Abstract
Heparin induced thrombocytopenia (HIT) is an immune mediated disorder caused by antibodies that recognize complexes of platelet factor 4 and heparin. Thrombosis is a central and unpredictable feature of this syndrome. Despite optimal management, disease morbidity and mortality from thrombosis remain high. The hypercoagulable state in HIT is biologically distinct from other thrombophilic disorders in that clinical complications are directly attributable to circulating ultra-large immune complexes (ULICs). In some individuals, ULICs elicit unchecked cellular procoagulant responses that culminate in thrombosis. To date, the clinical and biologic risk factors associated with thrombotic risk in HIT remain elusive. This review will summarize our current understanding of thrombosis in HIT with attention to its clinical features, cellular mechanisms, and its management.
Keywords: Heparin, thrombocytopenia, thrombosis, platelets, Basic, Translational, Clinical Research, Animal Models of Human Disease, Basic Science Research, Clinical Studies, Mechanisms, Pathophysiology, Platelets, Translational Studies
Graphical Abstract

INTRODUCTION
Unfractionated heparin (UFH) remains the mainstay of anticoagulation for indications for which there are no effective substitutes, such as anticoagulation for cardiopulmonary bypass (CPB) surgery, extracorporeal membrane oxygenator (ECMO) circuits, dialysis and mechanical prosthetic valves. The continued use of UFH in clinical practice places patients at risk for developing heparin induced thrombocytopenia (HIT), a potentially devastating immune disorder caused by antibodies to complexes of platelet factor 4 (PF4) and heparin (anti-PF4/H Abs).
Thrombosis is the most serious and life-threatening complication of HIT. Thrombosis occurs at presentation or complicates the course of illness in 20–64%.1, 2 Disease morbidity is further compounded by high rates of bleeding (~40%) associated with use of potent non-heparin anticoagulants for the prevention or treatment of HIT thrombosis. 2 Even with use of alternative anticoagulants, thrombosis contributes to fatal outcomes in ~6–26% of HIT patients.2–5
As thrombosis is central to disease morbidity and mortality in HIT, this review will summarize our current understanding of its risk factors, mechanisms, and treatment. For topics related to other aspects of the disease, such as the epidemiology3, 6, diagnosis7or laboratory testing8, 9 in HIT the reader is referred to corresponding reviews on these topics.
Clinical Features & Risk Factors for Thrombosis
HIT is primarily a disease of hospitalized or recently discharged patients. HIT occurs in 0.5–1% of patients exposed to UFH for medical and/or surgical indications with a markedly lower incidence (0.1–0.5%) in patients receiving LMWH.3, 6, 10, 11 While the impact of direct oral anticoagulants (DOACs) has yet to be directly investigated, observational data suggests that the incidence of HIT and its disease burden in recent years has not been significantly affected. Data from a publicly available US database indicate that the incidence of HIT has remained fairly stable between years 2009 and 2013, with a diagnostic prevalence between the initial and last year of the study period (2013) showing only a modest decrease.3 Of various populations exposed to heparin, those undergoing cardiac surgery appear to particularly high risk for HIT, despite the fact that only a subset of HIT antibodies generated are platelet-activating.12, 13 While isolated cases of spontaneous HIT have been reported in recent years,14–18 its incidence is likely rare and significantly less than that provoked by heparin exposure.
Clinical Features of Thrombosis
For the majority of patients, HIT presents in the context of recent exposure as a fall in platelet count, either as an absolute (<150,000/μL) or relative thrombocytopenia (>30% fall from baseline platelet count). Unlike other thrombocytopenic disorders, the low platelet counts in HIT do not increase bleeding risk, but rather serve as a marker of thrombotic risk. 19
The true incidence of thrombosis in HIT is uncertain due to few prospective studies of this disease and the challenges ascertaining HIT-related complications in critically ill patient populations with confounding risk factors. However, recent retrospective studies document the occurrence of thrombosis in ~20–64% of patients with HIT 1, 2 (See Table 1). New or progressive thromboses develop in ~19–40% of patients treated with alternative anticoagulants, while mortality varies depending on the study. Patients who develop isolated thrombocytopenia as initial disease presentation remain at significant risk for subsequent thrombosis occurring in 18–50%.5, 20
TABLE 1:
Clinical Features of Thrombosis in HIT
| Study | Type of Study (Total # of patients/# HIT patients) | Prevalence of thrombosis at time of diagnosis | Arterial v venous thrombosis events at presentation | Development of new/progressive thrombosis on treatment | Amputation | Death |
|---|---|---|---|---|---|---|
| Warkentin et al., 1996 20 | Single Center, Retrospective (127/127) | 51% | 1:4 | 76% | NR | 20% |
| Elalamy, et al., 20095 | Muticenter Retrospective (114/49) | 59% | 1:1 |
43% | 8% | 53% |
| Kuter et al., 20171 | Muticenter Retrospective (442/355) | 20% | NR* | 19% | 3% | 22% |
| Pishko et al., 2019 2] | Single Center Retrospective (310/42) | 64% | 1:4 |
36% | NR | 26% |
| Gruel et al., 20204 |
Muticenter Retrospective (144/144) | 40% | 1:3 | NR* | 4% | 6% |
Not recorded
Timing of Thrombosis
Whereas thrombocytopenia follows a predictable course after seroconversion, with platelet counts usually declining 2–4 days after seroconversion, thrombosis is less predictable and can occur at any time after detection of circulating anti-PF4/H Abs.21 In a large retrospective review of 408 patients with HIT, approximately two-thirds of patients developed thrombosis either concurrently (26%) or several days after thrombocytopenia (40%), while one-third experienced thrombosis prior to a fall in platelet counts. 19 Thrombotic risk in HIT extends for several weeks beyond duration of heparin exposure due to the presence of circulating anti-PF4/H Abs that recognize PF4 bound to endogenous glycosamginoglycans (GAGs) or other platelet components.22–24 Cross-reactivity of HIT antibodies with PF4 bound to cell-surface GAGs also explains why timing of heparin discontinuation has no measurable impact on disease progression in some patients.25 Indeed, PF4-dependent, but heparin-independent platelet activation26–29 may identify a subset of patients with “autoimmune” HIT whose disease manifestations are often delayed or severe.18
Types of Thrombosis
Thrombosis in HIT affects both arterial and venous beds, with venous thrombosis occurring three to four times more commonly than arterial thrombosis (Table 1).19 Deep venous thrombosis (DVT) and pulmonary embolism (PE) are the most common sites of venous thrombosis, while arterial thrombosis most often manifests as peripheral arterial embolism, followed by stroke and myocardial infarction. Arterial thrombosis may be more common after cardiac surgery,4 and spontaneous HIT may be more likely to be precipitated by recent orthopedic surgery.30 Distinctive, but uncommon, presentations of thrombosis include skin necrosis, venous limb gangrene, bilateral adrenal hemorrhage and cerebral vein thrombosis (CVT).31–35 Skin necrosis in HIT occurs at heparin injection sites and is one of the few clinical settings where HIT may present without accompanying thrombocytopenia.31 Venous limb gangrene is often precipitated by concurrent warfarin use and is caused by severe venous occlusion extending to venular beds.33, 36, 37 Adrenal hemorrhage is a thrombotic manifestation of HIT caused by occlusion of the sole adrenal central vein supplying the adrenal gland. This complication is most often seen in the post-operative setting and when bilateral, the patient presents with symptoms and signs of adrenal insufficiency (hypotension, nausea, vomiting and hyponatremia), if unrecognized, can progress to death from adrenal failure.32, 38Rare presentations of CVT have been reported in spontaneous 34, 35as well as drug-induced HIT. 39–42
Risk factors for Thrombosis
Numerous studies have examined biologic and clinical risk factors predisposing individuals to thrombotic complications in HIT. To date, there is no evidence to support a role for conventional thrombophilic abnormalities, such as Factor V Leiden or deficiencies of antithrombin, protein C or protein S.43, 44 As discussed below, polymorphic variation in the extracellular domain of the FcγIIA receptor (H131R; histidine to arginine at the 131 amino acid position) and its functional regulation by tyrosine phosphatases, CD148 and the T-Cell Ubiquitin Ligand-2 (TULA-2), have been identified as potential genetic causes influencing predisposition to thrombosis in HIT.45, 46 While genome wide association (GWAS) studies have identified several single nucleotide polymorphisms (SNPs) and HLA associations in small cohorts of HIT as compared to control patients, 47–50 these findings may be more germane to development of the immune response given the study design of comparing patients with HIT to those without. Other shortcomings of the GWAS studies, including differences in case ascertainment, lack of validation and absence of functional studies, presently limit the generalizability of these studies to understanding HIT pathogenesis.
