Abstract
MicroRNAs (miRNAs) are a class of short RNA molecules that mediate the regulation of gene activity through interactions with target mRNAs and subsequent silencing of gene expression. It has become increasingly clear the miRNAs regulate many diverse aspects of bone biology, including bone formation and bone resorption processes. The role of miRNAs specifically in osteoclasts has been of recent investigation, due to clinical interest in discovering new paradigms to control excessive bone resorption, as is observed in multiple conditions including aging, estrogen deprivation, cancer metastases or glucocorticoid use. Therefore understanding the role that miRNAs play during osteoclastic differentiation is of critical importance. In this review, we highlight and discuss general aspects of miRNA function in osteoclasts, including exciting data demonstrating that miRNAs encapsulated in extracellular vesicles (EVs) either originating from osteoclasts, or signaling to osteoclast from divergent sites, have important roles in bone homeostasis.
Keywords: miRNA, Osteoclast, Extracellular vesicles, Differentiation
1. Introduction
The continual maintenance of bone mass through the life-span involves balancing the coordinated activities of bone formation (via the osteoblasts) and bone resorption (via the osteoclasts) [1]. These activities are largely orchestrated by the osteocyte, which is a mature, osteoblastic, matrix-embedded cell type [2–4]. As the organism ages or during physiological or environmental stressors (i.e. glucocorticoid use, estrogen depletion, cancer metastases), alterations in how these cell-types communicate can lead to an unbalancing of the processes of bone resorption and bone formation resulting in significant bone loss [5,6]. This compromised state of bone homeostasis is categorized in a disease called osteoporosis, which is characterized by the local destruction of bone tissue leading to lowered bone mass or compromised bone quality, leading to the increased risk of life-debilitating events such as fracture [6,7]. This condition is responsible for approximately 2 million fractures per year and is positively associated with morbidity and mortality, mostly in the elderly population [8]. Therefore, understanding how these processes are regulated at the molecular level is of critical importance and will uncover new avenues to target therapeutics to improve, or even prevent, the negative consequences of osteoporosis.
Although the regulation of normal and pathologic bone metabolism is multi-factorial and involves coordination of all bone-related cell types, in this review we will focus on the state of knowledge regarding the function and biology of miRNAs as they related to the osteoclast cell lineage.
2. Osteoclast functions in bone metabolism
2.1. General osteoclast function and clinical relevance
Osteoclasts are multinucleated, bone-resorbing cells derived from the myeloid lineage and are crucial to normal skeletal development and homeostasis. The functional importance of osteoclasts has been elucidated in large part through the study of osteopetrosis, a class of diseases characterized by increased bone density caused by defective bone resorption [9]. There are several forms of osteopetrosis, depending on the specific causal mutations, and severity of disease ranges from mild bone sclerosis to fatality during infancy. In addition to increased bone density, patients can experience cranial or optic nerve compression, extramedullary hematopoiesis, anemia, and immunodeficiency. Despite increased bone density, impaired bone remodeling also leads to higher risk for pathological fractures in osteopetrotic patients. Patients can exhibit dental abnormalities, impaired tooth eruption and osteomyelitis [10]. Similar to genetic mutations that disrupt resorption, pharmacologic anti-resorptive therapies used in the treatment of osteoporosis or cancer-induced bone disease, such as bisphosphonates and denosumab, induce side effects by decreasing the number of functional osteoclasts. Most commonly, these drugs are associated with significant reductions in new bone formation due to the reduction in bone remodeling and have also been associated with medication related osteonecrosis of the jaw [11] and atypical femoral fractures [12].
2.2. Osteoclast differentiation
Differentiation of osteoclasts, which has been reviewed thoroughly [13–16], primarily requires two cytokines; macrophage colony stimulating factor (MCSF) that promotes proliferation and survival, and receptor activator of nuclear factor kappa-B ligand (RANKL) that induces differentiation. RANKL is produced by osteoblasts and osteocytes to stimulate bone resorption and initiate remodeling, at specific skeletal sites [17–19]. Osteoclast differentiation is further regulated by the expression of osteoprotegerin (OPG), a decoy receptor for RANKL, which inhibits the activation of osteoclast differentiation [20]. The binding of RANKL to the cell surface receptor RANK on osteoclast progenitors activates intracellular signaling pathways that stimulate nuclear factor kappa-light-chain-enhancer of activated B cells (NFKB), FOS and nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1) transcription factors [13]. Osteoclast differentiation induces expression of additional proteins crucial for cell fusion (i.e. dendrocyte expressed seven transmembrane protein [DCSTAMP]) [21,22] and adhesion to the bone surface (integrin subunit alpha V [ITGAV] and integrin subunit beta 3 [ITGB3]) [23]. RANKL activation of mature osteoclasts together with integrin signaling induces morphological changes in order to resorb bone. The actin cytoskeleton is rearranged to form the actin ring and seal off a zone between the basal membrane and the bone surface [24]. This space becomes the resorption lacunae, which is acidified through secretion of H+ and Cl− ions. In addition, fusion of lysosomal vesicles creates the characteristic osteoclast ruffled border while delivering enzymes to aid in digestion of the bone matrix [25]. The processes involved in cell fusion, adhesion, as well as vesicular trafficking are highly dependent on G protein signaling [26], which, in part, underlie the sensitivity of these cells to nitrogenous bisphosphonates [27]. By resorbing bone, osteoclasts help to shape bones during skeletal development, as well as create the bone marrow cavity required for hematopoiesis and B lymphocyte differentiation [28]. Following skeletal maturation, bone resorption continues to be essential for the maintenance of bone quality, removing weakened or damaged bone so that it can be replaced by new bone [29].
There is much evidence that osteoclasts promote subsequent osteoblast differentiation and bone formation at sites of bone resorption, a process known as the “coupling” of bone resorption to bone formation [30,31]. It is well established that osteoclast-mediated bone resorption precedes bone formation during bone remodeling. Further, serum markers of resorption closely correlate with markers of bone formation, and pharmacologic disruption of resorption (i.e. anti-resorptive therapy) leads to a concomitant reduction in bone formation [32].
During bone resorption, osteoclasts release bone-derived factors, including transforming growth factor-β (TGFB) [33] and insulin-like growth factor-1 (IGF1) [34] that may act in an autocrine or paracrine manner within the bone microenvironment to promote subsequent cycles of bone formation. However, as evidenced in osteopetrosis patients that maintain osteoclast numbers (“osteoclast-rich” osteopetrosis) with defective bone resorption but normal or increased bone formation, the presence of osteoclasts themselves promote bone formation [35,36]. Thus, osteoclasts are thought to also secrete factors, dubbed “clastokines” [37] that may signal to the bone microenvironment to promote angiogenesis [38] and osteoblast differentiation [35,39,40]. In addition to secreted factors, there is evidence that osteoclast-membrane bound proteins may directly interact with osteoprogenitors to regulate osteoblast differentiation. This interaction has been suggested for osteoclast-derived EFNB2 to promote osteoblast differentiation through osteoprogenitor-expressed EPHB4 [41]. Likewise, osteoclast expression of RANK can activate bidirectional signaling in osteoprogenitors downstream of membrane bound RANKL [42].
2.3. Osteoclasts and T-lymphocyte activation
Osteoclasts also have roles in modulating immune responses, specifically through regulation of T lymphocyte activation. Similar to other myeloid-derived immune cells (i.e. macrophages, dendritic cells), osteoclasts can express class I/II major histocompatibility complex (MHC) molecules and co-activators for antigen presentation and activation of T cell responses [43]. Kiesel et al. reported that murine osteoclasts induced FOXP3 expression in CD8+ T cells; these cells lacked cytolytic activity, suggesting activation of a T-regulatory (Treg) cell response [44]. Certain osteoclast progenitor and osteoclast populations have been shown to have myeloid suppressor function [45,46]. More recently, dendritic cell-derived inflammatory osteoclasts in a mouse model of colitis were described to activate inflammatory CD4+ T cell responses in contrast to activation of Treg responses in healthy mice [47]. Further study has shown that these inflammatory osteoclast populations show further functional heterogeneity, with differential effects on T cell activation. The ability of osteoclasts to prevent T lymphocyte activation, even in the setting of inflammation, may be crucial to skeletal homeostasis, especially considering the potential for immune responses to collagen peptides generated during bone resorption [48].
