Abstract
Background:
Individuals undergoing bariatric surgery report higher levels of suicidality than the general population, but it is unknown what mediates this phenomenon or how this compares to individuals with severe obesity not receiving surgery.
Objectives:
We evaluated suicidality in 131 individuals 12 years post surgery compared to 205 individuals with severe obesity that did not undergo surgery. Changes in health-related quality of life (HRQOL) and metabolic health were assessed as mediators of suicidality.
Setting:
University
Methods:
Suicidality was assessed with the Suicide Behaviors Questionnaire - Revised (SBQ-R) at 12 years. Metabolic health and HRQOL (Short-Form 36 (SF-36) Mental Component Summary score (MCS), Physical Component Summary score (PCS), and Impact of Weight on Quality of Life-Lite (IWQOL-Lite)) were assessed at baseline and two and six years. The effects of bariatric surgery on suicidality at 12 years were assessed through univariate and multivariate sequential moderated mediation models, with changes in metabolic health and HRQOL from 0–2 years and 2–6 years as mediators.
Results:
Suicidality was higher in the surgery group versus the non-surgery group (est.=0.708, SE=0.292, p<.05). Only the indirect pathways at two years after surgery for SF-36 MCS in the univariate models (est.=−0.172, SE=0.080, p<.05) and for SF-36 PCS in the multivariate model (est.=0.593, SE=0.281, p<.05) were significant.
Conclusion:
Individuals undergoing bariatric surgery reported higher levels of suicidality at 12 years, which was mediated by less improvement in the mental and physical components of HRQOL in the first two years after surgery, suggesting the need for additional clinical monitoring.
Keywords: Suicidality, Bariatric surgery, Mediation analysis, Suicide Behaviors Questionnaire-Revised (SBQ-R), Short-Form 36 (SF-36), Impact of Weight on Quality of Life-Lite (IWQOL-Lite), Utah Obesity Study
1. Introduction
Bariatric surgery is widely accepted as an effective clinical treatment for severe obesity and the chronic metabolic conditions that co-exist with severe obesity (1, 2). Following surgery, many patients experience remission of co-existing metabolic conditions, including hypertension, dyslipidemia, and type 2 diabetes (3–5). Long-term data have shown that these metabolic benefits, particularly remission of type 2 diabetes, may be maintained up to 12 years after surgery (6).
In addition to studying the physical and metabolic effects of bariatric surgery, numerous studies have focused on the effects of bariatric surgery on patient well-being and mental health. During the first 12 months after surgery, a majority of patients show a strong increase in health-related quality of life (HRQOL) (7, 8) and a decrease in depressive symptoms (9, 10) compared to pre-operative levels. Long-term post-operative data show similar findings, with depression rates (11–13) and HRQOL (13–15) both improving from baseline measurements, often with peak benefit seen around two years after surgery (11, 14).
Despite these findings, recent epidemiological studies report higher rates of intentional self-harm and overdose (16), self-harm emergencies (17), suicide-related thoughts and behaviors (18), and externally caused death, including suicide (19–21), among those who have undergone bariatric surgery. While the risk of suicide in patients after bariatric surgery is greater than in the general population (22), it is unknown what factors mediate (i.e., statistically account for) the heightened risk. Mitchell and colleagues (23) have proposed that unmet expectations regarding weight loss, metabolic health, mental health problems, and poor physical functioning—especially a loss of key improvements after surgery—may foster feelings of disappointment and failure that increase risk of suicide.
To date, no study has examined mediators of suicidality after bariatric surgery or whether post-bariatric surgery patients report higher suicidality (i.e., suicide-related thoughts and behaviors) relative to those with severe obesity who have not undergone bariatric surgery. The present study assesses suicidality 12 years after baseline in individuals from the Utah Obesity Study, which includes participants with obesity who have and have not undergone surgery. This study sought to answer three questions. (1) Is suicidality greater for individuals completing bariatric surgery than for individuals with severe obesity that do not undergo surgery? (2) Do changes in HRQOL, metabolic health, or weight (including initial weight loss and weight regain in the long term) mediate the differences in suicidality between these populations? (3) Do these mediating effects differ by baseline characteristics, including age and sex?
2. Methods
2.1. Participant Recruitment
Study participants were recruited from a single bariatric surgery center in Utah and from the Utah Health Family Tree program as part of the Utah Obesity Study (24). This large cohort comprises individuals who (1) underwent Roux-en-Y gastric bypass (surgery group) or (2) had severe obesity but did not undergo surgery (non-surgery group). This latter group includes individuals that sought but did not receive Roux-en-Y gastric bypass or were part of a random control sample of the Utah Health Family Tree program that were initially reported to be over 100 lb overweight (25). Participants in all groups had reported a body mass index (BMI) ≥ 40 kg/m2 or a BMI ≥ 35 kg/m2 with two or more comorbidities. Exclusion criteria included: previous gastric surgery for weight loss; active cancer within the last 5 years, with the exception of non-melanoma skin cancer; gastric or duodenal ulcers in the previous 6 months; abuse of alcohol or narcotics; and myocardial infarction in the previous 6 months. Though not an exclusion criteria in the larger Utah Obesity Study, for the purposes of the current study, individuals who were originally in the non-surgery group but received surgery for weight loss at a later date were excluded. Other selection criteria and information regarding recruitment have been published previously (3, 6, 24, 26).
Data from the present study were based on 131 participants from the surgery group and 205 participants from the non-surgery group that completed the initial HRQOL and metabolic health assessments and returned for follow-up at years two and six to assess metabolic health and HRQOL and again at year 12 to measure suicidality. The study was approved by the Institutional Review Board at the University of Utah, and all participants signed approved informed consent documents.
2.2. Measures
To assess HRQOL, both a general measure, Medical Outcomes Study Short Form-36 (SF-36, Version 2.0), and weight-specific measure, Impact of Weight on Quality of Life-Lite (IWQOL-Lite), were used.