Other clinical risk factors appear related to disease presentation. Several groups have identified cardiovascular disease and/or surgery as a major risk factors for arterial thrombosis.3, 4, 43, 51 Endothelial injury associated with central venous catheters places patients at increased risk for venous thrombosis.51, 52 Other clinical predictors of thrombotic risk include severe thrombocytopenia, with a >70% drop in platelet count increasing thrombotic risk by 8-fold, high antibody levels or titers in immunoassays or platelet activation assays and heparin-independent platelet activation seen in a subset of patients with autoimmune HIT which account for disease manifestations occurring after heparin discontinuation.53–56
Pathogenesis of Thrombosis in HIT
The intense hypercoagulable state in HIT is triggered by cellular activating anti-PF4/H Abs that principally engage cellular Fcγ receptor, FcγRIIA (on platelets, monocytes and neutrophils) 57, 58 or indirectly activate endothelial cells through non-FcγR mechanisms.59 The human FcγRIIA (CD32A) is a 40kDa, Type 1 transmembrane glycoprotein low affinity receptor for IgG that preferentially binds to IgG in immune complexes over monomeric IgG.60–62Clustering of the FcγRIIA receptors by immune complexes initiates phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) contained in the cytoplasmic tail of the receptor and activates downstream signaling via the spleen tyrosine kinase (Syk) resulting in release of intracellular Ca2+ stores, degranulation, cytokine production and cellular activation.60
Although FcγRIIA was implicated in cellular activation by HIT antibodies in the 1980’s,57 its essential role in disease pathogenesis was not proven until the development of the murine HIT model in 2001. As mice lack the human equivalent of FcγRIIA 63 and murine PF4 does not cross-react with HIT antibodies, 64 double transgenic mice, expressing hPF4 and hFcγRIIA, were generated to characterize the in vivo requirements for thrombosis. Single transgenic mice, expressing either the hPF4 or hFcγRIIA transgenes, did not develop thrombocytopenia or thrombosis when injected with a HIT like monoclonal antibody (KKO),64 but double transgenic mice developed severe thrombocytopenia (>80% drop in platelet counts) and thrombosis in multiple vascular beds (heart, liver, kidneys and lungs) in response to the monoclonal antibody.65 In addition to establishing the requirements for cellular FcγRIIA in HIT thrombosis, these murine studies were also first to demonstrate the vivo pathogenicity of circulating anti-PF4/H Abs.65
Cellular contributions to HIT Thrombosis
The following sections will describe the individual cellular contributions as well as the cellular interactions that promote and propagate thrombosis in HIT.
Platelets
Platelets play a prominent role in virtually all aspects of HIT, from their conspicuous involvement in disease expression (thrombocytopenia occurs in >94% of patients with HIT) 19 to their central role in functional assays often used for diagnostic confirmation.56 Early studies of HIT focused on effects of complement activation on platelet responses.66, 67 These studies demonstrated increased complement deposition on circulating platelets from HIT patients, 66 complement fixation by HIT IgG, 66, 67 and requirements for classical pathway components in platelet activation.66 Once platelet FcγRs were identified in 1987, 68 the field shifted its focus to investigations of this cellular receptor as a target for HIT immune complexes. Kelton et al.57 and Chong et al.58 demonstrated that platelet activation by HIT antibodies, as measured by the release reaction, required both F(ab’)2 and Fc portions of HIT IgG, was blocked by antibodies to FcγRII and did not involve the glycoprotein (GP) receptors, Ib/V/ and IIb/IIIa IX.57, 58 Other studies showed that while GP IIb/IIIa inhibitors could prevent platelet aggregation responses by HIT antibodies 69, 70, they did not prevent release of radiolabeled 14C-serotonin, 67, 71 which required ADP and P2Y12 or Gi-dependent signaling pathways.71, 72 In vivo studies using the murine HIT model have validated the importance of FcγRIIA signaling pathways by demonstrating the efficacy of the Syk kinase inhibitor, PRT-060318 (PRT318), in preventing HIT-induced thrombocytopenia and thrombosis.73
Platelets from healthy donors show considerable interindividual variation in responses to HIT ULICs; platelets from some healthy donors consistently activate in response to HIT ULICs while platelets from other donors are poorly responsive.74 This donor variability in platelet activation response is presumed to contribute to the heterogeneity seen in clinical disease. The mechanisms underlying this variability in platelet responses is not fully understood, but several recent studies have shown effects of genetic variants on FcγRIIA functional responses to HIT immune complexes. Of these genetic determinants, the FcγRIIA H131R polymorphism has garnered the most attention in the field. The H131R polymorphism shows differential affinity for binding the IgG2 subclass; the 131R allotype binds minimally to human IgG2 whereas the 131H binds IgG2 as well as other subclasses. Early clinical studies were largely inconclusive as to the thrombotic risk conferred by the H131R polymorphism.75–77 However, a recent study has revisited the functional consequences of the 131RR -allelic expression in the context of other endogenous IgG.4, 78 In these studies, Rollin and colleagues demonstrated that platelets from 131RR individuals have increased reactivity to HIT antibodies (generally, of the IgG1 subclass) due to inability of the RR isoform to bind endogenous IgG2. The 131HH allotype, on the other hand, binds native IgG2 and therefore offers fewer binding sites for platelet activating HIT IgG1.78 Consequently, platelets from individuals expressing the 131RR allele have greater reactivity to HIT IgG than platelets expressing other isoforms. In a prospective study of 144 HIT patients whose genotype was characterized for several genetic variants, including FcγRIIA H131R, HPA-1a/b dimorphism affecting glycoprotein IIIa, and platelet endothelial cell adhesion molecule-1 (L125V) thrombotic risk was increased only in individuals expressing the 131RR polymorphism, which was present in 38% of patients with thrombosis as compared to 18 % without thrombosis (OR 2.9).4 Other studies have also examined genetic variants regulating FcγRIIA signaling. Two polymorphisms on CD148 (276QQ and 326RR), a tyrosine phosphatase that can modulate FcγRIIA signaling were shown to be protective of thrombosis.79 Similar findings were noted in studies investigating TULA-2, a tyrosine phosphatase that dephosphorylates Syk.46, 80 In humans, TULA-2 expression was inversely correlated with platelet responsiveness to HIT antibodies, 80 while murine TULA-2 deficiency was associated with heightened platelet reactivity and severe thrombocytopenia in response to immune complexes.46
Once activated by HIT ULICs, platelets propagate thrombosis through generation of procoagulant microparticles, 81 upregulation of P-selectin that mediates formation of platelet leukocyte aggregates 82, 83 and release of intracellular PF4 and polyphosphates.84 Released PF4 binds to cellular GAGs on platelets and/or extruded von Willebrand factor on the endothelium to form antigenic sites for circulating HIT antibodies.85 See Figure 1.
Figure 1. Platelet contributions to thrombosis:
Binding of HIT ULICs to platelet FcγRIIA (1) initiates phosphorylation of ITAM motifs and downstream signaling via Syk kinase(2) leading to degranulation of alpha and dense granules leading to release of additional PF4, polyphosphate (PO4−n), ADP, and release of soluble P-selectin and microparticles (3). Released ADP binds to G-coupled receptors, P2Y12 initiating Gi-dependent intracellular signaling (4) resulting in further activation signals to generate “coated” platelets. Negative regulators include increased expression of TULA-2 and polymorphisms of the tyrosine phosphatase CD148 (5).
Monocytes
Findings of monocytes and neutrophil involvement in HIT pathogenesis could be traced to early studies by Kelton and colleagues 57 who showed that addition of monocytes and neutrophils to platelets, at physiologic ratios, inhibited platelet activation by HIT antibodies. These observations suggested competitive binding of HIT antibodies to cellular GAG or FcγR. Indeed, subsequent studies confirmed differential binding of HIT ULICs to monocytes as compared to platelets. This increased binding serves as an important physiologic reservoir for HIT antibodies and has a moderating effect on thrombocytopenia in vivo. In the murine HIT model, depletion of monocytes using clodronate liposomes or gadolinium chloride markedly exacerbates thrombocytopenia, as monocytes are not available to bind HIT antibodies.86 Increased ULIC binding to monocytes results in heightened procoagulant activity, as indicated by upregulation of TF mRNA, 87, 88 release of TF-bearing microparticles 89 and increased cell surface TF expression in response to KKO or HIT IgG.87–90 Monocyte involvement in thrombosis was also evident in the size of thrombi formed after cellular depletion. In the murine HIT model, monocyte depletion markedly inhibited thrombus formation after infusion of KKO,86 while ex vivo depletion of monocytes from human blood attenuated platelet deposition and fibrin generation.91 ULIC-mediated effects on monocyte activation were dependent on FcγRIIA, and downstream signaling via Syk kinases, as inhibition of this pathway markedly reduced thrombin and fibrin formation.88, 91 In turn, thrombin generated from monocyte TF activates platelet protease-activated receptor 1 (PAR-1).91 Dual engagement of platelet PAR-1 and FcγRIIA leads to generation of “coated” platelets, 91 a subpopulation of highly activated platelets formed by dual activation with thrombin and collagen or thrombin and FcγRIIA.92 Monocytes activated by HIT ULICs additionally upregulate CD11b (Mac-1) expression which promotes formation of platelet-monocyte aggregates.83 See Figure 2.
