Abstract
Extracellular vesicles (EVs) are a means of cell-to-cell communication and can facilitate the exchange of a broad array of molecules between adjacent or distant cells. Platelets are anucleate cells derived from megakaryocytes and are primarily known for their role in maintaining hemostasis and vascular integrity. Upon activation by a variety of agonists, platelets readily generate EVs, which were initially identified as procoagulant particles. However, as both platelets and their EVs are abundant in blood, the role of platelet EVs in hemostasis may be redundant. Moreover, findings have challenged the significance of platelet-derived EVs in coagulation. Looking beyond hemostasis, platelet EV cargo is incredibly diverse and can include lipids, proteins, nucleic acids, and organelles involved in numerous other biological processes. Furthermore, while platelets cannot cross tissue barriers, their EVs can enter lymph, bone marrow, and synovial fluid. This allows for the transfer of platelet-derived content to cellular recipients and organs inaccessible to platelets. This review highlights the importance of platelet-derived EVs in physiological and pathological conditions beyond hemostasis.
Introduction
Platelets were first described in the 1880s, and it was quickly understood that their main function was the prevention of bleeding. Following damage to blood vessels, platelets rapidly seal the breach to prevent blood loss. However, pathogens (bacteria, viruses, fungi) can take advantage of the loss of vascular integrity to invade the blood stream and disseminate. It is now reocognized that platelets express numerous inflammatory molecules and receptors capable of recruiting immune cells and limiting the risk of infection. Thus, although platelets are poised to play roles in immunity and inflammation, their primary functions remain restricted to the blood circulation due to their limited migratory capabilities.
Extracellular vesicles (EVs) are membrane vesicles released from the cellular plasma membrane (microvesicles or microparticles) or endosomal compartment (exosomes) of cells.1,2 They are produced by platelets upon activation and have been associated with both non-infectious chronic inflammatory diseases3 (e.g. atherosclerosis, diabetes mellitus, coronary artery disease, hypertension) and infectious diseases4,5 (e.g. influenza, COVID-19). Similarly to EVs from other cell types, platelet-derived EVs (pEVs) transport diverse cargo (e.g. RNA, lipids, proteins), which can be transferred to cellular recipients. Thus, because pEVs can reach organs and tissues inaccessible to platelets, they may contribute to more distant cellular communication. In this review, we present historical findings related to pEVs and hemostasis, and discuss how more recent findings point to a role for pEVs in intercellular communication under both physological and pathological conditions.
Historical findings on the role of platelet EVs
The earliest observation documenting activity that was later attributed to pEVs was by Chargaff and West6, who described coagulant “lipoproteins with a high particle weight” that were separated from platelets by differential centrifugation. Later, a platelet-like activity in serum was identified by a thrombin-generation test7, but was not yet associated with pEVs. The first observation of small particles fitting the current description of EVs was by Peter Wolf,8 who also noted platelet-derived particles that could be separated from platelets by differential centrifugation.8 These small particles were termed “platelet-dust” and displayed a procoagulant function that could shorten clotting time and promote thrombin generation.8 Shortly thereafter, electron microscopic analyses9 of alpha-granule release from platelets also imaged small vesicles being released. This release of vesicles, refered to as “microparticles”, from the platelet plasma membrane implicates the extrusion of platelet cytomembrane structures.10 A later study using electron microscopy11 provided a more detailed description of the two types of pEVs: small vesicles with a diameter of approximately 80 to 200 nm and larger vesicles with a diameter of 400 to 600 nm which retained procoagulant potential mediated by factor V-like activity and tissue factor. While PEVs had primarily been associated with procoagulant activity, another study offered the first evidence that pEVs may exert both pro- and anticoagulatory effects. This study showed that pEVs could support activation of prothrombin, but also inactivation of Factor Va12 through binding of protein S to the coagulation inhibitor protein C in some pEV preparations.13 These data provided an early indication that the function of pEVs can be modified post-release.
The procoagulant effects of pEVs have been largely linked to the surface exposure of negatively charged phospholipids (e.g. phosphatidylserine).14 However, the coagulant potential of pEVs may depend on their trigger of release from platelets.15 More specifically, highly procoagulant pEVs are produced when platelets are activated by a combination of collagen and thrombin, complement C5b-9, or the non-physiologic trigger calcium ionophore.15,16 However, the procoagulant activity is lower if platelets are activated by thrombin, ADP, or epinephrine.15,16 After in vitro platelet stimulation, with the exception of C5b-9-induced pEVs, only 25 to 30% of total procoagulant activity is associated with pEVs independent of platelets.15,16 This suggests that the procoagulant properties of pEVs may be eclipsed by platelets, and therefore may not be their sole function.
Detailed electron microscopic analysis of activated platelets17 connected the data from various publications and described the release of two different EV populations (termed ‘microvesicles’ and ‘exosomes’) triggered by platelet activation with the proteinase-activated receptor (PAR) agonist, thrombin receptor agonist peptide SFLLRN (TRAP), or α-thrombin. Microvesicles were defined as vesicles 100–1000 nm in diameter, phosphatidylserine-exposing (Annexin-V binding), which express αIIB-β3 and β1, GP1bα, and P-selectin, proteins that are present on (activated) platelets.17 Exosomes were defined as 40–100 nm in diameter, similar to internal vesicles in multivesicular bodies and alpha-granules, expose CD63, and are undetectable by flow cytometry.17 Factor X and prothrombin were able to bind to microvesicles, but not exosomes. Thus, it was suggested that the coagulant properties of pEVs are associated with microvesicles, but not exosomes.17 Some procoagulant activity of circulating pEVs was subsequently associated with tissue factor.18 Although platelets have not been conclusively demonstrated to express tissue factor19, studies suggest that they may acquire it from tissue factor-bearing EVs (from other cells) through fusion in a P-selectin glycoprotein ligand-1-dependent manner.20
Definitions of EVs and general considerations in the interpretation of pioneering studies
Historically, the two main types of EVs that have been identified have most often been referred to as microvesicles/microparticles and exosomes. However, the terminology has frequently been used in different contexts and the isolation protocols were not standardized leading to confusion, misinterpretation, and reproducibility issues. Unless experimental conditions permit capturing vesicle release as it occurs to discern whether they orginate from plasma membrane budding or intracellular compartments, the current consensus21 is that the umbrella term ‘extracellular vesicles’ (EVs) should be used. The term EV encompasses all different types of vesicles, including microvesicles/microparticles and exosomes. Moreover, a clear description of the preparation methodology and a detailed characterization of EVs is required when they are reported21. In particular, with regard to pEV isolation, attention should be paid to proper separation of pEVs and platelets,22 and their distinction from lipoproteins (chylomicrons, LDL, HDL).23 The exact concentrations of EVs (and pEVs) in healthy plasma is an ongoing matter of debate and concentrations ranging from 200 up to 109 EVs/µL have been reported.24 These discrepancies can likely be attributed to low sensitivity of detection methods or co-detection of contaminants.24,23 A conservative estimate of pEVs by cryo-electronmicroscopy determined their concentration to be close to 11,500/µL in healthy plasma.24 Considering these issues, it is prudent to include EV measurements from healthy control plasma in side-by-side comparisons with EVs obtained from a study group-of-interest to enable a direct comparison of EV quantities. Likewise, a meta-analysis of EV concentrations across different studies may have limited usefulness if it cannot guarantee comparable isolation and detection protocols.