The SF-36 health survey is a widely used tool to assess health-related quality of life and provides minimum psychometric standards that allow for group comparisons (27). The survey has been validated for internal consistency (28) and test-retest reliability (29) and has been validated for use among a wide range of groups, including persons with obesity (30). Of the eight HRQOL domains measured by the SF-36, four assess physical aspects of HRQOL (physical functioning, role-physical, bodily pain, and general health), and four assess mental aspects of HRQOL (vitality, social functioning, role emotional, and mental health) (27). A Physical Component Summary score (PCS) is calculated for the first four domains, and a Mental Component Summary score (MCS) is calculated for the second four domains. Scores are transformed on a scale of 0 to 100, where 100 represents the best HRQOL. Participants were asked to rate themselves on these items over the past 4 weeks unless otherwise indicated.
The IWQOL-Lite is a widely used tool to assess weight-related quality of life. Most items begin with the phrase “because of my weight” to capture the effects of weight on quality of life. This 31-item measure yields five domain scores (physical function, self-esteem, sexual life, public distress, and work) and a total score. Scores range from 0 to 100, where higher scores indicate better weight-related quality of life. The IWQOL-Lite has demonstrated excellent psychometric properties (31, 32).
The Suicide Behaviors Questionnaire - Revised (SBQ-R) was used to assess suicidality. The SBQ-R is a 4-item questionnaire used to identify several dimensions of suicide risk, such as lifetime suicide ideation and behaviors, which are a significant risk factor for future suicidal behaviors (33), the frequency of suicide ideation over the past 12 months, threats of suicide attempts, and the likelihood of future suicidal behaviors. In addition to its more extensive use than other comparable brief measures of suicidality (34), the SBQ-R has shown reliability with regard to internal consistency and has been validated for use in both clinical and non-clinical settings (34). In the present sample, the measure yielded good internal consistency (α = .793). The questionnaire was self-administered, and a summated score was generated based on their response to each question. Total scores range from 3–18, with higher scores representing higher levels of suicidality.
Metabolic health information was obtained through a standardized medical history and endpoints questionnaire with confirmation, in most cases, by laboratory assessment of metabolic parameters, as has been described previously (24). The criteria for type 2 diabetes was defined as having one or more of the following conditions: a fasting blood glucose level of at least 126 mg per deciliter (7.0 mmol per liter), a glycated hemoglobin level of at least 6.5%, or current use of any antidiabetic medication. The criteria for hypertension was defined as having a blood pressure of at least 140/90 mm Hg while seated and/or the reported use of antihypertensive medication. The criteria for dyslipidemia was defined as having one or more of the following conditions: a fasting low-density lipoprotein cholesterol level of at least 160 mg per deciliter (4.1 mmol per liter), a high-density lipoprotein cholesterol level of less than 40 mg per deciliter (1.0 mmol per liter), a triglyceride level of at least 200 mg per deciliter (2.3 mmol per liter), or current use of lipid-lowering medication.
2.3. Baseline Subject Study Examinations
Participants attended either the Huntsman General Clinical Research Center (GCRC), University of Utah Medical Center, SLC, UT., or our outpatient clinic to receive their baseline examination. During this visit, patients completed the SF-36 health survey and IWQOL-Lite, and metabolic health information was obtained as described above.
Participants in the surgery group received the Roux-en-Y gastric bypass operation within a year after their baseline examination. The operation was performed by either an open or laparoscopic approach, as previously described (35, 36). Participants from the non-surgery group did not receive a study-based intervention for weight loss, but they were not restricted from seeking out such treatment. However, as previously stated, participants from the non-surgery comparison group who went on to have surgery during the 12 years of follow-up were not included in this analysis (n = 70).
2.3. Subject Follow-up at 2 and 6 Years
Participants returned for follow-up examinations at years two and six, during which the SF-36 health survey, IWQOL-Lite and metabolic health measurements were repeated. Follow-up results from the metabolic health data (3, 26) and HRQOL data (37, 38) have been reported previously. Remission of type 2 diabetes, hypertension, and dyslipidemia were defined as the absence of the disease based on the criteria above. Relapse was defined as the reemergence of co-existing metabolic conditions following remission.
2.4. Subject Follow-up at 12 Years
Participants returned for a final examination at year 12. Suicidality was assessed only at this visit using the SBQ-R. Metabolic health and HRQOL measurements collected at the earlier visits were repeated at this time point but are not included in the present study. Findings from those data have been reported previously (6, 14).
2.5. Statistical Analyses
Both sequential moderated mediation analyses and sequential multiple moderated mediation analyses (See Figure 1) were conducted to assess whether SBQ-R score (suicidality) measured at the 12-year follow-up visit was mediated by changes in SF-36 MCS, SF-36 PCS, IWQOL-Lite, BMI, type 2 diabetes status, hypertension status, and dyslipidemia status. Both models measured the total effects of being in the surgery group versus the non-surgery group on SBQ-R score measured during the 12-year follow up visit, as well as the simultaneous indirect effects of the mediating variables. The total effect (c) measures the amount of variance in the suicidality score that is due to being in the surgery or comparison group and is the sum of the direct (c’) and total indirect (ab) effects. The total indirect effect measures the part of the total effect that is accounted for by the mediators. All analyses were performed using the PROCESS macro (39) within SPSS version 19.0.
Figure 1.