Figure 2. Monocyte contributions to thrombosis:
PF4 bound to cell-surface GAGs serves as an important physiologic reservoir for binding HIT antibodies (1). Circulating ULICs also engage cellular FcγRIIA (2) initiating downstream signaling via Syk kinase(3) leading to upregulation of cell surface TF and release of TF-bearing microparticles that promote thrombin (IIa) generation (4) which then activates platelet protease activated receptor-1 to generate coated-platelets. Activated monocytes upregulate CD11b/Mac-1 which binds to circulating platelets to form leukocyte-platelet aggregates (5).
Neutrophils
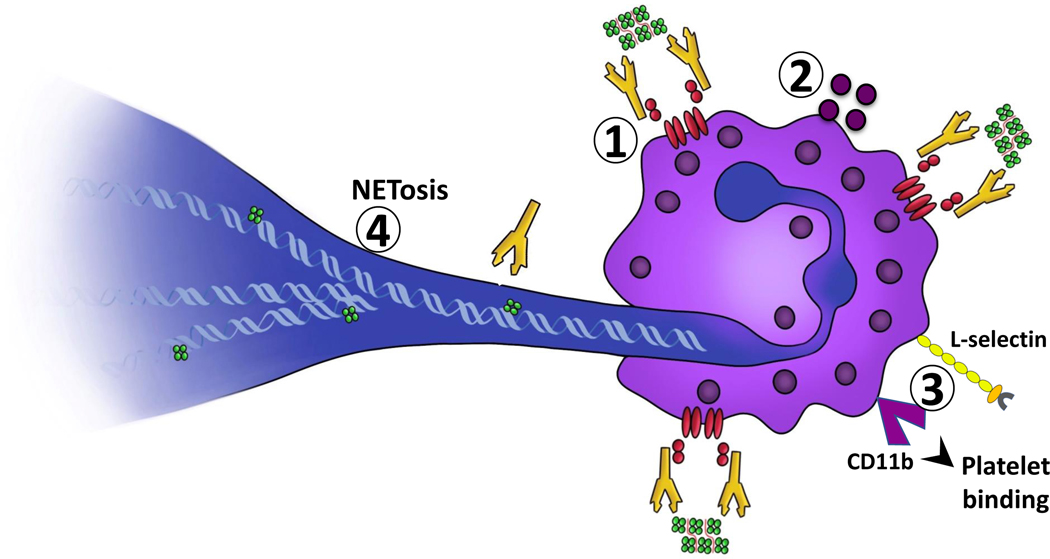
Similar to other FcγR expressing cells, neutrophils are readily activated by HIT ULICs. Cellular activation is accompanied by increased CD11b expression, 83, 93 degranulation 83, 93–95 and increased cell adhesion via L-selectin.93 These activation events, however, do not appear as essential for thrombus formation as the ability of activated cells to release neutrophil extracellular traps (NETs). NETs are extruded strands of deoxyribonucleic acid (DNA) and granular material that serve important functions in innate immunity, by ensnaring pathogens.96 NETs are also critical for thrombus formation,97 as they directly engage platelets, red blood cells, and circulating hemostatic proteins such as PF4, 98 fibronectin and von Willebrand Factor.99 Two recent studies by Gollomp et al.98 and Perdomo et al.95 showed that neutrophils promote thrombosis in HIT through formation of NETS. These investigators showed that patients with HIT, as compared to control subjects, have evidence of NETosis in plasma, as indicated by increased levels of cell free DNA, citrullinated histones and myeloperoxidase.95, 98 Further, in microfluidic studies using human blood, HIT plasma or IgG binds NETs,98 protects DNA from degradation by deoxyribonucleases95, 98 and induces neutrophil-dependent thrombus formation.95 Using a cremaster injury model, Gollomp and colleagues showed that there was significant neutrophil and platelet accumulation in venous, but not arterial thrombi, findings which were reinforced in HIT mice lacking peptidyl arginine deiminase 4, an enzyme essential for NETosis.98 Using a differing approach for assessing thrombosis, by evaluating spontaneous pulmonary embolism rather than laser-induced injury occlusion, Perdomo and colleagues found that inhibition of NETosis through chemical or genetic approaches abrogated thrombosis in HIT mice, a process dependent on neutrophil FcγRIIA, but appeared relatively independent of platelets.95 It should be noted that while platelets were not considered essential for NETosis in both murine studies, their presence was a critical determinant of thrombus size.95, 98 These murine studies not only demonstrating a critical role for neutrophil involvement in HIT, but also importantly furnish a mechanism (NETs) by which these cells promote thrombosis. See Figure 3.
Figure 3. Neutrophil contributions to thrombosis:
Circulating ULICs engage cellular FcγRIIA (1) initiating neutrophil activation, degranulation (2) upregulation of L-selectin and CD11b/Mac-1 to promote adhesion and formation of leukocyte platelet aggregates (3). Activated neutrophils extrude NETs which bind PF4 and HIT ULICs (4).
Endothelial Cells
While there is persuasive clinical and laboratory data indicating endothelial involvement in HIT, the mechanisms by which HIT ULICs activate these cells is less clear. Of endothelial cells, only dermal microvascular cells express the activating FcγRIIA,100 while a limited number of other endothelial beds (liver sinusoidal, placental and aortic endothelial cells) express the inhibitory receptor FcγRIIB.101 While there is no evidence for direct endothelial activation, there is ample support for ULIC-mediated bystander injury.
Histologic studies of the microvasculature from HIT patients undergoing limb amputations indicate multiple platelet thrombi, infiltrating endothelial cells and intraluminal hyperplasia of endothelial cells (ECs).102 Patient antibodies bind directly to ECs via cellular GAGs,59, 64, 103 promote complement deposition on cell surfaces,59 and induce procoagulant activity.59 EC activation by HIT serum or IgG is markedly enhanced by platelets, leading to increased expression of cellular adhesion proteins (E-selectin, ICAM-1, VCAM), cytokine release, (IL-1 β, IL6, PAI-1, and TNFα)and platelet deposition104. These cellular effects of platelets are attenuated by selective inhibition of platelet GPIIb/IIIa receptors or ADP.104 In microfluidic studies, KKO and/or HIT patient antibodies readily bind PF4-VWF complexes, promoting platelet adhesion and enlargement of thrombi within the microfluidic channels.85 In microfluidic chambers coated with human umbilical vein endothelial cells (HUVECs) or endothelial cells from human aortas, chemical EC injury is accompanied by release of vWF from the EC glycocalyx, 105 and increased PF4 binding to vWF strings leading to expression of antigenic sites for HIT antibodies.85, 105 In HIT transgenic mice, HIT antibodies exacerbate thrombus formation at peri-injury sites on the endothelium where released PF4 binds to the endothelium and thrombus occlusion can be modulated through use of ADAMTS13 or N-acetylcysteine.85 These latter studies are in keeping with clinical findings showing that sites of endothelial injury, such as vessels containing central venous catheters are particularly prone to thrombosis.51
Taken together, these studies suggest that the endothelium promotes thrombosis in HIT through responses to direct or indirect cellular injury. Direct physical injury, perhaps through catheterization, surgery or underlying atherosclerotic disease, releases hemostatic vWF multimers that support thrombosis through binding platelets and providing additional binding sites for HIT antibodies. Intact endothelium could be activated indirectly by complement activation and/or secreted products of FcγRIIA bearing cells, leading to expression of adhesion markers and/or TF expression. See Figure 4.
Figure 4. Endothelial cell contributions to thrombosis:
Circulating ULICs or HIT antibodies bind to endothelial cell GAGs (1) triggering complement activation, deposition and EC activation(2). ECs express TF (3), and in the presence of activated platelets, upregulate cell-surface adhesive markers, E-selectin, ICAM-1 and VCAM (4). Activated ECs release vWF strings that bind PF4 to serve as new antigenic targets for HIT antibodies(5).
Treatment
Treatment in HIT is directed at lowering the intense thrombin generation that accompanies disease.106 A number of on-label and off-label therapies have been used in the management of HIT and are briefly discussed below. For detailed information related to pharmacology, dosing and/or monitoring of therapies, the reader is referred to recent guidelines 107 and reviews.108, 109
Direct Thrombin Inhibitors (DTIs)
Argatroban and bivalirudin are FDA-approved medications for HIT management, the latter in the setting of percutaneous coronary interventions in HIT patients. In clinical trials leading to drug approval, argatroban reduced the risk of composite outcome (new thrombosis, death due to thrombosis, amputation due to thrombosis) compared to historical controls.110 Due to hepatic clearance, argatroban has a limited role in patients with severe liver disease. Bivalirudin, has a shorter half-life of approximately 25 min in patients with normal renal function. This agent has been studied in detail as a non-heparin alternative for cardiopulmonary bypass for patients with acute/subacute HIT requiring emergent cardiac surgery111, 112 but is not commonly used for this indication at most medical centers due to high rates of bleeding.