3. miRNA biology
3.1. General miRNA biogenesis and function
MicroRNAs (miRNAs or miRs) are a specialized family of small, non-coding RNA molecules (~19–25 nucleotides in length) that regulate gene expression through post-transcriptional degradation and/or translational repression of mRNAs [49,50]. They mediate this effect through specific base-pairing interactions with “seed” sequences (7–8 nucleotides) primarily located within the 3′ untranslated region (UTR) of target mRNAs [51]. Since the interaction between the miRNA and mRNA-UTR utilizes only short stretches of sequence information, most miRNAs can interact with and influence the expression of many mRNAs simultaneously (often numbering in the 100 s) [52–54]. Interestingly, this miRNA pathway of gene expression regulation has been uncovered in organisms throughout evolutionary history, suggesting this regulatory mechanism is highly conserved and is a deeply embedded biological regulatory mechanism [55,56]. They have been implicated in essentially all biological processes and in nearly every tissue, including in the development, homeostasis and aging of the skeleton [57–64].
3.2. Osteoclast phenotypes of disrupted miRNA processing
Many excellent reviews on the biosynthetic pathways of miRNA biogenesis and processing can already be found in the literature [49–51,56], therefore an extensive review will not be included here. However, the importance of the miRNA regulatory pathway in bone biology can be illustrated by examining the effects of conditional deletion of one of the important molecules involved in the production of mature miRNAs, namely DICER1. Briefly, DICER1 is a member of the RNase III family of enzymes, which serves to process pre-miRNAs that have been exported to the cytoplasm by trimming off the terminal loop resulting in a mature, double-stranded miRNA molecule that then is loaded in the RNA-induced silencing complex (RISC) and eventually leads to RNA silencing [65]. Gene silencing of DICER1 in osteoclast precursors results in an impairment of osteoclastic gene expression and function in vitro, including decreases in osteoclastogenesis and bone resorption [66]. Conditional deletion of DICER1 in osteoclast precursors, using Cd11b-Cre, results in a mild form of osteopetrosis characterized by decreases in overall osteoclast number [66]. Similarly, conditional DICER1 deletions using cathepsin K (Ctsk)-Cre, which is active in more mature osteoclastic cells, also results in a decrease in osteoclast number and leads to higher bone mass phenotype [67]. In a broader sense, deletion of DICER1 in osteoblast cells (using various osteoblastic promoters such as Col1a1, Bglap, Osx and Runx2) also affects bone homeostasis in various capacities [60,68,69]. These prior reports provide proof-of-principle arguments that loss of miRNA function in bone cells, including in osteoclasts, is important for the proper maintenance of bone homeostasis. Therefore, understanding how miRNAs regulate various cellular activities of osteoclasts is important.
4. miRNA functions in various aspects of osteoclast formation
During the course of osteoclastogenesis, many miRNAs are differentially expressed and regulate osteoclast differentiation and function (reviewed by Hrdlicka et al. and Lozano et al. [70,71]). In this section, we will highlight some of the miRNAs that are important in each stage of osteoclast differentiation (summarized in Fig. 1 and Table 1) and validated using in vivo studies.
Fig. 1.
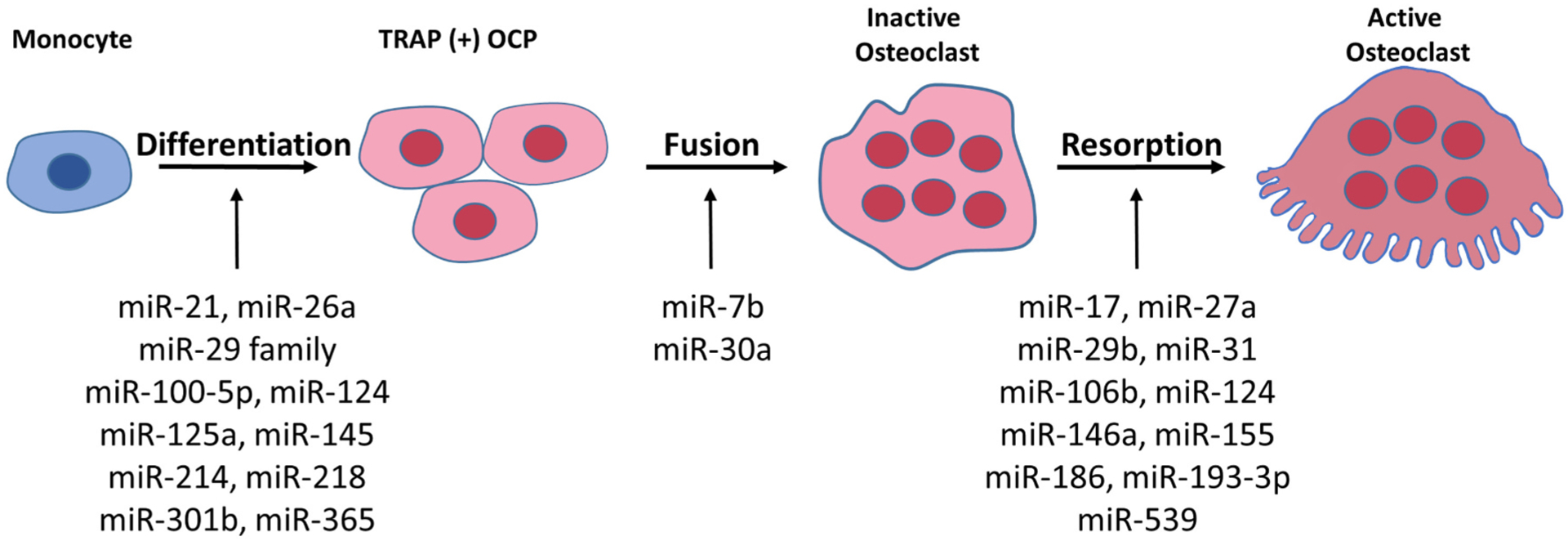
Depiction of miRNAs involved in the various stages of osteoclast differentiation.
Table 1.
Summary of featured miRNA regulators of genes involved in osteoclast (Ocl) differentiation and function.
| miRNA | Target mRNA(s) | Pathways/functions | Reference (s) |
|---|---|---|---|
| Positive regulators | |||
| mmu-miR-21 | Pten, Spry1, Pdcd4 | PI3K/AKT pathway, Ocl differentiation | [76,93,104] |
| mmu-miR-29 family | Nfia, Gpr85, Cd93, Srgap2, Cdc42, Calcr | Ocl differentiation | [83] |
| mmu-miR-31 | Rhoa | Ocl differentiation, bone resorption | [100] |
| mmu-miR-128 | Sirt1 | NFKB pathway, Ocl differentiation | [82] |
| mmu-miR-146a | – | Bone resorption | [92] |
| mmu-miR-214 | Pten, Atf4 | PI3K/AKT pathway, NFKB pathway, bone resorption | [75,77,95] |
| mmu-miR-301b | Cyld | NFKB pathway, Ocl differentiation | [80] |
| Negative regulators | |||
| mmu-miR-7b | Dcstamp | Ocl fusion | [85,86] |
| mmu-miR-17 | – | EphA4 signaling, bone resorption | [96] |
| mmu-miR-26a | Ctgf/Ccn2 | Ocl differentiation | [87] |
| mmu-miR-27a | Pparg/Apc | Ocl differentiation, bone resorption | [91] |
| hsa-miR-29b | – | Ocl differentiation, bone resorption | [84] |
| mmu-miR-30a | Dcstamp | FOS/NFATC1 pathway, Ocl differentiation, bone resorption | [88] |
| mmu-miR-100–5p | Fgf21 | Ocl differentiation | [94] |
| mmu-miR-106b | Rankl | Bone resorption | [98] |
| mmu-miR-124 | Nfatc1 | Ocl differentiation | [72–74] |
| hsa-miR-125a | Traf6 | NFATC1 pathway, Ocl differentiation | [78] |
| mmu-miR-145 | Smad3 | Ocl differentiation | [99] |
| mmu-miR-155 | Lepr | AMPK pathway, bone resorption | [97] |
| mmu-miR-186 | Ctsk | Bone resorption | [90] |
| mmu-miR-193–3p | Nfatc1 | NFATC1 pathway, bone resorption | [79] |
| mmu-miR-218 | Tnfrsf1a | NFKB pathway, Ocl differentiation | [81,82] |
| mmu-miR-365 | Mmp9 | Ocl differentiation | [89] |
| mmu-miR-539 | – | AXIN-dependent Wnt signaling, Ocl apoptosis | [106] |
Pten, Phosphatase and tensin homolog; Pdcd4, programmed cell death 4; Lepr, Leptin receptor; Ampk, Adenosine monophosphate activated protein kinase.