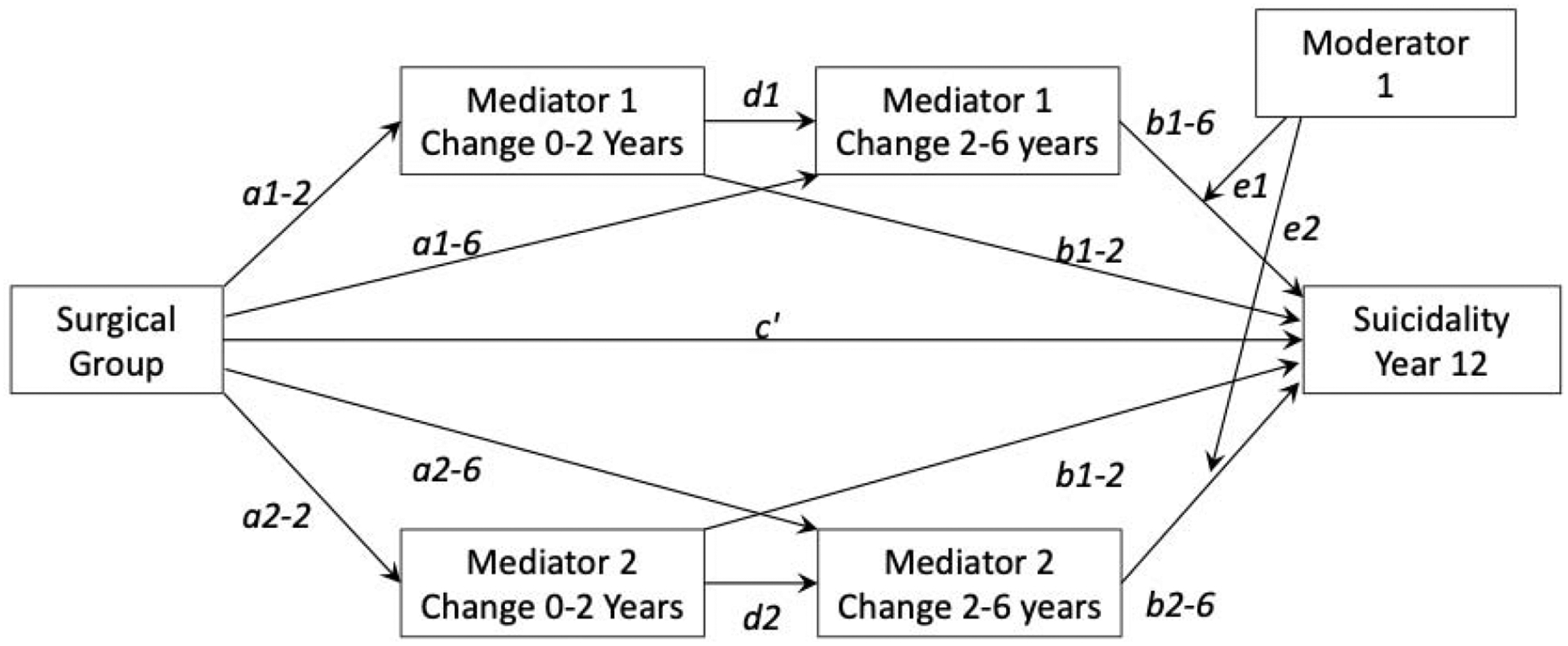
Reference sequential multiple moderated mediation model with two parallel mediators and one moderator. The a paths measure the effect of being in the surgery vs. non-surgery group on the change in mediators between years 0–2 or 2–6. The b paths measure the effect of change in the mediators from years 0–2 or 2–6 on suicidality measured at year 12. The d paths measure the effect of change in the mediators from years 0–2 on change in the mediators from years 2–6.
Sequential moderated mediation analyses were performed to assess each mediator individually. In the univariate models, the total indirect effect (ab) is comprised of three separate pathways: the indirect effect at year two (a2b2), the indirect effect at year six (a6b6), and the indirect effect at years two and six (a2db6). Sequential multiple moderated mediation analyses were conducted to evaluate the total indirect effect of all mediating variables on SBQ-R score at the 12-year follow-up visit. The same mediating variables from the univariate models were used. In the multivariate model, the total indirect effect (ab) reflects the sum of the individual indirect effects for each mediator. The moderating effect of age and sex (e) was only determined for mediators with significant indirect effects.
To meet the condition of temporal precedence for the mediating variables, change was represented as the residualized change from baseline and year two and the residualized change between years two and six. The status variables for type 2 diabetes, hypertension, and dyslipidemia were dichotomous “yes/no” reflecting whether or not the subject met the study’s criteria for the disease. In clinical terms, changes in these variables may reflect an initial diagnosis, remission, or relapse of the disease.
Results
3.1. Participant Characteristics
A cohort of 336 participants was included in the analysis, with 131 in the surgery group and 205 in the comparison group. Participant characteristics can be found in Table 1. Participants in both groups were predominantly female, White, and married. The surgery group, on average, was 3.72 years younger (p = .002) and weighed 25.23 kgs less (p < .001) than the comparison group at the 12-year follow-up examination. At baseline, the non-surgery group was less impaired on the IWQOL-Lite (p < .001) and SF-36 MCS (p = .042). Surgery patients used in the current study were significantly older (p = .005), had lower pre-surgical BMI (p = .036), and were less impaired on the IWQOL-Lite (p = .005) and the SF-36 MCS (p = .021) at the pre-surgical assessment than those surgery patients not included in these analyses. Similarly, the comparison group included in the current analyses were significantly older (p < .001), had lower pre-surgical BMI (p < .001), and were less impaired on the IWQOL-Lite (p < .001), SF-36 PCS (p = .019), and the SF-36 MCS (p < .001) at the pre-surgical assessment than those that did not receive surgery who were not included in these analyses.
Table 1.
Participant characteristics measured at the 12-year follow-up exam
| Characteristic | Surgery Group (n=131) | Non-surgery Comparison Group (n = 205) | Significance |
|---|---|---|---|
| Female, n (%) | 109 (83.2%) | 166 (81.0%) | p > 0.05 |
| Age, yr (mean ± SD) | 55.98 ± 10.80 | 59.70 ± 10.72 | p = 0.002 |
| Married, n (%) | 88 (67.2) | 137 (66.8) | p > 0.05 |
| White, n (%) | 127 (96.9%) | 204 (99.5%) | p > 0.05 |
| Weight, kg (mean ± SD) | 95.35 ± 25.43 | 120.58 ± 25.27 | p < 0.001 |
| Total body weight loss (%) | −27.66 ± 11.77 | −1.32 ± 13.55 | p < 0.001 |
| BMI, kg/m2 (mean ± SD) | 34.28 ± 8.06 | 43.96 ± 7.53 | p < 0.001 |
| Change in BMI (mean ± SD) | −11.76 ± 6.09 | 0.56 ± 5.91 | p < 0.001 |
| % Excess BMI Lost (mean ± SD) | −59.34 ± 28.25 | 5.46 ± 35.51 | p < 0.001 |
| Waist circumference, cm (mean ± SD) | 112.48 ± 17.44 | 133.14 ± 16.30 | p < 0.001 |
| Hip circumference, cm (mean ± SD) | 121.28 ± 17.50 | 138.28 ± 15.89 | p < 0.001 |
BMI = body mass index
3.2. Univariate Mediation Models
Results from seven univariate moderated mediation analyses can be found in Table 2. In each model, the total effect (c) was significant and revealed that suicidality was higher in the surgery group 12 years after surgery (Surgery group mean = 5.10, SD = 2.60; Non-surgery group mean = 4.45, SD = 2.33; d = .27). The direct effect (c’), the part of the total effect that is not accounted for by the mediators, was significant in models for SF-36 MCS, SF-36 PCS, IWQOL-Lite score, and dyslipidemia status. None of the total indirect effects, or the extent to which the mediators explain the variation in the SBQ-R score, were significant. Only the indirect pathway for SF-36 MCS at two years after surgery was significant (est. = −0.172, SE = 0.080, p < .05) (See Figure 2). This suggests that changes in mental components of HRQOL that occur in the first two years after surgery mediate higher suicidality 12 years after surgery, with those who experienced a lack of improvement or only minimal improvement in MCS having the highest suicidality in the long term. Age and sex did not significantly moderate this effect.