Despite their absolute quantities still being investigated, it is accepted that platelets and megakaryocytes (MKs) are the primary source of EVs in the blood circulation.25,26,24 While pEVs are released from platelets upon stimulation, MK EVs are consitutively released from MKs in the bone marrow into the blood. As such, MK EVs dominate in healthy individuals while pEVs increase in conditions with enhanced platelet activation. While both are positive for CD41, pEVs generally express the platelet activation markers P-selectin (CD62P) and phosphatidylserine (PS), while MK EVs do not.25,27 However, both pEV content and surface marker expression is dependent on the platelet agonist, and the resulting pEV populations are highly heterogenous.27,28 pEV numbers significantly increase in conditions with chronic inflammation and ongoing platelet activation, such as cardiovascular disease, cancer, and autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus (SLE).29,30,31,32,33 In addition to changes in pEV quantity, there are changes in pEV content in settings such as cardiovascular disease34, infections35, autoimmune diseases (multiple sclerosis36, rheumatoid arthritis37, and SLE38,39), and cancer40. As pEVs contain a subset of cargo packaged from platelets, this differential cargo could result from 1) plasma components directly endocytosed by platelets, 2) platelet changes at the level of their mother cells, MKs, or likely 3) a combination of these two mechanisms.
Is procoagulant activity the main function of platelet EVs?
With these diverse roles of pEVs in mind, it is prudent to revisit the assertion that their main function relates to propagation and support of procoagulant activity. A recent study by Berckmans et al.22 highlighted concerns regarding the interpretation of their earlier PEV studies. The authors re-evaluated their previous findings41 on the coagulant properties of pEVs in blood from healthy volunteers. In comparison to their earlier study41, they report up to 190- to 264-fold higher concentrations of pEVs in blood.22 Suprisingly, these pEVs display fibrinolytic activity in a plasmin generation assay22, rather than a procoagulant function as previously reported in a thrombin generation assay41. The authors argue that the collection method employed in the earlier study was suboptimal compared with more recent protocols, as the earlier separation of pEVs from platelets was performed by a single-step centrifugation protocol, which might have led to platelet contamination.22 In addition, platelets may have been activated when they were collected in glass tubes in past studies.22 In contrast, a different study confirmed the presence of procoagulant EVs in blood from healthy volunteers, this time using a more sensitive assay.42 As outlined in the first section, both procoagulant6,8,11,12,14,15,16,17,18,20 and anticoagulant12,13,15,16,43 properties are attributed to pEVs; these differences may be explained by the existence of different pEV subsets.17
The study of pEVs in human disease also provides information regarding their physiological role in coagulation. In Scott syndrome, platelets lack the ability to expose phosphatidylserine on their surface during activation and the number of circulating pEVs is drastically reduced.44 Although the reduced number of pEVs could account for the increased bleeding risk in these patients, phosphatidylserine exposure is deficient on platelets themselves and on other cells, which makes it difficult to determine the relative contribution of pEVs versus platelets to the observed bleeding phenotype. Moreover, these patients present with only a mild bleeding phenotype, indicating that pEVs may not be critically required for hemostasis.
Furthermore, Stormorken’s syndrome, also called “inverse Scott syndrome”45, is associated with either a gain-of-function mutation in the sensing protein stromal interaction molecule 1 gene (STIM1)46 or a loss-of-function in the calcium channel pore forming protein ORAI147. In Stormorken’s syndrome48, platelets appear to be hyperactivated and expose increased levels of phosphatidylserine on their surface. In addition, the concentration of circulating pEVs is higher in this disease. Of note is that the phenotype presents with a mild bleeding defect. Together, these in vivo and in vitro observations suggest that the role of pEVs in hemostasis may be minor, even though specific pro- and anticoagulant functions have been indirectly attributed to them.
Do platelet EVs have therapeutic potential?
EVs from various cell types are currently being explored as therapeutic tools.49 pEVs may have therapeutic potential, as they can support coagulation and angiogenesis in different animal models of bleeding and trauma.50,51,52,53 However, the effects of pEVs vary depending on the trigger of pEV generation; pEVs derived from resting platelets50,51 versus thrombin-activated platelets51 demonstrate mild or strong hemostatic properties (indicated by formation of smaller or larger aggregates), respectively. Moreover, exosomal pEVs have been found to be beneficial in treatment of chronic injuries and trauma.52,53 These studies suggest that careful production and characterization of PEVs is necessary before determining their utility in any in vivo applications. However, there is significant interest in development of pEVs for use in conflict or war zones where high rates of trauma and bleeding injuries are common.54,55 Since liquid platelet-rich plasma preparations only have a short half-life (~5 days) and are required to be kept at temperatures of 20–24°C, frozen pEV preparations are an attractive alternative.54,55 However, given the current knowledge of the diverse and seemingly contradictory functions of pEVs, reaching their full therapeutic potential will depend on clear separation of pEV subtypes and careful development of best-practice protocols for pEV generation and isolation.
Platelet EVs as inflammatory agents
Aside from their potential roles in coagulation, pEVs have significant inflammatory properties. For example, platelets activated by staphylococcal superantigen-like protein 5 release pEVs capable of inducing leukocyte aggregation.56 Moreover, pEVs carry molecules such as cytokines (e.g. IL-1β57,58), lipid mediators59, and damage-associated molecular patterns (DAMP, e.g. HMGB160), pointing to their role in the transfer of inflammatory signals. In addition, pEVs can modify the pentameric C reactive protein (CRP) into its inflammatory form.61 This change implicates the binding of pentameric CRP to phosphocholine on pEVs.61 Conversely, it was found that in an inflammatory milieu, peptidylarginine deiminase 4 (PAD4) could citrullinate proteins on the surface of pEVs and thereby promote their antigenicity.62
However, pEVs are not always proinflammatory, and may also have immune regulatory potential. For instance, pEVs can provide 12-lipoxygenase to mast cells, which enhances the production of lipoxin A4, a stimulator of the resolution of inflammation.63 Furthermore, pEVs shed by stored human platelets can polarize macrophages to an anti-inflammatory state.64 This effect may result from the depletion of complement proteins (C1q, CFH, C3d) by pEVs in plasma.64 Platelet EVs also regulate adaptive immunity: they can induce anti-inflammatory signaling in plasmacytoid dendritic cells65 and inhibit differentiation of regulatory T-cells into proinflammatory cells through a mechanism involving P-selectin66.
