Abstract
Background: Interleukin-6 (IL-6) is known to be detrimental in coronavirus disease 2019 (COVID-19) because of its involvement in driving cytokine storm. This systematic review and meta-analysis aimed to assess the safety and efficacy of anti-IL-6 signaling (anti-IL6/IL-6R/JAK) agents on COVID-19 based on the current evidence.
Methods: Studies were identified through systematic searches of PubMed, EMBASE, ISI Web of Science, Cochrane library, ongoing clinical trial registries (clinicaltrials.gov), and preprint servers (medRxiv, ChinaXiv) on August 10, 2020, as well as eligibility checks according to predefined selection criteria. Statistical analysis was performed using Review Manager (version 5.3) and STATA 12.0.
Results: Thirty-one studies were included in the pooled analysis of mortality, and 12 studies were identified for the analysis of risk of secondary infections. For mortality analysis, 5630 COVID-19 cases including 2,132 treated patients and 3,498 controls were analyzed. Anti-IL-6 signaling agents plus standard of care (SOC) significantly decreased the mortality rate compared to SOC alone (pooled OR = 0.61, 95% CI 0.45–0.84, p = 0.002). For the analysis of secondary infection risk, 1,624 patients with COVID-19 including 639 treated patients and 985 controls were included, showing that anti-IL-6 signaling agents did not increase the rate of secondary infections (pooled OR = 1.21, 95% CI 0.70–2.08, p = 0.50). By contrast, for patients with critical COVID-19 disease, anti-IL-6 signaling agents failed to reduce mortality compared to SOC alone (pooled OR = 0.75, 95% CI 0.42–1.33, p = 0.33), but they tended to increase the risk of secondary infections (pooled OR = 1.85, 95% CI 0.95–3.61, p = 0.07). A blockade of IL-6 signaling failed to reduce the mechanical ventilation rate, ICU admission rate, or elevate the clinical improvement rate.
Conclusion: IL-6 signaling inhibitors reduced the mortality rate without increasing secondary infections in patients with COVID-19 based on current studies. For patients with critical disease, IL-6 signaling inhibitors did not exhibit any benefit.
Keywords: COVID-19, anti-IL-6 signaling agents, mortality, secondary infections, mechanical ventilation, ICU admission, clinical improvement
Introduction
The COVID-19 pandemic has resulted in numerous deaths, and continues to circulate worldwide (World Health Organization, 2019). Effective treatment is still urgently needed. In addition to novel therapeutic regimes, repurposing available pharmaceuticals based on key pathological changes is extremely important for the treatment of COVID-19.
After invading the body, SARS-CoV-2 binds to angiotensin-converting enzyme 2 on cell membranes, enters cells by membrane infusion, completes self-replication, and triggers complex responses. Inflammatory responses induced in immune cells, and epithelial and endothelial cells were demonstrated to be crucial in triggering “cytokine storm” and lead to severe injury in the context of COVID-19 (Azkur et al., 2020). Cytokine storm was also proven to play an important role in severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (Liu et al., 2020a), characterized by robust amplification of proinflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and others (Liu et al., 2020b; Chen et al., 2020; Huang et al., 2020). Cytokine storm further leads to inflammatory filtration (e.g., exudative lung lesions), tissue destruction, and even multi-organ dysfunction. Cytokine storm is more destructive than the virus itself. The latest World Health Organization (WHO) guideline recommends systemic corticosteroids for the treatment of severe and critical COVID-19 patients (World Health Organization, 2020). Corticosteroids are powerful drugs used to fight cytokine storm, but its side effects should not be ignored, including secondary infections, hyperglycemia, peptic ulcer, sterile necrosis of the femoral head, and so on. Antibodies that can accurately combat cytokine storm might be a perspective choice.
IL-6 is thought to be a key mediator of cytokine storm, causing tissue injury and the progression of COVID-19. Levels of serum IL-6 and IL-6 receptors (IL-6R) were significantly elevated in patients with COVID-19 (Liu et al., 2020a), and were closely associated with respiratory failure, acute respiratory distress syndrome, secondary infections, and death (Somers et al., 2020). A meta-analysis of 21 studies involving 3,377 patients with COVID-19 supported the notion that IL-6 was a significant indicator for the severity of COVID-19 (Henry et al., 2020).
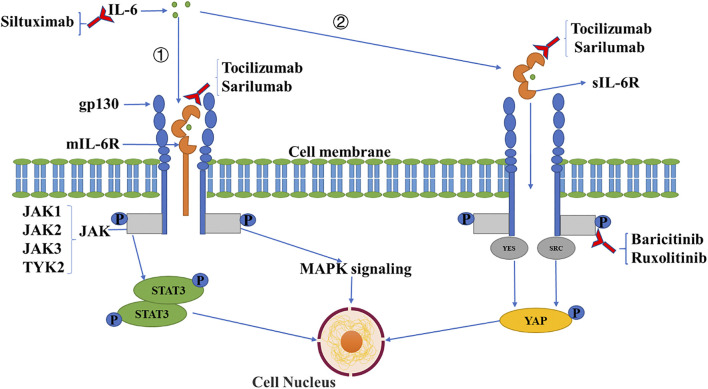
IL-6 functions via a signaling network to amplify inflammation and induce other damage. Under stimuli, IL-6 can be generated from various types of cells including monocytes, macrophages, T cells, B cells, epithelial and endothelial cells, and fibroblasts (Tanaka et al., 2014). There are two types of IL-6 receptors: membrane-bound IL-6 receptors (mIL-6R) and soluble IL-6 receptors (sIL-6R). Membrane-bound IL-6R is localized mainly in immune cells, while soluble IL-6R is produced through the cleavage of mIL-6R, which displays a biological effect in various ways (Kang et al., 2019; Tanaka et al., 2016; Moore & June, 2020). When IL-6 binds to mIL-6R or sIL-6R, the complex triggers the dimerization of transmembrane glycol protein 130 (gp130), followed by the activation of Janus kinases (JAK) that subsequently initiates intracellular signaling, including mitogen-activated protein kinases (MAPK) and signal transducers and activators of transcription (STAT) pathways (Heinrich et al., 2003). The activation of the MAPK cascade, including Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38, mediates the expression of multiple pro-inflammatory genes as transcriptional factors (Arthur and Ley, 2013). For example, JNK signaling upregulates the transcription of M1 macrophage-specific genes including CD86, iNOS, and IL-1β in bone marrow-derived macrophages. The ERK pathway triggers the transcription of cytokine genes such as IL-1β, IL-8, and TNF-α in macrophages and mesenchymal stem cells. p38 signaling controls the strength and duration of inflammatory responses by MAPK activated kinase 2. The activation of STAT3 is essential for the transformation of Th0 cells to Th17 cells, which can produce IL-17, IL-6, and TNF-α which promotes an inflammatory response (Luo et al., 2020a). (Figure 1). Previous studies demonstrated that IL-6, together with IL-1β and TNF-α, damage the cellular basement membrane and extracellular matrix, leading to high tissue permeability and edema via upregulating trypsin and activating matrix metalloproteinases (Indalao et al., 2017). Moreover, IL-6 was found to be related to the exhaustion of CD4+ and CD8+ T cells in patients with COVID-19, evidenced by a negative association of IL-6 with the numbers of T lymphocytes (Zhang et al., 2020).