Table 2.
Univariate mediaton model (n = 336): SBQ-R
| A. SF-36 MCS Effect | Variable | Coeff. | SE | p |
|---|---|---|---|---|
| Total effect of surgery versus non-surgery on SBQ-R total score | c | −0.660 | 0.263 | 0.012 |
| Direct effect of surgery versus non-surgery on SBQ-R total score | c′ | 0.723 | 0.274 | 0.008 |
| Total indirect effect of surgery versus non-surgery on SBQ-R total score | ab | −0.063 | 0.090 | 0.486 |
| Indirect effect at 2 years after surgery | a2b2 | −0.172 | 0.080 | 0.031 |
| Indirect effect at 6 years after surgery | a6b6 | 0.089 | 0.052 | 0.086 |
| Indirect effect at 2 and 6 years after surgery | a2db6 | 0.021 | 0.014 | 0.131 |
| Individual Paths | a2 | 4.925 | 1.133 | <0.001 |
| a6 | −2.809 | 1.144 | 0.014 | |
| b2 | −0.035 | 0.013 | 0.009 | |
| b6 | −0.032 | 0.012 | 0.010 | |
| d | −0.133 | 0.059 | 0.023 | |
| B. SF-36 PCS Effect | Variable | Coeff. | SE | p |
| Total effect of surgery versus non-surgery on SBQ-R total score | c | 0.668 | 0.260 | 0.010 |
| Direct effect of surgery versus non-surgery on SBQ-R total score | c′ | 1.128 | 0.386 | 0.004 |
| Total indirect effect of surgery versus non-surgery on SBQ-R total score | ab | −0.460 | 0.235 | 0.050 |
| Indirect effect at 2 years after surgery | a2b2 | −0.451 | 0.233 | 0.053 |
| Indirect effect at 6 years after surgery | a6b6 | −0.060 | 0.051 | 0.241 |
| Indirect effect at 2 and 6 years after surgery | a2db6 | 0.050 | 0.038 | 0.182 |
| Individual Paths | a2 | 13.195 | 0.868 | <0.001 |
| a6 | 2.644 | 1.335 | 0.048 | |
| b2 | −0.034 | 0.018 | 0.056 | |
| b6 | −0.023 | 0.014 | 0.104 | |
| d | −0.169 | 0.068 | 0.012 | |
| C. IWQOL-Lite Effect | Variable | Coeff. | SE | p |
| Total effect of surgery versus non-surgery on SBQ-R total score | c | 0.645 | 0.256 | 0.012 |
| Direct effect of surgery versus non-surgery on SBQ-R total score | c′ | 1.096 | 0.389 | 0.005 |
| Total indirect effect of surgery versus non-surgery on SBQ-R total score | ab | −0.451 | 0.293 | 0.124 |
| Indirect effect at 2 years after surgery | a2b2 | −0.493 | 0.292 | 0.091 |
| Indirect effect at 6 years after surgery | a6b6 | 0.042 | 0.050 | 0.402 |
| Indirect effect at 2 and 6 years after surgery | a2db6 | 0.000 | 0.041 | 0.997 |
| Individual Paths | a2 | 38.304 | 1.564 | <0.001 |
| a6 | −2.359 | 2.301 | 0.305 | |
| b2 | −0.013 | 0.008 | 0.092 | |
| b6 | −0.018 | 0.009 | 0.052 | |
| d | 0.000 | 0.053 | 0.997 | |
| D. BMI Effect | Variable | Coeff. | SE | p |
| Total effect of surgery versus non-surgery on SBQ-R total score | c | 0.638 | 0.254 | 0.012 |
| Direct effect of surgery versus non-surgery on SBQ-R total score | c′ | 0.406 | 0.543 | 0.455 |
| Total indirect effect of surgery versus non-surgery on SBQ-R total score | ab | 0.232 | 0.500 | 0.642 |
| Indirect effect at 2 years after surgery | a2b2 | 0.176 | 0.492 | 0.721 |
| Indirect effect at 6 years after surgery | a6b6 | 0.105 | 0.111 | 0.343 |
| Indirect effect at 2 and 6 years after surgery | a2db6 | −0.049 | 0.062 | 0.431 |
| Individual Paths | a2 | −15.330 | 0.429 | <0.001 |
| a6 | 3.215 | 1.080 | 0.003 | |
| b2 | −0.011 | 0.032 | 0.721 | |
| b6 | 0.033 | 0.033 | 0.322 | |
| D | 0.097 | 0.065 | 0.136 | |
| E. Type 2 Diabetes Status Effect | Variable | Coeff. | SE | p |