Thus, similar to observations made in coagulation studies, pEVs appear to have both pro- and anti-inflammatory roles. We again suggest that these different roles might be played by pEV subtypes that are generated after platelet stimulation with different agonists.
Platelet EVs as a means to exchange platelet cargo with other cells
EVs are a direct way to foster cell-to-cell communication to non-adjacent cells, and pEVs are no exception. Transcription factors67, messenger RNA, and non-coding RNA (e.g. microRNA) are packaged into and transported by pEVs.68 Several studies have shown that pEV-derived miRNAs are incorporated into target cells and can signal with varying effects.69 For instance, upon infiltration of solid tumor tissue in mice and humans, pEVs can promote apoptosis of tumor cells by transfer of miR-24.70 Furthermore, pEVs have been shown to transfer miR-223 in complex with Argonaute 2 to endothelial cells71 and miR-223 and miR-126 to breast cancer cells72, which directly modify respective recipient cell functions. However, it should be noted that significant amounts of circulating RNAs are also associated with small, non-vesicular particles73, which are likely to be lipoproteins74, suggesting that pEVs are not the sole source of extracellular RNA molecules in blood.
Mitochondria can be released from platelets and other cells as free mitochondria or as cargo in EVs75,76,77,78. Circulating mitochondria are generally considered a source of potential DAMPs79, promoting inflammation once outside the cell, which may be pathogenic in situations like trauma-induced injury.80 Moreover, mitochondria released by platelets indirectly contribute to inflammation via the liberation of inflammatory mediators upon secreted phospholipase A2 IIA (sPLA2-IIA) -catalyzed hydrolysis.78 In addition, mitochondria in the circulation may serve as a source of auto-antigens as demonstrated in SLE.81 However, the role of circulating mitochondria is complicated by the fact that mitochondria released in association with EVs have been reported to be proinflammatory77, non-inflammatory, and potentially cytoprotective75,76. Indeed, while extracellular mitochondria released from endotoxin-stimulated monocytic cells can activate endothelial cells, mitochondria released from resting monocytes were unable to induce inflammatory effects. These differences may be due to the activation state of the cellular source of mitochondria. As mitochondria in pEVs are functional78, the transportation of mitochondria by pEVs could play a role in reprogramming the metabolism of the cellular recipient. This process is already recognized for mesenchymal stem cells76 and bone-marrow-derived stromal cells75, which are capable of transfering mitochondria embedded in EVs to other cells, thereby improving the bioenergetics of the recipient.76,75
In summary, although pEVs are produced by an anucleated cell, they bear components capable of regulating the transcription, RNA stability, translation, and metabolism of their target cells.
Change of location – Looking beyond the blood
Taken together, pEVs can perform a wide range of functions in the circulation, including (anti)coagulant or (anti)inflammatory effects and are involved in intercellular communication between blood cells. However, these roles of pEVs are shared, or overlap, with platelet function, with the importance of platelets in coagulation being undoubtedly superior. Combined with the challenges of physically separating pEVs from platelets, it may be more relevant and meaningful to examine pEVs in spatially different contexts than platelets.
Platelet EVs in the synovial fluid
Platelet EVs have been identified in synovial fluid 58,82 and are elevated in rheumatoid arthritis82. Typically platelets are rarely found in, or are absent from, synovial fluid. Under inflammatory conditions, pEVs may cross over into the synovial fluid where they become the target of autoantibodies against citrullinated proteins62,83. These pEVs are proinflammatory, as they induce cytokine responses in synovial fibroblasts mediated by interleukin-1, thereby potentially contributing to the disease.58 Moreover, pEVs may serve as a substrate for sPLA2-IIA, which is overexpressed in synovial fluid.67 Neovascularization is thought to be detrimental in arthritis84, and pEVs might contribute to neovascularization both indirectly by promoting inflammation58,62,83 or directly by supporting angiogenesis85,86. It has been shown in vitro that pEVs can promote endothelial cell proliferation, survival, migration, and tube formation.85 In vivo angiogenesis and post-ischemic revascularization are also promoted by pEVs.86 A potential mode of pro-angiogenic action is the induction of matrix metalloproteinases (MMPs) in the target endothelial cells. This is supported by data showing that pEVs can mediate increased expression of MMP-2 and MMP-9 mRNA and protein in human umbilical vein endothelial cells, despite the absence of these enzymes in pEVs.87 Moreover, pEVs can support early outgrowth of endothelial cells after vascular injury88, and promote proliferation of smooth muscle cells89 and hematopoietic cells90. As such, these roles of pEVs may enhance tissue remodeling in chronic inflammatory joint disease, or promote healing following tissue injury. Increased vascular permeability in inflamed joints may additionally support the crossover of pEVs into the synovial fluid. Nonetheless, it cannot be excluded that platelets may release pEV locally through activation by the subendothelial matrix if they are transported by leukocytes, or could undergo migration.91,92
Platelet EVs in the lymph
Interstitial fluid—rich in leukocytes, proteins, and EVs93—is drained through the lymphatic system away from tissue and into the blood. Platelet EVs circulate in lymph in the absence of inflammation, suggesting that this fluid, absent of any platelets, is used by pEVs to reach tissue locations inaccessible to platelets themselves.93,94 While one animal study found that platelets are essentially absent within solid tumors, pEVs could reach tumor cells and reprogram them with their microRNA content70, potentially due to their circulation through the lymphatics. Moreover, inflammation can lead to increased access of pEVs to lymph, such as in atherosclerosis and rheumatoid arthritis.93,94 In rheumatoid arthritis, pEV egress into lymph involves serotonin-mediated vascular permeablity, as pEVs in lymph were was reduced in mice lacking peripheral serotonin.94 In contrast to blood pEVs, the pEVs that accumulate in lymph in autoimmune arthritis do not contribute to coagulation94, suggesting that pEVs in this fluid may play a non-redundant role with platelets. As the lymphatic system connects lymphoid organs, the presence of pEVs in lymph might suggest that pEVs participate in key immune activities. Furthermore, platelets contribute to lymphatic vessel development by CLEC-2-Podoplanin interactions.95 Specifically in blood-lymphatic vessel separation96, platelets become activated on contact with lymphatic endothelium in a CLEC-2-dependent manner resulting in the formation of a lymphovenous clot preventing blood from entering lymph.96 Intriguingly, the presence of CLEC-2 expressing pEVs in lymph94 offers the possibility of pEV-mediated effects on lymphatic development independently of their parental platelets. Of note, phosphatidylserine expression and comparable levels of miR-451 and miR-223 could be detected in lymph pEVs compared with blood pEVs. However, the absence of mitochondria in lymph pEVs may represent a feature distinguishing these from blood pEVs.
