Abstract
ADAM17 is a transmembrane protease expressed by most cells in humans and mice that cleaves cell surface substrates primarily in a cis manner, a process referred to as ectodomain shedding. ADAM17 has numerous substrates and plays a broad role in various physiological processes, including as a key regulator of inflammation. At this time, little is known about ADAM17 expression and function in dogs. A well-established ADAM17 substrate is the leukocyte adhesion protein CD62L (L-selectin). We show that a selective inhibitor of ADAM17, but not an inhibitor of its most closely related family member ADAM10, blocks CD62L shedding upon canine neutrophil activation. We also tested several anti-human ADAM17 monoclonal antibodies (mAbs) for staining canine neutrophils. Although most did not recognize canine neutrophils, the mAbs MEDI3622 and D1(A12) did. They also blocked the downregulation of CD62L upon neutrophil activation. MEDI3622 is a human IgG antibody and we found that a canine chimeric version of this mAb also blocked CD62L shedding by canine leukocytes. Taken together, our findings provide the first direct evidence of ADAM17 expression and sheddase activity in dogs, establishing a potential therapeutic target for various inflammatory disorders.
Keywords: Neutrophil, metalloprotease, inflammation, canine
Introduction
ADAM17 (a disintegrin and metalloproteinase 17) is a transmembrane protease that is constitutively expressed by most cells in the body (Lambrecht et al., 2018). It cleaves its substrates on the cell surface typically in a cis manner at a specific extracellular site, a process referred to as ectodomain shedding. Its catalytic activity is highly inducible upon cell activation by a broad range of stimuli. This process occurs very rapidly and involves ADAM17 transitioning from a low to high activity state through a conformational change and intermolecular interactions (Mishra et al., 2017). ADAM17 substrates have a key role in regulating inflammatory and immune processes and include various cytokines, their receptors, and adhesion molecules (Lambrecht et al., 2018; Mishra et al., 2017; Zunke and Rose-John, 2017). ADAM17 has been well-characterized in human and mouse neutrophils where it directly regulates various effector functions, including their activation, migration, and phagocytosis of antibody-opsonized targets (Mishra et al., 2017). Aberrant ADAM17 induction, however, has pathological consequences (Lambrecht et al., 2018; Mishra et al., 2017; Zunke and Rose-John, 2017). Using models of sepsis, we have reported that mice with ADAM17-null leukocytes have significantly reduced mortality (Long et al., 2012; Long et al., 2010; Mishra et al., 2016). This was associated with increased neutrophil recruitment, reduced bacteria levels at sites of infection, and markedly reduced systemic levels of proinflammatory cytokines, establishing a central role of ADAM17 in sepsis pathogenesis (Long et al., 2012; Long et al., 2010; Mishra et al., 2016). Moreover, in human patients, ADAM17 activity and a functional polymorphism of its gene corresponded with sepsis progression (Kermarrec et al., 2005; Shao et al., 2016).
One of the best studied substrates of mouse and human ADAM17 is the leukocyte adhesion protein CD62L (L-selectin) (Ivetic, 2018; Mishra et al., 2017). Canine CD62L has been characterized at the protein, cellular, and functional levels and is very similar to human CD62L (Abbassi et al., 1991; Crockett-Torabi and Fantone, 1997). Similar to human and murine CD62L (Jutila et al., 1990; Kishimoto et al., 1989; Kishimoto et al., 1990), canine CD62L also undergoes a rapid downregulation in expression upon neutrophil activation, suggesting it is similarly regulated by ectodomain shedding (Abbassi et al., 1991). At this time, the function of ADAM17 in canine neutrophils has not been reported. We demonstrate its expression and that blocking its activity disrupts the downregulation of CD62L expression. Given that ADAM17 has a broad immunomodulatory role in humans (Lambrecht et al., 2018; Mishra et al., 2017; Zunke and Rose-John, 2017), this sheddase may be a key therapeutic target in dogs for different inflammatory disorders.
Materials and methods
Animals
Peripheral blood was collected from 15 healthy pet dogs with owner consent. All animals received routine veterinary examinations and vaccines. The dog breeds represented in this study include four mixed breed dogs, three Labrador retrievers, one beagle, one Weimaraner, and one German shorthaired pointer. Breed information was not readily available for five donors due to client de-identification at time of collection. Blood collection was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Minnesota (Protocol Number: 21903–36913A).
