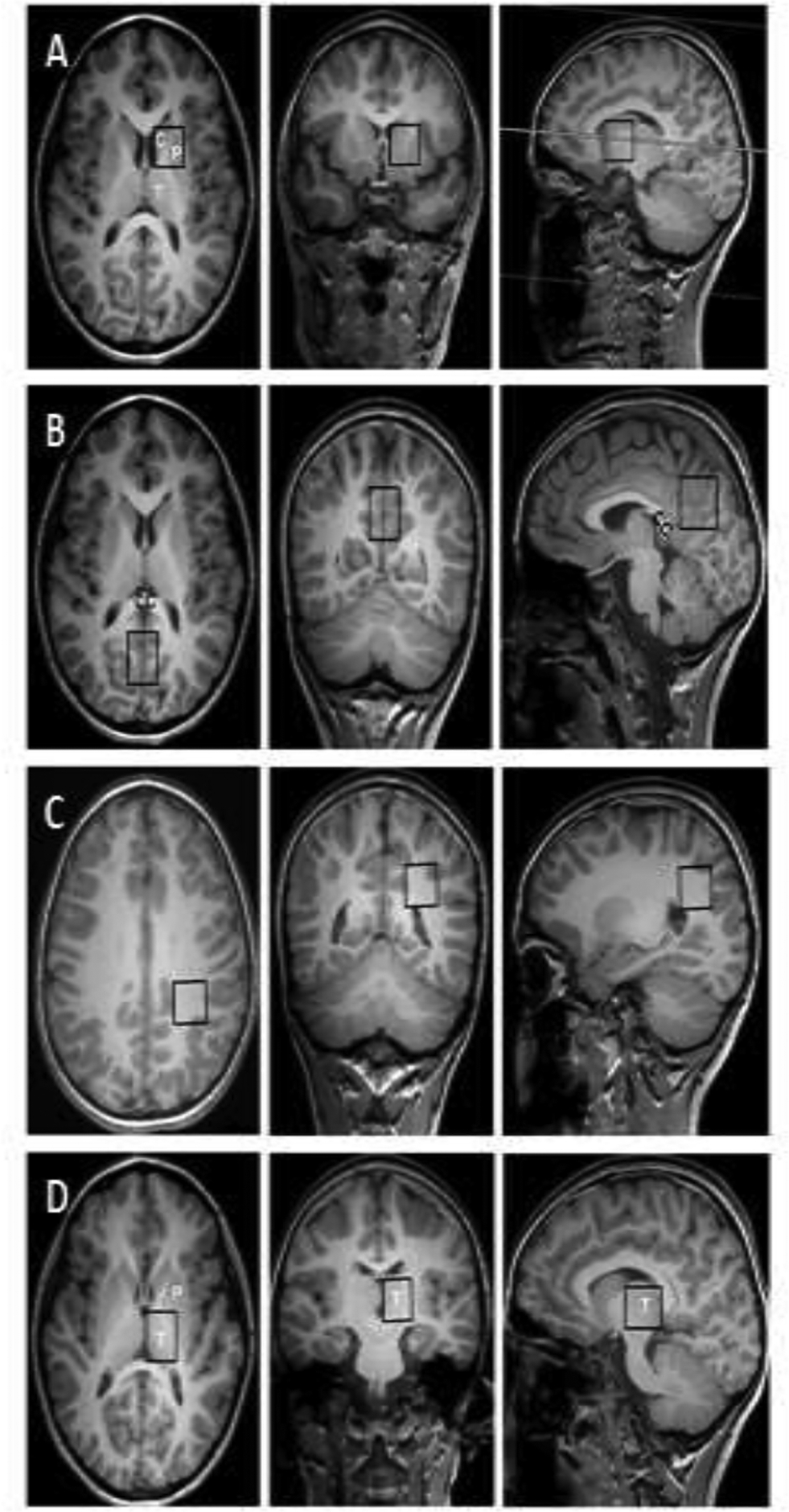
Magnetic resonance spectroscopy regions of interest. A) Basal ganglia: caudate (C) and putamen (P); B) Parietooccipital gray matter; C) Parietal white matter; and D) Thalamus (T). CC, corpus callosum.
Raw MRS data files were transferred off-line for fully-automated post-processing using LCModel software (LCModel©, Stephen Provencher Inc., Ontario, Canada)18. Absolute concentrations of Lac and NAA were determined using the unsuppressed water signal as internal reference. LCModel processing provides objective measures for the signal-to-noise ratio and the spectral linewidth (full width at have maximum = FWHM) for objective quality assessment. Spectra with a low signal-to-noise ratio of > 2 standard deviations below the mean were discarded. Also, spectra with poor linewidth > 2 standard deviations above the mean were not included in the analysis. These guidelines for quality control were adapted from LCModel manual (LCModel User’s Manual, June 15, 2014, LCModel version 6.3–1J) (http://s-provencher.com/pub/LCModel/manual/manual.pdf (accessed 09/09/2014)). MRS ROI placements were verified by an experienced board certified pediatric neuroradiologist. Cardiac arrest results in a global hypoxic-ischemic injury; therefore, MRS measurements were performed on one hemisphere only.