FIGURE 1.
IL-6 signaling pathway and inhibitory agents. IL-6 binds to mIL-6R (①) or sIL-6R (②), which triggers the dimerization of transmembrane gp130, followed by the activation of JAK which induces intracellular signaling including MAPK and STAT signaling pathways, and the activation of the SRC family kinase (YES, SRC), with the YES-associated protein signaling pathway subsequently induced. Three types of antibodies have been applied clinically against IL-6 signaling including anti-IL-6 (siltuximab), anti-IL-6R (tocilizumab, sarilumab), and anti-JAK (baricitinib, ruxolitinib) monoclonal antibodies.
To date, three categories of antibodies have been developed against IL-6 signaling, including anti-IL-6, anti-IL-6R, and anti-JAK monoclonal antibodies. These are used to treat autoimmune diseases such as rheumatoid arthritis. Based on the crucial role of IL-6 in SARS-CoV-2-induced injury, investigation of the safety and efficacy of anti-IL-6 signaling agents is extremely important for patients with COVID-19.
In this meta-analysis and systemic review, we pooled data from all available studies with acceptable quality to assess the effects of anti-IL-6/IL-6R/JAK antibodies on COVID-19.
Materials and Methods
Literature Search
We identified studies involving antibodies that block IL-6 signaling for the treatment of patients confirmed to have COVID-19 using a systematic search on PubMed, EMBASE, ISI Web of Science, Cochrane library, ongoing clinical trial registries (clinicaltrials.gov), and preprint servers including medRxiv, ChinaXiv on August 10, 2020. The search algorithm combining relevant key words were as follows: “anti-interleukin 6 receptor OR anti-interleukin 6 OR anti-IL-6R OR anti-IL-6 OR anti-JAK OR tocilizumab OR sarilumab OR siltuximab OR sirukumab OR olokizumab OR clazakizumab OR sylvant OR EBI-031 OR actemra OR kevzara NI-1201 OR vobarilizumab OR olamkicept OR tofacitinib OR XELJANZ OR ruxolitinib OR jakavi OR filgotinib OR baricitinib OR olumiant OR upadacitinib OR Rinvoq OR PF-04965842” AND “COVID-19 OR coronavirus OR SARS-CoV-2 OR COVID”. We did not limit our search by language. The aim of our systematic review and meta-analysis was to assess clinical outcomes and adverse events in patients with COVID-19 treated with anti-IL-6/IL-6R/JAK signaling antibodies.
Inclusion Criteria
Eligibility was checked carefully in accordance with predefined selection criteria by reading titles, abstracts, and full manuscripts. We included studies that investigated the safety and efficacy of anti-IL-6/IL-6R/JAK antibodies on adult patients with confirmed SARS-CoV-2 infections. Controlled studies, including randomized controlled trials, cohort studies, and case-control studies, were incorporated in the meta-analysis. Uncontrolled studies were descriptively reviewed. We excluded case reports, letters without original data, reviews, systematic reviews, protocols, non-human studies, duplicates, and studies without complete data.
Data Extraction and Quality Assessment
Two independent investigators reviewed the full texts of relevant studies, extracting essential features of each study, including the name of the first author, type of study, performed country, the number of patients, and baseline characteristics of participants such as age, sex, comorbidities including hypertension, chronic kidney disease, diabetes, obesity, heart and lung diseases, and clinical outcomes. The primary endpoint of interest was the mortality rate in patients confirmed with COVID-19. Secondary outcomes included the requirement of mechanical ventilation, intensive care unit (ICU) admission, clinical improvement, and secondary infections. The methodological quality of case-control studies and cohort studies were assessed using the Newcastle-Ottawa Scale (NOS) by two independent investigators. Cochrane’s bias risk assessment tool was used to evaluate the quality of the randomized controlled trial. The MINORS index was used to evaluate the quality of single-arm research.
Data Synthesis and Analysis
We used the Mantel-Haenszel method for the analysis of categorical variables. Statistical heterogeneity among studies was measured using Cochrane Q statistics and heterogeneity index I 2. If the heterogeneity was high (I 2 > 50%), we used the random-effects model. Otherwise, the fixed-effects model was used for analysis. The subgroup analyses were implemented according to performed continents, the severity of COVID-19 and the type of anti-IL-6 signaling agent. Sensitivity analysis was carried out by excluding studies one by one and observing whether the heterogeneity changed. Publication bias was evaluated using funnel plot analysis, which was considered to indicate no statistical difference if p > 0.05 in Begg’s and Egger’s tests. Statistical analysis was performed using Review Manager (version 5.3) and STATA 12.0.
Definitions
Clinical improvement was defined as discharge from hospital, a decrease of at least two points from baseline on the seven-category ordinal scale, or both. The seven-category ordinal scale as recommended by the WHO R&D Blueprint Group (https://www.who.int/teams/blueprint/covid-19) is as follows: 1) not hospitalized and able to resume normal activities; 2) not hospitalized, but unable to resume normal activities; 3) hospitalized, not requiring supplemental oxygen; 4) hospitalized, requiring supplemental oxygen; 5) hospitalized, requiring nasal high-flow oxygen therapy, non-invasive ventilation, or both; 6) hospitalized, requiring extracorporeal membrane oxygenation, invasive mechanical ventilation, or both; and 7) death.
The severity of disease has no pre-defined and unified definition. We accepted the classification of severity in each included study. The definition of SOC was also according to every specific study.