Antibodies
D1(A12) (MilliporeSigma, St. Louis, MO) is an anti-human ADAM17 mAb and has been shown to block its proteolytic activity (Tape et al., 2011). MEDI3622 is also an anti-human ADAM17 mAb that has been shown to block its proteolytic activity (Peng et al., 2016; Rios-Doria et al., 2015). For this study, MEDI3622 was generated as human IgG (Syd Labs, Natick, MA) and canine IgG (Creative Biolabs, Shirley, NY) using its variable heavy and variable light chain sequences. The anti-human ADAM17 mAbs 111633 and 111623 were purchased from R&D Systems (Minneapolis, MN). Anti-CD4 FITC (clone YKIX302.9) was purchased from eBioscience (San Diego, CA). Anti-CD5 PE (clone YKIX322.3), anti-CD3 PE (clone CA17.2A12), anti-CD8 APC (clone YCATE55.9), and anti-CD45 APC (clone YKIX716.13) were purchased from Bio-Rad Laboratories (Hercules, CA). The mAb CL2/6, which recognizes canine CD62L (Abbassi et al., 1991), and anti-CD22 FITC (clone RFB-4) were purchased from (ThermoFisher Scientific, Waltham, MA). The mouse anti-human ADAM17 mAb M220 (generously provided by Dr. R. Black, Immunex, Seattle, WA) has been previously described (Doedens and Black, 2000). The mAb R15.7 (generously provided by Dr. R. Rothlein, Boehringer Ingelheim, Ridgefield, CT) has been previously shown to recognize canine CD18 (Entman et al., 1990). Isotype-matched negative control mouse and human antibodies were purchased from R&D Systems and MilliporeSigma, respectively. Allophycocyanin-conjugated F(ab’)2 goat anti-mouse or donkey anti-human IgG (H+L) secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Leukocyte isolation and treatment
Canine peripheral blood was collected from healthy donors in K2-EDTA blood collection tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). Whole blood was diluted ten-fold with red blood cell lysis buffer (ammonium chloride, 155 mM; potassium bicarbonate, 10 mM; and disodium EDTA, 0.1 mM) and incubated for 10 minutes at room temperature. Leukocytes were washed in PBS buffer (without Ca+2 and Mg+2) (Lonza, Walkersville, MD) and cell viabilities were assessed using trypan blue exclusion, which was ≥ than 95%. Leukocytes (2.5×106/ml in PBS) were stimulated with 1–15ng/mL phorbol-12-myristate-13-acetate (PMA) (MilliporeSigma), 1μg/mL formyl peptide receptor-like 1 agonist (MilliporeSigma), or 10μg/mL Pseudomonas lipopolysaccharide (LPS) (MilliporeSigma), as we have previously described (Mishra et al., 2015). Cells were stimulated for 30 minutes at 37°C, which was stopped by washing three times with ice cold PBS. Some cells were pre-incubated for 30 minutes on ice with ADAM10 and ADAM17 hydroxymate-based small molecule inhibitors or function blocking antibodies prior to activation. The ADAM17 inhibitor BMS566394 (MedKoo Biosciences, Morrisville, NC), referred to previously as inhibitor 32 (Ott et al., 2008), selectively blocks ADAM17 at 5 μM (Wang et al., 2013); the ADAM10 inhibitor GI254023X (R&D Systems) is 10-fold more selective for ADAM10 than ADAM17 in cellular assays and preferentially blocks ADAM10 in cells at a concentration ranging from 0.2–1 μM (Hundhausen et al., 2003; Wang et al., 2010). GI254023X was used at 0.5 μM, as we have previously described (Wang et al., 2013). INCB3619 (MedKoo Biosciences) blocks the activity of ADAM10 and ADAM17 (Zhou et al., 2006). INCB3619 was used at 10μM, as previously described (Wang et al., 2013). All inhibitors were resuspended in DMSO and used at the indicated concentrations. The anti-ADAM17 monoclonal antibodies MEDI3622 and D1(A12) were used at 10 μg/ml.
Cell staining and flow cytometric analysis
For cell staining, Fc receptor and nonspecific antibody binding sites were blocked for 30 minutes using 25% canine serum and 25% FBS in PBS prior to their staining with antibodies. All cell staining was analyzed on FACSCanto or FACSCelesta instruments (BD Biosciences, San Jose, CA). For controls, fluorescence minus one was used as well as appropriate isotype-matched antibodies since the cells of interest expressed Fc receptors. An FSC-A/SSC-A plot was used to set an electronic gate on leukocyte populations and an FSC-A/FSC-H plot was used to set an electronic gate on single cells. Fixable viability dyes eFluor 506 (eBioscience) or Zombie Violet (BioLegend, San Diego, CA) were used to assess live vs. dead cells, as per the manufacturers’ instructions. Canine neutrophils were identified based on their forward and side light-scattering characteristics (Weiss et al., 2000; Weiss et al., 2004). Canine neutrophils express high levels of CD4 (Moore et al., 1992) and their identification was confirmed based on CD4 staining and lack of CD22, CD5, and CD8 staining (data not shown).
Pairwise Sequence Alignment
To identify the MEDI3622 binding site for ADAM17 across several species, amino acid sequences of human, mouse, rat, canine (predicted), feline (predicted), and rabbit (predicted) ADAM17 were retrieved from UniProtKB. Amino acid sequences were aligned using Clustal Omega (Dublin, Ireland).
Statistical analyses
All experiments were performed using three biological replicates. Flow cytometry data was analyzed using FlowJo. Statistical significance was determined between two groups by using an unpaired Student’s t test.
Results and Discussion
Effects of selective ADAM17 inhibitors on CD62L downregulation by activated canine neutrophils.
The mAb CL2/6 recognizes canine CD62L and it has been reported that canine neutrophils rapidly downregulate CD62L upon their activation (Abbassi et al., 1991). We observed that neutrophil staining with this mAb rapidly and efficiently decreased upon their treatment with various stimuli, as determined by flow cytometry (Fig 1A,B). To investigate the involvement of ADAM17 in the downregulation of canine CD62L, we utilized several small molecule hydroxamate-based metalloproteinase inhibitors. These compounds were used at concentrations previously established to have selective inhibitory activity, as described in the Materials and Methods. INCB3619 is a selective inhibitor of ADAM10 and ADAM17 (Zhou et al., 2006). ADAM10 is the most similar ADAM family member to ADAM17 in terms of amino acid sequence and structure (Takeda, 2016). INCB3619 was found to block CD62L downregulation following canine neutrophil activation with PMA (Fig 1A). GI254023X is a well described selective ADAM10 inhibitor (Hundhausen et al., 2003), but it was not found to significantly block the downregulation of CD62L following neutrophil activation (Fig 1A). BMS566394 has a potency orders of magnitude higher for ADAM17 than ADAM10 (Ott et al., 2008), and this compound did effectively block CD62L downregulation upon neutrophil activation (Fig 1A). BMS566394 also blocked the downregulation CD62L following canine neutrophil activation with physiological stimuli, such as the bacterial components formyl peptide and LPS (Fig 1B). Our findings based on the use of selective small molecule inhibitors suggests a role by ADAM17 but not ADAM10 in shedding canine CD62L. In humans, ADAM17 cleaves CD62L at a specific extracellular site proximal to the cell membrane (Kahn et al., 1994). The cleavage region of human CD62L is 87.5% identical and 100% homologous at the amino acid level to the membrane proximal region of canine CD62L (Fig 1C), which suggests that CD62L in canine leukocytes may be cut at the same location by ADAM17. However, we were not able to successfully immunoblot membrane or soluble CD62L from canine neutrophils, and thus were unable to confirm the presence and molecular mass of soluble CD62L or its membrane-associated cleavage fragment.