Important considerations when using DTIs is the concept of anticoagulation confounding due to baseline elevations of the activated partial thromboplastin time (aPTT) or international normalized ratio (INR). Baseline elevation of aPTT or INR can be caused by DTIs or HIT-associated complications of disseminated intravascular coagulation and contribute to under-dosing of DTI therapy leading to subsequent treatment failure from subtherapeutic anticoagulation.113
There is increasing recognition that bleeding complications from use of alternative anticoagulants also contributes to disease morbidity in HIT. Indeed, recent studies indicate bleeding complications occur in 38–44% of patients treated with non-heparin anticoagulants. 1, 2 The prothrombotic environment of HIT does not mitigate bleeding risk, as several studies show that HIT patients appear as susceptible to bleeding complications as those without disease. In a large retrospective study of ~300 patients treated with alternative anticoagulation for suspected or confirmed HIT, bleeding rates were similar in patients with or without disease, while critical illness, renal dysfunction and platelet counts <25 × 109/L thrombocytopenia were more predictive of bleeding risk. 2
Heparin-derivatives
LMWH is contraindicated in HIT due to high-rates of cross-reactivity with HIT antibodies.114 Fondaparinux, a synthetic pentasaccharide containing the heparin binding sequence of antithrombin, shows minimal cross-reactivity with HIT antibodies in vivo 115 and by itself, is a rare cause of HIT.116 Fondaparinux is often used off-label in patients with HIT, 117 but is of limited utility in patients with renal disease due to renal clearance and a long half-life (15–20 hours).118 Danaparoid is a heparinoid that comprises primarily of heparan sulfate, but is not available in the United States.119 In addition to its anticoagulant effect, the drug inhibits formation of PF4-heparin complexes which may additionally contribute to its efficacy.120
Direct oral anticoagulants (DOACs)
DOACs are increasingly being used off-label for the treatment of HIT. Of these drugs, rivaroxaban has the most published experience. A prospective observational study by Linkins and colleagues on the use of rivaroxaban for treatment of serologically confirmed HIT, while encouraging, had to close prematurely due to low patient accrual.121 A recent literature review of published and post-trial experience of DOACs by the same Hamilton group indicate that these agents are safe and effective for use in acute HIT, with findings of no complications of bleeding and thrombosis occurring in only 1/46 (2.2%) patients treated with rivaroxaban.122 Comparable findings were noted in patients treated with apixaban and dabigatran. Based on this and similar reports in the literature, 123 the American Society of Hematology guideline panel on HIT provided conditional recommendations for use of DOACs in acute HIT in clinically stable patients who are considered average risk for bleeding.107
Non-anticoagulant therapies
Despite maximal anticoagulation, some HIT patients develop refractory disease, characterized by severe and persistent thrombocytopenia, new and/or progressive thrombosis. Some of these patients respond to additional therapies directed at the immune response through treatment with intravenous immunoglobulin (IVIG) 124 or therapeutic plasma exchange (TPE). IVIG was first reported as an adjunctive therapy in 1989 for thrombocytopenia.125 Subsequent reports have shown that IVIG disrupts platelet activation by HIT antibodies by interfering with FcγRIIA- dependent platelet activation 126, 127 and is effective for treatment of thrombosis 124, 127, 128 and/or refractory disease.127, 129, 130 While there are concerns about thrombotic risk with IVIG 131, 132, a recent report utilizing a large inpatient health care database suggests that treatment may be safe.133 TPE is another modality that is often employed as an adjunctive therapy for management of acute or subacute HIT, particularly for management of emergent cardiac surgery 134 or as salvage therapy for refractory disease.135 The efficacy of TPE in HIT is presumed to be secondary to removal of anti-PF4/H Abs, 136 but drawbacks include only transient effects on antibody removal and limited availability in the community setting. Emerging therapies at the pre-clinical stage include bacterial proteases to cleave IgG (IdeS or IgG‐degrading enzyme of Streptococcus pyogenes 137 Syk 73 and tyrosine kinase inhibitors 62 as well as PF4 tetramerization inhibitors.138, 139
Conclusion
Thrombosis in HIT represents an orchestrated multicellular response to HIT ULICs. HIT ULICs trigger cell-specific procoagulant responses that are mutually reinforcing to generate a profoundly hypercoagulable state. At present, the reasons why some seropositive patients develop this potent cellular response, while most patients do not, remain elusive. While some of these differences can be explained by genetic variation in various effector mechanisms, 45, 46, 78, 80 they do not fully account for heterogeneity of disease expression. Additional studies focused on less characterized aspects of the disease, such as complement activation, structural studies of antigenic complexes and characterization of pathogenic and non-pathogenic antibodies are needed. New insights into mechanisms of thrombosis in HIT should translate to an improved understanding of other immune-mediate thrombotic disorders, such as the anti-phospholipid antibody syndrome and/or systemic lupus erythematosus.
Highlights:
Heparin induced thrombocytopenia (HIT) remains a clinically relevant medical problem due to continued use of unfractionated heparin in hospitalized patients.
The last two decades have witnessed significant progress in our understanding of the clinical manifestations and biologic mechanisms underlying thrombotic complications in HIT.
The profound hypercoagulable state in HIT is generated by cellular activating antibodies that initiate cell-specific prothrombotic responses.
Treatment outcomes in HIT remain suboptimal as current therapies do not interfere with cellular activating effects of HIT antibodies.
Acknowledgements
a) Acknowledgments: Both authors provide written acknowledgment of their approval of the submitted article. The authors wish to thank Ms. Evelyn Freel for her artwork.
b) Sources of Funding: Supported by the National Institutes of Health HL136512 (GMA), HL151730 (GMA) and K08 HL133479 (AP).
ABBREVIATIONS
- anti-PF4/H
Abs anti-platelet factor 4 antibodies
- DOAC
direct oral anticoagulant
- EC
endothelial cell
- GAG
glycosaminoglycan
- HIT
heparin induced thrombocytopenia
- HUVEC
human umbilical vein cells
- IL
interleukin
- IVIG
intravenous immunoglobulin
- NET
neutrophil extracellular traps
- PF4
platelet factor 4
- Syk
spleen tyrosine kinase
- TF
tissue factor
- ULIC
ultra-large immune complexes
- vWFvon
willebrand factor
Footnotes
c) Disclosures: GMA receives royalties from Biokit manufacturer of a HIT diagnostic assay and is a consultant for Novartis, Apotex and Veralox therapeutics. AP is on the advisory board of Veralox therapeutics, equity ownership in Retham Technologies and has pending patents assigned to Versiti inc and Retham Technologies.