4.1. Role of miRNAs in osteoclast differentiation
As a master regulator of osteoclastogenesis, NFATc1 is a critical component of osteoclast differentiation. There are several miRNAs that regulate osteoclast differentiation by targeting Nfatc1 mRNA. MiR-124 regulates RANKL-dependent and -independent osteoclast differentiation by suppressing Nfatc1 expression [72–74]. MiR-214 and miR-21 promote osteoclastogenesis by targeting the PTEN/PI3K/AKT pathway [75,76], while downregulating osteoclastogenesis by blocking the NFKB pathway [77]. MiR-125a dramatically suppresses osteoclastogenesis in human CD14+ peripheral blood mononuclear cells (PBMCs) through a novel tumor necrosis factor (TNF) receptor-associated factor 6 (Traf6)/Nfatc1/miR-125a regulatory feedback loop [78]. Overexpression of miR-193–3p has an osteoprotective effect in ovariectomized (OVX) mice by suppressing NFATc1 pathways [79]. MiR-301b knockout mice exhibited markedly increased bone mass by reducing osteoclastogenesis by directly targeting cylindromatosis (Cyld) leading to phosphorylated NFKB pathway. Further, these authors found that OVX-induced osteoclastogenesis was abrogated by osteoclastic miR-301b ablation [80]. MiR-218 negatively modulates osteoclast differentiation by downregulating the NFKB pathway and targeting TNF receptor superfamily member 1A (Tnfrsf1a) [81]. In addition, myeloid lineage restricted expression of miR-218 enhanced trabecular bone volume and decreased osteoclast number in vivo and protected from OVX-induced bone loss by targeting sirtuin 1 (Sirt1) and NFKB pathway signaling [82].
During early osteoclast differentiation, the miR-29 family is critical for osteoclast precursor commitment by directly targeting nuclear factor I/A (Nfia), G-protein coupled receptor 85 (Gpr85) and CD93 [83]. Contrary to this result, in human PBMCs, miR-29b overexpression inhibits osteoclast formation [84]. However, our unpublished data using global expression of miR-29 decoy, which down-regulates the miR-29 activity, indicates that expression of miR-29 in multiple organs and cells (e.g., osteoclasts and osteoblasts) makes it difficult to determine the role of miR-29 in bone as these mice, which express miR-29 decoy globally, did not exhibit alteration of bone mass (Lee and Delany, unpublished data).
4.2. miRNAs regulating pre-osteoclast maturation and fusion
As described earlier in this review, committed osteoclast precursors differentiate into multinucleated giant cells by fusion. Osteoclast fusion is regulated by the Rho-GTPase family members ras homolog family member A (RHOA), cell division cycle 42 (CDC42) and rac family small GTPase 1 (RAC1), as well as the miR-29 family, miR-7b, miR-26a and let-7e. The miR-29 family regulates actin remodeling by targeting the Rho-GTPase Cdc42 and SLIT-ROBO-GTPase activating protein-2 (SRGAP2) [83]. DCSTAMP, which is a critical for osteoclast fusion, has been reported to be a target for miR-7b [85,86], while upregulated miR-26a inhibits DCSTAMP protein expression during osteoclast differentiation by suppressing connective tissue growth factor/CCN family 2 (Ctgf/Ccn2) expression [87]. MiR-30a attenuates osteoclastogenesis via suppression of DCSTAMP/FOS/NFATc1 signaling pathways [88].
4.3. miRNAs regulating osteoclast function, survival and apoptosis
MiR-365 and miR-186 negatively regulates matrix metallopeptidase (Mmp9) and Ctsk, respectively, to modulate osteoclastic bone resorption [89,90]. MiR-27a has been reported to be involved in regulating estrogen-inhibited osteoclast bone resorption by targeting peroxisome proliferator-activated receptor gamma (PPARγ) and adenomatous polyposis coli (APC) [91]. Global deletion of miR-146a in mice exhibited no distinct trabecular or cortical bone phenotype with high bone turnover (increased osteoblast and osteoclast numbers). However, miR-146a deletion protects OVX-induced bone loss by causing impaired osteoclast bone resorption [92]. MiR-21 global knockout mice exhibit decreased osteoclast function and number in vivo and result in increased trabecular bone volume [93], while overexpression of miR-100–5p suppressed in vivo bone resorption by targeting fibroblast growth factor (Fgf21) [94]. In mice that have osteoclast-specific overexpression of miR-214 using the acid phosphatase 5, tartrate resistant (Acp5) promoter, trabecular bone volume is significantly decreased due to enhanced bone resorption by targeting activating transcription factor 4 (Atf4) mRNA (an important transcription factor for osteoblast differentiation) [75,95]. In addition, miR-128 deletion in osteoclast lineage using LysM-cre reveals a dramatic increase in trabecular bone volume, and an accompanying reduced bone resorption which prevents OVX-induced bone loss [82]. These authors also found that miR-128 specifically targets Sirt1 mRNA post-transcriptionally. Downregulation of miR-17 activates bone resorption by stabilizing protein tyrosine phosphatase receptor type O (Ptp-oc or Ptpro) mRNA and thereby downregulating EPHA4 receptor signaling, indicating the involvement in coupling [96]. Further, downregulation of miR-155 inhibits bone resorption [97], while upregulation of miR-106b inhibits bone resorption [98]. MiR-145 agomir treatment inhibits osteoclast activity in OVX mice by downregulating Smad3 expression [99], while miR-31 antagomir treatment significantly reduces bone resorption by controlling cytoskeleton organization by targeting RhoA [100]. miR-193–3p agomir treatment of OVX mice markedly reduces OVX-induced bone loss/resorption by inhibiting Nfatc1 expression and its downstream osteoclastic target proteins CTSK, ACP5 and carbonic anhydrase 2 (CAR2) [79].
Several studies implicate the critical role of miR-29 in bone. miR-29a overexpressing transgenic mice driven by a phosphoglycerate kinase 1 (Pgk1) promoter show significantly impaired bone resorption in vivo and a reduction in glucocorticoid-induced osteoclastic erosion [101]. Similar results that miR-29a protects glucocorticoid-induced bone loss, have been reported in rats [102]. Interestingly, our data indicated that miR-29–3p has a differential role in conditions of homeostasis versus inflammation, as its expression in myeloid cells impairs bone resorption and enhances trabecular and cortical bone volume by increasing calcitonin (CALCR) and CTSK expression, while miR-29–3p targets Tnfrsf1a mRNA during TNF-induced inflammation (Delany and Lee, unpublished).
Some miRNA have been implicated in osteoclast apoptosis and fas cell surface death receptor (FAS)/FAS ligand (FASL) interactions have been reported to be critical [103]. MiR-21 promotes cell survival targeting Fasl [104]. Mice deficient for miR-21 globally exhibited increased trabecular bone volume by decreasing osteoclast activity without altering osteoblast or bone formation [93]. Another miRNA, miR-29b which has been known to be expressed in multiple organs and cells, enhances osteoclast survival by targeting B-cell lymphoma 2 (BCL2) modifying factor (Bmf) [105]. In addition, overexpression of miR-539 accelerated osteoclastic apoptosis in an osteoporotic rat model [106].