Results
Search Results and Characteristics of Identified Studies
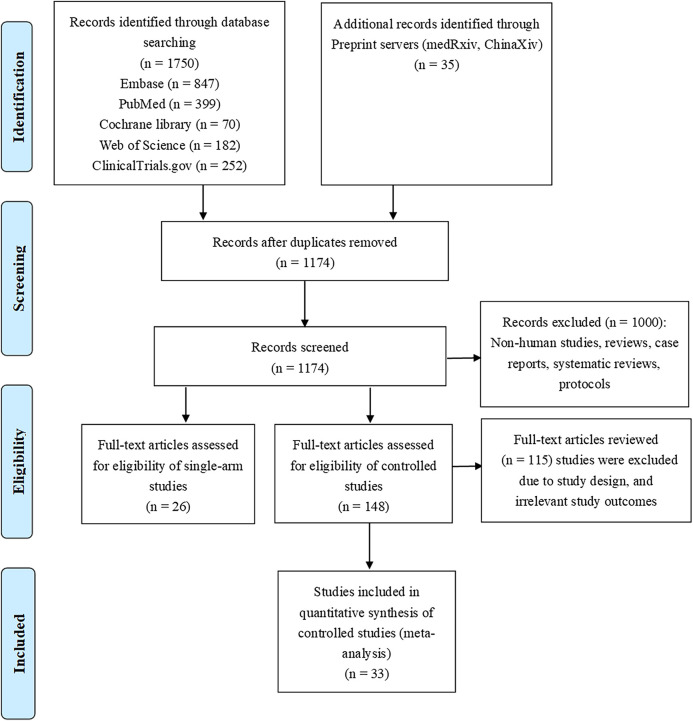
We identified 59 studies according to the selection criteria. We performed meta-analysis with one randomized controlled trial and 32 controlled studies. Others were single-arm studies, which we did not use in the production of conclusions, but only briefly described as part of the systematic review to introduce readers to the progress in this field. The flowchart of the literature search and screening process are listed in Figure 2.
FIGURE 2.
Flowchart of literature selection process.
The characteristics of the 33 controlled studies are shown in Table 1. Regarding types of anti-IL-6 signaling agents, 29 studies used anti-IL-6R antibodies (tocilizumab in 28 studies and sarilumab in one study). One study involved an anti-IL-6 antibody (siltuximab), and three studies involved anti-JAK-1/2 antibodies (baricitinib in two studies and ruxolitinib in one study).
TABLE 1.
Characteristics of the controlled studies included in the meta-analysis.
| Study | Study type | Setting | Country | Severity of participants | Study drug | Dosage | Sample size | Follow-up duration | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Study drug | SOC | |||||||||
| 1 | Cao et al. (Cao et al., 2020) | RCT | Multi-center | China | Severe | Ruxolitinib | 5 mg po bid | 20 | 21 | 28 days |
| 2 | Colaneri et al. (Colaneri et al., 2020) | Cohort study | Single-center | Italy | Severe | Tocilizumab | 8 mg/kg iv drip (max. 800 mg) | 21 | 91 | 7 days |
| 3 | Somers et al. (Somers et al., 2020) | Cohort study | Single-center | USA | Critical | Tocilizumab | 8 mg/kg iv drip (max. 800 mg) | 78 | 76 | Median 47 days (range 28–67) |
| 4 | Perrone et al. (Perrone et al., 2020) | Cohort study | Multi-center | Italy | All | Tocilizumab | 8 mg/kg iv drip (max. 800 mg) | 180 | 121 | 30 days |
| 5 | Mikulska et al. (Mikulska et al., 2020) | Case-control study | Single-center | Italy | Non-critical | Tocilizumab | 8 mg/kg iv drip or 162 mg ih | 85 | 66 | Median 49 days (range 4–70, IQR 30–56) |
| 6 | Cantini et al. (Cantini et al., 2020a) | Cohort study | Single-center | Italy | Moderate | Baricitinib | 4 mg po qd | 12 | 12 | 14 days |
| 7 | Della-Torre et al. (Della-Torre et al., 2020) | Cohort study | Single-center | Italy | Severe | Sarilumab | 400 mg iv drip | 28 | 28 | 28 days |
| 8 | Gritti et al. (Gritti et al., 2020) | Cohort study | Single-center | Italy | Severe or critical | Siltuximab | 11 mg/kg iv drip | 30 | 30 | 30 days |
| 9 | Guaraldi et al. (Guaraldi et al., 2020) | Cohort study | Multi-center | Italy | Severe | Tocilizumab | 8 mg/kg iv drip (max. 800 mg) or 162 mg ih | 179 | 365 | Median 9 days (IQR 4–15) |
| 10 | Quartuccio et al. (Quartuccio et al., 2020) | Case-control study | Single-center | Italy | Severe or critical | Tocilizumab | 8 mg/kg iv drip | 42 | 69 | 30 days |
| 11 | Campochiaro et al. (Campochiaro et al., 2020) | Cohort study | Single-center | Italy | Severe | Tocilizumab | Two dosage of 400 mg/24 h iv drip | 32 | 33 | 28 days |
| 12 | Capra et al. (Capra et al., 2020) | Cohort study | Single-center | Italy | Severe | Tocilizumab | 400 mg iv drip or 324 mg ih | 62 | 23 | Study drug median 9 days (IQR 5–19) vs. SOC 28 days |
| 13 | Potere et al. (Potere et al., 2020a) | Case-control study | Single-center | Italy | Severe | Tocilizumab | 324 mg ih | 40 | 40 | 35 days |
| 14 | Rojas-Marte et al. (Rojas-Marte et al., 2020) | Case-control study | Single-center | USA | All | Tocilizumab | 8 mg/kg iv drip | 96 | 97 | Mean (±SD), 15.3 ± 9.9 days |
| 15 | Garcia et al. (Moreno Garcia et al., 2020) | Cohort study | Single-center | Spain | Severe | Tocilizumab | 400 or 600 mg/24 h iv drip | 77 | 94 | Mean (±SD), 13.1 ± 9.0 days |
| 16 | Rossi et al. (Rossi et al., 2020) | Cohort study | Single-center | France | Severe | Tocilizumab | 400 mg iv drip | 106 | 140 | 28 days |
| 17 | Martinez-Sanz et al. (Martinez-Sanz et al., 2020) | Cohort study | Multi-center | Spain | Non-critical | Tocilizumab | Median dose of 600 mg iv drip | 260 | 969 | 30 days |
| 18 | Ip et al. (Ip et al., 2020) | Cohort study | Multi-center | USA | Critical | Tocilizumab | 96% received 400 mg iv drip | 134 | 413 | 30 days |