Fig 1. ADAM17 activity in canine neutrophils.
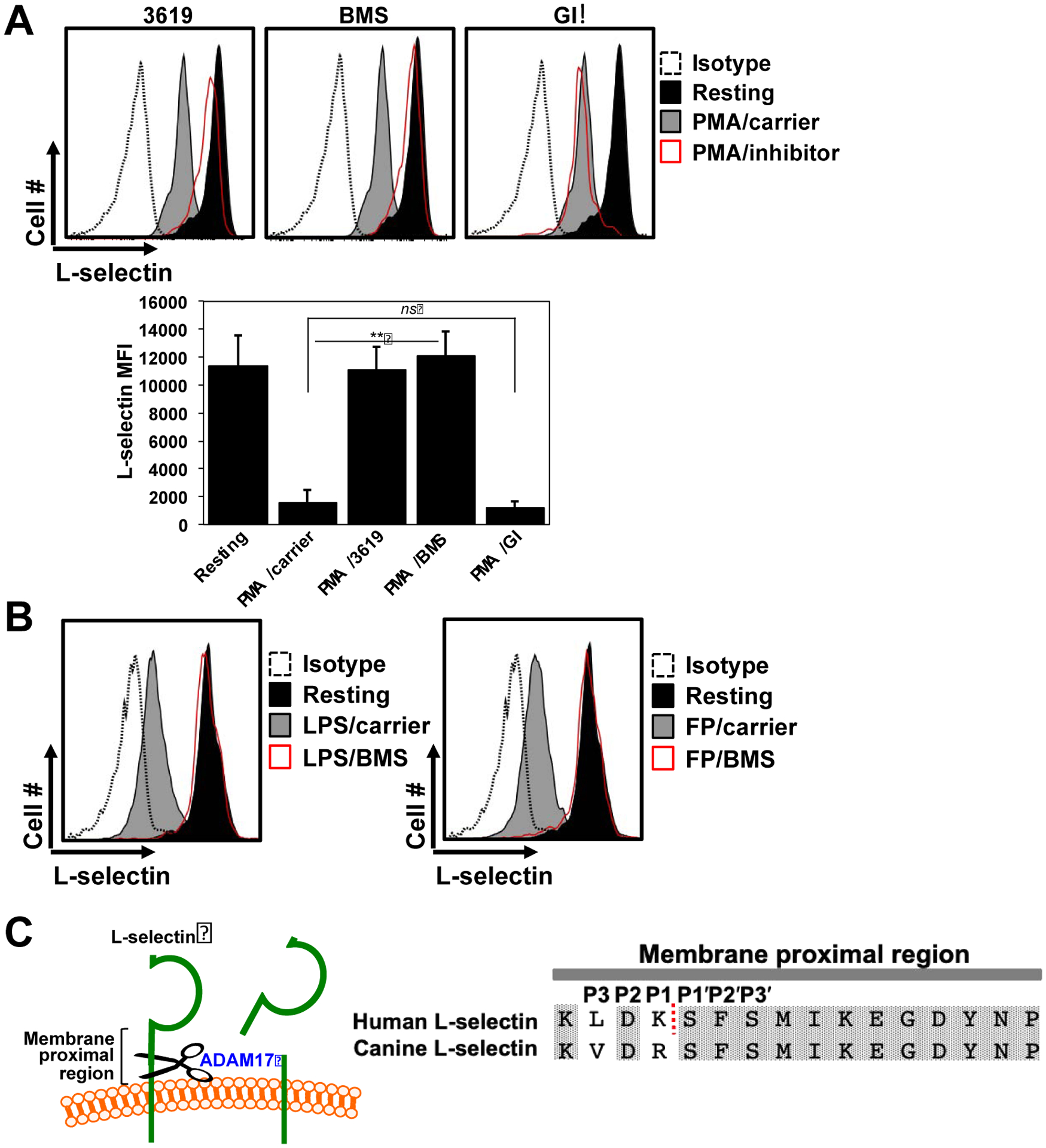
A. Canine peripheral blood leukocytes were treated with or without PMA in the presence or absence of the ADAM10 and ADAM17 inhibitor INCB3619 (3619), the ADAM17 inhibitor BMS566394 (BMS), the ADAM10 inhibitor GI254023X (GI), or DMSO carrier for 30 minutes at 37°C, as indicated. The leukocytes were then stained with an anti-CD62L mAb or an appropriate isotype negative control antibody and assessed by flow cytometry. Neutrophils were gated based on their characteristic forward and side light scatters. All histograms show representative data and the bar graphs show mean ± SD with at least 3 animals per treatment. The x-axis of all histograms = Log 10 fluorescence. Statistical significance is indicated as **p < 0.01 PMA/carrier vs. PMA/3619 and PMA/BMS. PMA/carrier vs. PMA/GI was not significant (ns). MFI = Mean fluorescence intensity. B. Leukocytes were treated with the ADAM17 inhibitor BMS as described above and LPS or formyl peptide (FP) were used as stimuli. C. CD62L undergoes ectodomain shedding by ADAM17 within a membrane proximal region, as indicated. Alignment of the extracellular membrane proximal regions of human and canine CD62L. The dashed line indicates the site of cleavage in human CD62L (Kahn et al., 1994). The amino acid sequences of human and canine CD62L are from the NCBI reference sequences NM_000655.4 and XM_537201.4, respectively.