5. miRNAs in human bone diseases
5.1. miRNAs in osteopetrosis
Osteopetrosis in humans is commonly caused by genetic mutations to genes affecting osteoclast differentiation (e.g., TNF superfamily member 11 [TNFSF11]) or osteoclast resorptive function (e.g., T-cell immune regulator 1, ATPase H+ transporting V0 subunit A3 [TCIRG1] or chlo-ride voltage-gated channel 7 [CLCN7]). While miRNAs have been shown to contribute to the regulation of osteoclast differentiation and function as discussed, there have been no causal miRNA-specific mutations identified in human osteopetrotic patients. However, it remains possible that disruption of miRNAs in response to osteopetrotic mutations could contribute to disease pathogenesis. Using PBMCs from healthy versus osteopetrotic patients (CLCN7, rs387907576: A > G), Ou et al. identified 123 differentially expressed miRNAs [107]. The most abundant miRNA was miR-23a, which is suppressor of osteogenic differentiation. In contrast, osteopetrotic PBMCs showed a significant decrease in miR-29b-3p, which stimulates osteoblast differentiation. It is possible that these miRNAs are dysregulated in response to increased bone formation relative to impaired resorption exhibited by these patients. The authors further detected 117 computationally predicted miRNA-protein target pairs that were reciprocally expressed in the osteopetrotic PBMCs. Of these pairs, miR-320a and ADP ribosylation factor 1 (ARF1) were further evaluated due to the known relationship between ARF family members and CLCN7. MiR-320a was significantly decreased in the osteopetrotic PBMCs, leading to increased ARF1 protein, potentially to compensate for insufficient CLCN7 [107]. While it is unlikely that these specific miRNAs play a causal role in osteoclast dysfunction contributing to osteopetrosis, it is possible that this bioinformatics strategy could be further used to identify novel therapeutic targets.
5.2. miRNAs in osteoporosis
Osteoporosis, in contrast to osteopetrosis, is a disease of low bone mass caused by excess bone resorption as compared to bone formation. While osteopetrosis is caused by mutations specifically affecting bone resorption, osteoporosis can result from altered osteoclast activity or changes to the osteoblast lineage. In a study designed to identify miRNAs associated with elevated osteoclast resorption in postmenopausal osteoporosis, Wang et al. performed a miRNA expression array on circulating monocytes derived from women with either high or low bone mineral density. They identified miR-133a as a potential biomarker for osteoporosis [108]. It remains unclear whether miR-133a has a functional effect on osteoclasts and will require further experimentation.
5.3. Potential for miRNA-based clinical therapies
There has been some investigation into potential miRNA-based therapies to promote bone formation in conditions such as osteoporosis; however, most of these studies have aimed to use miRNAs to modulate osteoblast differentiation and bone formation and not in osteoclast functions. Song et al. showed that endothelial cell-derived exosomes delivering miR-155 modulate osteoclastogenesis in vitro and prevented OVX-induced bone loss. These endothelial cell-derived exosomes showed improved localization to bone as compared to osteoblast lineage-derived exosomes, suggesting that these exosomes could have therapeutic potential for targeting the osteoclast lineage to treat osteoporosis [109]. More research is needed to identify miRNA-dependent pathways that could modulate the function of osteoclasts in particular in these human bone diseases.
6. Involvement of miRNA-containing EVs in osteoclast biology
6.1. General EV biology
EVs are a broad collection of secreted, membrane-bound phospholipid particles involved in cellular communication with nearby cells (autocrine or paracrine) or distant cells (endocrine) through traversing the circulation [110]. They are formed intracellularly as intraluminal vesicles, are sorted and routed through the endosomal pathway and eventually released from the cell by budding off of the plasma membrane [111]. EVs are loosely categorized based on size of the particle, with exosomes and small EVs in the range of 30–200 nm in diameter, and larger vesicles in the range of 200–1000 nm (and even up to 5000 nm when considering apoptotic bodies) [112,113]. These particles can carry a veritable treasure trove of cargo, including proteins and various enzymatic activities, DNA and mRNAs, including non-coding RNAs such as lncRNAs and miRNAs [112]. Due to their nature for carrying cargo from their incipient cell to distant targets through the circulation, EVs have been used as biomarkers for both the detection and prognosis in many conditions and diseases [110,114], which will be covered elsewhere in this Special Issue. For more detailed information on the biogenesis and activities of EVs in various cellular contexts and disease states, a number of excellent reviews already exist [115–119] and therefore will not be covered in detail herein. Fig. 2 summarizes the involvement of EVs secreted from osteoclasts that affect multiple other cell/tissue types and those EVs that affect osteoclast function (where the osteoclast is the target cell of the exogenous EV).
Fig. 2.
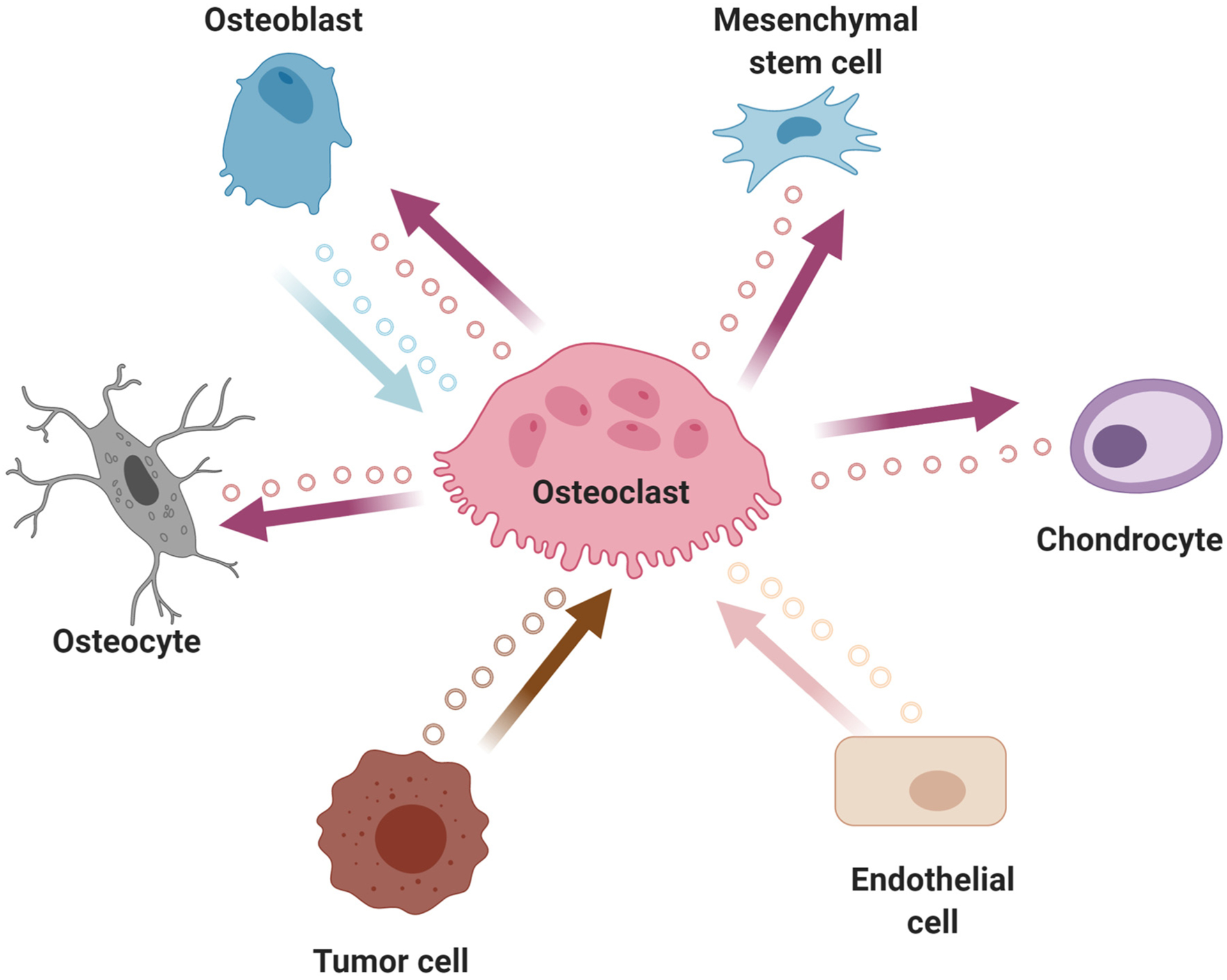
Depiction of extracellular vesicle (EVs; small circles) communication from osteoclasts to other distant cell lineages. Highlighted is the bi-directional EV communication between osteoclasts and osteoblasts.