Platelet EVs in the bone marrow
In many inflammatory conditions platelet counts rise, resulting in thrombocytosis. What initiates this up-regulation is not well understood and has largely been attributed to an inflammatory response and increased cytokine release. However, EVs released by platelets during states of ongoing inflammation can leave the circulation and penetrate into the bone marrow space.27 Once there, pEVs rapidly bind to bone marrow cells, including MKs and their progenitors (CD41+ cells). Of note, ex vivo treatment of bone marrow from mice lacking the TPO receptor (cMpl knockout) with wildtype pEVs can restore MK differentiation, showing pEV-dependent functional reprogramming. These data show that pEVs are a unique delivery system that can penetrate the bone marrow and deliver concentrated, targeted plasma cargo that alters MK function and phenotype. In this way, pEVs may act as sentinels and messengers, communicating changes happening in the plasma milieu directly back to cells in the bone marrow. Further in vivo studies examining the role that pEVs play in altering bone marrow cell populations in varying inflammatory states will help elucidate their functional impact on disease pathology.
Could platelet EVs migrate into other tissues or body fluids?
Various types of EVs in different tissues and body fluids have been discussed elsewehere97, including but not limited to blood, urine, saliva, breast milk, and cerebrospinal and synovial fluid. Of yet, it is not known if pEVs are found in tissues or body fluids other than the blood, synovial fluid, lymph, and bone marrow. However, the potential of EVs to be transferred across biological barriers has also been observed for the blood-brain-barrier.98,99,100 Interestingly, the transfer was bidirectional: i) transfer of glioma-derived EVs or pro-coagulant mitochondria containing EVs across the BBB to the blood100,101 and ii) intravenously injected EVs from blood across the BBB to neuronal cells102. It is unknown if this also applies to pEVs. Considering that several physical and chemical properties are shared between EVs of different origins, it is possible that pEVs may be transferred across tissue barriers such as the BBB, especially in inflammatory conditions.
How do platelet EVs cross intact barriers?
Platelets themselves show significant, but limited migratory capacities.92 They not only adhere to the inflamed vessel wall, but also migrate against the blood flow and probe the surrounding area for microbes.92 Whether this migratory ability enables platelets to move across the endothelial barrier and leave the circulation has not been shown. However, platelet-leukocyte aggregates have been reported in various inflammatory and hematologic pathologies.103,104,105 Interactions of platelets and leukocytes can be mediated by αIIbβ3106, CD62P (P-Selectin)107,108, and glycoprotein Ib109. These proteins can also be expressed on pEVs17, and may be involved in mediating pEV-leukocyte interactions.110,111,112 While it is difficult to distinguish platelet-leukocyte and pEV-leukocyte aggregates in vivo113, such associations have the potential to offer platelets and pEVs a piggyback ride across the vascular barrier. In addition, studies have also found that pEVs are enhanced in the extravascular space under conditions with enhanced vascular permeability.91,94 This leads to the hypothesis that the small size of pEVs may allow them to migrate into certain organs under these condtions. However, there remains much work to be done on the mechanisms of how pEVs can accessed these privledged spaces.
Summary
Platelet EVs were historically identified as procoagulant particles released by activated platelets. Over time, it has become clear that their roles are more diverse. In researching both their roles in coagulation and beyond, one of the most important challenges is distinguishing the functions of pEVs from those mediated by their platelets of origin. The ability to do this will likely depend on careful isolation and characteriztion of the different subtypes of pEVs created after platelet stimulation with various agonists. Moreover, accumulating evidence points to the importance of pEVs in intercellular communication not only within circulating cells but also beyond to other organs. Given that pEVs may permeate tissues that are inaccessible to platelets— such as joints, lymph, and bone marrow— the dissemination of platelet components into tissues and organs beyond the blood may be among their significant functions. Taken together, these observations suggest that future studies may reveal pEV abilities that extend beyond coagulation and inflammation, and into tissue barriers impenetrable to platelets.
Figure 1.