| Total effect of surgery versus non-surgery on SBQ-R total score | c | 0.645 | 0.260 | 0.013 |
| Direct effect of surgery versus non-surgery on SBQ-R total score | c′ | 0.513 | 0.268 | 0.056 |
| Total indirect effect of surgery versus non-surgery on SBQ-R total score | ab | 0.132 | 0.111 | 0.236 |
| Indirect effect at 2 years after surgery | a2b2 | 0.054 | 0.159 | 0.732 |
| Indirect effect at 6 years after surgery | a6b6 | 0.025 | 0.061 | 0.679 |
| Indirect effect at 2 and 6 years after surgery | a2db6 | 0.052 | 0.126 | 0.680 |
| Individual Paths | a2 | −0.249 | 0.038 | <0.001 |
| a6 | −0.101 | 0.030 | 0.001 | |
| b2 | −0.218 | 0.632 | 0.730 | |
| b6 | −0.251 | 0.592 | 0.672 | |
| d | 0.834 | 0.035 | <0.001 | |
| F. Dyslipidemia Status Effect | Variable | Coeff. | SE | p |
| Total effect of surgery versus non-surgery on SBQ-R total score | c | 0.637 | 0.254 | 0.012 |
| Direct effect of surgery versus non-surgery on SBQ-R total score | c′ | 0.628 | 0.296 | 0.034 |
| Total indirect effect of surgery versus non-surgery on SBQ-R total score | ab | 0.009 | 0.161 | 0.958 |
| Indirect effect at 2 years after surgery | a2b2 | −0.035 | 0.187 | 0.851 |
| Indirect effect at 6 years after surgery | a6b6 | 0.011 | 0.042 | 0.799 |
| Indirect effect at 2 and 6 years after surgery | a2db6 | 0.033 | 0.118 | 0.779 |
| Individual Paths | a2 | −0.521 | 0.045 | <0.001 |
| a6 | −0.105 | 0.051 | 0.038 | |
| b2 | 0.067 | 0.354 | 0.849 | |
| b6 | −0.101 | 0.356 | 0.776 | |
| d | 0.624 | 0.051 | <0.001 | |
| G. Hypertension Status Effect | Variable | Coeff. | SE | p |
| Total effect of surgery versus non-surgery on SBQ-R total score | c | 0.653 | 0.256 | 0.011 |
| Direct effect of surgery versus non-surgery on SBQ-R total score | c′ | 0.509 | 0.273 | 0.062 |
| Total indirect effect of surgery versus non-surgery on SBQ-R total score | ab | 0.143 | 0.108 | 0.186 |
| Indirect effect at 2 years after surgery | a2b2 | 0.073 | 0.155 | 0.640 |
| Indirect effect at 6 years after surgery | a6b6 | 0.009 | 0.024 | 0.710 |
| Indirect effect at 2 and 6 years after surgery | a2db6 | 0.062 | 0.114 | 0.587 |
| Individual Paths | a2 | −0.360 | 0.050 | <0.001 |
| a6 | −0.038 | 0.038 | 0.329 | |
| b2 | −0.202 | 0.424 | 0.634 | |
| b6 | −0.235 | 0.420 | 0.576 | |
| d | 0.729 | 0.042 | <0.001 |
SBQ-R = Suicide Behaviors Questionnaire – Revised; SF-36 MCS = Short-Form 36 Mental Component Summary score; SF-36 PCS = Short-Form 36 Physical Component Summary score; IWQOL-Lite = Impact of Weight on Quality of Life-Lite; BMI = body mass index
Figure 2.
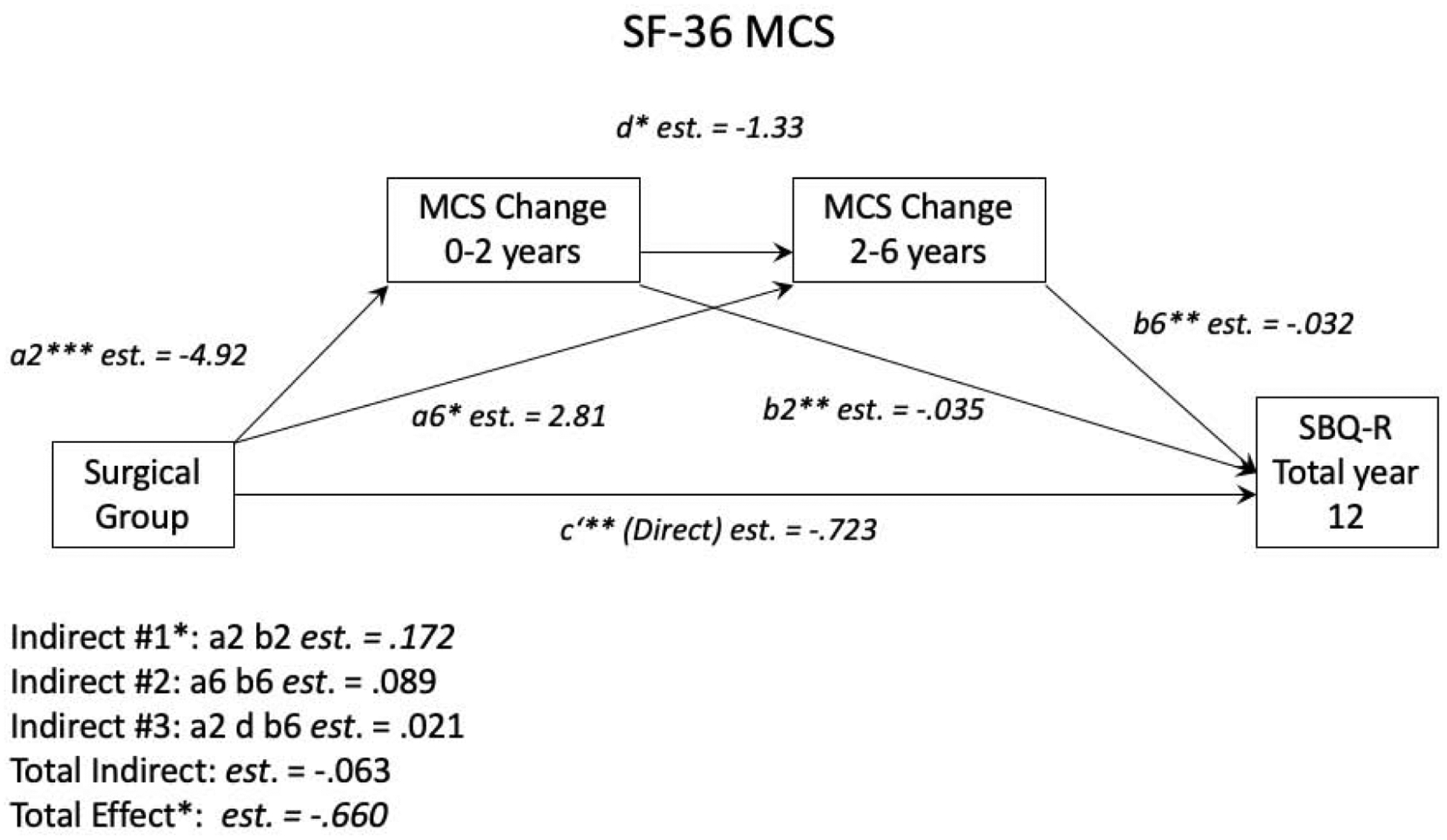
SF-36 MCS Univariate Mediation Model. *p < 0.05, **p < 0.01, ***p < 0.001. a2 = the effect of surgery vs. no surgery on MCS change between baseline and year 2. a6 = the effect of surgery vs. no surgery on MCS change between year 2 and year 6. b2 = the effect of MCS change between baseline and year 2 on SBQ-R score. b6 = the effect of MCS change between year 2 and year 6 on SBQ-R score. d = the effect of MCS change between baseline and year 2 on MCS change between year 2 and 6. c’ = the part of the total effect of surgery vs. no surgery on SBQ-R score that isn’t accounted for by the mediators.