References
- 1.Kuter DJ, Konkle BA, Hamza TH, et al. Clinical outcomes in a cohort of patients with heparin-induced thrombocytopenia. Am J Hematol. 2017;92:730–738 [DOI] [PubMed] [Google Scholar]
- 2.Pishko AM, Lefler DS, Gimotty P, Paydary K, Fardin S, Arepally GM, Crowther M, Rice L, Vega R, Cines DB, Guevara JP, Cuker A. The risk of major bleeding in patients with suspected heparin-induced thrombocytopenia. J Thromb Haemost. 2019;17:1956–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhakal B, Kreuziger LB, Rein L, Kleman A, Fraser R, Aster RH, Hari P, Padmanabhan A. Disease burden, complication rates, and health-care costs of heparin-induced thrombocytopenia in the USA: A population-based study. Lancet Haematol. 2018;5:e220–e231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruel Y, Vayne C, Rollin J, et al. Comparative analysis of a French prospective series of 144 patients with heparin-induced thrombocytopenia (FRIGTIH) and the literature. Thromb Haemost. 2020;120:1096–1107 [DOI] [PubMed] [Google Scholar]
- 5.Elalamy I, Tardy-Poncet B, Mulot A, de Maistre E, Pouplard C, Nguyen P, Cleret B, Gruel Y, Lecompte T, Tardy B, Group GHS. Risk factors for unfavorable clinical outcome in patients with documented heparin-induced thrombocytopenia. Thromb Res. 2009;124:554–559 [DOI] [PubMed] [Google Scholar]
- 6.Aguayo E, Sanaiha Y, Seo YJ, Mardock A, Bailey K, Dobaria V, Benharash P. Heparin-induced thrombocytopenia in cardiac surgery: Incidence, costs, and duration of stay. Surgery. 2018;164:1377–1381 [DOI] [PubMed] [Google Scholar]
- 7.Favaloro EJ, McCaughan G, Mohammed S, Lau KKE, Gemmell R, Cavanaugh L, Donikian D, Kondo M, Brighton T, Pasalic L. Hit or miss? A comprehensive contemporary investigation of laboratory tests for heparin induced thrombocytopenia. Pathology. 2018;50:426–436 [DOI] [PubMed] [Google Scholar]
- 8.Husseinzadeh HD, Gimotty PA, Pishko AM, Buckley M, Warkentin TE, Cuker A. Diagnostic accuracy of igg-specific versus polyspecific enzyme-linked immunoassays in heparin-induced thrombocytopenia: A systematic review and meta-analysis. J Thromb Haemost. 2017;15:1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tardy B, Lecompte T, Mullier F, Vayne C, Pouplard C . Detection of platelet-activating antibodies associated with heparin-induced thrombocytopenia. J Clin Med. 2020;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D, Warkentin TE, Zytaruk N, Crowthe M, Geerts W, Cooper DJ, Vallance S, Qushmaq I, Rocha M, Berwanger O, Vlahakis NE. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364:1305–1314 [DOI] [PubMed] [Google Scholar]
- 11.Girolami B, Prandoni P, Stefani PM, Tanduo C, Sabbion P, Eichler P, Ramon R, Baggio G, Fabris F, Girolami A. The incidence of heparin-induced thrombocytopenia in hospitalized medical patients treated with subcutaneous unfractionated heparin: A prospective cohort study. Blood. 2003;101:2955–2959 [DOI] [PubMed] [Google Scholar]
- 12.Pouplard C, May MA, Iochmann S, Amiral J, Vissac AM, Marchand M, Gruel Y. Antibodies to platelet factor 4-heparin after cardiopulmonary bypass in patients anticoagulated with unfractionated heparin or a low-molecular-weight heparin : Clinical implications for heparin-induced thrombocytopenia. Circulation. 1999;99:2530–2536 [DOI] [PubMed] [Google Scholar]
- 13.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using pf4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6:1304–1312 [DOI] [PubMed] [Google Scholar]
- 14.Mallik A, Carlson KB, DeSancho MT. A patient with ‘spontaneous’ heparin-induced thrombocytopenia and thrombosis after undergoing knee replacement. Blood Coagul Fibrinolysis. 2011;22:73–75 [DOI] [PubMed] [Google Scholar]
- 15.Perrin J, Barraud D, Toussaint-Hacquard M, Bollaert PE, Lecompte T. Rapid onset heparin-induced thrombocytopenia (hit) without history of heparin exposure: A new case of so-called ‘spontaneous’ hit. Thromb Haemost. 2012;107:795–797 [DOI] [PubMed] [Google Scholar]
- 16.Warkentin TE, Basciano PA, Knopman J, Bernstein RA. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood. 2014;123:3651–3654 [DOI] [PubMed] [Google Scholar]
- 17.Okata T, Miyata S, Miyashita F, Maeda T, Toyoda K. Spontaneous heparin-induced thrombocytopenia syndrome without any proximate heparin exposure, infection, or inflammatory condition: Atypical clinical features with heparin-dependent platelet activating antibodies. Platelets. 2015;26:602–607 [DOI] [PubMed] [Google Scholar]
- 18.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15:2099–2114 [DOI] [PubMed] [Google Scholar]
- 19.Greinacher A, Farner B, Kroll H, Kohlmann T, Warkentin TE, Eichler P. Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408 patients. Thromb Haemost. 2005;94:132–135 [DOI] [PubMed] [Google Scholar]
- 20.Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996;101:502–507 [DOI] [PubMed] [Google Scholar]
- 21.Warkentin TE, Sheppard JA, Moore JC, Cook RJ, Kelton JG. Studies of the immune response in heparin-induced thrombocytopenia. Blood. 2009;113:4963–4969 [DOI] [PubMed] [Google Scholar]
- 22.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001;135:502–506 [DOI] [PubMed] [Google Scholar]
- 23.Rice L, Attisha WK, Drexler A, Francis JL. Delayed-onset heparin-induced thrombocytopenia. Ann Intern Med. 2002;136:210–215 [DOI] [PubMed] [Google Scholar]
- 24.Padmanabhan A, Jones CG, Bougie DW, Curtis BR, McFarland JG, Wang D, Aster RH. Heparin-independent, pf4-dependent binding of hit antibodies to platelets: Implications for hit pathogenesis. Blood. 2015;125:155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallis DE, Workman DL, Lewis BE, Steen L, Pifarre R, Moran JF. Failure of early heparin cessation as treatment for heparin-induced thrombocytopenia. Am J Med. 1999;106:629–635 [DOI] [PubMed] [Google Scholar]
- 26.Padmanabhan A, Jones CG, Curtis BR, Bougie DW, Sullivan MJ, Peswani N, McFarland JG, Eastwood D, Wang D, Aster RH. A novel pf4-dependent platelet activation assay identifies patients likely to have heparin-induced thrombocytopenia/thrombosis (HIT). Chest. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuelson-Bannow BT, Warad D, Jones C, et al. A prospective, blinded study of a pf4-dependent assay for hit diagnosis. Blood. 2020. (First Edition). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones CG, Pechauer SM, Curtis BR, Bougie DW, Irani MS, Dhakal B, Pierce B, Aster RH, Padmanabhan A. A platelet factor 4-dependent platelet activation assay facilitates early detection of pathogenic heparin-induced thrombocytopenia antibodies. Chest. 2017;152:e77–e80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irani M, Siegal E, Jella A, Aster R, Padmanabhan A. Use of intravenous immunoglobulin g to treat spontaneous heparin-induced thrombocytopenia. Transfusion. 2019;59:931–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poudel DR, Ghimire S, Dhital R, Forman DA, Warkentin TE. Spontaneous hit syndrome post-knee replacement surgery with delayed recovery of thrombocytopenia: A case report and literature review. Platelets. 2017;28:614–620 [DOI] [PubMed] [Google Scholar]
- 31.Handschin AE, Trentz O, Kock HJ, Wanner GA. Low molecular weight heparin-induced skin necrosis-a systematic review. Arch Surg 2005;390:249–254 [DOI] [PubMed] [Google Scholar]
- 32.Rosenberger LH, Smith PW, Sawyer RG, Hanks JB, Adams RB, Hedrick TL. Bilateral adrenal hemorrhage: The unrecognized cause of hemodynamic collapse associated with heparin-induced thrombocytopenia. Crit Care Med. 2011;39:833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warkentin TE, Elavathil LJ, Hayward CP, Johnston MA, Russett JI, Kelton JG. The pathogenesis of venous limb gangrene associated with heparin-induced thrombocytopenia. Ann Int Med. 1997;127:804–812 [DOI] [PubMed] [Google Scholar]
- 34.Hwang SR, Wang Y, Weil EL, Padmanabhan A, Warkentin TE, Pruthi RK. Cerebral venous sinus thrombosis associated with spontaneous heparin-induced thrombocytopenia syndrome after total knee arthroplasty. Platelets. 2020:1–5 [DOI] [PubMed] [Google Scholar]