Statistical Analyses:
All analyses were conducted using SAS® 9.2 (Copyright © [2002–2008] SAS Institute Inc., Cary, NC, USA). Frequencies and percentages were determined for categorical variables. Data are presented as median (interquartile range [IQR]) if non-parametric or mean and standard deviation if parametric. All p-values were 2-sided, and a p <0.05 was considered statistically significant. Group differences were compared using the Wilcoxon Rank Sum test. Differences in categorical data were assessed using chi-square analysis with Fisher’s Exact test when appropriate. Agreement between neuroradiologist grading of conventional MRI scores was analyzed using Cohen’s Kappa test in Stata 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
RESULTS
Patients
Twenty-three children underwent neuroimaging and were included in the present study (Figure 2). Patients had a median (interquartile range [IQR]) age of 1.5 (0.3–4.0) years (Table 1). Cardiac arrest events occurred out-of-hospital in 21 (91%) children and 20 (87%) cases were due to asphyxia. Ten (44%) children had favorable outcome and 3 (13%) children died by hospital discharge. More children with witnessed status (70% versus 8%) were in the favorable versus unfavorable groups, p<.05.
Figure 2.
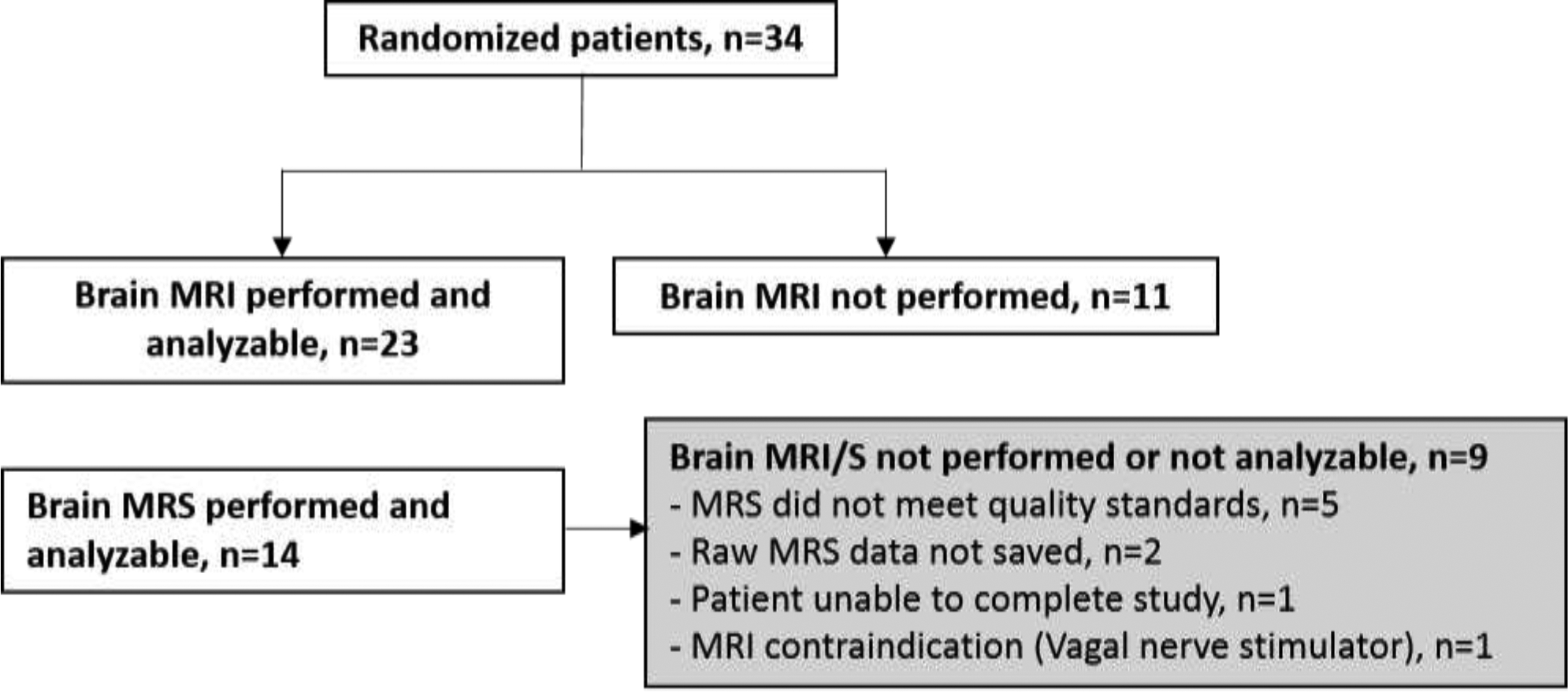
CONSORT diagram of patients who received imaging after enrollment into the parent randomized, controlled trial.
Table 1.
Child and cardiac arrest characteristics overall and by outcome at hospital discharge.
| Overall N=23 | Favorable outcome n=10 (43.5%) | Unfavorable outcome n=13 (56.5%) | p-value | |
|---|---|---|---|---|
| Age, years | 1.5 (0.3–4.0) | 2.3 (0.3–13.3) | 1.5 (0.3–2.4) | .738 |
| Female Sex, n (%) | 14 (61) | 6 (60) | 8 (62) | 1.000 |
| Location of cardiac arrest | 1.000 | |||
| In-Hospital | 2 (9) | 1 (10) | 1 (8) | |
| Out-of-hospital | 21 (91) | 9 (90) | 12(92) | |
| Etiology | 0.068 | |||
| Asphyxia | 20 (87) | 7 (70) | 13(100) | |
| Cardiac | 3 (13) | 3 (30) | 0 (0) | |
| Epinephrine doses | 3.0 (1.0–4.0) | 2.5 (0.0–5.0) | 3.0 (2.0–3.0) | 0.889 |
| First documented rhythm | n=22 | n=10 | n=12 | 0.056 |
| Pulseless electrical activity | 12 (55) | 6 (60) | 6 (50) | |
| Asystole | 7 (32) | 1 (10) | 6 (50) | |
| Sinus | 2 (9) | 2 (20) | 0 (0) | |
| Ventricular tachycardia or fibrillation | 1 (5) | 1 (10) | 0 (0) | |
| Witnessed | 8 (35) | 7 (70) | 1 (8) | 0.006 |
| Bystander CPR | 18 (78) | 7 (70) | 11 (85) | 0.618 |
| Hospital length of stay, days | 24.0 (14.0–37.0) | 21.0 (9.0–26.0) | 33.0 (23.0–43.0) | 0.037 |
| ICU length of stay, days | 15.0 (9.0–33.0) | 10.0 (6.0–13.0) | 25.0 (15.0–37.0) | 0.002 |
| Died by hospital discharge | 3 (13) | 0 (0) | 3 (23) | 0.229 |
| CA to brain MRI, days | 6.0 (4.0–8.0) | 5.5 (4.0–7.0) | 6.0 (4.0–8.0) | 0.771 |
The values are expressed as n (%) and median interquartile range (IQR)
The p-values are based on Fisher’s exact test for categorical variables and Wilcoxon test for median
All variables have complete data unless specified.