3.3. Multivariate Mediation Model
Results from the multivariate mediation model can be found in Table 3. The total effect was significant (est. = 0.708, SE = 0.292, p < .05), indicating that suicidality was higher in the surgery group. Neither the direct effect nor the total indirect effect was significant. In this model, only the indirect effect at two years for SF-36 PCS was significant (est. = 0.593, SE = 0.281, p < .05), with the indirect effect at two years for SF-36 MCS being just beyond the threshold for significance (est. = −0.192, SE = 0.099, p = .053). These results indicate that less improvement in the physical components of HRQOL in the first two years after surgery is associated with higher suicidality at 12 years post-surgery. Those who did not have improvements or experienced only modest improvements in PCS in the short term had the highest levels of suicidality 12 years after surgery. Similar to our findings in the univariate models, age and sex did not significantly moderate this effect.
Table 3.
Multivariate mediation model (n = 336): SBQ-R
| Effect | Variable | Coeff. | SE | p |
|---|---|---|---|---|
| Total effect of surgery versus non-surgery on SBQ-R total score | c | 0.708 | 0.292 | 0.015 |
| Direct effect of surgery versus non-surgery on SBQ-R total score | c′ | 0.628 | 0.671 | 0.349 |
| Total indirect effect of surgery versus non-surgery on SBQ-R total score | ab | 0.080 | 0.625 | 0.898 |
| Indirect effect at 2 years after surgery | ||||
| IWQOL-Lite | a1–2 b1–2 | 0.191 | 0.453 | 0.674 |
| PCS | a2–2 b2–2 | 0.593 | 0.281 | 0.035 |
| MCS | a3–2 b3–2 | −0.192 | 0.099 | 0.053 |
| BMI | a4–2 b4–2 | 0.370 | 0.584 | 0.527 |
| Type 2 Diabetes Status | a5–2 b5–2 | 0.091 | 0.167 | 0.584 |
| Dyslipidemia Status | a6–2 b6–2 | 0.029 | 0.215 | 0.893 |
| Hypertension Status | a7–2 b7–2 | 0.002 | 0.189 | 0.991 |
| Indirect effect at 6 years after surgery | ||||
| IWQOL-Lite | a1–6 b1–6 | 0.015 | 0.050 | 0.761 |
| PCS | a2–6 b2–6 | −0.057 | 0.060 | 0.341 |
| MCS | a3–6 b3–6 | 0.088 | 0.060 | 0.143 |
| BMI | a4–6 b4–6 | −0.061 | 0.157 | 0.700 |
| Type 2 Diabetes Status | a5–6 b5–6 | 0.006 | 0.069 | 0.925 |
| Dyslipidemia Status | a6–6 b6–6 | −0.013 | 0.052 | 0.811 |
| Hypertension Status | a7–6 b7–6 | 0.016 | 0.032 | 0.606 |
| Indirect effect at 2 and 6 years after surgery | ||||
| IWQOL-Lite | a1–2 d1 b1–6 | 0.000 | 0.033 | 0.999 |
| PCS | a2–2 d2 b2–6 | 0.048 | 0.045 | 0.282 |
| MCS | a3–2 d3 b3–6 | 0.021 | 0.015 | 0.176 |
| BMI | a4–2 d4 b4–6 | 0.028 | 0.088 | 0.751 |
| Type 2 Diabetes Status | a5–2 d5 b5–6 | 0.013 | 0.142 | 0.926 |
| Dyslipidemia Status | a6–2 d6 b6–6 | −0.039 | 0.148 | 0.794 |
| Hypertension Status | a7–2 d7 b7–6 | 0.115 | 0.144 | 0.426 |
SBQ-R = Suicide Behaviors Questionnaire – Revised; SF-36 MCS = Short-Form 36 Mental Component Summary score; SF-36 PCS = Short-Form 36 Physical Component Summary score; IWQOL-Lite = Impact of Weight on Quality of Life-Lite; BMI = body mass index
4. Discussion
The present study assessed mediators of suicidality in individuals 12 years after bariatric surgery relative to a comparison group of individuals with severe obesity who did not receive surgery. Our results revealed that participants who underwent bariatric surgery had, on average, higher levels of suicidality than those in the comparison group that did not receive surgery. Both the presence of a comparison group with severe obesity and the follow-up more than 10 years after surgery make this a novel finding. Our findings are consistent with those of Gordon and colleagues (18) from the LABS-2 observational cohort study, the only other long-term study of suicidality after bariatric surgery. Using the SBQ-R and Beck Depression Inventory-Version 1 (BDI-1) to measure suicidality five years after surgery, they found that patients had rates of suicidality well above those of the general population. The higher suicidality we observed in the surgery group is also consistent with previous reports describing a higher prevalence of suicidal ideation in individuals seeking bariatric surgery (40, 41) and with a well-documented increased risk of suicide after bariatric surgery (19–22).