- 35.Moores G, Warkentin TE, Farooqi MAM, Jevtic SD, Zeller MP, Perera KS. Spontaneous heparin-induced thrombocytopenia presenting as cerebral venous sinus thrombosis. Neurology: Clinical Practice. 2020: 10.1212/CPJ.0000000000000805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warkentin TE, Kelton JG, Crowther MA, Heddle NM, Hayward CP, Warkentin T. Venous limb gangrene during warfarin treatment of cancer-associated deep venous thrombosis. Ann Int Med. 2001;135:589–593 [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan AF, Rice L, Bartholomew JR, Rangaswamy C, La Perna L, Thompson JE, Murphy S, Baker KR. Warfarin-induced skin necrosis and venous limb gangrene in the setting of heparin-induced thrombocytopenia. Arch Intern Med. 2004;164:66–70 [DOI] [PubMed] [Google Scholar]
- 38.Warkentin TE, Safyan EL, Linkins LA. Heparin-induced thrombocytopenia presenting as bilateral adrenal hemorrhages. N Engl J Med. 2015;372:492–494 [DOI] [PubMed] [Google Scholar]
- 39.Richard S, Perrin J, Lavandier K, Lacour JC, Ducrocq X. Cerebral venous thrombosis due to essential thrombocythemia and worsened by heparin-induced thrombocytopenia and thrombosis. Platelets. 2011;22:157–159 [DOI] [PubMed] [Google Scholar]
- 40.Randi ML, Tezza F, Scapin M, Duner E, Scarparo P, Scandellari R, Fabris F. Heparin-induced thrombocytopenia in patients with philadelphia-negative myeloproliferative disorders and unusual splanchnic or cerebral vein thrombosis. Acta Haematol. 2010;123:140–145 [DOI] [PubMed] [Google Scholar]
- 41.Meýer-Lindenberg A, Quenzel EM, Bierhoff E, Wolff H, Schindler E, Biniek R. Fatal cerebral venous sinus thrombosis in heparin-induced thrombotic thrombocytopenia. Eur Neurol. 1997;37:191–192 [DOI] [PubMed] [Google Scholar]
- 42.Kyritsis AP, Williams EC, Schutta HS. Cerebral venous thrombosis due to heparin-induced thrombocytopenia. Stroke. 1990;21:1503–1505 [DOI] [PubMed] [Google Scholar]
- 43.Boshkov LK, Warkentin TE, Hayward CP, Andrew M, Kelton JG. Heparin-induced thrombocytopenia and thrombosis: Clinical and laboratory studies. British Journal of Haematology. 1993;84:322–328 [DOI] [PubMed] [Google Scholar]
- 44.Lee DH, Warkentin TE, Denomme GA, Lagrotteria DD, Kelton JG. Factor v leiden and thrombotic complications in heparin-induced thrombocytopenia. Thromb Haemost. 1998;79:50–53 [PubMed] [Google Scholar]
- 45.Rollin J, Pouplard C, Gratacap MP, Leroux D, May MA, Aupart M, Gouilleux-Gruart V, Payrastre B, Gruel Y. Polymorphisms of protein tyrosine phosphatase cd148 influence fcgammariia-dependent platelet activation and the risk of heparin-induced thrombocytopenia. Blood. 2012;120:1309–1316 [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y, Abraham S, Renna S, Edelstein LC, Dangelmaier CA, Tsygankov AY, Kunapuli SP, Bray PF, McKenzie SE. Tula-2 (t-cell ubiquitin ligand-2) inhibits the platelet fc receptor for igg iia (fcgammariia) signaling pathway and heparin-induced thrombocytopenia in mice. Arterioscler Thromb Vasc Biol. 2016;36:2315–2323 [DOI] [PubMed] [Google Scholar]
- 47.Witten A, Bolbrinker J, Barysenka A, Huber M, Rühle F, Nowak-Göttl U, Garbe E, Kreutz R, Stoll M. Targeted resequencing of a locus for heparin-induced thrombocytopenia on chromosome 5 identified in a genome-wide association study. J Mol Med (Berl). 2018;96:765–775 [DOI] [PubMed] [Google Scholar]
- 48.Karnes JH, Cronin RM, Rollin J, et al. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost. 2015;113:772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karnes JH, Shaffer CM, Cronin R, Bastarache L, Gaudieri S, James I, Pavlos R, Steiner HE, Mosley JD, Mallal S, Denny JC, Phillips EJ, Roden DM. Influence of human leukocyte antigen (hla) alleles and killer cell immunoglobulin-like receptors (kir) types on heparin-induced thrombocytopenia (hit). Pharmacotherapy. 2017;37:1164–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pouplard C, Cornillet-Lefebvre P, Attaoua R, Leroux D, Lecocq-Lafon C, Rollin J, Grigorescu F, Nguyen P, Gruel Y. Interleukin-10 promoter microsatellite polymorphisms influence the immune response to heparin and the risk of heparin-induced thrombocytopenia. Thromb Res. 2012;129:465–469 [DOI] [PubMed] [Google Scholar]
- 51.Hong AP, Cook DJ, Sigouin CS, Warkentin TE. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood. 2003;101:3049–3051 [DOI] [PubMed] [Google Scholar]
- 52.Mian H, Warkentin TE, Sheppard JI, MacDonald A, Linkins LA, Benger A, Foley R. Autoimmune hit due to apheresis catheter heparin flushes for stem cell harvesting before autotransplantation for myeloma. Blood. 2017;130:1679–1682 [DOI] [PubMed] [Google Scholar]
- 53.Alberio L, Kimmerle S, Baumann A, Taleghani BM, Biasiutti FD, Lammle B. Rapid determination of anti-heparin/platelet factor 4 antibody titers in the diagnosis of heparin-induced thrombocytopenia. Am J Med. 2003;114:528–536 [DOI] [PubMed] [Google Scholar]
- 54.Baroletti S, Hurwitz S, Conti NA, Fanikos J, Piazza G, Goldhaber SZ. Thrombosis in suspected heparin-induced thrombocytopenia occurs more often with high antibody levels. Am J Med. 2012;125:44–49 [DOI] [PubMed] [Google Scholar]
- 55.Zwicker JI, Uhl L, Huang WY, Shaz BH, Bauer KA. Thrombosis and elisa optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost. 2004;2:2133–2137 [DOI] [PubMed] [Google Scholar]
- 56.Warkentin TE, Sheppard J-AI, Moore JC, Moore KM, Sigouin CS, Kelton JG. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: How much class do we need? J Lab Clin Med. 2005;146:341–346 [DOI] [PubMed] [Google Scholar]
- 57.Kelton JG, Sheridan D, Santos A, Smith J, Steeves K, Smith C, Brown C, Murphy WG. Heparin-induced thrombocytopenia: Laboratory studies. Blood. 1988;72:925–930 [PubMed] [Google Scholar]
- 58.Chong BH, Fawaz I, Chesterman CN, Berndt MC. Heparin-induced thrombocytopenia: Mechanism of interaction of the heparin-dependent antibody with platelets. Br J Haematol. 1989;73:235–240 [DOI] [PubMed] [Google Scholar]
- 59.Cines DB, Tomaski A, Tannenbaum S. Immune endothelial-cell injury in heparin-associated thrombocytopenia. N Engl J Med. 1987;316:581–589 [DOI] [PubMed] [Google Scholar]
- 60.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234 [DOI] [PubMed] [Google Scholar]
- 61.Arman M, Krauel K. Human platelet igg fc receptor fcgammariia in immunity and thrombosis. J Thromb Haemost. 2015;13:893–908 [DOI] [PubMed] [Google Scholar]
- 62.Goldmann L, Duan R, Kragh T, Wittmann G, Weber C, Lorenz R, von Hundelshausen P, Spannagl M, Siess W. Oral bruton tyrosine kinase inhibitors block activation of the platelet fc receptor cd32a (fcgammariia): A new option in hit? Blood Adv. 2019;3:4021–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKenzie SE, Taylor SM, Malladi P, Yuhan H, Cassel DL, Chien P, Schwartz E, Schreiber AD, Surrey S, Reilly MP. The role of the human fc receptor fc gamma riia in the immune clearance of platelets: A transgenic mouse model. J Immunol 1999;162:4311–4318 [PubMed] [Google Scholar]
- 64.Arepally GM, Kamei S, Park KS, Kamei K, Li ZQ, Liu W, Siegel DL, Kisiel W, Cines DB, Poncz M. Characterization of a murine monoclonal antibody that mimics heparin-induced thrombocytopenia antibodies. Blood. 2000;95:1533–1540 [PubMed] [Google Scholar]
- 65.Reilly MP, Taylor SM, Hartman NK, Arepally GM, Sachais BS, Cines DB, Poncz M, McKenzie SE. Heparin-induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcgammaRIIa. Blood. 2001;98:2442–2447 [DOI] [PubMed] [Google Scholar]
- 66.Cines DB, Kaywin P, Bina M, Tomaski A, Schreiber AD. Heparin-associated thrombocytopenia. New Engl J Med. 1980;303:788–795 [DOI] [PubMed] [Google Scholar]
- 67.Chong BH, Grace CS, Rozenberg MC. Heparin-induced thrombocytopenia: Effect of heparin platelet antibody on platelets. Br J Haematol. 1981;49:531–540 [DOI] [PubMed] [Google Scholar]
- 68.Stricker RB, Reyes PT, Corash L, Shuman MA. Evidence that a 210,000-molecular-weight glycoprotein (gp 210) serves as a platelet fc receptor. J Clin Invest. 1987;79:1589–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liem TK, Teel R, Shukla S, Silver D. The glycoprotein iib/iiia antagonist c7e3 inhibits platelet aggregation in the presence of heparin-associated antibodies. J Vasc Surg. 1997;25:124–130 [DOI] [PubMed] [Google Scholar]
- 70.Jeske WP, Walenga JM, Szatkowski E, Ero M, Herbert JM, Haas S, Bakhos M. Effect of glycoprotein iib/iiia antagonists on the HIT serum induced activation of platelets. Thromb Res. 1997;88:271–281 [DOI] [PubMed] [Google Scholar]