CPR, cardiopulmonary resuscitation; ICU, intensive care unit; CA, cardiac arrest; MRI, magnetic brain imaging
Children who had a brain MRI were more likely to be alive by hospital discharge (73% vs. 13%, p=.001) and had longer hospital (24 [14–37] vs. 3 [2–16] days) and ICU lengths of stay (15 [9–33] v. 3 [2–11] days) compared to children who did not undergo a brain MRI (Supplemental Table 1).
Brain MRI and Child Outcomes
Brain MRI/S occurred on hospital day 5.5 (4.0–7.0) for children with favorable vs. 6.0 (4.0–8.0) in children with unfavorable outcome post-cardiac arrest, p=0.771. The kappa agreement for MRI scoring was 0.98.
Regional lesions on T2-weighted imaging most commonly occurred in the lentiform 15 (68%) and caudate (55%) nuclei of the basal ganglia and thalamus (50%). On DWI, lesions were most common in the lentiform nucleus (41%), thalamus (32%) and frontal and parietal lobes (30%) each (Table 2). White matter injury in the posterior limb of the internal capsule (n=1) and centrum semi-ovale (n=1) were uncommon.
Table 2.
Magnetic resonance imaging results by randomized group and outcome at hospital discharge.
| Overall N=23 | 24 h HT N=12 | 72 h HT N=11 | p | Favorable outcome N=10 | Unfavorable outcome N=13 | p | ||
|---|---|---|---|---|---|---|---|---|
| ROSC to MRI, days | 6.0 (4.0–8.0) | 5.0 (3.5–6.5) | 7.0 (5.0–10.0) | 0.126 | 5.5 (4.0–7.0) | 6.0 (4.0–8.0) | 0.771 | |
| T2 | ||||||||
| Frontal lobe | 5 (22) | 3 (25) | 2 (18) | 1.000 | 1 (10) | 4 (31) | 0.339 | |
| Temporal lobe | 3 (13) | 2 (17) | 1 (9) | 1.000 | 0 (0) | 3 (23) | 0.229 | |
| Parietal lobe | 5 (22) | 3 (25) | 2 (18) | 1.000 | 1 (10) | 4 (31) | 0.339 | |
| Occipital lobe | 4 (17) | 3 (25) | 1 (9) | 0.590 | 1 (10) | 3 (23) | 0.604 | |
| Lentiform | 15 (68), n=22 | 9 (82), n=11 | 6 (55) | 0.361 | 3 (33), n=9 | 12 (92) | 0.007 | |
| Caudate | 12 (55), n=22 | 8 (73), n=11 | 4 (36) | 0.198 | 3 (33), n=9 | 9 (69) | 0.192 | |
| Thalamus | 11 (50), n=22 | 7 (64), n=11 | 4 (36) | 0.395 | 3 (33), n=9 | 8 (62) | 0.387 | |
| Cerebellum | 1 (44) | 1 (8) | 0 (0) | 1.000 | 1 (10) | 0 (0) | 0.435 | |
| Brain stem | 0 (0) | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA | |
| Posterior limb of the internal capsule | 1 (5); n=22 | 1 (9); n=11 | 0 (0) | 1.000 | 1 (11); n=9 | 0 (0) | 0.409 | |
| Centrum semi-ovale | 1 (5); n=22 | 1 (9); n=11 | 0 (0) | 1.000 | 0 (0); n=9 | 1 (8) | 1.000 | |
| Diffusion-weighted imaging | ||||||||
| Frontal lobe | 7 (30) | 6 (50) | 1 (9) | 0.069 | 2 (20) | 5 (39) | 0.405 | |
| Temporal lobe | 4 (17) | 4 (33) | 0 (0) | 0.093 | 1 (10) | 3 (23) | 0.604 | |
| Parietal lobe | 7 (30) | 6 (50) | 1 (9) | 0.069 | 2 (20) | 5 (39) | 0.405 | |
| Occipital lobe | 6 (26) | 5 (42) | 1 (9) | 0.155 | 1 (10) | 5 (39) | 0.179 | |
| Lentiform | 9 (41); n=22 | 6 (55); n=11 | 3 (27); n=11 | 0.387 | 2 (22); n=9 | 7 (54) | 0.203 | |
| Caudate | 6 (27); n=22 | 4 (36); n=11 | 2 (18); n=11 | 0.635 | 1 (11); n=9 | 5 (39) | 0.333 | |
| Thalamus | 7 (32); n=22 | 5 (46); n=11 | 2 (18); n=11 | 0.361 | 2 (22); n=9 | 5 (39) | 0.648 | |
| Cerebellum | 0 (0) | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA | |
| Brain stem | 1 (4) | 1 (8) | 0 (0) | 1.000 | 1 (10) | 0 (0) | 0.435 | |
| Posterior limb of the internal capsule | 1 (5); n=22 | 0 (0); n=11 | 1 (9); n=11 | 1.000 | 0 (0); n=9 | 1 (8) | 1.000 | |
| Centrum semi-ovale | 2 (9); n=22 | 1 (9); n=11 | 1 (9); n=11 | 1.000 | 0 (0); n=9 | 2 (15) | 0.494 | |
| Generalized edema | 3 (14); n=22 | 3 (27); n=11 | 0 (0); n=11 | 0.214 | 0 (0); n=9 | 3 (23) | 0.240 | |
| Brain herniation | 0 (0) | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
Comparisons between lesions and randomized or outcome group analyzed with Fisher exact test
ROSC, return of spontaneous circulation; MRI, magnetic resonance imaging; HT, hypothermia; NA, not applicable
Children with favorable outcomes had fewer lesions in the lentiform nuclei on T2-weighted imaging (p<0.05). Although no child with favorable outcome had evidence of generalized edema contrasting children with unfavorable outcome, this difference was not statistically significant (0% vs. 23%, p=0.24) (Figures 3 and 4). There were no differences in regional brain lesion location by randomized group.