6.2. Osteoclast-secreted EVs
Osteoclasts secrete factors to promote the coupling of bone resorption to bone formation and until recently, these studies have focused primarily on secreted proteins. However it is plausible that osteoclast-derived miRNAs in EVs may also contribute to the coupling of resorption and formation, and as such serve as “EV-associated coupling factors”. One such miRNA is miR-214–3p, which has been found to be present in osteoclast-derived exosomes [95,120,121]. These miR-214–3p-containing exosomes have been demonstrated to target pre-osteoblastic cells in a paracrine fashion, and through direct interaction with and repression of Atf4 mRNA, inhibits bone formation [122]. Similarly, exosomes derived from RANKL-induced, osteoclastic RAW 264.7 cells contain miR-23a-5p that downregulates Runx2, an essential transcription factor for bone formation [123], to inhibit osteogenic differentiation [124]. Osteoclast-derived exosomes also contain let-7a-5p, which targets the SMAD2/TGFB-induced pathway to inhibit chondrogenic differentiation and therefore promotes hypertrophic differentiation of chondrocytes [125]. Thus, it remains an exciting possibility that osteoclast-derived exosomes, which possess targeting for osteoprogenitors, may be loaded with anabolic miRNAs for therapeutic purposes to drive osteogenic responses.
6.3. Systemically-derived EVs affecting osteoclasts
Slightly more is known about systemically-derived EVs that target osteoclasts to influence the regulation of bone mass. Many of these miRNA-containing exosomes originate from tumor cells from various sources, and are often called “tumor-derived exosomes”, which can influence various aspects of osteoclastic activity, depending on their particular target mRNAs [126]. These miRNA-containing exosomes arise from diverse sources including breast cancer cells [127], prostate cancer cells [77,128], multiple myeloma plasma cells [129–131], osteosarcoma cells [132] and lung adenocarcinoma cells [133]. The particular miRNAs found in these tumor-derived exosomes often directly enhance osteoclastic activity to cause autolytic bone destruction and overall poor health of the bone leading to potentially life-threatening fractures (especially in these cancer patients). Other sources of EVs that directly target osteoclasts also existing, arising from such diverse sources as endothelial cells [109,134] and aging bone marrow stromal cells [135] (covered elsewhere in this Special Issue).
7. Conclusions and future directions
In the past few years knowledge concerning the functions of miRNAs both in osteoclast biology and the effects of miRNAs from EVs on osteoclasts has dramatically increased, although many significant gaps in our understanding still exist. A majority of these studies were conducted using in vitro or ex vivo (e.g., cell culture) systems. Indeed, more in vivo studies are necessary to confirm the functionality of miRNAs in a more biologically relevant system to greater understanding of their role(s) in bone homeostasis and for the potential for the use of miRNAs (or encapsulated miRNAs in EVs) in therapeutic options for bone diseases such as osteoporosis and osteopetrosis.
Acknowledgments
This work was supported by NIH grants R01 AR068275 (DGM), R01 AG063707 (DGM), P01 AG004875 (DGM), K01 AR070281 (MMW), R01 AR064867 (SKL).
Footnotes
Declaration of competing interest
The authors have nothing to disclose.
References
- [1].Almeida M, O’Brien CA, Basic biology of skeletal aging: role of stress response pathways, J. Gerontol. A Biol. Sci. Med. Sci 68 (2013) 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bonewald LF, The amazing osteocyte, J. Bone Miner. Res 26 (2011) 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bonewald LF, The role of the osteocyte in bone and nonbone disease, Endocrinol. Metab. Clin. N. Am 46 (2017) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jilka RL, O’Brien CA, The role of osteocytes in age-related bone loss, Curr Osteoporos Rep 14 (2016) 16–25. [DOI] [PubMed] [Google Scholar]
- [5].Khosla S, Pathogenesis of age-related bone loss in humans, J. Gerontol. A Biol. Sci. Med. Sci 68 (2013) 1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. , The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine, J. Bone Miner. Res 29 (2014) 2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lespessailles E, Cortet B, Legrand E, Guggenbuhl P, Roux C, Low-trauma fractures without osteoporosis, Osteoporos. Int 28 (2017) 1771–1778. [DOI] [PubMed] [Google Scholar]
- [8].General S, Bone Health and Osteoporosis: A Report of the Surgeon General, Rockville, MD, U.S. Department of Health and Human Services, Office of the Surgeon General, 2004. [PubMed] [Google Scholar]
- [9].Palagano E, Menale C, Sobacchi C, Villa A, Genetics of Osteopetrosis, Curr Osteoporos Rep 16 (2018) 13–25. [DOI] [PubMed] [Google Scholar]
- [10].Stark Z, Savarirayan R, Osteopetrosis, Orphanet J Rare Dis 4 (2009) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Otto S, Pautke C, Van den Wyngaert T, Niepel D, Schiodt M, Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases, Cancer Treat. Rev 69 (2018) 177–187. [DOI] [PubMed] [Google Scholar]
- [12].Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. , Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research, J. Bone Miner. Res 29 (2014) 1–23. [DOI] [PubMed] [Google Scholar]
- [13].Asagiri M, Takayanagi H, The molecular understanding of osteoclast differentiation, Bone 40 (2007) 251–264. [DOI] [PubMed] [Google Scholar]
- [14].Boyle WJ, Simonet WS, Lacey DL, Osteoclast differentiation and activation, Nature 423 (2003) 337–342. [DOI] [PubMed] [Google Scholar]
- [15].Ikeda K, Takeshita S, The role of osteoclast differentiation and function in skeletal homeostasis, J. Biochem 159 (2016) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Park JH, Lee NK, Lee SY, Current understanding of RANK signaling in osteoclast differentiation and maturation, Mol Cells 40 (2017) 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fumoto T, Takeshita S, Ito M, Ikeda K, Physiological functions of osteoblast lineage and T cell-derived RANKL in bone homeostasis, J. Bone Miner. Res 29 (2014) 830–842. [DOI] [PubMed] [Google Scholar]
- [18].Takeshita S, Fumoto T, Naoe Y, Ikeda K, Age-related marrow adipogenesis is linked to increased expression of RANKL, J. Biol. Chem 289 (2014) 16699–16710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA, Matrix-embedded cells control osteoclast formation, Nat. Med 17 (2011) 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. , Osteoprotegerin: a novel secreted protein involved in the regulation of bone density, Cell 89 (1997) 309–319. [DOI] [PubMed] [Google Scholar]
- [21].Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, et al. , RANKL-induced DC-STAMP is essential for osteoclastogenesis, J. Exp. Med 200 (2004) 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, et al. , DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells, J. Exp. Med 202 (2005) 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Duong LT, Rodan GA, Integrin-mediated signaling in the regulation of osteoclast adhesion and activation, Front. Biosci 3 (1998) d757–d768. [DOI] [PubMed] [Google Scholar]
- [24].Lakkakorpi PT, Vaananen HK, Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro, J. Bone Miner. Res 6 (1991) 817–826. [DOI] [PubMed] [Google Scholar]
- [25].Stenbeck G, Formation and function of the ruffled border in osteoclasts, Semin. Cell Dev. Biol 13 (2002) 285–292. [DOI] [PubMed] [Google Scholar]