| 19 | Ramaswamy et al. (Ramaswamy et al., 2020) | Case-control study | Multi-center | USA | Severe | Tocilizumab | 400 mg iv drip or 8 mg/kg iv drip (max. 800 mg) | 21 | 65 | 30 days |
| 20 | Roumier et al. (Roumier et al., 2020) | Case-control study | Single-center | France | Severe | Tocilizumab | 8 mg/kg iv drip | 30 | 29 | Median 8 days (IQR 6.0–9.75) |
| 21 | Kimmig et al. (Kimmig et al., 2020) | Case-control study | Single-center | USA | Critical | Tocilizumab | 400 mg or 800 mg iv drip | 48 | 63 | ND |
| 22 | Narain et al. (Narain et al., 2020) | Cohort study | Multi-center | USA | ND | Tocilizumab | ND | 364 | 1,505 | 40 days |
| 23 | Wadud et al. (Wadud et al., 2020) | Case-control study | Single-center | USA | Critical | Tocilizumab | ND | 44 | 50 | Mean 17.9 days |
| 24 | Kewan et al. (Kewan et al., 2020) | Cohort study | Single-center | USA | Severe or critical | Tocilizumab | 8 mg/kg plus 400 mg iv drip | 28 | 23 | 21 days |
| 25 | Klopfenstein et al. (Klopfenstein et al., 2020) | Case-control study | Single-center | France | Severe | Tocilizumab | 1 or 2 doses | 20 | 25 | Mean, range, SD Study drug 13 (4–32)±7 days vs. SOC 17 (5–41)±12 days |
| 26 | Cantini et al. (Cantini et al., 2020b) | Cohort study | Multi-center | Italy | Moderate | Baricitinib | 4 mg po qd | 113 | 78 | 14 days |
| 27 | Moreno-Perez et al. (Moreno-Pérez et al., 2020) | Cohort study | Single-center | Spain | Severe | Tocilizumab | 600 mg, with a second or third dose (400 mg) | 77 | 159 | Median 83.0 days (IQR 78.0–86.5) |
| 28 | Tsai et al. (Tsai et al., 2020) | Cohort study | Single-center | USA | Severe | Tocilizumab | 400\600\800 mg iv drip | 66 | 66 | ND |
| 29 | Canziani et al. (Canziani et al., 2020) | Case-control study | Multi-center | Italy | Severe or critical | Tocilizumab | 8 mg/kg iv drip | 64 | 64 | 30 days |
| 30 | Potere et al. (Potere et al., 2020b) | Cohort study | Single-center | Italy | Moderate with hyperinflammation | Tocilizumab | 324 mg ih | 10 | 10 | 35 days |
| 31 | Gokhale et al. (Gokhale et al., 2020) | Cohort study | Single-center | India | Severe | Tocilizumab | 400 mg iv drip | 70 | 91 | Median 16 days (IQR 4.5–50) |
| 32 | Eimer et al. (Eimer et al., 2020) | Cohort study | Single-center | Sweden | Critical | Tocilizumab | 8 mg/kg iv drip | 29 | 58 | 30 days |
| 33 | Patel et al. (Patel et al., 2020a) | Cohort study | Single-center | USA | Severe or critical | Tocilizumab | ND | 42 | 41 | 7 days |
RCT, randomized controlled trial; SOC, standard of care; IQR, interquartile range; SD, standard deviation; Max., maximum; ND, no data; iv drip, intravenous drip; po, oral intake; ih, subcutaneous injection; qd, once a day; bid, twice a day.
For the countries where the studies were implemented, European countries were involved in 21 studies (Italy 14, France three, Spain three, and Sweden one), the US conducted ten studies, and Asian countries conducted two studies (China one and India one).
Outcomes
Mortality
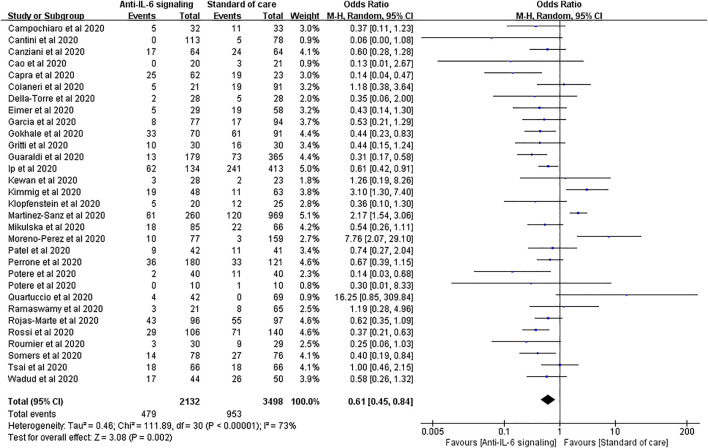
Thirty-one studies reported mortality as an outcome, involving 2,132 treated patients and 3,498 controls. The results of pooled analysis showed that anti-IL-6 signaling agents plus SOC significantly decreased the mortality rate relative to SOC alone in patients with COVID-19 (pooled OR = 0.61, 95% CI 0.45–0.84, p = 0.002). However, the heterogeneity of these studies was high (I 2 = 73%, p < 0.00001) (Figure 3).
FIGURE 3.
Pooled odds ratio and forest plot of mortality between the anti-IL-6 signaling treatment and standard of care (SOC) groups among patients with COVID-19. Thirty-one studies including 5,630 patients with COVID-19 were included in the statistical analysis, with 2,132 treated patients and 3,498 controls. The result showed that anti-IL-6 signaling agents plus SOC significantly decreased mortality relative to SOC alone in patients with COVID-19 (pooled OR = 0.61, 95% CI 0.45–0.84, p = 0.002).
To reduce the high heterogeneity, we performed sensitivity analysis and found that the study by Martinez-Sanz et al. had a substantial effect (Supplementary Figure S1). When this study was excluded, statistically significant decreases in mortality were still observed in the treated group compared with the SOC group, with lower heterogeneity (pooled OR = 0.57, 95% CI 0.44–0.74, p < 0.0001; I 2 = 54%, p = 0.0002).