Figure 3.
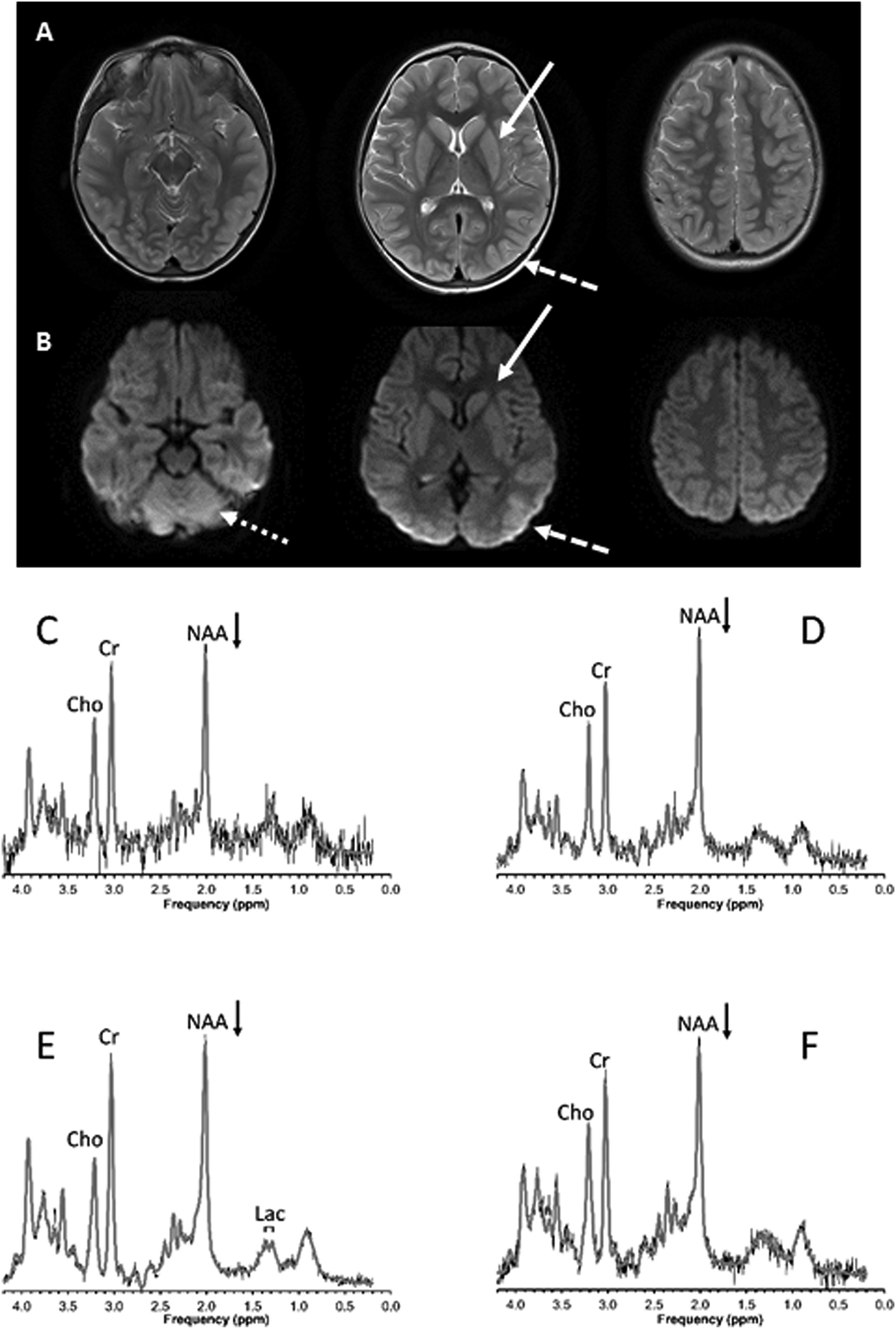
A previously well 3-year old child with severe asthma, respiratory failure, and asphyxia cardiac arrest peri-intubation in the intensive care unit. A) Axial T2-weighted and B) Diffusion-weighted MRI 9 days post-arrest showing injury to the basal ganglia (solid arrow), occipital lobe (dashed arrow), and cerebellum (dotted arrow). Spectra from the C) basal ganglia, D) thalamus, E) occipitoparietal gray and F) parietal white matter regions of interest show possibly mildly decreased N-acetyl-aspartate (NAA, arrows) and a small lactate (Lac) signal. Signals from creatine (Cr) and choline (Cho) are also labeled. Shown are the unprocessed data (thin black lines) with the superimposed fit (thick gray lines) used for quantitation. The patient had mild neurologic disability at hospital discharge and no noticeable disability at 6 months follow-up.