- [26].Touaitahuata H, Blangy A, Vives V, Modulation of osteoclast differentiation and bone resorption by Rho GTPases, Small GTPases 5 (2014), e28119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodan GA, Reszka AA, Bisphosphonate mechanism of action, Curr. Mol. Med 2 (2002) 571–577. [DOI] [PubMed] [Google Scholar]
- [28].Panaroni C, Wu JY, Interactions between B lymphocytes and the osteoblast lineage in bone marrow, Calcif. Tissue Int 93 (2013) 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zaidi M, Skeletal remodeling in health and disease, Nat. Med 13 (2007) 791–801. [DOI] [PubMed] [Google Scholar]
- [30].Martin TJ, Sims NA, Osteoclast-derived activity in the coupling of bone formation to resorption, Trends Mol. Med 11 (2005) 76–81. [DOI] [PubMed] [Google Scholar]
- [31].Matsuo K, Irie N, Osteoclast-osteoblast communication, Arch. Biochem. Biophys 473 (2008) 201–209. [DOI] [PubMed] [Google Scholar]
- [32].Weivoda MM, Chew CK, Monroe DG, Farr JN, Atkinson EJ, Geske JR, et al. , Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism, Nat. Commun 11 (2020) 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, et al. , TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation, Nat. Med 15 (2009) 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, et al. , Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells, Nat. Med 18 (2012) 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lotinun S, Kiviranta R, Matsubara T, Alzate JA, Neff L, Luth A, et al. , Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation, J. Clin. Invest 123 (2013) 666–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thudium CS, Moscatelli I, Flores C, Thomsen JS, Bruel A, Gudmann NS, et al. , A comparison of osteoclast-rich and osteoclast-poor osteopetrosis in adult mice sheds light on the role of the osteoclast in coupling bone resorption and bone formation, Calcif. Tissue Int 95 (2014) 83–93. [DOI] [PubMed] [Google Scholar]
- [37].Charles JF, Aliprantis AO, Osteoclasts: more than ‘bone eaters’, Trends Mol. Med 20 (2014) 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, et al. , PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis, Nat. Med 20 (2014) 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim BJ, Lee YS, Lee SY, Baek WY, Choi YJ, Moon SA, et al. , Osteoclast-secreted SLIT3 coordinates bone resorption and formation, J. Clin. Invest 128 (2018) 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Weivoda MM, Ruan M, Pederson L, Hachfeld C, Davey RA, Zajac JD, et al. , Osteoclast TGF-beta receptor signaling induces Wnt1 secretion and couples bone resorption to bone formation, J. Bone Miner. Res 31 (2016) 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, et al. , Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis, Cell Metab. 4 (2006) 111–121. [DOI] [PubMed] [Google Scholar]
- [42].Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, et al. , Coupling of bone resorption and formation by RANKL reverse signalling, Nature 561 (2018) 195–200. [DOI] [PubMed] [Google Scholar]
- [43].Li H, Hong S, Qian J, Zheng Y, Yang J, Yi Q, Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells, Blood 116 (2010) 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kiesel JR, Buchwald ZS, Aurora R, Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells, J. Immunol 182 (2009) 5477–5487. [DOI] [PubMed] [Google Scholar]
- [45].Charles JF, Hsu LY, Niemi EC, Weiss A, Aliprantis AO, Nakamura MC, Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function, J. Clin. Invest 122 (2012) 4592–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Grassi F, Manferdini C, Cattini L, Piacentini A, Gabusi E, Facchini A, et al. , T cell suppression by osteoclasts in vitro, J. Cell. Physiol 226 (2011) 982–990. [DOI] [PubMed] [Google Scholar]
- [47].Ibanez L, Abou-Ezzi G, Ciucci T, Amiot V, Belaid N, Obino D, et al. , Inflammatory osteoclasts prime TNFalpha-producing CD4(+) T cells and express CX3 CR1, J. Bone Miner. Res 31 (2016) 1899–1908. [DOI] [PubMed] [Google Scholar]
- [48].Madel MB, Ibanez L, Ciucci T, Halper J, Rouleau M, Boutin A, et al. , Dissecting the Phenotypic and Functional Heterogeneity of Mouse Inflammatory Osteoclasts by the Expression of Cx3cr1, Elife 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bartel DP, MicroRNAs: genomics, biogenesis, mechanism, and function, Cell 116 (2004) 281–297. [DOI] [PubMed] [Google Scholar]
- [50].Kim VN, Nam JW, Genomics of microRNA, Trends Genet. 22 (2006) 165–173. [DOI] [PubMed] [Google Scholar]
- [51].Bartel DP, MicroRNAs: target recognition and regulatory functions, Cell 136 (2009) 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP, The impact of microRNAs on protein output, Nature 455 (2008) 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N, Widespread changes in protein synthesis induced by microRNAs, Nature 455 (2008) 58–63. [DOI] [PubMed] [Google Scholar]
- [54].Friedman RC, Farh KK, Burge CB, Bartel DP, Most mammalian mRNAs are conserved targets of microRNAs, Genome Res. 19 (2009) 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee CT, Risom T, Strauss WM, Evolutionary conservation of microRNA regulatory circuits: an examination of microRNA gene complexity and conserved microRNA-target interactions through metazoan phylogeny, DNA Cell Biol. 26 (2007) 209–218. [DOI] [PubMed] [Google Scholar]
- [56].Dexheimer PJ, Cochella L, MicroRNAs: from mechanism to organism, Front Cell Dev Biol 8 (2020) 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kronenberg HM, PTHrP and skeletal development, Ann. N. Y. Acad. Sci 1068 (2006) 1–13. [DOI] [PubMed] [Google Scholar]
- [58].Kobayashi T, Lu J, Cobb BS, Rodda SJ, McMahon AP, Schipani E, et al. , Dicer-dependent pathways regulate chondrocyte proliferation and differentiation, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].He X, Eberhart JK, Postlethwait JH, MicroRNAs and micromanaging the skeleton in disease, development and evolution, J. Cell. Mol. Med 13 (2009) 606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gaur T, Hussain S, Mudhasani R, Parulkar I, Colby JL, Frederick D, et al. , Dicer inactivation in osteoprogenitor cells compromises fetal survival and bone formation, while excision in differentiated osteoblasts increases bone mass in the adult mouse, Dev. Biol 340 (2010) 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhang Y, Xie RL, Gordon J, LeBlanc K, Stein JL, Lian JB, et al. , Control of mesenchymal lineage progression by microRNAs targeting skeletal gene regulators Trps1 and Runx2, J. Biol. Chem 287 (2012) 21926–21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, et al. , MicroRNA control of bone formation and homeostasis, Nat Rev Endocrinol 8 (2012) 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dole NS, Delany AM, MicroRNA variants as genetic determinants of bone mass, Bone 84 (2016) 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, et al. , MicroRNA-183–5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence, Tissue Eng Part A 23 (2017) 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Song MS, Rossi JJ, Molecular mechanisms of dicer: endonuclease and enzymatic activity, Biochem. J 474 (2017) 1603–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sugatani T, Hruska KA, Impaired micro-RNA pathways diminish osteoclast differentiation and function, J. Biol. Chem 284 (2009) 4667–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mizoguchi F, Izu Y, Hayata T, Hemmi H, Nakashima K, Nakamura T, et al. , Osteoclast-specific dicer gene deficiency suppresses osteoclastic bone resorption, J. Cell. Biochem 109 (2010) 866–875. [DOI] [PubMed] [Google Scholar]