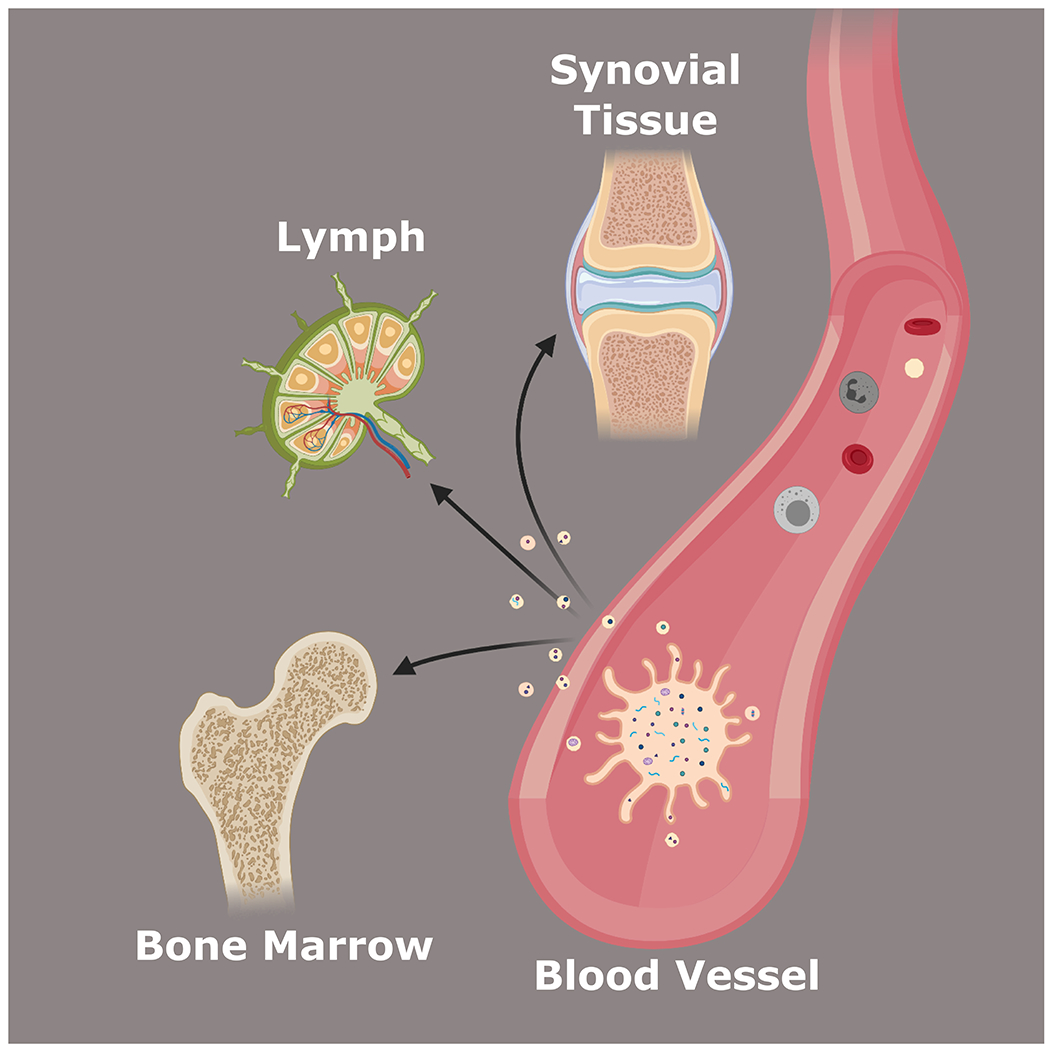
Platelet-derived extracellular vesicles can leave circulation and penetrate privileged organs such as the synovium, lymph, and bone marrow.
Highlights.
Upon activation by a variety of agonists, platelets readily generate extracellular vesicles, which were initially identified as procoagulant particles.
As both platelets and their extracellular vesicles are abundant in blood, the role of platelet extracellular vesicles in hemostasis may be redundant.
Recent findings have challenged the significance of platelet-derived extracellular vesicles in hemostasis.
Platelet extracellular vesicle cargo and function is incredibly diverse and can affect many different cell types.
In contrast to platelets, platelet extracellular vesicles can cross tissue barriers, extending their abilities beyond the blood.
Acknowledgements
Sources of Funding: FP is recipient of a postdoctoral fellowship from FRQS. EB is recipient of senior award from the Fonds de Recherche du Québec en Santé (FRQS). KRM is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K01DK111515) at the National Institutes of Health, and is an American Society of Hematology Scholar.
Abbreviations:
- CRP
C reactive protein
- DAMPs
Damage-associated molecular patterns
- EVs
Extracellular vesicles
- IL
Interleukin
- MMPs
Matrix metalloproteinases
- MK
Megakaryocyte
- PS
Phosphatidylserine
- PAD4
Peptidylarginine deiminase 4
- pEV
Platelet-derived extracellular vesicle
- CD62P
P-selectin
- sPLA2-IIA
Secreted phospholipase A2 IIA
- SLE
Systemic lupus erythematosus
Footnotes
Conflict of interest disclosures: The authors have nothing to disclose
Disclosure
None.
References
- 1.Sedgwick AE, D’Souza-Schorey C. The biology of extracellular microvesicles. Traffic. 2018;19:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lötvall J, Hill AF, Hochberg F, Buzás EI, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell vesicles. 2014;3:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Li G, Liu ML. Microvesicles as Emerging Biomarkers and Therapeutic Targets in Cardiometabolic Diseases. Genomics, Proteomics Bioinforma. 2018;16:50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boilard E, Paré G, Rousseau M, Cloutier N, Dubuc I, Lévesque T, Borgeat P, Flamand L. Influenza virus H1N1 activates platelets through FcγRIIA signaling and thrombin generation. Blood. 2014;123:2854–63. [DOI] [PubMed] [Google Scholar]
- 5.Zaid Y, Puhm F, Allaeys I, et al. Platelets Can Associate with SARS-Cov-2 RNA and Are Hyperactivated in COVID-19. Circ Res. 2020;CIRCRESAHA. 120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CHARGAFF E, WEST R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–97. [PubMed] [Google Scholar]
- 7.O’Brien JR. The Platelet-like Activity of Serum. Br J Haematol. 1955;1:223–228. [PubMed] [Google Scholar]
- 8.Wolf P The Nature and Significance of Platelet Products in Human Plasma. Br J Haematol. 1967;13:269–288. [DOI] [PubMed] [Google Scholar]
- 9.Webber AJ, Johnson SA. Platelet participation in blood coagulation aspects of hemostasis. Am J Pathol. 1970;60:19–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford N The Presence of Contractile Proteins in Platelet Microparticles Isolated from Human and Animal Platelet-free Plasma. Br J Haematol. 1971;21:53–69. [DOI] [PubMed] [Google Scholar]
- 11.Sandberg H, Bode AP, Dombrose FA, Hoechli M, Lentz BR. Expression of coagulant activity in human platelets: Release of membranous vesicles providing platelet factor 1 and platelet factor 3. Thromb Res. 1985;39:63–79. [DOI] [PubMed] [Google Scholar]
- 12.Tans G, Rosing J, Thomassen MC, Heeb MJ, Zwaal RF, Griffin JH. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood. 1991;77:2641–8. [PubMed] [Google Scholar]
- 13.Dahlbaeck B, Wiedmer T, Sims PJ. Binding of anticoagulant vitamin K-dependent protein S to platelet-derived microparticles. Biochemistry. 1992;31:12769–12777. [DOI] [PubMed] [Google Scholar]
- 14.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–604. [DOI] [PubMed] [Google Scholar]
- 15.Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJSJ. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: an isolated defect in platelet procoagulant activity. J Biol Chem. 1989;264:17049–57. [PubMed] [Google Scholar]
- 16.Zwaal RFA, Comfurius P, Bevers EM. Platelet procoagulant activity and microvesicle formation. Its putative role in hemostasis and thrombosis. Biochim Biophys Acta - Mol Basis Dis. 1992;1180:1–8. [DOI] [PubMed] [Google Scholar]
- 17.Heijnen HFG, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived From Exocytosis of Multivesicular Bodies and Granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 18.Mallat Z, Hugel B, Ohan J, Lesèche G, Freyssinet JM, Tedgui A. Shed Membrane Microparticles With Procoagulant Potential in Human Atherosclerotic Plaques. Circulation. 1999;99:348–353. [DOI] [PubMed] [Google Scholar]
- 19.Østerud B, Bouchard BA. Detection of tissue factor in platelets: why is it so troublesome? Platelets. 2019;30:957–961. [DOI] [PubMed] [Google Scholar]
- 20.Del Conde I, Shrimpton CN, Thiagarajan P, López JA, López JA. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. [DOI] [PubMed] [Google Scholar]
- 21.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berckmans RJ, Lacroix R, Hau CM, Sturk A, Nieuwland R. Extracellular vesicles and coagulation in blood from healthy humans revisited. J Extracell Vesicles. 2019;8:1688936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnsen KB, Gudbergsson JM, Andresen TL, Simonsen JB. What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer. Biochim Biophys Acta - Rev Cancer. 2019;1871:109–116. [DOI] [PubMed] [Google Scholar]
- 24.Arraud N, Linares R, Tan S, Gounou C, Pasquet J-M, Mornet S, Brisson AR. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614–27. [DOI] [PubMed] [Google Scholar]