Our results also indicate that changes to mental and physical components of HRQOL within two years after surgery, as measured by the SF-36 questionnaire, mediate suicidality more than a decade after surgery. For both aspects of HRQOL, individuals in the surgery group that experienced the least improvement in the short term were the most likely to have higher levels of suicidality more than a decade after surgery. This finding provides support for the detection of a similar trend in the LABS-2 study: patients who experienced less improvement or a worsening of general health (one of the four domains in the SF-36 PCS) had a higher risk of suicide-related thoughts and behaviors after surgery (18). A meta-analysis of SF-36 MCS and PCS after bariatric surgery previously found that both components of HRQOL improve dramatically during the first post-operative year (7), which our results, as indicated by the a1 paths in both the univariate and multivariate mediation models, support. Although there are, on average, strong improvements to HRQOL after surgery, this same meta-analysis reported that there was considerable heterogeneity within the sample. The high variability indicates that not all patients experience robust improvements. Mitchell and colleagues (23) proposed that a subset of individuals who receive bariatric surgery and experience unchanged or worsening HRQOL may be at a higher risk of suicide due to their sense of disappointment or failure. Our findings suggest that ongoing deficiencies in HRQOL after surgery may mediate higher levels of suicidal thoughts and behaviors and thus the risk for suicide. Consequently, it may be important for surgeons and other health care providers to monitor changes in HRQOL in the short term after surgery. Patients who do not experience any improvements or only minor increases in HRQOL may benefit from additional monitoring of suicidal thoughts and behaviors.
Neither weight changes (poor initial weight loss or long-term weight regain) nor the relapse of type 2 diabetes, hypertension, or dyslipidemia mediated higher suicidality among those in the surgery group. Weight regain following peak weight loss in bariatric surgery is a well-documented phenomenon (42) and may be related to the recurrence of the chronic metabolic conditions that coexist with severe obesity (43). Recent reports suggest that weight regain after surgery may also be associated with long-term increases in depressive symptoms (44), which have a strong association with an increased risk of suicidality in this population (18). It has been proposed that weight regain and the reemergence of these chronic metabolic conditions after surgery, as well as unmet expectations regarding weight loss, may foster feelings of failure and contribute to suicide risk (23, 45, 46), which our findings do not support. Our results are consistent, however, with previous research that found no relation between suicidality and weight loss within the first post-operative year (47) or weight change measured five years after surgery (18). While our findings in this cohort indicate that changes in metabolic health do not directly influence suicidality, it is possible that changes in this area may impact HRQOL and thus affect suicidality, as long-term HRQOL changes after surgery have been shown to resemble changes in metabolic health during a long-term follow-up of patients in the Utah Obesity Study (6, 14).
Strengths of this study include a comparison group with severe obesity, a 12-year longitudinal design with assessments at four time points, and the use of both generic and weight-specific measures of HRQOL. A limitation of the present study was that suicidality and depression were not measured at baseline. Consequently, it was impossible to control for baseline depression in our analyses or to assess whether the surgery group had higher levels of suicidality before surgery. This may have influenced our findings, given that a history of suicidality is a strong predictor of suicidality in the short term after bariatric surgery (47). Additionally, all participants were recruited from a single site, and not all participants enrolled in the Utah Obesity Study were included in the current analysis. While this subsample of the cohort who completed all questionnaires at each time point also differed slightly in age, initial BMI, and quality of life at their initial assessment from those who were not included, the pattern of differences was similar between each subsample and their counterparts in the Utah Obesity Study, suggesting minimal additional selection bias when comparing surgery with non-surgery participants within this subsample of the parent study. Because participants were only included in the sample if they completed each visit and all necessary assessments, the seven individuals who committed suicide from the larger Utah Obesity Study cohort (all of whom underwent bariatric surgery) were not included in our analyses (6). Consequently, our findings may underestimate the true level of suicidality of those who received bariatric surgery.
5. Conclusion
In conclusion, this study is the first to assess suicidality after bariatric surgery relative to a comparison group of individuals with severe obesity and the first to report suicidality more than a decade after surgery. Individuals who underwent bariatric surgery reported higher levels of suicidality, which was mediated by changes in both mental and physical components of HRQOL in the first two years after surgery. Those who experienced the least improvements in these areas were the most likely to have higher suicidality at our long-term follow-up and may benefit from additional monitoring by their health care providers.
Highlights.
Suicidality was higher in the surgery group compared to the non-surgery group at 12 years.
Changes in health-related quality of life within 2 years after surgery mediated higher suicidality.
Higher suicidality in the surgery group was not mediated by changes in weight and metabolic health.
Acknowledgements:
We are grateful for the long-term support of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (DK-55006).
Conflict of Interest: Dr. Hunt received grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health during the conduct of the study. Dr. Crosby is a paid statistical consultant for Health Outcomes Solutions, Winter Park, Florida, USA. The other authors declare they have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Longitudinal Assessment of Bariatric Surgery Consortium, Flum DR, Belle SH, et al. Perioperative Safety in the Longitudinal Assessment of Bariatric Surgery. N Engl J Med. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sjostrom L, Lindroos A, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. [DOI] [PubMed] [Google Scholar]
- [3].Adams TD, Pendleton RC, Strong MB, et al. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity (Silver Spring). 2010;18:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59:945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sjostrom L Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–34. [DOI] [PubMed] [Google Scholar]
- [6].Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377:1143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Magallares A, Schomerus G. Mental and physical health-related quality of life in obese patients before and after bariatric surgery: a meta-analysis. Psychol Health Med. 2015;20:165–76. [DOI] [PubMed] [Google Scholar]