- [68].Liu P, Baumgart M, Groth M, Wittmann J, Jack HM, Platzer M, et al. , Dicer ablation in osteoblasts by Runx2 driven cre-loxP recombination affects bone integrity, but not glucocorticoid-induced suppression of bone formation, Sci. Rep 6 (2016) 32112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bendre A, Moritz N, Vaananen V, Maatta JA, Dicer1 ablation in osterix positive bone forming cells affects cortical bone homeostasis, Bone 106 (2018) 139–147. [DOI] [PubMed] [Google Scholar]
- [70].Hrdlicka HC, Lee SK, Delany AM, MicroRNAs are critical regulators of osteoclast differentiation, Curr Mol Biol Rep 5 (2019) 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lozano C, Duroux-Richard I, Firat H, Schordan E, Apparailly F, MicroRNAs: key regulators to understand osteoclast differentiation? Front. Immunol 10 (2019) 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lee Y, Kim HJ, Park CK, Kim YG, Lee HJ, Kim JY, et al. , MicroRNA-124 regulates osteoclast differentiation, Bone 56 (2013) 383–389. [DOI] [PubMed] [Google Scholar]
- [73].Zhao N, Han D, Liu Y, Li Y, Zeng L, Wang Y, et al. , DLX3 negatively regulates osteoclastic differentiation through microRNA-124, Exp. Cell Res 341 (2016) 166–176. [DOI] [PubMed] [Google Scholar]
- [74].Ohnuma K, Kasagi S, Uto K, Noguchi Y, Nakamachi Y, Saegusa J, et al. , MicroRNA-124 inhibits TNF-alpha- and IL-6-induced osteoclastogenesis, Rheumatol. Int 39 (2019) 689–695. [DOI] [PubMed] [Google Scholar]
- [75].Zhao C, Sun W, Zhang P, Ling S, Li Y, Zhao D, et al. , miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway, RNA Biol. 12 (2015) 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang S, Liu Z, Wang J, Ji X, Yao Z, Wang X, miR21 promotes osteoclastogenesis through activation of PI3K/Akt signaling by targeting Pten in RAW264.7 cells, Mol. Med. Rep 21 (2020) 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Duan Y, Tan Z, Yang M, Li J, Liu C, Wang C, et al. , PC-3-derived Exosomes inhibit osteoclast differentiation by downregulating miR-214 and blocking NF-kappaB signaling pathway, Biomed. Res. Int 2019 (2019) 8650846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Guo LJ, Liao L, Yang L, Li Y, Jiang TJ, MiR-125a TNF receptor-associated factor 6 to inhibit osteoclastogenesis, Exp. Cell Res 321 (2014) 142–152. [DOI] [PubMed] [Google Scholar]
- [79].Li X, Yang L, Guo Z, miR-193–3p ameliorates bone resorption in ovariectomized mice by blocking NFATc1 signaling, Int. J. Clin. Exp. Pathol 12 (2019) 4077–4086. [PMC free article] [PubMed] [Google Scholar]
- [80].Zhu J, Wang H, Liu H, Osteoclastic miR-301-b knockout reduces ovariectomy (OVX)-induced bone loss by regulating CYDR/NF-kappaB signaling pathway, Biochem. Biophys. Res. Commun 529 (2020) 35–42. [DOI] [PubMed] [Google Scholar]
- [81].Wang W, Yang L, Zhang D, Gao C, Wu J, Zhu Y, et al. , MicroRNA-218 negatively regulates osteoclastogenic differentiation by repressing the nuclear factor-kappaB signaling pathway and targeting tumor necrosis factor receptor 1, Cell. Physiol. Biochem 48 (2018) 339–347. [DOI] [PubMed] [Google Scholar]
- [82].Shen G, Ren H, Shang Q, Zhang Z, Zhao W, Yu X, et al. , miR-128 plays a critical role in murine osteoclastogenesis and estrogen deficiency-induced bone loss, Theranostics 10 (2020) 4334–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Franceschetti T, Kessler CB, Lee SK, Delany AM, miR-29 promotes murine osteoclastogenesis by regulating osteoclast commitment and migration, J. Biol. Chem 288 (2013) 33347–33360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rossi M, Pitari MR, Amodio N, Di Martino MT, Conforti F, Leone E, et al. , miR-29b negatively regulates human osteoclastic cell differentiation and function: implications for the treatment of multiple myeloma-related bone disease, J. Cell. Physiol 228 (2013) 1506–1515. [DOI] [PubMed] [Google Scholar]
- [85].Dou C, Zhang C, Kang F, Yang X, Jiang H, Bai Y, et al. , MiR-7b directly targets DC-STAMP causing suppression of NFATc1 and c-Fos signaling during osteoclast fusion and differentiation, Biochim. Biophys. Acta 1839 (2014) 1084–1096. [DOI] [PubMed] [Google Scholar]
- [86].Dou C, Ding N, Luo F, Hou T, Cao Z, Bai Y, et al. , Graphene-based MicroRNA transfection blocks preosteoclast fusion to increase bone formation and vascularization, Adv Sci (Weinh) 5 (2018) 1700578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kim K, Kim JH, Kim I, Lee J, Seong S, Park YW, et al. , MicroRNA-26a regulates RANKL-induced osteoclast formation, Mol Cells 38 (2015) 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yin Y, Tang L, Chen J, Lu X, MiR-30a attenuates osteoclastogenesis via targeting DC-STAMP-c-Fos-NFATc1 signaling, Am. J. Transl. Res 9 (2017) 5743–5753. [PMC free article] [PubMed] [Google Scholar]
- [89].Li G, Bu J, Zhu Y, Xiao X, Liang Z, Zhang R, Curcumin improves bone microarchitecture in glucocorticoid-induced secondary osteoporosis mice through the activation of microRNA-365 via regulating MMP-9, Int. J. Clin. Exp. Pathol 8 (2015) 15684–15695. [PMC free article] [PubMed] [Google Scholar]
- [90].Ma Y, Yang H, Huang J, Icariin ameliorates dexamethasoneinduced bone deterioration in an experimental mouse model via activation of microRNA186 inhibition of cathepsin K, Mol. Med. Rep 17 (2018) 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Guo L, Chen K, Yuan J, Huang P, Xu X, Li C, et al. , Estrogen inhibits osteoclasts formation and bone resorption via microRNA-27a targeting PPARgamma and APC, J. Cell. Physiol 234 (2018) 581–594. [DOI] [PubMed] [Google Scholar]
- [92].Zhao J, Huang M, Zhang X, Xu J, Hu G, Zhao X, et al. , MiR-146a deletion protects from bone loss in OVX mice by suppressing RANKL/RANKL and M-CSF in bone microenvironment, J. Bone Miner. Res 34 (2019) 2149–2161. [DOI] [PubMed] [Google Scholar]
- [93].Hu CH, Sui BD, Du FY, Shuai Y, Zheng CX, Zhao P, et al. , miR-21 deficiency inhibits osteoclast function and prevents bone loss in mice, Sci. Rep 7 (2017) 43191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhou L, Song HY, Gao LL, Yang LY, Mu S, Fu Q, MicroRNA1005p inhibits osteoclastogenesis and bone resorption by regulating fibroblast growth factor 21, Int. J. Mol. Med 43 (2019) 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sun Y, Kuek V, Liu Y, Tickner J, Yuan Y, Chen L, et al. , MiR-214 is an important regulator of the musculoskeletal metabolism and disease, J. Cell. Physiol 234 (2018) 231–245. [DOI] [PubMed] [Google Scholar]
- [96].Lau KW, Sheng MH, A novel miR17/protein tyrosine phosphatase-oc/EphA4 regulatory axis of osteoclast activity, Arch. Biochem. Biophys 650 (2018) 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mao Z, Zhu Y, Hao W, Chu C, Su H, MicroRNA-155 inhibition up-regulates LEPR to inhibit osteoclast activation and bone resorption via activation of AMPK in alendronate-treated osteoporotic mice, IUBMB Life 71 (2019) 1916–1928. [DOI] [PubMed] [Google Scholar]
- [98].Wang T, Yin H, Wang J, Li Z, Wei H, Liu Z, et al. , MicroRNA-106b inhibits osteoclastogenesis and osteolysis by targeting RANKL in giant cell tumor of bone, Oncotarget 6 (2015) 18980–18996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yu FY, Xie CQ, Sun JT, Peng W, Huang XW, Overexpressed miR-145 inhibits osteoclastogenesis in RANKL-induced bone marrow-derived macrophages and ovariectomized mice by regulation of Smad3, Life Sci. 202 (2018) 11–20. [DOI] [PubMed] [Google Scholar]