- 25.Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli E, Klement GL, Sola-Visner M, Italiano JE. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aatonen MT, Ohman T, Nyman TA, Laitinen S, Grönholm M, Siljander PR-M. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.French SL, Butov KR, Allaeys I, et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020;4:3011–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milioli M, Ibáñez-Vea M, Sidoli S, Palmisano G, Careri M, Larsen MR. Quantitative proteomics analysis of platelet-derived microparticles reveals distinct protein signatures when stimulated by different physiological agonists. J Proteomics. 2015;121:56–66. [DOI] [PubMed] [Google Scholar]
- 29.Abrams CS, Ellison N, Budzynski AZ, Shattil SJ. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood. 1990;75:128–38. [PubMed] [Google Scholar]
- 30.Varon D, Shai E. Role of platelet-derived microparticles in angiogenesis and tumor progression. Discov Med. 2009;8:237–41. [PubMed] [Google Scholar]
- 31.Mobarrez F, Vikerfors A, Gustafsson JT, Gunnarsson I, Zickert A, Larsson A, Pisetsky DS, Wallén H, Svenungsson E. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): phenotypic characterization and clinical associations. Sci Rep. 2016;6:36025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiva-Blanch G, Laake K, Myhre P, Bratseth V, Arnesen H, Solheim S, Badimon L, Seljeflot I. Platelet-, monocyte-derived and tissue factor-carrying circulating microparticles are related to acute myocardial infarction severity. PLoS One. 2017;12:e0172558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gitz E, Pollitt AY, Gitz-Francois JJ, Alshehri O, Mori J, Montague S, Nash GB, Douglas MR, Gardiner EE, Andrews RK, Buckley CD, Harrison P, Watson SP. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood. 2014;124:2262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nomura S, Uehata S, Saito S, Osumi K, Ozeki Y, Kimura Y. Enzyme immunoassay detection of platelet-derived microparticles and RANTES in acute coronary syndrome. Thromb Haemost. 2003;89:506–12. [PubMed] [Google Scholar]
- 35.Joop K, Berckmans R, Nieuwland R, Berkhout J, Romijn F, Hack CE, Sturk A. Microparticles from Patients with Multiple Organ Dysfunction Syndrome and Sepsis Support Coagulation through Multiple Mechanisms. Thromb Haemost. 2001;85:810–820. [PubMed] [Google Scholar]
- 36.Sheremata WA, Jy W, Horstman LL, Ahn YS, Alexander JS, Minagar A. Evidence of platelet activation in multiple sclerosis. J Neuroinflammation. 2008;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knijff-Dutmer EAJ, Koerts J, Nieuwland R, Kalsbeek-Batenburg EM, van de Laar MAFJ. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 2002;46:1498–503. [DOI] [PubMed] [Google Scholar]
- 38.Østergaard O, Nielsen CT, Iversen L V, Tanassi JT, Knudsen S, Jacobsen S, Heegaard NHH. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis Rheum. 2013;65:2680–90. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen CT, Østergaard O, Stener L, Iversen L V, Truedsson L, Gullstrand B, Jacobsen S, Heegaard NHH. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 2012;64:1227–36. [DOI] [PubMed] [Google Scholar]
- 40.Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M, Ratajczak MZ. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006;46:1199–209. [DOI] [PubMed] [Google Scholar]
- 41.Berckmans RJ, Nieuwland R, Böing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–46. [PubMed] [Google Scholar]
- 42.Vallier L, Bouriche T, Bonifay A, Judicone C, Bez J, Franco C, Guervilly C, Hisada Y, Mackman N, Houston R, Poncelet P, Dignat-George F, Lacroix R. Increasing the sensitivity of the human microvesicle tissue factor activity assay. Thromb Res. 2019;182:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berckmans RJ, Sturk A, Van Tienen LM, Schaap MCL, Nieuwland R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011;117:3172–3180. [DOI] [PubMed] [Google Scholar]
- 44.Satta N, Toti F, Fressinaud E, Meyer D, Freyssinet J-M. Scott syndrome: an inherited defect of the procoagulant activity of platelets. Platelets. 1997;8:117–124. [DOI] [PubMed] [Google Scholar]
- 45.Ridger VC, Boulanger CM, Angelillo-Scherrer A, et al. Microvesicles in vascular homeostasis and diseases. Thromb Haemost. 2017;117:1296–1316. [DOI] [PubMed] [Google Scholar]
- 46.Misceo D, Holmgren A, Louch WE, Holme PA, Mizobuchi M, Morales RJ, De Paula AM, Stray-Pedersen A, Lyle R, Dalhus B, Christensen G, Stormorken H, Tjønnfjord GE, Frengen E. A Dominant STIM1 Mutation Causes Stormorken Syndrome. Hum Mutat. 2014;35:556–564. [DOI] [PubMed] [Google Scholar]
- 47.Nesin V, Wiley G, Kousi M, Ong E-C, Lehmann T, Nicholl DJ, Suri M, Shahrizaila N, Katsanis N, Gaffney PM, Wierenga KJ, Tsiokas L. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc Natl Acad Sci. 2014;111:4197–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stormorken H, Holmsen H, Sund R, Sakariassen KS, Hovig T, Jellum E, Solum O. Studies on the haemostatic defect in a complicated syndrome. An inverse Scott syndrome platelet membrane abnormality? Thromb Haemost. 1995;74:1244–51. [PubMed] [Google Scholar]
- 49.Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez E, Srivastava AK, Burchfield J, Wang Y-W, Cardenas JC, Togarrati PP, Miyazawa B, Gonzalez E, Holcomb JB, Pati S, Wade CE. Platelet-derived-Extracellular Vesicles Promote Hemostasis and Prevent the Development of Hemorrhagic Shock. Sci Rep. 2019;9:17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JH, Jung H, Song J, Choi ES, You G, Mok H. Activated Platelet-Derived Vesicles for Efficient Hemostatic Activity. Macromol Biosci. 2020;20:e1900338. [DOI] [PubMed] [Google Scholar]
- 52.Guo S-C, Tao S-C, Yin W-J, Qi X, Yuan T, Zhang C-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayon Y, Dashevsky O, Shai E, Brill A, Varon D, Leker R R, Leker RR. Platelet Microparticles Induce Angiogenesis and Neurogenesis after Cerebral Ischemia. Curr Neurovasc Res. 2012;9:185–192. [DOI] [PubMed] [Google Scholar]