CD18 integrins are additional adhesion molecules expressed by neutrophils that are also important for their attachment and transmigration through the vascular endothelium (Ley et al., 2007). CD62L and Mac-1 (CD11b/CD18) surface levels are inversely regulated upon neutrophil activation (Kishimoto et al., 1989). Mac-1 resides in intracellular granules that are very rapidly translocated to the cell surface after neutrophil stimulation, providing a sensitive and quantifiable measure of cell activation (Berger et al., 1984). We found that neither INCB3619 nor BMS566394 treatment of canine neutrophils affected the upregulation of CD18 expression upon their stimulation (Fig 2). These findings thus indicate that the inhibitors at the concentrations used did not overtly impair neutrophil activation and prevent CD62L downregulation in that manner.
Fig 2. ADAM10 and ADAM17 inhibitors do not affect Mac-1 upregulation.
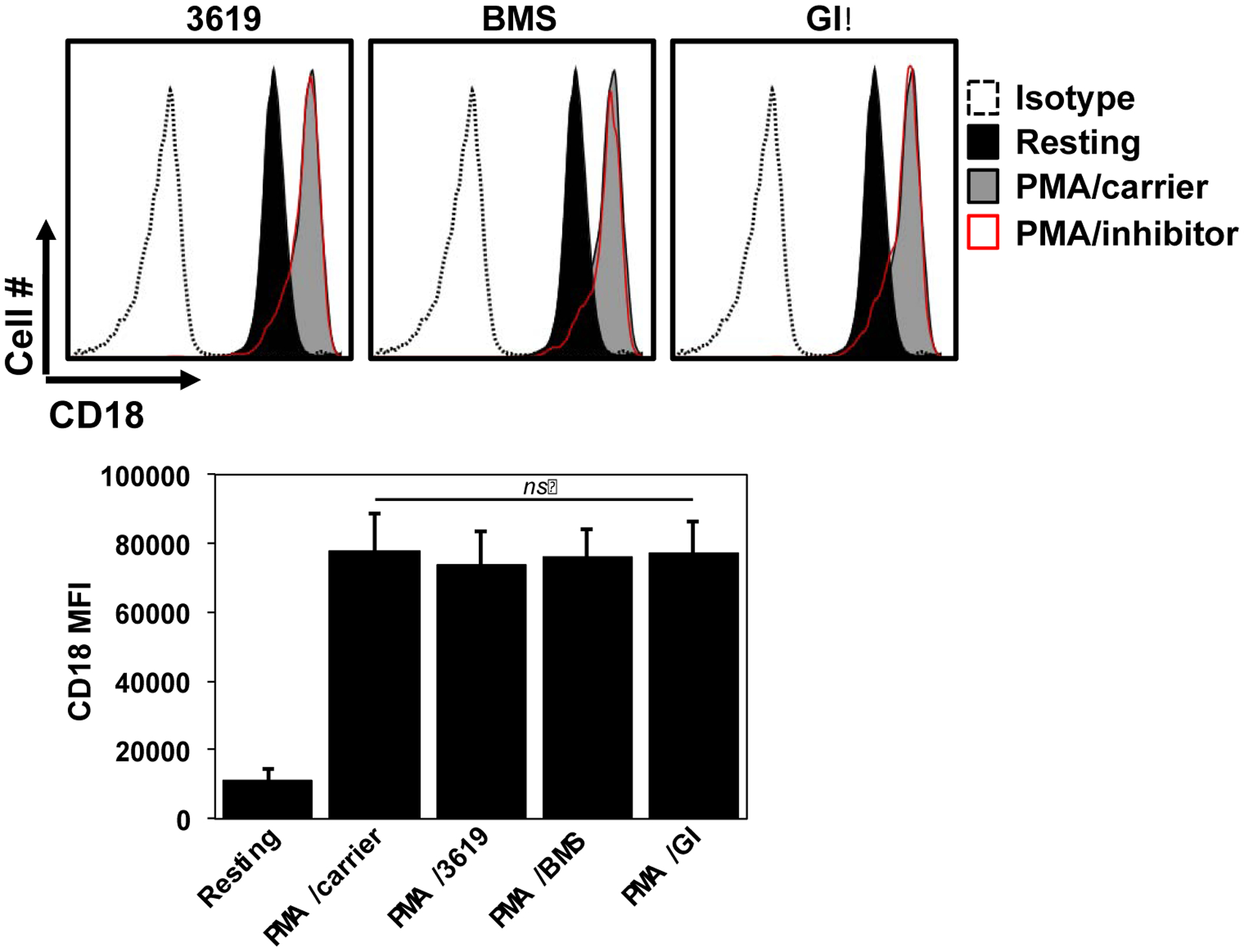
Canine peripheral blood leukocytes were treated with or without PMA in the presence or absence of ADAM10 and/or ADAM17 inhibitors, as described in Figure 1. Relative cell-staining levels of CD18 were determined by flow cytometry. Neutrophils were gated based on their characteristic forward and side light scatters. The x-axis of all histograms = Log 10 fluorescence. All histograms show representative data and the bar graphs show mean ± SD with at least 3 animals per treatment.
ADAM17 expression and function on canine neutrophils.
To date, antibodies that detect canine ADAM17 have not been described. The predicted amino acid sequence of canine ADAM17 based on cDNA sequence reveals a 96% identity to human ADAM17 (Sarasa et al., 2010). Therefore, we examined the reactivity of several anti-human ADAM17 mAbs with canine neutrophils. We have previously demonstrated that the anti-ADAM17 mAbs 111633, 111623, and m220 all stain ADAM17 on human cells by flow cytometry (Buchanan et al., 2017; Jing et al., 2015; Walcheck et al., 2006; Wang et al., 2010). In Figure 3A, these mAbs are shown to stain human peripheral blood neutrophils, but not canine peripheral blood neutrophils above that of an isotype-matched negative control antibody (Fig 3B).