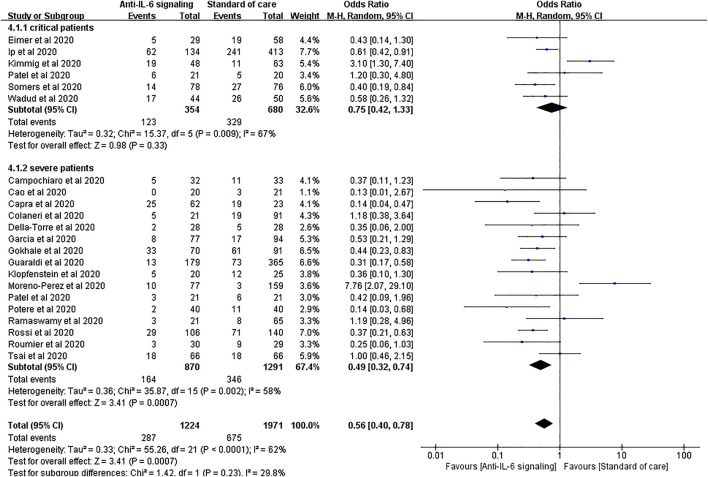
We performed a subgroup analysis according to the severity of COVID-19 by sorting severe and critical patients. For patients with severe COVID-19, anti-IL-6 signaling agents displayed remarkable advantages in reducing mortality compared to SOC, consistent with the result above (pooled OR = 0.49, 95% CI 0.32–0.74, p = 0.0007). Notably, for patients with critical disease, anti-IL-6 signaling agents failed to reduce the mortality rate compared to SOC (pooled OR = 0.75, 95% CI 0.42–1.33, p = 0.33) (Figure 4).
FIGURE 4.
Subgroup analysis according to severity of patients with COVID-19 for pooled odds ratio of mortality. Subgroup analysis compared the risk of mortality in groups of severe or critical patients with COVID-19 separately. Six studies were included in the subgroup of critical patients, including 354 treated patients and 680 controls. There was no statistical difference in the risk of mortality between the two groups of critical patients (pooled OR = 0.75, 95% CI 0.42–1.33, p = 0.33). Sixteen studies were included in the subgroup of severe patients, including 870 treated patients and 1,291 controls. The mortality rate in the anti-IL-6 signaling treatment group was remarkably reduced (pooled OR = 0.49, 95% CI 0.32–0.74, p = 0.0007) compared to the SOC group in patients with severe disease.
According to the types of anti-IL-6 signaling drugs (i.e., IL-6 neutralizing, IL-6 receptor blockers, and JAK inhibitors), we did a subgroup analysis showing that both IL-6 receptor blockers and JAK inhibitors showed superiority in reducing death rate compared to SOC (OR = 0.64, 95% CI 0.47–0.89, p = 0.007; OR = 0.09, 95% CI 0.01–0.70, p = 0.02, respectively), while IL-6 neutralizing drug tended to reduce deaths but did not reach a significant difference (OR = 0.44, 95% CI 0.15–1.24, p = 0.12) (Supplementary Figure S2).
Subgroup analysis according to various continents where these studies were implemented showed that studies in American patients did not support the effectiveness of anti-IL-6 signaling agents on mortality from COVID-19 (Supplementary Figure S3).
Secondary Infections
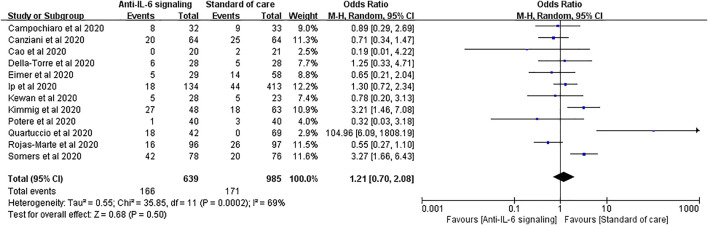
Secondary infections are among the most reported adverse effects of anti-IL-6 signaling agents. Twelve studies reported secondary infections, involving 639 treated patients and 985 controls. No significant difference in the rate of secondary infections was found between the two groups (pooled OR = 1.21, 95% CI 0.70–2.08, p = 0.50) (Figure 5).
FIGURE 5.
Pooled odds ratio and forest plot of secondary infections between the anti-IL-6 signaling treatment group and standard of care group in patients with COVID-19. Twelve studies were included in the statistical analysis on secondary infections, including 639 patients treated with IL-6 signaling inhibitors and 985 patients with SOC. There was no significant difference in terms of secondary infection rates between patients with COVID-19 treated with anti-IL-6 signaling agents and those treated with SOC (pooled OR = 1.21, 95% CI 0.70–2.08, p = 0.50).
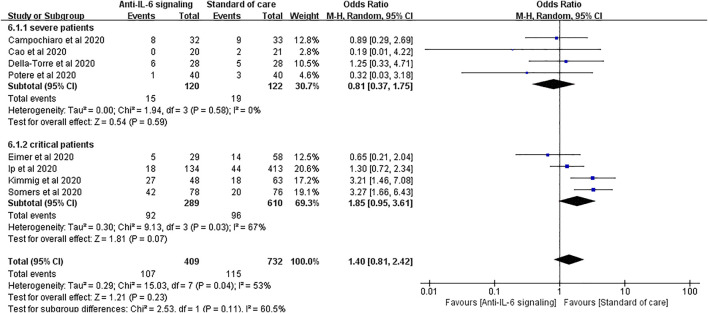
We performed a further subgroup analysis according to the severity of disease. For patients with severe disease, the risk of secondary infections did not substantially increase with anti-IL-6 signaling treatment compared to SOC alone (pooled OR = 0.81, 95% CI 0.37–1.75, p = 0.59). Again, it is noteworthy that the rate of secondary infections tended to increase in the group treated with anti-IL-6 signaling agents compared to the SOC group in patients with critical disease (pooled OR = 1.85, 95% CI 0.95–3.61, p = 0.07), despite not reaching statistical difference (Figure 6).
FIGURE 6.
Subgroup analysis according to severity of patients with COVID-19 for pooled odds ratio of secondary infections. Four studies were included in the subgroup of patients with severe disease, including 120 patients treated with IL-6 signaling inhibitors and 122 patients treated with SOC. There was no significant difference in terms of risk of infection between groups (pooled OR = 0.81, 95% CI 0.37–1.75, p = 0.59). Four studies were included in the subgroup of patients with critical disease, including 289 patients treated with IL-6 signaling inhibitors and 610 patients treated with SOC. The risk of secondary infections tended to be greater in the subgroup of patients with critical disease treated with anti-IL-6 signaling agents than in those treated with SOC (pooled OR = 1.85, 95% CI 0.95–3.61, p = 0.07), though it did not reach statistical significance.