- 54.Hess JR, Lelkens CCM, Holcomb JB, Scalea TM. Advances in military, field, and austere transfusion medicine in the last decade. Transfus Apher Sci. 2013;49:380–6. [DOI] [PubMed] [Google Scholar]
- 55.Miyazawa B, Trivedi A, Togarrati PP, et al. Regulation of endothelial cell permeability by platelet-derived extracellular vesicles. J Trauma Acute Care Surg. 2019;86:931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bei J-J, Liu C, Peng S, Liu C-H, Zhao W-B, Qu X-L, Chen Q, Zhou Z, Yu Z-P, Peter K, Hu H-Y. Staphylococcal SSL5-induced platelet microparticles provoke proinflammatory responses via the CD40/TRAF6/NFKB signalling pathway in monocytes. Thromb Haemost. 2016;115:632–645. [DOI] [PubMed] [Google Scholar]
- 57.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1β synthesis. J Cell Biol. 2001;154:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boilard E, Nigrovic PA, Larabee K, Watts GFM, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets Amplify Inflammation in Arthritis via Collagen-Dependent Microparticle Production. Science (80-). 2010;327:580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boilard E Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA. J Lipid Res. 2018;59:2037–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maugeri N, Capobianco A, Rovere-Querini P, et al. Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Sci Transl Med. 2018;10:eaao3089. [DOI] [PubMed] [Google Scholar]
- 61.Braig D, Nero TL, Koch H-G, et al. Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nat Commun. 2017;8:14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cloutier N, Tan S, Boudreau LH, Cramb C, Subbaiah R, Lahey L, Albert A, Shnayder R, Gobezie R, Nigrovic P a., Farndale RW, Robinson WH, Brisson A, Lee DM, Boilard E. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle‐associated immune complexes. EMBO Mol Med. 2013;5:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang K, Liu J, Yang Z, Zhang B, Zhang H, Huang C, Ma J, Shen G-X, Ye D, Huang B. Microparticles mediate enzyme transfer from platelets to mast cells: A new pathway for lipoxin A4 biosynthesis. Biochem Biophys Res Commun. 2010;400:432–436. [DOI] [PubMed] [Google Scholar]
- 64.Sadallah S, Eken C, Martin PJ, Schifferli JA. Microparticles (Ectosomes) Shed by Stored Human Platelets Downregulate Macrophages and Modify the Development of Dendritic Cells. J Immunol. 2011;186:6543–6552. [DOI] [PubMed] [Google Scholar]
- 65.Ceroi A, Delettre FA, Marotel C, Gauthier T, Asgarova A, Biichle S, Duperrier A, Mourey G, Perruche S, Lagrost L, Masson D, Saas P. The anti-inflammatory effects of platelet-derived microparticles in human plasmacytoid dendritic cells involve liver X receptor activation. Haematologica. 2016;101:e72–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dinkla S, van Cranenbroek B, van der Heijden WA, He X, Wallbrecher R, Dumitriu IE, van der Ven AJ, Bosman GJCGM, Koenen HJPM, Joosten I. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood. 2016;127:1976–1986. [DOI] [PubMed] [Google Scholar]
- 67.Duchez A-C, Boudreau LH, Naika GS, et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A 2 -IIA. Proc Natl Acad Sci. 2015;112:E3564–E3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melki I, Tessandier N, Zufferey A, Boilard E. Platelet microvesicles in health and disease. Platelets. 2017;28:214–221. [DOI] [PubMed] [Google Scholar]
- 69.Lazar S, Goldfinger LE. Platelet Microparticles and miRNA Transfer in Cancer Progression: Many Targets, Modes of Action, and Effects Across Cancer Stages. Front. Cardiovasc. Med 2018;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michael J V, Wurtzel JGT, Mao GF, et al. Platelet microparticles infiltrating solid tumors transfer miRNAs that suppress tumor growth. Blood. 2017;130:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laffont B, Corduan A, Plé H, Duchez A-C, Cloutier N, Boilard E, Provost P. Activated platelets can deliver mRNA regulatory Ago2•microRNA complexes to endothelial cells via microparticles. Blood. 2013;122:253–261. [DOI] [PubMed] [Google Scholar]
- 72.Gasperi V, Vangapandu C, Savini I, Ventimiglia G, Adorno G, Catani MV. Polyunsaturated fatty acids modulate the delivery of platelet microvesicle-derived microRNAs into human breast cancer cell lines. J Nutr Biochem. 2019;74:108242. [DOI] [PubMed] [Google Scholar]
- 73.Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell. 2019;177:428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Puhm F, Afonyushkin T, Resch U, et al. Mitochondria Are a Subset of Extracellular Vesicles Released by Activated Monocytes and Induce Type I IFN and TNF Responses in Endothelial Cells. Circ Res. 2019;125:43–52. [DOI] [PubMed] [Google Scholar]
- 78.Boudreau LH, Duchez A-CC, Cloutier N, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Becker Y, Loignon R-C, Julien A-S, et al. Anti-mitochondrial autoantibodies in systemic lupus erythematosus and their association with disease manifestations. Sci Rep. 2019;9:4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.György B, Szabó TG, Turiák L, et al. Improved Flow Cytometric Assessment Reveals Distinct Microvesicle (Cell-Derived Microparticle) Signatures in Joint Diseases. PLoS One. 2012;7:e49726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Villar-Vesga J, Grajales C, Burbano C, Vanegas–García A, Muñoz–Vahos CH, Vásquez G, Rojas M, Castaño D. Platelet-derived microparticles generated in vitro resemble circulating vesicles of patients with rheumatoid arthritis and activate monocytes. Cell Immunol. 2019;336:1–11. [DOI] [PubMed] [Google Scholar]
- 84.Elshabrawy HA, Chen Z, Volin M V, Ravella S, Virupannavar S, Shahrara S The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18:433–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim HK, Song KS, Chung J-H, Lee KR, Lee S-N. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–384. [DOI] [PubMed] [Google Scholar]