Fig 3. Anti-ADAM17 mAbs stain human but not canine neutrophils.
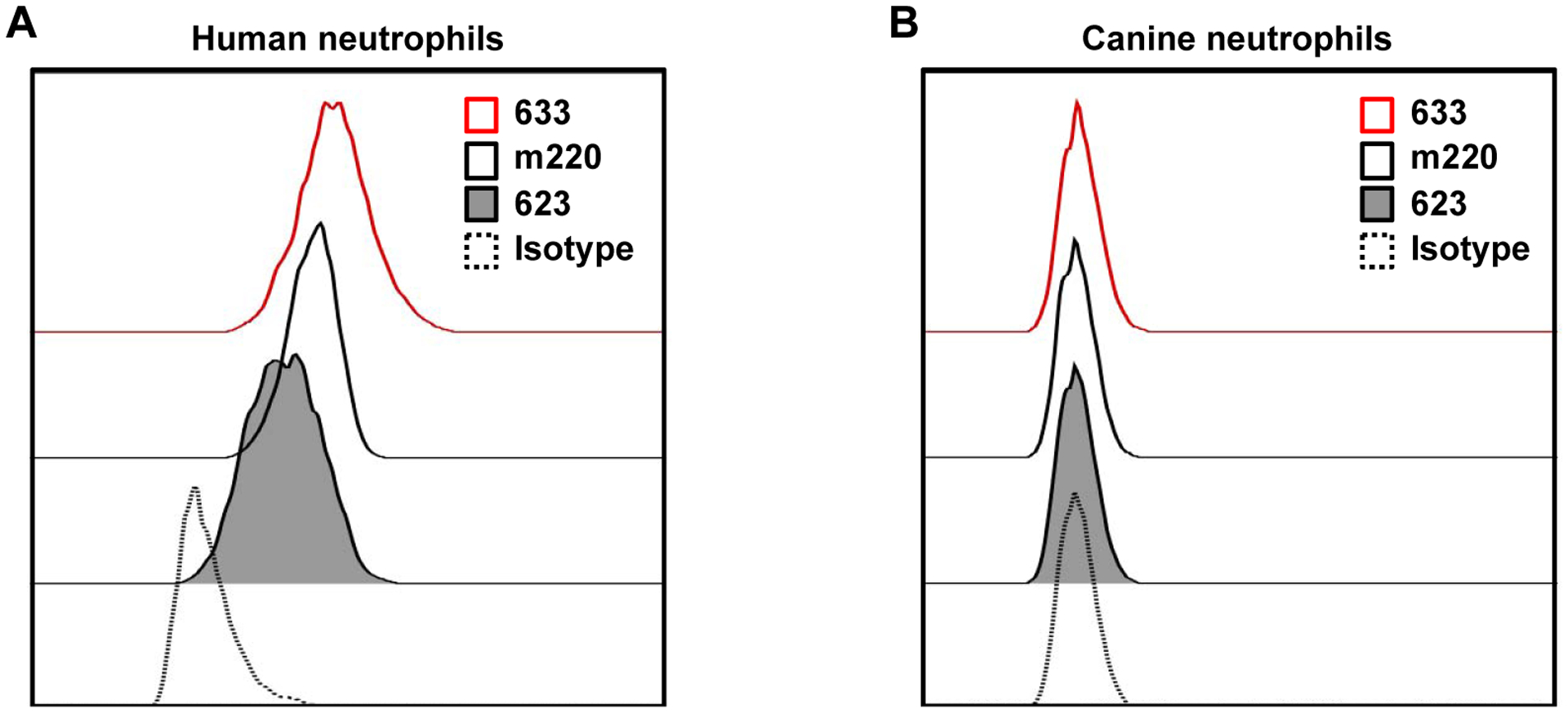
Human (A) and canine (B) peripheral blood leukocytes were stained by different anti-ADAM17 mAbs and examined by flow cytometry, as indicated. Neutrophils were gated based on their characteristic forward and side light scatters. The y-axis of all histograms = cell number and the x-axis = Log 10 fluorescence. Data is representative of at least 3 independent experiments using leukocytes from separate human and canine donors.
MEDI3622 is a human IgG mAb that specifically recognizes ADAM17 and is well characterized at blocking its function (Mishra et al., 2020; Mishra et al., 2018; Rios-Doria et al., 2015). MEDI3622 also blocks the downregulation of CD62L on human leukocytes upon their activation (Mishra et al., 2018). Its epitope has been mapped to a surface loop that is located within the catalytic domain of ADAM17, between amino acids P366-N381, which does not occur in other ADAM family members (Peng et al., 2016). Alignment of this region with the predicted amino acid sequence for canine ADAM17 shows it to be nearly identical to human ADAM17. Additionally, this region is also conserved in other animal species, including mouse, rat, feline, and rabbit (Fig 4A). Indeed, MEDI3622 has been shown to recognize mouse ADAM17 (Mishra et al., 2018; Rios-Doria et al., 2015). We found that it also stained canine neutrophils and blocked the downregulation of CD62L upon their activation (Fig 4B,C).
Fig 4. ADAM17 mAbs block the function of canine ADAM17.