Mechanical Ventilation, ICU Admission, and Clinical Improvement Rates
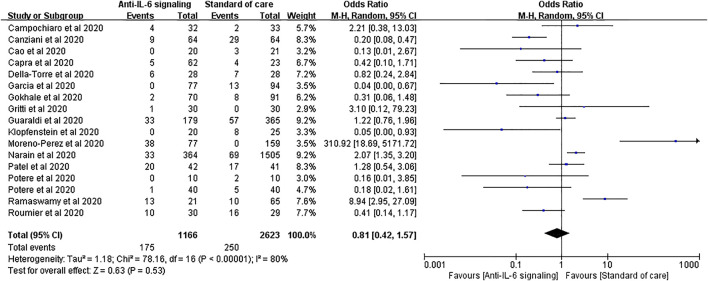
Seventeen studies applied mechanical ventilation rate as an outcome, with 1,166 treated cases and 2,623 controls. The pooled analysis showed that anti-IL-6 signaling agents did not reduce the rate of mechanical ventilation relative to SOC for patients with severe disease (pooled OR = 0.81, 95% CI 0.42–1.57, p = 0.53) (Figure 7).
FIGURE 7.
Pooled odds ratio and forest plot of mechanical ventilation rate between the anti-IL-6 signaling treatment group and the standard of care group among patients with COVID-19. Seventeen studies were included in the statistical analysis of the mechanical ventilation rate including 1,166 patients treated with IL-6 signaling inhibitors and 2,623 patients treated with SOC. Anti-IL-6 signaling treatment did not have a beneficial effect on the mechanical ventilation rate relative to SOC (pooled OR = 0.81, 95% CI 0.42–1.57, p = 0.53).
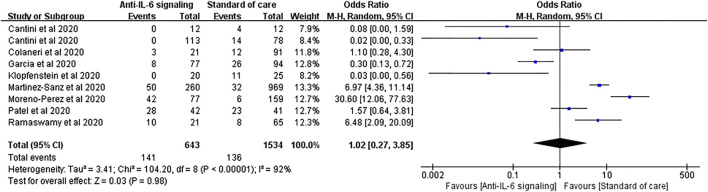
Similar results were found in the outcomes of ICU admission. Nine studies with 643 treated patients and 1,534 controls were pooled for analysis, showing no statistical difference between the two groups (pooled OR = 1.02, 95% CI 0.27–3.85, p = 0.98) (Figure 8). However, in patients treated with JAK inhibitors, the ICU admission rate was significantly reduced compared to SOC (OR = 0.04, 95% CI 0.00–0.29, p = 0.002) (Supplementary Figure S2).
FIGURE 8.
Pooled odds ratio and forest plot of ICU admission rate between the anti-IL-6 signaling treatment group and the standard of care group among patients with COVID-19. Nine studies were included in the statistical analysis with 643 patients treated with IL-6 signaling inhibitors and 1,534 patients with SOC. Anti-IL-6 signaling treatment did not show a beneficial effect on the ICU admission rate compared to SOC in patients with COVID-19 (pooled OR = 1.02, 95% CI 0.27–3.85, p = 0.98).
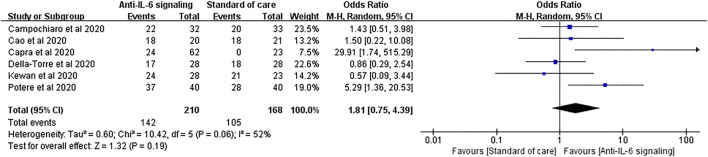
For the clinical improvement rate, pooled analysis of six studies involving 210 treated patients and 168 controls showed that 67.6% and 62.5% patients in the anti-IL-6 signaling and the SOC groups, respectively, displayed clinical improvement (pooled OR = 1.81, 95% CI 0.75–4.39, p = 0.19) (Figure 9).
FIGURE 9.
Pooled odds ratio and forest plot of clinical improvement rate between the anti-IL-6 signaling treatment group and the standard of care group among patients with COVID-19. Six studies were included in the analysis of clinical improvement, including 210 patients treated with IL-6 signaling inhibitors and 168 patients treated with SOC. Anti-IL-6 signaling treatment did not show any beneficial effects on the clinical improvement rate compared to SOC in patients with COVID-19 (pooled OR = 1.81, 95% CI 0.75–4.39, p = 0.19).
Quality Assessment
Randomized and non-randomized controlled trials were assessed using Cochrane’s bias risk assessment tool and NOS, respectively (Supplementary Table S1). We found that all the controlled studies were of satisfactory high quality for meta-analysis.
Publication Bias
We utilized the funnel plot to analyze publication bias in the 31 studies included in the pooled analysis of mortality. The dots symbolizing the studies mostly clustered in the middle and at the top of the funnel plot, with both sides nearly symmetrical (Figure 10). Only eight articles presented outside the funnel plot. Begg’s and Egger’s tests further demonstrated no significant publication bias (Begg’s test, p = 0.892; Egger’s test, p = 0.158).
FIGURE 10.
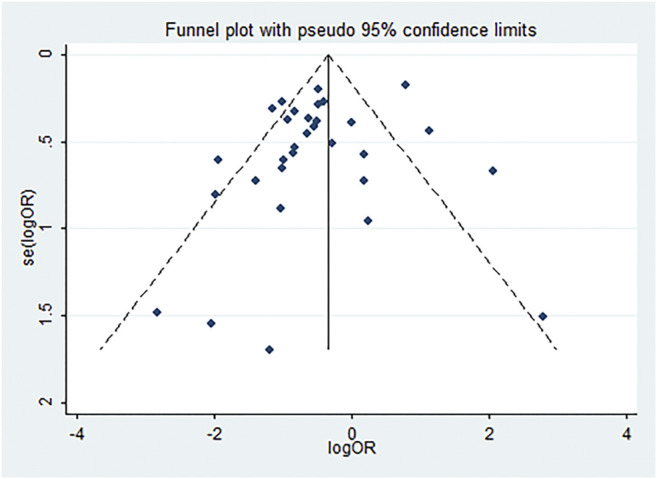
Funnel plot analysis of publication bias. Publication bias of the 31 studies. Dots symbolizing studies mostly clustered in the middle and at the top of the funnel plot; both sides are nearly symmetrical, and only eight articles are outside the funnel plot.
A Brief Review of Single-Arm Studies
We reviewed 26 single-arm studies including 1,565 patients. Characteristics of the studies are shown in Supplementary Table S2. Two individual researchers performed quality evaluation with the MINORS index shown in Supplementary Table S1.