Figure 4.
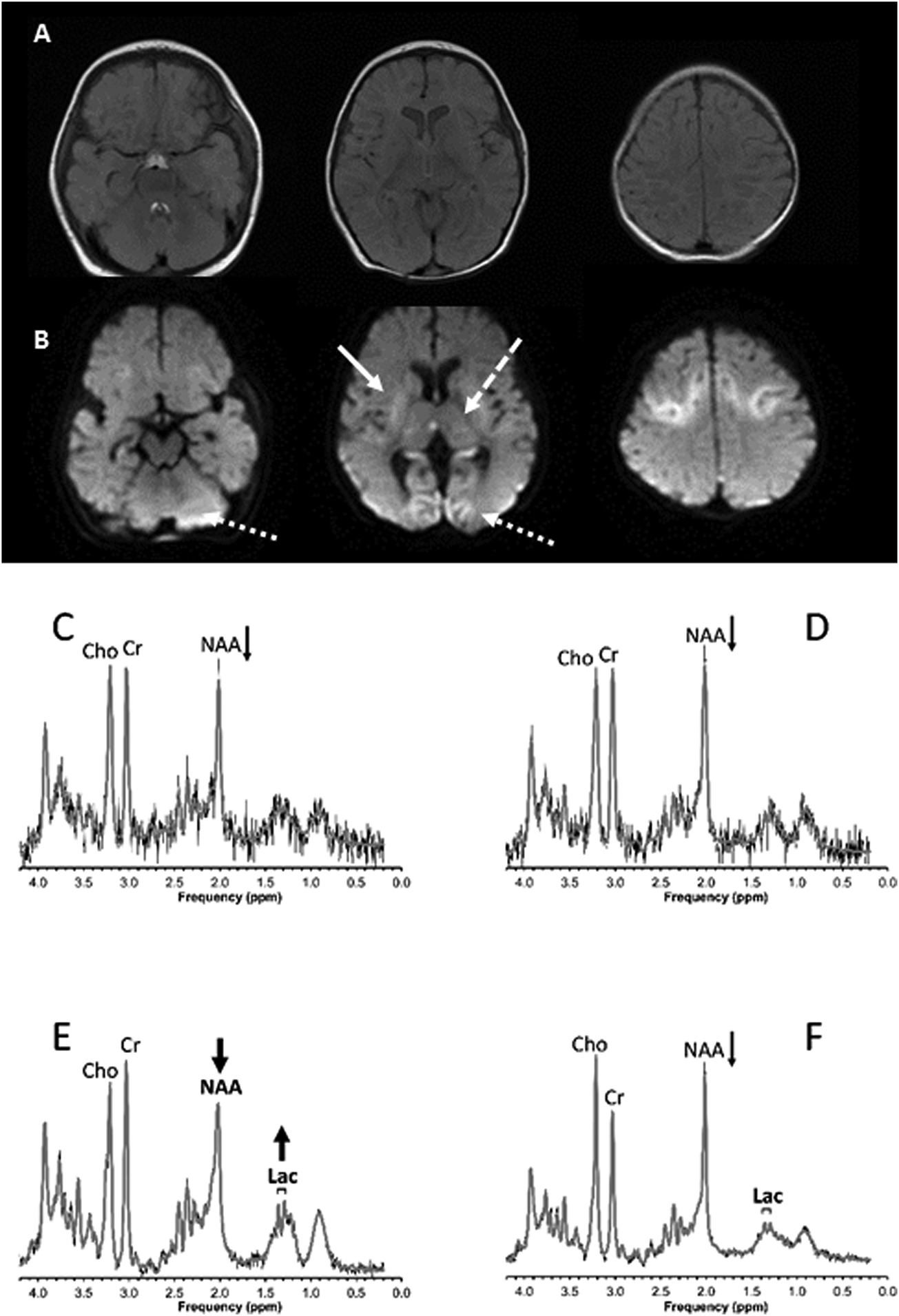
A previously well 4-month old child with asphyxial cardiac arrest at home. A) Axial T2-weighted and B) Diffusion-weighted brain MRI 3 days post-arrest showing injury to the basal ganglia (solid arrow), thalamus (dashed arrow), occipital lobe (dotted arrow), and cerebellum (dotted arrow). Spectroscopy studies performed in the C) basal ganglia, D) thalamus, E) occipital/parietal gray matter and F) parietal white matter regions of interest show decreased NAA in all regions being particularly pronounced in gray matter. In addition, lactate (and underlying lipids) is increased in gray matter consistent with acute and significant hypoxic ischemic brain injury in this region. The patient died following withdrawal of support due to severe neurologic injury.
No child in the 72-hour hypothermia group had generalized edema on brain MRI while 3 (27%) children had generalized edema in the 24-hour hypothermia group, p=.214.
Brain MR Spectroscopy and Child Outcomes
Fourteen of 23 (61%) children with brain MRI performed also had a brain MRS that met imaging quality criteria for inclusion in this analysis. Fewer children had MRS as compared to MRI due to variability in clinical protocols prior to May 2009 and data loss associated with a scanner upgrade.
Lactate was increased in the occipital-parietal gray matter ROI in children with unfavorable versus favorable outcome (1.83 [0.90–2.68 vs. 0.74 [0.68–0.92]], p=0.043) (Table 3). NAA was decreased in children with unfavorable versus favorable outcome in both the occipital-parietal gray (3.54 [2.52–4.82] vs. 9.73 [7.88–10.85], p=0.005) and white matter (6.81 [4.62–7.50] vs. 9.33 [8.58–10.10], p=0.032) regions of interest. There were no significant differences in regional MRS NAA or lactate concentrations by randomized group.