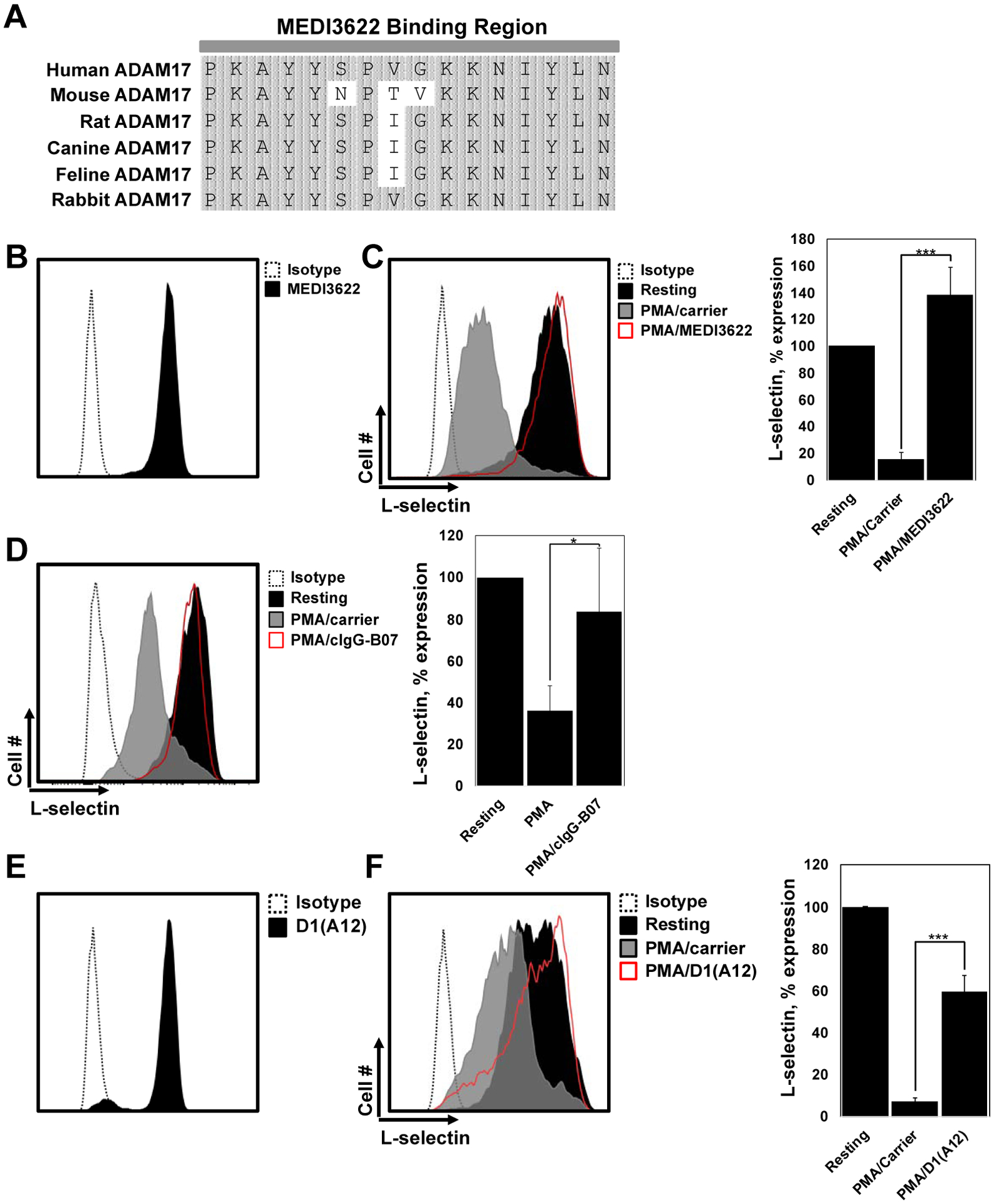
A. Alignment of amino acid sequences for ADAM17 across several species shows a high level of conservation in the MEDI3622 binding site, which occurs between amino acids P366-N381. Primary accession number (UniProt) for amino acid sequences are as follows: human (P78536), mouse (Q9Z0F8), rat (Q9Z1K9), canine predicted (M1V4E8), feline predicted (M3W8I1), and rabbit predicted (G1SZC8). B. Canine peripheral blood neutrophils were stained with MEDI3622 or an IgG1 isotype negative control antibody and assessed by flow cytometry. C and D. Canine peripheral blood neutrophils were treated with or without PMA in the presence of MEDI3622 (C) or cIgG-B07 (D). E. Canine peripheral blood neutrophils were stained with D1(A12) or an IgG1 isotype negative control antibody and assessed by flow cytometry. F. Canine peripheral blood neutrophils were treated with or without PMA in the presence of D1(A12). CD62L shedding was determined via flow cytometry. The y-axis of all histograms = cell number and the x-axis = Log 10 fluorescence. Data is representative of at least 3 independent experiments using leukocytes from separate animals. The bar graph shows mean ± SD with at least 3 animals per treatment. Statistical significance is indicated as *p <0.001 PMA/carrier vs. PMA/cIgG-B07, ***p <0.001 PMA/carrier vs. PMA/MEDI3622 or PMA/carrier vs PMA/D1(A12).
MEDI3622 was generated by screening human scFv phage libraries with human ADAM17 (Peng et al., 2016; Rios-Doria et al., 2015). Using corresponding nucleotide sequences of the amino acids comprising the variable heavy and light regions of MEDI3622, we generated a chimeric antibody consisting of the constant heavy and light regions of canine IgG (D isotype), referred to as cIgG-B07, which also blocked the downregulation of surface CD62L expression upon canine neutrophil activation (Fig 4D).
D1(A12) is another anti-human ADAM17 mAb that blocks its function (Tape et al., 2011). We found this mAb also stained canine neutrophils and blocked the downregulation of CD62L upon their activation (Fig 4E,F), but not as effectively as MEDI3622 (Fig 4C). However, though MEDI3622 and D1(A12) were used at saturating concentrations, a comprehensive comparison of the inhibitory activity of these mAbs was not performed. D1(A12) is a human mAb engineered from phage display and its variable heavy and light chains were designed to recognize distinct epitopes in different regions of ADAM17 (Tape et al., 2011). To our knowledge, the specific locations of these epitopes have not been determined. D1(A12) has been reported to not recognize mouse ADAM17 (Tape et al., 2011), indicating less cross-reactivity than MEDI3622. The apparent decreased efficiency of blocking canine CD62L shedding by D1(A12) compared to MEDI3622 may be the result of either its heavy or light chain not effectively recognizing its respective epitope on canine ADAM17.