- 71.Polgár J, Eichler P, Greinacher A, Clemetson KJ. Adenosine diphosphate (adp) and adp receptor play a major role in platelet activation/aggregation induced by sera from heparin-induced thrombocytopenia patients. Blood. 1998;91:549–554 [PubMed] [Google Scholar]
- 72.Gratacap MP, Herault JP, Viala C, Ragab A, Savi P, Herbert JM, Chap H, Plantavid M, Payrastre B. Fcgammariia requires a gi-dependent pathway for an efficient stimulation of phosphoinositide 3-kinase, calcium mobilization, and platelet aggregation. Blood. 2000;96:3439–3446 [PubMed] [Google Scholar]
- 73.Reilly MP, Sinha U, Andre P, Taylor SM, Pak Y, Deguzman FR, Nanda N, Pandey A, Stolla M, Bergmeier W, McKenzie SE. Prt-060318, a novel syk inhibitor, prevents heparin-induced thrombocytopenia and thrombosis in a transgenic mouse model. Blood. 2011;117:2241–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warkentin TE, Hayward CP, Smith CA, Kelly PM, Kelton JG. Determinants of donor platelet variability when testing for heparin-induced thrombocytopenia. J Lab Clin Med. 1992;120:371–379 [PubMed] [Google Scholar]
- 75.Arepally G, McKenzie SE, Jiang XM, Poncz M, Cines DB. Fc gamma RIIA H/R 131 polymorphism, subclass-specific IgG anti-heparin/platelet factor 4 antibodies and clinical course in patients with heparin-induced thrombocytopenia and thrombosis. Blood. 1997;89:370–375. [PubMed] [Google Scholar]
- 76.Carlsson LE, Santoso S, Baurichter G, Kroll H, Papenberg S, Eichler P, Westerdaal NA, Kiefel V, van de Winkel JG, Greinacher A. Heparin-induced thrombocytopenia: new insights into the impact of the FcgammaRIIa-RH131 polymorphism. Blood. 1998;92:1526–1531. [PubMed] [Google Scholar]
- 77.Trikalinos TA, Karassa FB, Ioannidis JP. Meta-analysis of the association between low-affinity fcgamma receptor gene polymorphisms and hematologic and autoimmune disease. Blood. 2001;98:1634–1635 [DOI] [PubMed] [Google Scholar]
- 78.Rollin J, Pouplard C, Sung HC, Leroux D, Saada A, Gouilleux-Gruart V, Thibault G, Gruel Y. Increased risk of thrombosis in fcgammariia 131rr patients with hit due to defective control of platelet activation by plasma IgG2. Blood. 2015;125:2397–2404 [DOI] [PubMed] [Google Scholar]
- 79.Leroux D, Canepa S, Viskov C, Mourier P, Herman F, Rollin J, Gruel Y, Pouplard C. Binding of heparin-dependent antibodies to pf4 modified by enoxaparin oligosaccharides: Evaluation by surface plasmon resonance and serotonin release assay. J Thromb Haemost. 2012;10:430–436 [DOI] [PubMed] [Google Scholar]
- 80.Zhou Y, Abraham S, Andre P, Edelstein LC, Shaw CA, Dangelmaier CA, Tsygankov AY, Kunapuli SP, Bray PF, McKenzie SE. Anti-miR-148a regulates platelet FcγRIIA signaling and decreases thrombosis in vivo in mice. Blood. 2015;126:2871–2881. doi: 10.1182/blood-2015-02-631135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warkentin TE, Hayward CP, Boshkov LK, Santos AV, Sheppard JA, Bode AP, Kelton JG. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: An explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood. 1994;84:3691–3699 [PubMed] [Google Scholar]
- 82.Khairy M, Lasne D, Brohard-Bohn B, Aiach M, Rendu F, Bachelot-Loza C. A new approach in the study of the molecular and cellular events implicated in heparin-induced thrombocytopenia. Formation of leukocyte-platelet aggregates. Thromb Haemost. 2001;85:1090–1096 [PubMed] [Google Scholar]
- 83.Khairy M, Lasne D, Amelot A, Crespin M, Rendu F, Aiach M, Bachelot-Loza C. Polymorphonuclear leukocyte and monocyte activation induced by plasma from patients with heparin-induced thrombocytopenia in whole blood. Thromb Haemost. 2004;92:1411–1419 [DOI] [PubMed] [Google Scholar]
- 84.Cines DB, Yarovoi SV, Zaitsev SV, et al. Polyphosphate/platelet factor 4 complexes can mediate heparin-independent platelet activation in heparin-induced thrombocytopenia. Blood Adv. 2016;1:62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnston I, Sarkar A, Hayes V, Koma GT, Arepally GM, Chen J, Chung DW, Lopez JA, Cines DB, Rauova L, Poncz M. Recognition of pf4-vwf complexes by heparin-induced thrombocytopenia antibodies contributes to thrombus propagation. Blood. 2020;135:1270–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rauova L, Hirsch JD, Greene TK, Zhai L, Hayes VM, Kowalska MA, Cines DB, Poncz M. Monocyte-bound pf4 in the pathogenesis of heparin-induced thrombocytopenia. Blood. 2010;116:5021–5031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pouplard C, Iochmann S, Renard B, Herault O, Colombat P, Amiral J, Gruel Y. Induction of monocyte tissue factor expression by antibodies to heparin-platelet factor 4 complexes developed in heparin-induced thrombocytopenia. Blood. 2001;97:3300–3302 [DOI] [PubMed] [Google Scholar]
- 88.Lhermusier T, van Rottem J, Garcia C, Xuereb JM, Ragab A, Martin V, Gratacap MP, Sie P, Payrastre B. The syk-kinase inhibitor r406 impairs platelet activation and monocyte tissue factor expression triggered by heparin-pf4 complex directed antibodies. J Thromb Haemost. 2011;9:2067–2076 [DOI] [PubMed] [Google Scholar]
- 89.Kasthuri RS, Glover SL, Jonas W, McEachron T, Pawlinski R, Arepally GM, Key NS, Mackman N. Pf4/heparin-antibody complex induces monocyte tissue factor expression and release of tissue factor positive microparticles by activation of FcγRI Blood. 2012;119:5285–5293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arepally GM, Mayer IM. Antibodies from patients with heparin-induced thrombocytopenia stimulate monocytic cells to express tissue factor and secrete interleukin-8. Blood. 2001;98:1252–1254 [DOI] [PubMed] [Google Scholar]
- 91.Tutwiler V, Madeeva D, Ahn HS, Andrianova I, Hayes V, Zheng XL, Cines DB, McKenzie SE, Poncz M, Rauova L. Platelet transactivation by monocytes promotes thrombosis in heparin-induced thrombocytopenia. Blood. 2016;127:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Batar P, Dale GL. Simultaneous engagement of thrombin and fcγriia receptors results in platelets expressing high levels of procoagulant proteins. J Lab Clin Med. 2001;138:393–402 [DOI] [PubMed] [Google Scholar]
- 93.Xiao Z, Visentin GP, Dayananda KM, Neelamegham S. Immune complexes formed following the binding of anti-platelet factor 4 (cxcl4) antibodies to cxcl4 stimulate human neutrophil activation and cell adhesion. Blood. 2008;112:1091–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duarte M, Kuchibhatla M, Khandelwal S, Arepally GM, Lee GM. Heterogeneity in neutrophil responses to immune complexes. Blood Adv. 2019;3:2778–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perdomo J, Leung HHL, Ahmadi Z, Yan F, Chong JJH, Passam FH, Chong BH. Neutrophil activation and netosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun. 2019;10:1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 97.Martinod K, Wagner DD. Thrombosis: Tangled up in nets. Blood. 2014;123:2768–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gollomp K, Kim M, Johnston I, Hayes V, Welsh J, Arepally GM, Kahn M, Lambert MP, Cuker A, Cines DB, Rauova L, Kowalska MA, Poncz M. Neutrophil accumulation and net release contribute to thrombosis in hit. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr., Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Groger M, Sarmay G, Fiebiger E, Wolff K, Petzelbauer P. Dermal microvascular endothelial cells express cd32 receptors in vivo and in vitro. J Immunol. 1996;156:1549–1556 [PubMed] [Google Scholar]
- 101.Anderson CL, Ganesan LP, Robinson JM. The biology of the classical fcγ receptors in non-hematopoietic cells. Immunol Rev. 2015;268:236–240 [DOI] [PubMed] [Google Scholar]
- 102.Kwaan HC, Sakurai S. Endothelial cell hyperplasia contributes to thrombosis in heparin-induced thrombocytopenia. Semin Thromb Hemost. 1999;25 Suppl 1:23–27 [PubMed] [Google Scholar]
- 103.Visentin GP, Ford SE, Scott JP, Aster RH. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J Clin Invest. 1994;93:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herbert JM, Savi P, Jeske WP, Walenga JM. Effect of sr121566a, a potent gp iib-iiia antagonist, on the hit serum/heparin-induced platelet mediated activation of human endothelial cells. Thromb Haemost. 1998;80:326–331 [PubMed] [Google Scholar]