- [100].Mizoguchi F, Murakami Y, Saito T, Miyasaka N, Kohsaka H, miR-31 controls osteoclast formation and bone resorption by targeting RhoA, Arthritis Res Ther 15 (2013) R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wu RW, Lian WS, Chen YS, Kuo CW, Ke HC, Hsieh CK, et al. , MicroRNA-29a Counteracts Glucocorticoid Induction of Bone Loss through Repressing TNFSF13b Modulation of Osteoclastogenesis, Int J Mol Sci 20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wang FS, Chuang PC, Lin CL, Chen MW, Ke HJ, Chang YH, et al. , MicroRNA-29a protects against glucocorticoid-induced bone loss and fragility in rats by orchestrating bone acquisition and resorption, Arthritis Rheum. 65 (2013) 1530–1540. [DOI] [PubMed] [Google Scholar]
- [103].Wu X, McKenna MA, Feng X, Nagy TR, McDonald JM, Osteoclast apoptosis: the role of Fas in vivo and in vitro, Endocrinology 144 (2003) 5545–5555. [DOI] [PubMed] [Google Scholar]
- [104].Sugatani T, Hruska KA, Down-regulation of miR-21 biogenesis by estrogen action contributes to osteoclastic apoptosis, J. Cell. Biochem 114 (2013) 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sul OJ, Rajasekaran M, Park HJ, Suh JH, Choi HS, MicroRNA-29b enhances osteoclast survival by targeting BCL-2-modifying factor after lipopolysaccharide stimulation, Oxidative Med. Cell. Longev 2019 (2019) 6018180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhu XB, Lin WJ, Lv C, Wang L, Huang ZX, Yang SW, et al. , MicroRNA-539 promotes osteoblast proliferation and differentiation and osteoclast apoptosis through the AXNA-dependent Wnt signaling pathway in osteoporotic rats, J. Cell. Biochem 119 (2018) 8346–8358. [DOI] [PubMed] [Google Scholar]
- [107].Ou M, Zhang X, Dai Y, Gao J, Zhu M, Yang X, et al. , Identification of potential microRNA-target pairs associated with osteopetrosis by deep sequencing, iTRAQ proteomics and bioinformatics, Eur. J. Hum. Genet 22 (2014) 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang Y, Li L, Moore BT, Peng XH, Fang X, Lappe JM, et al. , MiR-133a in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis, PLoS One 7 (2012), e34641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Song H, Li X, Zhao Z, Qian J, Wang Y, Cui J, et al. , Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible Exosomes, Nano Lett. 19 (2019) 3040–3048. [DOI] [PubMed] [Google Scholar]
- [110].Colombo M, Raposo G, Thery C, Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles, Annu. Rev. Cell Dev. Biol 30 (2014) 255–289. [DOI] [PubMed] [Google Scholar]
- [111].Kowal J, Tkach M, Thery C, Biogenesis and secretion of exosomes, Curr. Opin. Cell Biol 29 (2014) 116–125. [DOI] [PubMed] [Google Scholar]
- [112].Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H, New technologies for analysis of extracellular vesicles, Chem. Rev 118 (2018) 1917–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. , Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines, J Extracell Vesicles 7 (2018) 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Shah R, Patel T, Freedman JE, Circulating extracellular vesicles in human disease, N. Engl. J. Med 379 (2018) 958–966. [DOI] [PubMed] [Google Scholar]
- [115].Abels ER, Breakefield XO, Introduction to extracellular vesicles: biogenesis, RNA Cargo Selection, Content, Release, and Uptake, Cell Mol Neurobiol 36 (2016) 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Doyle LM, Wang MZ, Overview of Extracellular Vesicles, their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis, Cells 8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Maas SLN, Breakefield XO, Weaver AM, Extracellular vesicles: unique intercellular delivery vehicles, Trends Cell Biol. 27 (2017) 172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tkach M, Thery C, Communication by extracellular vesicles: where we are and where we need to go, Cell 164 (2016) 1226–1232. [DOI] [PubMed] [Google Scholar]
- [119].van Niel G, D’Angelo G, Raposo G, Shedding light on the cell biology of extracellular vesicles, Nat Rev Mol Cell Biol 19 (2018) 213–228. [DOI] [PubMed] [Google Scholar]
- [120].Li D, Liu J, Guo B, Liang C, Dang L, Lu C, et al. , Osteoclast-derived exosomal miR-214–3p inhibits osteoblastic bone formation, Nat. Commun 7 (2016) 10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sun W, Zhao C, Li Y, Wang L, Nie G, Peng J, et al. , Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity, Cell Discov 2 (2016) 16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang X, Guo B, Li Q, Peng J, Yang Z, Wang A, et al. , miR-214 targets ATF4 to inhibit bone formation, Nat. Med 19 (2013) 93–100. [DOI] [PubMed] [Google Scholar]
- [123].Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G, Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation, Cell 89 (1997) 747–754. [DOI] [PubMed] [Google Scholar]
- [124].Yang JX, Xie P, Li YS, Wen T, Yang XC, Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2, Cell. Signal 70 (2020) 109504. [DOI] [PubMed] [Google Scholar]
- [125].Dai J, Dong R, Han X, Li J, Gong X, Bai Y, et al. , Osteoclast-derived exosomal let-7a-5p targets Smad2 to promote the hypertrophic differentiation of chondrocytes, Am J Physiol Cell Physiol (2020). [DOI] [PubMed] [Google Scholar]
- [126].Dai J, Dong R, Han X, Li J, Gong X, Bai Y, et al. , Osteoclast-derived exosomal let-7a-5p targets Smad2 to promote the hypertrophic differentiation of chondrocytes, Am J Physiol Cell Physiol (2020), 10.1152/ajpcell.00039.2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [127].Guo L, Zhu Y, Li L, Zhou S, Yin G, Yu G, et al. , Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1, Cancer Med 8 (2019) 5687–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Karlsson T, Lundholm M, Widmark A, Persson E, Tumor cell-derived exosomes from the prostate cancer cell line TRAMP-C1 impair osteoclast formation and differentiation, PLoS One 11 (2016), e0166284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Faict S, Muller J, De Veirman K, De Bruyne E, Maes K, Vrancken L, et al. , Exosomes play a role in multiple myeloma bone disease and tumor development by targeting osteoclasts and osteoblasts, Blood Cancer J 8 (2018) 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Moloudizargari M, Abdollahi M, Asghari MH, Zimta AA, Neagoe IB, Nabavi SM, The emerging role of exosomes in multiple myeloma, Blood Rev. 38 (2019) 100595. [DOI] [PubMed] [Google Scholar]
- [131].Raimondi L, De Luca A, Amodio N, Manno M, Raccosta S, Taverna S, et al. , Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation, Oncotarget 6 (2015) 13772–13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Raimondi L, De Luca A, Gallo A, Costa V, Russelli G, Cuscino N, et al. , Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs, Carcinogenesis 41 (2020) 666–677. [DOI] [PubMed] [Google Scholar]
- [133].Xu Z, Liu X, Wang H, Li J, Dai L, Li J, et al. , Lung adenocarcinoma cell-derived exosomal miR-21 facilitates osteoclastogenesis, Gene 666 (2018) 116–122. [DOI] [PubMed] [Google Scholar]
- [134].Cui Y, Fu S, Sun D, Xing J, Hou T, Wu X, EPC-derived exosomes promote osteoclastogenesis through LncRNA-MALAT1, J. Cell. Mol. Med 23 (2019) 3843–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Xu R, Shen X, Si Y, Fu Y, Zhu W, Xiao T, et al. , MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment, Aging Cell 17 (2018), e12794. [DOI] [PMC free article] [PubMed] [Google Scholar]