In summary, we demonstrate for the first time the function of ADAM17 in canine neutrophils and its role in the downregulation of CD62L upon their activation. We show that selective small molecule inhibitors of ADAM17, but not ADAM10, as well as the function-blocking ADAM17 mAbs MEDI3622 and D1(A12) block canine CD62L shedding from the neutrophil surface. ADAM17 has an important role in regulating the release of assorted soluble factors and the cell surface density of receptors and adhesion molecules; however, its excessive or prolonged induction has pathological consequences (Lambrecht et al., 2018; Mishra et al., 2017; Zunke and Rose-John, 2017). Indeed, systemic CD62L shedding by circulating neutrophils impairs their critical recruitment to sites of infection in animal models of sepsis and in septic patients (Kermarrec et al., 2005; Long et al., 2012; Long et al., 2010; Mishra et al., 2016). Neutrophil dysfunction is also a characteristic of the early stages of sepsis in dogs (Webb et al., 2007). ADAM17 therefore may serve as an important therapeutic target to reduce hyperinflammation and maintain proper leukocyte function during sepsis or other inflammatory disorders (Mishra et al., 2017).
Acknowledgments
We would like to thank Kathy Stuebner and Amber Winter from the University of Minnesota, College of Veterinary Medicine, Clinical Investigation Center for assistance in acquiring canine peripheral blood samples, Taylor DePauw with flow cytometry assistance, Dr. Jianming Wu for his assistance with the figures and copy editing the manuscript. This work was supported by the National Institutes of Health [grant number HL128580]. KS was supported by a Howard Hughes Medical Institute and Burroughs Wellcome Fund Medical Research Fellowship. CAM and KS were supported by the Office of the Director of the NIH, award number T35OD011118.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- Abbassi O, Lane CL, Krater S, Kishimoto TK, Anderson DC, McIntire LV, Smith CW, 1991. Canine neutrophil margination mediated by lectin adhesion molecule-1 in vitro. J Immunol 147, 2107–2115. [PubMed] [Google Scholar]
- Berger M, O'Shea J, Cross AS, Folks TM, Chused TM, Brown EJ, Frank MM, 1984. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. The Journal of clinical investigation 74, 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan PC, Boylan KLM, Walcheck B, Heinze R, Geller MA, Argenta PA, Skubitz APN, 2017. Ectodomain shedding of the cell adhesion molecule Nectin-4 in ovarian cancer is mediated by ADAM10 and ADAM17. J Biol Chem 292, 6339–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett-Torabi E, Fantone JC, 1997. L-selectin stimulation of canine neutrophil initiates calcium signal secondary to tyrosine kinase activation. Am J Physiol 272, H1302–1308. [DOI] [PubMed] [Google Scholar]
- Doedens JR, Black RA, 2000. Stimulation-induced down-regulation of tumor necrosis factor-alpha converting enzyme. J Biol Chem 275, 14598–14607. [DOI] [PubMed] [Google Scholar]
- Entman ML, Youker K, Shappell SB, Siegel C, Rothlein R, Dreyer WJ, Schmalstieg FC, Smith CW, 1990. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J Clin Invest 85, 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A, 2003. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 102, 1186–1195. [DOI] [PubMed] [Google Scholar]
- Ivetic A, 2018. A head-to-tail view of L-selectin and its impact on neutrophil behaviour. Cell Tissue Res 371, 437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Ni Z, Wu J, Higgins L, Markowski TW, Kaufman DS, Walcheck B, 2015. Identification of an ADAM17 cleavage region in human CD16 (FcgammaRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS One 10, e0121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutila MA, Kishimoto TK, Butcher EC, 1990. Regulation and lectin activity of the human neutrophil peripheral lymph node homing receptor. Blood 76, 178–183. [PubMed] [Google Scholar]
- Kahn J, Ingraham RH, Shirley F, Migaki GI, Kishimoto TK, 1994. Membrane proximal cleavage of L-selectin: identification of the cleavage site and a 6-kD transmembrane peptide fragment of L-selectin. J Cell Biol 125, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermarrec N, Selloum S, Plantefeve G, Chosidow D, Paoletti X, Lopez A, Mantz J, Desmonts JM, Gougerot-Pocidalo MA, Chollet-Martin S, 2005. Regulation of peritoneal and systemic neutrophil-derived tumor necrosis factor-alpha release in patients with severe peritonitis: role of tumor necrosis factor-alpha converting enzyme cleavage. Crit Care Med 33, 1359–1364. [DOI] [PubMed] [Google Scholar]
- Kishimoto TK, Jutila MA, Berg EL, Butcher EC, 1989. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science 245, 1238–1241. [DOI] [PubMed] [Google Scholar]
- Kishimoto TK, Jutila MA, Butcher EC, 1990. Identification of a human peripheral lymph node homing receptor: A rapidly down-regulated adhesion molecule. Proceedings of the National Academy of Sciences of the United States of America 87, 2244–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Vanderkerken M, Hammad H, 2018. The emerging role of ADAM metalloproteinases in immunity. Nature reviews. Immunology [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S, 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews. Immunology 7, 678–689. [DOI] [PubMed] [Google Scholar]