Table 3.
Magnetic resonance spectroscopy results by randomized group and outcome at hospital discharge.
| Overall N=14 | 24 hr hypothermia N=8 | 72 hr hypothermia N=6 | p | Favorable outcome N=6 | Unfavorable outcome N=8 | p | |
|---|---|---|---|---|---|---|---|
| Basal Ganglia | |||||||
| Lactate | 1.18 (0.89–2.03) | 1.67 (1.07–3.29) | 0.96 (0.47–1.19) | 0.181 | 0.75 (0.21–1.41) | 1.56 (1.07–4.47) | 0.108 |
| N-acetylaspartate | 6.99 (4.49–8.21) | 6.99 (4.24–7.74) | 7.48 (4.85–9.13) | 0.414 | 8.36 (7.29–9.13) | 5.60 (4.09–7.04) | 0.059 |
| Lactate/NAA Ratio | 0.19 (0.10–0.35) | 0.27 (0.15–0.68) | 0.14 (0.05–0.19) | 0.282 | 0.08 (0.03–0.28) | 0.23 (0.18–1.10) | 0.081 |
| Parietooccipital gray matter | |||||||
| Lactate | 0.93 (0.69–1.98) | 1.53 (0.81–2.68) | 0.82 (0.63–0.92) | 0.108 | 0.74 (0.68–0.92) | 1.83 (0.90–2.68) | 0.043 |
| N-acetylaspartate | 4.82 (3.42–8.86) | 3.81 (3.03–7.79) | 6.90 (4.70–10.85) | 0.228 | 9.73 (7.88–10.85) | 3.54 (2.52–4.82) | 0.005 |
| Lactate/NAA Ratio | 0.16 (0.09–0.83) | 0.40 (0.10–0.93) | 0.11 (0.09–0.18) | 0.228 | 0.09 (0.06–0.09) | 0.64 (0.16–0.93) | 0.005 |
| Parietal white matter | |||||||
| Lactate | 0.99 (0.70–1.78); n=9 | 1.06 (0.67–1.58); n=4 | 0.99 (0.70–1.78); n=5 | 0.905 | 0.74 (0.64–1.27); n=4 | 1.35 (0.99–1.81); n=5 | 0.286 |
| N-acetylaspartate | 8.07 (6.81–9.10); n=9 | 8.07 (7.44–8.82); n=4 | 7.50 (4.62–9.10); n=5 | 0.730 | 9.33 (8.58–10.10); n=4 | 6.81 (4.62–7.50); n=5 | 0.032 |
| Lactate/NAA Ratio | 0.20 (0.08–0.23); n=9 | 0.14 (0.08–0.21); n=4 | 0.20 (0.09–0.30); n=5 | 0.730 | 0.08 (0.07–0.14); n=4 | 0.23 (0.20–0.30); n=5 | 0.032 |
| Thalamus | |||||||
| Lactate | 1.31 (0.97–1.95); n=13 | 1.35 (0.97–2.66); n=7 | 1.15 (0.72–1.95); n=6 | 0.534 | 1.10 (0.54–1.95) | 1.35 (1.07–4.23); n=7 | 0.295 |
| N-acetylaspartate | 8.12 (6.20–9.77) | 8.12 (6.20–10.20) | 8.30 (6.19–9.77) | 0.945 | 9.69 (8.12–10.20) | 6.97 (4.16–9.76) | 0.138 |
| Lactate/NAA Ratio | 0.16 (0.12–0.20) | 0.16 (0.12–0.43) | 0.15 (0.10–0.20) | 0.836 | 0.12 (0.05–0.20) | 0.17 (0.13–1.02) | 0.295 |
Comparisons between metabolites and randomized or outcome group analyzed with Wilcoxon rank sum test
NAA, N-acetyl-aspartate
DISCUSSION
The primary findings of this study include the following: 1) two-thirds of children admitted to the PICU following cardiac arrest and enrolled in a research study had a clinical brain MRI/S performed within the first two weeks; 2) lesions in the lentiform nuclei (putamen and globus pallidus) on MRI were associated with unfavorable outcome at hospital discharge; and 3) Increased lactate and decreased NAA concentrations in the occipital-parietal gray matter and decreased NAA in the occipital-parietal white matter regions of interest were associated with unfavorable outcomes at hospital discharge.
Brain MRI is one of several tools recommended for clinical prognostication following cardiac arrest19,20. Conventional MRI sequences, T1- and T2- and DWI, are largely standardized across centers and are seen as essential to evaluating hypoxic-ischemic injury following cardiac arrest in adults21,22. Injury to brain regions selectively vulnerable to hypoxia-ischemia due to cardiac arrest, including the basal ganglia, thalamus, and cortical lobes, are associated with unfavorable neurological outcomes9–13,23. In our study, lesions in the lentiform nuclei (putamen and globus pallidus) on T2 were associated with unfavorable outcome at hospital discharge, in agreement with a prior non-randomized pilot study at our institution and other observational studies in pediatric cardiac arrest12,13. Although no brain regions were associated with outcome as assessed using on DWI sequences in our cohort, Oualha et al found associations between quantitative ADC in regions with diffusion abnormalities and outcome in children following cardiac arrest24. Although not statistically significant, generalized brain edema on MRI may add predictive utility as all three children with generalized brain edema had unfavorable outcomes, suggesting edema as a potential therapeutic target. It is important to note that presence of brain MRI lesions following cardiac arrest does not prohibit survival with favorable outcome13. Further, although MRI lesions often becomes apparent within a day of the cardiac arrest event, lesions evolve over days to weeks; thus, timing of imaging may impact interpretation25.