In these single-arm studies, mortality ranged from 0 to 43% in patients with COVID-19 treated with anti-IL-6 signaling agents, and the raw overall mortality was 15.27%, which was significantly lower than the pooled mortality of the SOC group (15.27 vs. 27.24%, p < 0.0001) and also the anti-IL-6 signaling group (15.27 vs. 22.47%, p < 0.0001) in the meta-analysis. These differences may be partially derived from the different baseline characteristics of patients with COVID-19. Six single-arm studies reported secondary bacterial infections, with an overall rate of 14.35%, which was also remarkably lower than that of the SOC group (14.35 vs. 17.36%, p < 0.0001) and the anti-IL-6 signaling group (14.35 vs. 25.98%, p < 0.0001) in the meta-analysis. Four single-arm studies reported increased hepatic enzyme levels, and other studies reported increased creatinine, thrombocytopenia, or neutropenia.
The largest single-arm study was reported by Sinha et al. with 255 patients with COVID-19 enrolled; the overall mortality was 10.98%. Patients with COVID-19 were divided into two groups according to the fraction of inspiration O2 (FiO2 < 45% or >45%) at the time of administration of IL-6R antibody treatment (sarilumab or tocilizumab). The mortality rate was significantly lower in the FiO2 < 45% group compared to the FiO2 > 45% group (adjusted hazard ration 0.24, 95% CI 0.08–0.74). Similarly, patients in the FiO2 < 45% group had a higher discharge rate and a lower intubation rate. This suggests that the early use of IL-6R antibodies in less severe patients was more beneficial.
Discussion
In this meta-analysis and systematic review, we analyzed 33 controlled studies, including 31 studies for pooled analysis of mortality (5,630 patients with COVID-19), 12 studies for pooled analysis of secondary infections (1,624 patients with COVID-19), and appropriate studies for the assessment of mechanical ventilation, ICU admission, and clinical improvement. Our results showed that a blockade of IL-6 signaling by anti-IL-6/IL-6R/JAK antibodies plus SOC significantly reduced the mortality rate but did not increase the risk of secondary infections compared to SOC alone in patients with COVID-19. By contrast, in patients with critical disease, the beneficial effect of anti-IL-6/IL-6R/JAK antibodies on mortality became indistinct, and resulted in an evident trend of increased secondary infection rates. Blockade of IL-6 signaling did not show a beneficial effect on reducing the mechanical ventilation rate, decreasing ICU admission, or increasing clinical improvement.
IL-6 plays a key role in immune cell proliferation and differentiation through the IL-6/IL-6R/JAK pathway. To date, clinically available antibodies targeting the IL-6/IL-6R/JAK pathway include anti-IL-6 antibodies (e.g., siltuximab), anti-IL-6R antibodies (e.g., tocilizumab and sarilumab), and anti-JAK antibodies (e.g., ruxolitinib and baricitinib), all of which have been successfully used to treat autoimmune diseases such as rheumatoid arthritis. Tocilizumab and sarilumab are both humanized anti-IL-6 receptor monoclonal antibodies, capable of binding to either mIL-6R or sIL-6R. Tocilizumab has been approved in several countries since 2005 and is currently used to treat rheumatoid arthritis, giant cell arteritis, and juvenile idiopathic arthritis (Tanaka et al., 2014). Sarilumab was approved in the USA for the treatment of refractory rheumatoid arthritis in 2017 (Lamb and Deeks, 2018). Siltuximab is an anti-IL-6 immunoglobulin G-k monoclonal antibody that specifically binds to IL-6 and neutralizes its biological activity; it was approved in the USA in 2014 with restriction to the treatment of Castleman disease (Lyseng-Williamson, 2015). Baricitinib and ruxolitinib, both targeting JAK1 and JAK2 which are the dominant kinases downstream of IL-6, have been approved for the treatment of myelofibrosis and rheumatoid arthritis, respectively (Garbers et al., 2018).
Mortality was set as the primary outcome in this meta-analysis. Except for two studies that reported no specific data concerning death rate, 31 controlled studies were involved in the pooled analysis of mortality, including 5,630 patients with COVID-19. We found that anti-IL-6 signaling agents significantly decreased mortality in patients with COVID-19. There was high heterogeneity (I 2 = 73%, p < 0.00001), partially due to varying study designs, patient severity, agent dosages, and follow-up durations. In the sensitivity analysis, removal of the largest study (Martinez-Sanz et al.) generated lower heterogeneity with no substantial alteration of the pooled OR.
Strikingly, we found that blockade of IL-6 signaling failed to effectively reduce mortality in patients with critical disease. Previous studies reported that serum IL-6 levels were elevated in patients with critical disease in comparison with severe patients in the early stages of SARS-CoV-2 infection (Cummings et al., 2020; Han et al., 2020). One study illustrated the predictive ability of IL-6 for prognosis in patients with COVID-19 (Han et al., 2020). Therefore, we speculated that IL-6 in critical patients was not sufficiently neutralized. On the other hand, more complex pathophysiological changes may occur in the critical stage that limit the therapeutic effect of anti-IL-6 signaling agents. This supports the early application of IL-6 blockade treatment for patients with COVID-19.
Of note, Marfella et al. found that patients with COVID-19 who had hyperglycemia at admission had five-fold higher plasma IL-6 levels than non-hyperglycemia cases, and hyperglycemia diminished the effect of tocilizumab on disease progression, possibly through the upregulation of IL-6 (Marfella et al., 2020; Sardu et al., 2020a; Sardu et al., 2020b). Sardu et al. discovered that an early glucose control within the first 24 hours significantly slowed COVID-19 progression and improved survival (Sardu et al., 2020c). In our hands, critical patients have a higher rate of previously diagnosed diabetes than severe patients (311/940, 33.09% vs. 429/1971, 21.77%, p < 0.0001), and in severe patients who received tocilizumab treatment, diabetic patients tended to have higher mortality compared to non-diabetic patients, but without statistical significance (OR = 1.64, 95% CI 0.81–3.32, p = 0.17) (Supplementary Figure S4). Therefore, hyperglycemia may contribute to the unsatisfactory effect of tocilizumab on patients with critical disease, and early glucose management could be a perspective strategy to improve the effect of tocilizumab on critical cases. It was also shown that patients with critical COVID-19 had higher morbidity of hypertension than severe cases (570/940, 60.64% vs. 794/1971, 40.28%, p < 0.0001), and in severe patients who received tocilizumab treatment, hypertensive patients with COVID-19 tended to have a high death rate compared to non-hypertensive patients (OR = 1.85, 95% CI 0.93–3.67, p = 0.08) (Supplementary Figure S5). However, whether hypertension has a negative effect on tocilizumab function still needs to be confirmed (Sardu et al., 2020d). Moreover, ABO blood types might be associated with outcomes in patients with critical disease. As reported by Sardu et al., non-O blood types in hypertensive patients were closely related to pro-thrombotic status, higher rate of cardiac injury, and deaths compared to O blood type patients with COVID-19 (Sardu et al., 2020e). Finally, how to achieve the optimal effect of anti-IL-6 signaling agents in patients with critical COVID-19 is still worth further study.