- 86.BRILL A, DASHEVSKY O, RIVO J, GOZAL Y, VARON D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–38. [DOI] [PubMed] [Google Scholar]
- 87.Sun C, Feng S-B, Cao Z-W, Bei J-J, Chen Q, Zhao W-B, Xu X-J, Zhou Z, Yu Z-P, Hu H-Y. Up-Regulated Expression of Matrix Metalloproteinases in Endothelial Cells Mediates Platelet Microvesicle-Induced Angiogenesis. Cell Physiol Biochem. 2017;41:2319–2332. [DOI] [PubMed] [Google Scholar]
- 88.Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, Müller-Newen G, Soehnlein O, Weber C. Platelet Microparticles Enhance the Vasoregenerative Potential of Angiogenic Early Outgrowth Cells After Vascular Injury. Circulation. 2010;122:495–506. [DOI] [PubMed] [Google Scholar]
- 89.Weber A-A, Köppen HO, Schrör K. Platelet-Derived Microparticles Stimulate Coronary Artery Smooth Muscle Cell Mitogenesis by a PDGF-Independent Mechanism. Thromb Res. 2000;98:461–466. [DOI] [PubMed] [Google Scholar]
- 90.Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30:450–459. [DOI] [PubMed] [Google Scholar]
- 91.Cloutier N, Paré A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S, Boilard E. Platelets can enhance vascular permeability. Blood. 2012;120:1334–1343. [DOI] [PubMed] [Google Scholar]
- 92.Gaertner F, Ahmad Z, Rosenberger G, et al. Migrating Platelets Are Mechano-scavengers that Collect and Bundle Bacteria. Cell. 2017;171:1368–1382.e23. [DOI] [PubMed] [Google Scholar]
- 93.Milasan A, Tessandier N, Tan S, Brisson A, Boilard E, Martel C. Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis. J Extracell Vesicles. 2016;5:31427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tessandier N, Melki I, Cloutier N, et al. Platelets Disseminate Extracellular Vesicles in Lymph in Rheumatoid Arthritis. Arterioscler Thromb Vasc Biol. 2020;40:929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bertozzi CC, Schmaier AA, Mericko P, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Welsh JD, Kahn ML, Sweet DT. Lymphovenous hemostasis and the role of platelets in regulating lymphatic flow and lymphatic vessel maturation. Blood. 2016;128:1169–73. [DOI] [PubMed] [Google Scholar]
- 97.Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27:31–9. [DOI] [PubMed] [Google Scholar]
- 98.András IE, Leda A, Contreras MG, Bertrand L, Park M, Skowronska M, Toborek M. Extracellular vesicles of the blood-brain barrier: Role in the HIV-1 associated amyloid beta pathology. Mol Cell Neurosci. 2017;79:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N, Shi M, Banks WA, Zhang J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun. 2017;5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao Z, Wang M, Tian Y, Hilton T, Salsbery B, Zhou EZ, Wu X, Thiagarajan P, Boilard E, Li M, Zhang J, Dong J. Cardiolipin-mediated procoagulant activity of mitochondria contributes to traumatic brain injury–associated coagulopathy in mice. Blood. 2016;127:2763–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.García-Romero N, Carrión-Navarro J, Esteban-Rubio S, et al. DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget. 2017;8:1416–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. [DOI] [PubMed] [Google Scholar]
- 103.Brambilla M, Camera M, Colnago D, Marenzi G, De Metrio M, Giesen PL, Balduini A, Veglia F, Gertow K, Biglioli P, Tremoli E. Tissue factor in patients with acute coronary syndromes: expression in platelets, leukocytes, and platelet-leukocyte aggregates. Arterioscler Thromb Vasc Biol. 2008;28:947–53. [DOI] [PubMed] [Google Scholar]
- 104.Jensen MK, de Nully Brown P, Lund B V, Nielsen OJ, Hasselbalch HC. Increased circulating platelet-leukocyte aggregates in myeloproliferative disorders is correlated to previous thrombosis, platelet activation and platelet count. Eur J Haematol. 2001;66:143–51. [DOI] [PubMed] [Google Scholar]
- 105.Irving PM, Macey MG, Shah U, Webb L, Langmead L, Rampton DS. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:361–72. [DOI] [PubMed] [Google Scholar]
- 106.Constantinescu-Bercu A, Grassi L, Frontini M, Salles-Crawley II, Woollard K, Crawley JTB. Activated αIIbβ3 on platelets mediates flow-dependent NETosis via SLC44A2. Elife. 2020;9:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ehlers R, Ustinov V, Chen Z, Zhang X, Rao R, Luscinskas FW, Lopez J, Plow E, Simon DI. Targeting platelet-leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibalpha. J Exp Med. 2003;198:1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han P, Hanlon D, Arshad N, et al. Platelet P-selectin initiates cross-presentation and dendritic cell differentiation in blood monocytes. Sci Adv. 2020;6:eaaz1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Furie B, Furie BC. The molecular basis of platelet and endothelial cell interaction with neutrophils and monocytes: role of P-selectin and the P-selectin ligand, PSGL-1. Thromb Haemost. 1995;74:224–7. [PubMed] [Google Scholar]
- 110.Lo S-C, Hung C-Y, Lin D-T, Peng H-C, Huang T-F. Involvement of platelet glycoprotein Ib in platelet microparticle mediated neutrophil activation. J Biomed Sci. 2006;13:787–96. [DOI] [PubMed] [Google Scholar]
- 111.Salanova B, Choi M, Rolle S, Wellner M, Luft FC, Kettritz R. Beta2-integrins and acquired glycoprotein IIb/IIIa (GPIIb/IIIa) receptors cooperate in NF-kappaB activation of human neutrophils. J Biol Chem. 2007;282:27960–9. [DOI] [PubMed] [Google Scholar]
- 112.Kuravi SJ, Harrison P, Rainger GE, Nash GB. Ability of Platelet-Derived Extracellular Vesicles to Promote Neutrophil-Endothelial Cell Interactions. Inflammation. 2019;42:290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Loguinova M, Pinegina N, Kogan V, Vagida M, Arakelyan A, Shpektor A, Margolis L, Vasilieva E. Monocytes of Different Subsets in Complexes with Platelets in Patients with Myocardial Infarction. Thromb Haemost. 2018;118:1969–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