- [8].Hachem A, Brennan L. Quality of Life Outcomes of Bariatric Surgery: A Systematic Review. Obes Surg. 2016;26:395–409. [DOI] [PubMed] [Google Scholar]
- [9].Masheb RM, White MA, Toth CM, et al. The prognostic significance of depressive symptoms for predicting quality of life 12 months after gastric bypass. Compr Psychiatry. 2007;48:231–6. [DOI] [PubMed] [Google Scholar]
- [10].Alabi F, Guilbert L, Villalobos G, et al. Depression before and after bariatric surgery in low-Income patients: the utility of the beck depression inventory. Obes Surg. 2018. [DOI] [PubMed] [Google Scholar]
- [11].Ribeiro G, Giapietro HB, Belarmino LB, Salgado-Junior W. Depression, anxiety, and binge eating before and after bariatric surgery: problems that remain. Arq Bras Cir Dig. 2018;31:e1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vangoitsenhoven R, Frederiks P, Gijbels B, et al. Long-term effects of gastric bypass surgery on psychosocial well-being and eating behavior: not all that glitters is gold. Acta Clin Belg. 2016;71:395–402. [DOI] [PubMed] [Google Scholar]
- [13].Aasprang A, Andersen JR, Vage V, Kolotkin RL, Natvig GK. Five-year changes in health-related quality of life after biliopancreatic diversion with duodenal switch. Obes Surg. 2013;23:1662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kolotkin RL, Kim J, Davidson LE, et al. 12-year trajectory of health-related quality of life in gastric bypass patients versus comparison groups. Surg Obes Relat Dis. 2018. [DOI] [PubMed] [Google Scholar]
- [15].Andersen JR, Aasprang A, Karlsen TI, et al. Health-related quality of life after bariatric surgery: a systematic review of prospective long-term studies. Surg Obes Relat Dis. 2015;11:466–73. [DOI] [PubMed] [Google Scholar]
- [16].Lent MR, Avakoff E, Hope N, et al. Clinical characteristics of Roux-en-Y gastric bypass patients with death from accidental overdose or intentional self-harm: a descriptive study. Obes Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bhatti JA, Nathens AB, Thiruchelvam D, et al. Self-harm emergencies after bariatric surgery: a population-based cohort study. JAMA Surg. 2016;151:226–32. [DOI] [PubMed] [Google Scholar]
- [18].Gordon KH, King WC, White GE, et al. A longitudinal examination of suicide-related thoughts and behaviors among bariatric surgery patients. Surg Obes Relat Dis. 2019;15:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Adams TD, Gress R, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007:753–61. [DOI] [PubMed] [Google Scholar]
- [20].Davidson LE, Adams TD, Kim J, et al. Association of patient age at gastric bypass surgery with long-term all-cause and cause-specific mortality. JAMA Surg. 2016;151:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Omalu BI, Ives DG, Buhari AM, et al. Death rates and causes of death after bariatric surgery for Pennsylvania residents, 1995 to 2004. Arch Surg. 2007;142:923–8; discussion 9. [DOI] [PubMed] [Google Scholar]
- [22].Tindle HA, Omalu B, Courcoulas A, et al. Risk of suicide after long-term follow-up from bariatric surgery. Am J Med. 2010;123:1036–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mitchell JE, Crosby R, de Zwaan M, et al. Possible risk factors for increased suicide following bariatric surgery. Obesity (Silver Spring). 2013;21:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Adams TD, Avelar E, Cloward T, et al. Design and rationale of the Utah obesity study. A study to assess morbidity following gastric bypass surgery. Contemp Clin Trials. 2005;26:534–51. [DOI] [PubMed] [Google Scholar]
- [25].Williams RR, Hunt SC, Barlow GK, et al. Health family trees: a tool for finding and helping young family members of coronary and cancer prone pedigrees in Texas and Utah. Am J Public Health. 1988;78:1283–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Adams TD, Davidson LE, Litwin S, et al. Health benefits of gastric bypass surgery after 6 years. Journal of American Medical Association. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ware JE Jr., Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–12. [DOI] [PubMed] [Google Scholar]
- [28].Ware JE Jr., Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. Boston: Health Institute, New England Medical Center; 1993. [Google Scholar]
- [29].Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Corica F, Corsonello A, Apolone G, et al. Construct validity of the Short Form-36 Health Survey and its relationship with BMI in obese outpatients. Obesity (Silver Spring). 2006;14:1429–37. [DOI] [PubMed] [Google Scholar]
- [31].Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11:157–71. [DOI] [PubMed] [Google Scholar]
- [32].Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–11. [DOI] [PubMed] [Google Scholar]
- [33].Osman A, Bagge CL, Gutierrez PM, et al. The Suicidal Behaviors Questionnaire-Revised (SBQ-R): validation with clinical and nonclinical samples. Assessment. 2001;8:443–54. [DOI] [PubMed] [Google Scholar]
- [34].Batterham PJ, Ftanou M, Pirkis J, et al. A systematic review and evaluation of measures for suicidal ideation and behaviors in population-based research. Psychol Assess. 2015;27:501–12. [DOI] [PubMed] [Google Scholar]
- [35].Smith SC, Edwards CB, Goodman GN, Halversen RC, Simper SC. Open vs laparoscopic Roux-en-Y gastric bypass: comparison of operative morbidity and mortality. Obes Surg. 2004;14:73–6. [DOI] [PubMed] [Google Scholar]
- [36].Smith SC, Goodman GN, Edwards CB. Roux-en-Y gastric bypass: a 7-year retrospective review of 3,855 Patients. Obes Surg. 1995;5:314–8. [DOI] [PubMed] [Google Scholar]
- [37].Kolotkin RL, Crosby RD, Gress RE, Hunt SC, Adams TD. Two-year changes in health-related quality of life in gastric bypass patients compared with severely obese controls. Surg Obes Relat Dis. 2009;5:250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kolotkin RL, Davidson LE, Crosby RD, Hunt SC, Adams TD. Six-year changes in health-related quality of life in gastric bypass patients versus obese comparison groups. Surg Obes Relat Dis. 2012;8:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. NY: Guilford Press; 2013. [Google Scholar]
- [40].Chen EY, Fettich KC, McCloskey MS. Correlates of suicidal ideation and/or behavior in bariatric-surgery-seeking individuals with severe obesity. Crisis. 2012;33:137–43. [DOI] [PubMed] [Google Scholar]
- [41].Windover AK, Merrell J, Ashton K, Heinberg LJ. Prevalence and psychosocial correlates of self-reported past suicide attempts among bariatric surgery candidates. Surg Obes Relat Dis. 2010;6:702–6. [DOI] [PubMed] [Google Scholar]
- [42].Karmali S, Brar B, Shi X, et al. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23:1922–33. [DOI] [PubMed] [Google Scholar]
- [43].Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Freire CC, Zanella MT, Segal A, et al. Associations between binge eating, depressive symptoms and anxiety and weight regain after Roux-en-Y gastric bypass surgery. Eat Weight Disord. 2020. [DOI] [PubMed] [Google Scholar]
- [45].Jones-Corneille LR, Wadden TA, Sarwer DB. Risk of Depression and suicide in patients with extreme obesity who seek bariatric surgery. Obes Manag. 2007;3:255–60. [Google Scholar]
- [46].Polovina S, Micic D. Lifestyle, depression and metabolic/bariatric surgery. J Depress Anxiety. 2019;S13:1–3. [Google Scholar]
- [47].Wnuk S, Parvez N, Hawa R, Sockalingam S. Predictors of suicidal ideation one-year post-bariatric surgery: Results from the Toronto Bari-Psych Cohort Study. Gen Hosp Psychiatry. 2018. [DOI] [PubMed] [Google Scholar]