- 105.Hayes V, Johnston I, Arepally GM, McKenzie SE, Cines DB, Rauova L, Poncz M. Endothelial antigen assembly leads to thrombotic complications in heparin-induced thrombocytopenia. J Clin Invest. 2017;127:1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Greinacher A, Eichler P, Lubenow N, Kwasny H, Luz M. Heparin-induced thrombocytopenia with thromboembolic complications: Meta-analysis of 2 prospective trials to assess the value of parenteral treatment with lepirudin and its therapeutic aPTT range. Blood. 2000;96:846–851 [PubMed] [Google Scholar]
- 107.Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, Linkins LA, Rodner SB, Selleng S, Warkentin TE, Wex A, Mustafa RA, Morgan RL, Santesso N. American society of hematology 2018 guidelines for management of venous thromboembolism: Heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelton JG, Arnold DM, Bates SM. Nonheparin anticoagulants for heparin-induced thrombocytopenia. New Engl J Med. 2013;368:737–744 [DOI] [PubMed] [Google Scholar]
- 109.Hogan M, Berger JS. Heparin-induced thrombocytopenia (hit): Review of incidence, diagnosis, and management. Vasc Med. 2020;25:160–173 [DOI] [PubMed] [Google Scholar]
- 110.Lewis BE, Wallis DE, Hursting MJ, Levine RL, Leya F. Effects of argatroban therapy, demographic variables, and platelet count on thrombotic risks in heparin-induced thrombocytopenia. Chest. 2006;129:1407–1416 [DOI] [PubMed] [Google Scholar]
- 111.Dyke CM, Koster A, Veale JJ, Maier GW, McNiff T, Levy JH. Preemptive use of bivalirudin for urgent on-pump coronary artery bypass grafting in patients with potential heparin-induced thrombocytopenia. Ann Thorac Surg. 2005;80:299–303 [DOI] [PubMed] [Google Scholar]
- 112.Dyke CM, Smedira NG, Koster A, Aronson S, McCarthy HL 2nd, Kirshner R, Lincoff AM, Spiess BD. A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: The evolution-on study. J Thorac Cardiovasc Surg. 2006;131:533–539 [DOI] [PubMed] [Google Scholar]
- 113.Warkentin TE. Anticoagulant failure in coagulopathic patients: PTT confounding and other pitfalls. Expert Opin Drug Saf. 2014;13:25–43 [DOI] [PubMed] [Google Scholar]
- 114.Vun CM, Evans S, Chong BH. Cross-reactivity study of low molecular weight heparins and heparinoid in heparin-induced thrombocytopenia. Thromb Res. 1996;81:525–532 [DOI] [PubMed] [Google Scholar]
- 115.Savi P, Chong BH, Greinacher A, Gruel Y, Kelton JG, Warkentin TE, Eichler P, Meuleman D, Petitou M, Herault JP, Cariou R, Herbert JM. Effect of fondaparinux on platelet activation in the presence of heparin-dependent antibodies: A blinded comparative multicenter study with unfractionated heparin. Blood. 2005;105:139–144 [DOI] [PubMed] [Google Scholar]
- 116.Warkentin TE, Maurer BT, Aster RH. Heparin-induced thrombocytopenia associated with fondaparinux. N Engl J Med. 2007;356:2653–2655. [DOI] [PubMed] [Google Scholar]
- 117.Kang M, Alahmadi M, Sawh S, Kovacs MJ, Lazo-Langner A. Fondaparinux for the treatment of suspected heparin-induced thrombocytopenia: A propensity score-matched study. Blood. 2015;125:924–929 [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y, Zhang M, Tan L, Pan N, Zhang L. The clinical use of fondaparinux: A synthetic heparin pentasaccharide. Prog Mol Biol Transl Sci. 2019;163:41–53 [DOI] [PubMed] [Google Scholar]
- 119.Chong BH, Gallus AS, Cade JF, Magnani H, Manoharan A, Oldmeadow M, Arthur C, Rickard K, Gallo J, Lloyd J, Seshadri P, Chesterman CN, Australian HITSG. Prospective randomised open-label comparison of danaparoid with dextran 70 in the treatment of heparin-induced thrombocytopaenia with thrombosis: A clinical outcome study. Thromb Haemost. 2001;86:1170–1175 [PubMed] [Google Scholar]
- 120.Krauel K, Fürll B, Warkentin TE, Weitschies W, Kohlmann T, Sheppard JI, Greinacher A. Heparin-induced thrombocytopenia--therapeutic concentrations of danaparoid, unlike fondaparinux and direct thrombin inhibitors, inhibit formation of platelet factor 4-heparin complexes. J Thromb Haemost. 2008;6:2160–2167 [DOI] [PubMed] [Google Scholar]
- 121.Linkins LA, Warkentin TE, Pai M, Shivakumar S, Manji RA, Wells PS, Wu C, Nazi I, Crowther MA. Rivaroxaban for treatment of suspected or confirmed heparin-induced thrombocytopenia study. J Thromb Haemost. 2016 [DOI] [PubMed] [Google Scholar]
- 122.Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of hit: Update of hamilton experience and literature review. Blood. 2017;130:1104–1113 [DOI] [PubMed] [Google Scholar]
- 123.Davis KA, Davis DO. Direct acting oral anticoagulants for the treatment of suspected heparin-induced thrombocytopenia. Eur J Haematol. 2017;99:332–335 [DOI] [PubMed] [Google Scholar]
- 124.Warkentin TE. High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: A review. Expert Rev Hematol. 2019;12:685–698 [DOI] [PubMed] [Google Scholar]
- 125.Frame JN, Mulvey KP, Phares JC, Anderson MJ. Correction of severe heparin-associated thrombocytopenia with intravenous immunoglobulin. Ann Int Med. 1989;111:946–947 [DOI] [PubMed] [Google Scholar]
- 126.Greinacher A, Liebenhoff U, Kiefel V, Presek P, Mueller-Eckhardt C. Heparin-associated thrombocytopenia: The effects of various intravenous igg preparations on antibody mediated platelet activation--a possible new indication for high dose IVIG. Thromb Haemost. 1994;71:641–645 [PubMed] [Google Scholar]
- 127.Padmanabhan A, Jones CG, Pechauer SM, Curtis BR, Bougie DW, Irani MS, Bryant BJ, Alperin JB, Deloughery TG, Mulvey KP, Dhakal B, Wen R, Wang D, Aster RH. Ivig for treatment of severe refractory heparin-induced thrombocytopenia. Chest. 2017;152:478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Winder A, Shoenfeld Y, Hochman R, Keren G, Levy Y, Eldor A. High-dose intravenous gamma-globulins for heparin-induced thrombocytopenia: A prompt response. J Clin Immunol. 1998;18:330–334 [DOI] [PubMed] [Google Scholar]
- 129.Park BD, Kumar M, Nagalla S, De Simone N, Aster RH, Padmanabhan A, Sarode R, Rambally S. Intravenous immunoglobulin as an adjunct therapy in persisting heparin-induced thrombocytopenia. Transfus Apher Sci. 2018;57:561–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arcinas LA, Manji RA, Hrymak C, Dao V, Sheppard JI, Warkentin TE. Autoimmune heparin-induced thrombocytopenia and venous limb gangrene after aortic dissection repair: In vitro and in vivo effects of intravenous immunoglobulin. Transfusion. 2019;59:1924–1933 [DOI] [PubMed] [Google Scholar]
- 131.Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: Risk of precipitating thromboembolic events. Neurology. 1994;44:223–226 [DOI] [PubMed] [Google Scholar]
- 132.Woodruff RK, Grigg AP, Firkin FC, Smith IL. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet. 1986;2:217–218 [DOI] [PubMed] [Google Scholar]
- 133.Dhakal B, Rein L, Szabo A, Padmanabhan A. Use of intravenous immunoglobulin G in HIT patients is not associated with increased rates of thrombosis: A population-based study. Chest. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Warkentin TE, Sheppard JA, Chu FV, Kapoor A, Crowther MA, Gangji A. Plasma exchange to remove hit antibodies: Dissociation between enzyme-immunoassay and platelet activation test reactivities. Blood. 2015;125:195–198 [DOI] [PubMed] [Google Scholar]
- 135.Jaben EA, Torloni AS, Pruthi RK, Winters JL. Use of plasma exchange in patients with heparin-induced thrombocytopenia: A report of two cases and a review of the literature. J Clin Apher. 2011;26:219–224 [DOI] [PubMed] [Google Scholar]
- 136.Robinson JA, Lewis BE. Plasmapheresis in the management of heparin-induced thrombocytopenia. Semin Hematol. 1999;36:29–32 [PubMed] [Google Scholar]
- 137.Kizlik-Masson C, Deveuve Q, Zhou Y, Vayne C, Thibault G, McKenzie SE, Pouplard C, Loyau S, Gruel Y, Rollin J. Cleavage of anti-pf4/heparin IgG by a bacterial protease and potential benefit in heparin-induced thrombocytopenia. Blood. 2019;133:2427–2435 [DOI] [PubMed] [Google Scholar]
- 138.Sachais BS, Rux AH, Cines DB, Yarovoi SV, Garner LI, Watson SP, Hinds JL, Rux JJ. Rational design and characterization of platelet factor 4 antagonists for the study of heparin-induced thrombocytopenia. Blood. 2012;119:5955–5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cai Z, Yarovoi SV, Zhu Z, Rauova L, Hayes V, Lebedeva T, Liu Q, Poncz M, Arepally G, Cines DB, Greene MI. Atomic description of the immune complex involved in heparin-induced thrombocytopenia. Nat Commun. 2015;6:8277. [DOI] [PMC free article] [PubMed] [Google Scholar]