- Long C, Hosseinkhani MR, Wang Y, Sriramarao P, Walcheck B, 2012. ADAM17 activation in circulating neutrophils following bacterial challenge impairs their recruitment. J Leukoc Biol 92, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Wang Y, Herrera AH, Horiuchi K, Walcheck B, 2010. In vivo role of leukocyte ADAM17 in the inflammatory and host responses during E. coli-mediated peritonitis. J Leukoc Biol 87, 1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra HK, Johnson TJ, Seelig DM, Walcheck B, 2016. Targeting ADAM17 in leukocytes increases neutrophil recruitment and reduces bacterial spread during polymicrobial sepsis. J Leukoc Biol 100, 999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra HK, Long C, Bahaie NS, Walcheck B, 2015. Regulation of CXCR2 expression and function by a disintegrin and metalloprotease-17 (ADAM17). J Leukoc Biol 97, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra HK, Ma J, Mendez D, Hullsiek R, Pore N, Walcheck B, 2020. Blocking ADAM17 Function with a Monoclonal Antibody Improves Sepsis Survival in a Murine Model of Polymicrobial Sepsis. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra HK, Ma J, Walcheck B, 2017. Ectodomain Shedding by ADAM17: Its Role in Neutrophil Recruitment and the Impairment of This Process during Sepsis. Front Cell Infect Microbiol 7, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra HK, Pore N, Michelotti EF, Walcheck B, 2018. Anti-ADAM17 monoclonal antibody MEDI3622 increases IFNgamma production by human NK cells in the presence of antibody-bound tumor cells. Cancer Immunol Immunother 67, 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PF, Rossitto PV, Danilenko DM, Wielenga JJ, Raff RF, Severns E, 1992. Monoclonal antibodies specific for canine CD4 and CD8 define functional T-lymphocyte subsets and high-density expression of CD4 by canine neutrophils. Tissue Antigens 40, 75–85. [DOI] [PubMed] [Google Scholar]
- Ott GR, Asakawa N, Lu Z, Anand R, Liu RQ, Covington MB, Vaddi K, Qian M, Newton RC, Christ DD, Trzaskos JM, Duan JJ, 2008. Potent, exceptionally selective, orally bioavailable inhibitors of TNF-alpha Converting Enzyme (TACE): novel 2-substituted-1H-benzo[d]imidazol-1-yl)methyl)benzamide P1’ substituents. Bioorg Med Chem Lett 18, 1577–1582. [DOI] [PubMed] [Google Scholar]
- Peng L, Cook K, Xu L, Cheng L, Damschroder M, Gao C, Wu H, Dall’Acqua WF, 2016. Molecular basis for the mechanism of action of an anti-TACE antibody. MAbs 8, 1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Doria J, Sabol D, Chesebrough J, Stewart D, Xu L, Tammali R, Cheng L, Du Q, Schifferli K, Rothstein R, Leow CC, Heidbrink-Thompson J, Jin X, Gao C, Friedman J, Wilkinson B, Damschroder M, Pierce AJ, Hollingsworth RE, Tice DA, Michelotti EF, 2015. A Monoclonal Antibody to ADAM17 Inhibits Tumor Growth by Inhibiting EGFR and Non-EGFR-Mediated Pathways. Mol Cancer Ther 14, 1637–1649. [DOI] [PubMed] [Google Scholar]
- Sarasa L, Gallego C, Monleon I, Olvera A, Canudas J, Montanes M, Pesini P, Sarasa M, 2010. Cloning, sequencing and expression in the dog of the main amyloid precursor protein isoforms and some of the enzymes related with their processing. Neuroscience 171, 1091–1101. [DOI] [PubMed] [Google Scholar]
- Shao Y, He J, Chen F, Cai Y, Zhao J, Lin Y, Yin Z, Tao H, Shao X, Huang P, Yin M, Zhang W, Liu Z, Cui L, 2016. Association Study Between Promoter Polymorphisms of ADAM17 and Progression of Sepsis. Cell Physiol Biochem 39, 1247–1261. [DOI] [PubMed] [Google Scholar]
- Takeda S, 2016. ADAM and ADAMTS Family Proteins and Snake Venom Metalloproteinases: A Structural Overview. Toxins (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tape CJ, Willems SH, Dombernowsky SL, Stanley PL, Fogarasi M, Ouwehand W, McCafferty J, Murphy G, 2011. Cross-domain inhibition of TACE ectodomain. Proc Natl Acad Sci U S A 108, 5578–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcheck B, Herrera AH, St Hill C, Mattila PE, Whitney AR, Deleo FR, 2006. ADAM17 activity during human neutrophil activation and apoptosis. Eur J Immunol 36, 968–976. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu J, Newton R, Bahaie NS, Long C, Walcheck B, 2013. ADAM17 cleaves CD16b (FcgammaRIIIb) in human neutrophils. Biochim Biophys Acta 1833, 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang AC, Ni Z, Herrera A, Walcheck B, 2010. ADAM17 activity and other mechanisms of soluble L-selectin production during death receptor-induced leukocyte apoptosis. J Immunol 184, 4447–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C, McCord K, Dow S, 2007. Neutrophil function in septic dogs. J Vet Intern Med 21, 982–989. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Blauvelt M, Sykes J, McClenahan D, 2000. Flow cytometric evaluation of canine bone marrow differential cell counts. Vet Clin Pathol 29, 97–104. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Welle M, Mortiz A, Walcheck B, 2004. Evaluation of leukocyte cell surface markers in dogs with septic and nonseptic inflammatory diseases. Am J Vet Res 65, 59–63. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Peyton M, He B, Liu C, Girard L, Caudler E, Lo Y, Baribaud F, Mikami I, Reguart N, Yang G, Li Y, Yao W, Vaddi K, Gazdar AF, Friedman SM, Jablons DM, Newton RC, Fridman JS, Minna JD, Scherle PA, 2006. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell 10, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunke F, Rose-John S, 2017. The shedding protease ADAM17: Physiology and pathophysiology. Biochim Biophys Acta Mol Cell Res 1864, 2059–2070. [DOI] [PubMed] [Google Scholar]