Brain MRS is a promising tool to aid in prognostication following pediatric brain injury14,26. While MRI utilizes hydrogen ions attached to water molecules to generate an anatomical map of brain tissue, proton MRS, as used in this study, measures signals from hydrogen nuclei attached to other molecules, to generate a biochemical profile from a prespecified ROI27. NAA is present in neurons and oligodendrocytes and thus serves as a marker of neuron density while lactate is a marker of anaerobic metabolism and potentially necrotic tissue27. Ashwal et al found that the detection of lactate in a combined ROI in the occipital gray and parietal white matter was associated with serious morbidity or death following pediatric brain injury14. Further, NAA/Creatine ratios were lower in children with lactate peaks. In our study, we were able to individually quantitate both NAA and lactate in four distinct regions of interest using LC Model software14,18. We found that although each of the regions of interest had similar patterns of MRS metabolite concentrations by patient outcome, the occipito-parietal region, a selectively vulnerable region following ischemia, was the most discriminatory between favorable and unfavorable outcome28.
Although we found signals for the benefit of longer duration of hypothermia, none were significant. Neonates with varying severity of hypoxic-ischemic encephalopathy in several trials who received hypothermia for 72 hours had less injury on brain MRI/S than neonates randomized to normothermia29–31. Future trials in pediatric cardiac arrest should consider including brain MRI/S and other MR sequences as a therapeutic evaluation tool to discern global and regional effects along with further exploration of the impact of a 72-h duration of hypothermia.
Our findings need to be validated in adequately powered prospective studies to inform optimal implementation into clinical prognostication guidelines. Once validated, clinicians and families will have more clarity on how brain MRI/S can best be incorporated into an individual child’s care plan to inform decision-making. For example, knowledge about the location and severity of brain injury can be used to anticipate approach to early rehabilitation interventions and future needs for successful reintegration into school. It is widely accepted that prognostication accuracy would be optimized using multiple tools together – the child’s neurological examination, brain MRI and other adjunctive testing and biomarkers, possibly at multiple time points32. Until then, recent publications from the American Heart Association suggest that brain MRI within the first 7 days post cardiac arrest may be useful for prognostication19,20,33.
Study limitations
The small sample size of this study prevents multivariate analysis of patient outcome. Due to the small number of deaths (3) our study was unable to analyze the predictive capability of brain MRI/S for prognostication of mortality. Given the number of statistical tests performed, there is a possibility for type II error, in which results stated as being statistically significant could be false. Our study analyzed associations with outcome at hospital discharge. Future validation studies should conduct analyses using outcomes post-hospital discharge to account for functional gains. In our study, one-third of children did not undergo MRI examination, and timing of MRI was dependent on clinical need. Children who did not undergo clinical MRI in this study had higher mortality and much shorter lengths of stay; conversely, of the three children surviving to hospital discharge, two had favorable outcome and may have been perceived as “too well” to benefit from brain MRI/S examination. This is a reflection of our center’s clinical approach to MRI in which imaging is delayed or not pursued for children who are too ill for intra-hospital transport, children who are unstable for intra-hospital transport, disqualified due to positive screening for contraindications such as body metal, or well-appearing, and in whom the benefit of an MRI evaluation is less well defined34. However, whether MRI/S and other MR sequences may help inform prognostication and clinical and school decision-making for children who are grossly well-appearing immediately post-arrest but at risk for cognitive or other sequelae remains to be defined. PCPC was assigned by the study PI (a pediatric intensivist), who was aware of the duration of hypothermia. The primary investigator is a pediatric intensivist that provides care for children following cardiac arrest and was not blinded to the original MRI findings. However, the PI had no input into pediatric neuroradiologist readings and scoring of MRIs.
CONCLUSION
Brain MRI/S biomarkers were associated for neurological outcome prognostication in pediatric cardiac arrest and should undergo validation testing in a larger sample.
Supplementary Material
ACKNOWLEGEMENTS
Special thanks to the UPMC Children’s Hospital of Pittsburgh Radiology staff especially Dennis Willaman, Gino Volpe for their safe and expert facilitation of this study and to Julia Wallace for her expertise in imaging data management. We are grateful to the staff, nurses, and physicians of the ICU for their efforts children and families who generously participated in this study.
Funding Source: NICHD K12 HD047349 (E.L.F.), NINDS K23 NS065132 (E.L.F.), Laerdal Foundation (E.L.F.), Department of Critical Care Medicine, University of Pittsburgh (E.L.F.) and NICHD K23HD099309 (JLW). This publication was also made possible by Grant Number 5UL1 RR024153-04 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. This project is registered with clinicaltrials.gov (NCT00797680) as “Duration of Hypothermia for Neuroprotection after Pediatric Cardiac Arrest: A Randomized, Controlled Trial”
Abbreviations:
- PICU
Pediatric intensive care unit
- RCT
Randomized controlled trial
- PCPC
Pediatric Cerebral Performance Category score
- NAA
N-acetyl-aspartate
- MRI/S
Magnetic resonance imaging/spectroscopy
- ROI
Regions of interest
- DWI
Diffusion weighted imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None
Conflict of Interest Disclosures: None
REFERENCES
- 1.Moler FW, Donaldson AE, Meert K, et al. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39(1):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke (Part II). Int Emerg Nurs. 2010;18(1):8–28. [DOI] [PubMed] [Google Scholar]
- 3.Slomine BS, Silverstein FS, Christensen JR, et al. Neurobehavioral Outcomes in Children After Out-of-Hospital Cardiac Arrest. Pediatrics. 2016;137(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119(11):1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get With the Guidelines-Resuscitation. Circulation. Cardiovascular quality and outcomes. 2013;6(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meert KL, Slomine BS, Christensen JR, et al. Family Burden After Out-of-Hospital Cardiac Arrest in Children. Pediatr Crit Care Med. 2016;17(6):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronco R, King W, Donley DK, Tilden SJ. Outcome and cost at a children’s hospital following resuscitation for out-of-hospital cardiopulmonary arrest. Arch Pediatr Adolesc Med. 1995;149(2):210–214. [DOI] [PubMed] [Google Scholar]
- 8.Mortamet G, Kossorotoff M, Baptiste A, et al. Description and Contribution of Brain Magnetic Resonance Imaging in Nontraumatic Critically Ill Children. J Child Neurol. 2016;31(14):1584–1590. [DOI] [PubMed] [Google Scholar]
- 9.Hogler S, Sterz F, Sipos W, et al. Distribution of neuropathological lesions in pig brains after different durations of cardiac arrest. Resuscitation. 2010;81(11):1577–1583. [DOI] [PubMed] [Google Scholar]
- 10.Back T, Hemmen T, Schuler OG. Lesion evolution in cerebral ischemia. J Neurol. 2004;251(4):388–397. [DOI] [PubMed] [Google Scholar]
- 11.Fink EL, Alexander H, Marco CD, et al. An experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatr Crit Care Med. 2004;5:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink EL, Panigrahy A, Clark RS, et al. Regional brain injury on conventional and diffusion weighted MRI is associated with outcome after pediatric cardiac arrest. Neurocrit Care. 2013;19(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christophe C, Fonteyne C, Ziereisen F, et al. Value of MR imaging of the brain in children with hypoxic coma. AJNR Am J Neuroradiol. 2002;23(4):716–723. [PMC free article] [PubMed] [Google Scholar]
- 14.Ashwal S, Holshouser BA, Tomasi LG, et al. 1H-magnetic resonance spectroscopy-determined cerebral lactate and poor neurological outcomes in children with central nervous system disease. Ann Neurol. 1997;41(4):470–481. [DOI] [PubMed] [Google Scholar]
- 15.Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999;20(8):1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 16.Fink EL, Clark RSB, Berger RP, et al. 24 vs. 72 hours of hypothermia for pediatric cardiac arrest: A pilot, randomized controlled trial. Resuscitation. 2018;126:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. [DOI] [PubMed] [Google Scholar]
- 18.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. [DOI] [PubMed] [Google Scholar]
- 19.Topjian AA, de Caen A, Wainwright MS, et al. Pediatric Post-Cardiac Arrest Care: A Scientific Statement From the American Heart Association. Circulation. 2019;140(6):e194–e233. [DOI] [PubMed] [Google Scholar]
- 20.Geocadin RG, Callaway CW, Fink EL, et al. Standards for Studies of Neurological Prognostication in Comatose Survivors of Cardiac Arrest: A Scientific Statement From the American Heart Association. Circulation. 2019;140(9):e517–e542. [DOI] [PubMed] [Google Scholar]
- 21.Ho ML, Campeau NG, Ngo TD, Udayasankar UK, Welker KM. Pediatric brain MRI part 1: basic techniques. Pediatr Radiol. 2017;47(5):534–543. [DOI] [PubMed] [Google Scholar]
- 22.Maciel CB, Barden MM, Youn TS, Dhakar MB, Greer DM. Neuroprognostication Practices in Postcardiac Arrest Patients: An International Survey of Critical Care Providers. Crit Care Med. 2019. [DOI] [PubMed] [Google Scholar]
- 23.Fink EL, Manole MD, Clark RSB. Rogers Textbook of Pediatric Intensive Care. 4 ed. Philadelphia, PA: Lippincott Williams & Williams; 2015. [Google Scholar]
- 24.Oualha M, Gatterre P, Boddaert N, et al. Early diffusion-weighted magnetic resonance imaging in children after cardiac arrest may provide valuable prognostic information on clinical outcome. Intensive Care Med. 2013;39(7):1306–1312. [DOI] [PubMed] [Google Scholar]
- 25.Greer D, Scripko P, Bartscher J, et al. Serial MRI changes in comatose cardiac arrest patients. Neurocrit Care. 2011;14(1):61–67. [DOI] [PubMed] [Google Scholar]
- 26.Ho ML, Campeau NG, Ngo TD, Udayasankar UK, Welker KM. Pediatric brain MRI, Part 2: Advanced techniques. Pediatr Radiol. 2017;47(5):544–555. [DOI] [PubMed] [Google Scholar]
- 27.Panigrahy A, Nelson MD Jr., Bluml S Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr Radiol. 2010;40(1):3–30. [DOI] [PubMed] [Google Scholar]
- 28.Graham DI. Pathology of hypoxic brain damage in man. J Clin Pathol Suppl (R Coll Pathol). 1977;11:170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shankaran S, Barnes PD, Hintz SR, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montaldo P, Lally PJ, Oliveira V, et al. Therapeutic hypothermia initiated within 6 hours of birth is associated with reduced brain injury on MR biomarkers in mild hypoxic-ischaemic encephalopathy: a non-randomised cohort study. Arch Dis Child Fetal Neonatal Ed. 2019;104(5):F515–F520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9(1):32–39. [DOI] [PubMed] [Google Scholar]
- 33.Topjian AA, de Caen A, Wainwright MS, et al. Pediatric Post-Cardiac Arrest Care: A Scientific Statement From the American Heart Association. Circulation. 2019:CIR0000000000000697. [DOI] [PubMed] [Google Scholar]
- 34.Tocchio S, Kline-Fath B, Kanal E, Schmithorst VJ, Panigrahy A. MRI evaluation and safety in the developing brain. Semin Perinatol. 2015;39(2):73–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