Subgroup analysis also revealed that studies in the USA did not support the effectiveness of anti-IL-6 signaling agents on mortality in patients with COVID-19, which was different from the pooled results of the European studies or Asian studies. This may result from disparate patient severities, diverse SOC regimens, and varying study methods.
The issue of the secondary infection rate was prioritized in this meta-analysis. IL-6/IL-6R/JAK blockade therapeutics were reported to increase the risk of secondary bacterial infection (Rose-John et al., 2017). Both anti-IL-6R antibodies tocilizumab and sarilumab received black box warnings regarding the risks of serious infections for patients with pulmonary diseases, issued by the U.S. Food and Drug Administration (Food and Drug Administration, 2010; Food and Drug Administration, 2018). Studies indicated that approximately 8% of rheumatoid arthritis patients treated with tocilizumab had serious infections (Smolen et al., 2007; Campbell et al., 2011). Thus, whether the off-label use of anti-IL-6 signaling agents for COVID-19 could increase secondary infections is indeed a matter of concern. We analyzed 12 studies that collected data about secondary infections, and found no significant difference between anti-IL-6 signaling agents and SOC.
However, in a subgroup analysis in patients with severe and critical disease, we found a substantial tendency toward an increased rate of secondary infections owing to anti-IL-6 signaling treatment in the critical group. This might be interpreted as immunosuppression status giving a higher probability of occurrence and higher severity in patients with critical disease. This gives us a hint that, for patients with critical disease, treatment with IL-6 inhibitors needs to be considered in the context of immune status.
Our study has several limitations. One limitation is that only one randomized controlled study was included, because the implementation of this type of study is confronted with many obstacles in the pandemic situation. The second limitation is the high heterogeneity in some results. Non-randomized methods, diverse severities, different dosages and routes of administration of anti-IL-6 signaling agents, and various regimens of SOC (e.g., anti-viral agents and corticosteroids) may contribute to the heterogeneity of the studies. Third, baseline characteristics such as age, gender, hypertension, and obesity of the two groups of patients did not match (Table 2). Therefore, randomized trials with relatively large sample sizes and rational designs are still needed. Moreover, due to the absence of data, our study did not consider or analyze the baseline levels of IL-6; therefore, we could not illustrate the specific function of IL-6/IL-6R/JAK pathway antibodies on patients with COVID-19 with different baseline IL-6 levels.
TABLE 2.
Baseline characteristics of patients with COVID-19 included in the meta-analysis.
| Baseline characteristics | Anti-IL-6 signaling group (N = 2,508) | SOC group (N = 5,015) | p value |
|---|---|---|---|
| Age, mean (±SD) (9 studies) | 61.78 ± 13.62 | 64.10 ± 16.13 | 0.0048 |
| Male, N (%) (32 studies) | 1809/2,488 (72.71%) | 3,121/4,990 (62.55%) | <0.0001 |
| Comorbidities, N (%) | |||
| Diabetes (29 studies) | 559/2,273 (24.59%) | 1,255/4,766 (26.33%) | 0.1258 |
| Hypertension (30 studies) | 848/2015 (42.08%) | 1,269/3,394 (37.39%) | 0.0007 |
| Heart diseases (10 studies) | 119/709 (16.78%) | 216/1,069 (20.21%) | 0.0727 |
| Coronary artery disease (15 studies) | 144/1,254 (11.48%) | 344/3,367 (10.22%) | 0.2333 |
| Obesity (7 studies) | 76/268 (28.36%) | 170/433 (39.26%) | 0.0034 |
| Lung diseases (6 studies) | 83/579 (14.34%) | 166/1,405 (11.81%) | 0.1359 |
| COPD (14 studies) | 95/1,008 (9.42%) | 189/2,452 (7.71%) | 0.1089 |
| Stroke (3 studies) | 9/165 (5.45%) | 15/225 (6.67%) | 0.6751 |
| Smoking (14 studies) | 196/1,000 (19.60%) | 440/2,545 (17.29%) | 0.1175 |
| CKD (13 studies) | 107/1,292 (8.28%) | 364/3,681 (9.89%) | 0.1006 |
N, number; SD, standard deviation; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
Despite these limitations, our review and meta-analysis summarized the available studies to some extent, and provided some basis for clinical practice and further large-scale studies. The forthcoming results of a phase III randomized controlled study sponsored by Hoffmann-La Roche (COVACTA, NCT04320615) may provide more valid clinical evidence and understanding of anti-IL-6 signaling agent usage in patients with COVID-19.
Conclusion
Under conditions where no specific medication proves to be efficacious, anti-IL-6 signaling agents are promising for the treatment of COVID-19. However, for patients with critical disease, the risk-benefit ratio of IL-6 signaling blockade agents should be carefully evaluated. Randomized controlled trials, trials on patients with critical disease, and studies on the effect of a blockade of IL-6 signaling on laboratory parameters remain in urgent need.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
QH and MG contributed to the literature research and data extraction. QH and YuZ contributed to the quality evaluation. YiZ, CX, and LZ helped with the literature search. RS, YD, YiL, and YaL helped with data extraction. FX provided valuable advice for the methods and manuscript writing. QH and YuZ wrote the first version of the manuscript. JP and YC contributed to the study design and manuscript revision.
Funding
This study was supported by the National Key R&D Program of China (2020YFC0846600), the Shandong Provincial Key R&D Program (2020SFXGFY03), the National Natural Science Foundation of China (81701952), the Taishan Pandeng Scholar Program of Shandong Province (tspd20181220), the Taishan Young Scholar Program of Shandong Province (tsqn20161065, tsqn201812129), and the Qilu Young Scholar Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All authors would like to pay tribute to all healthcare workers for their arduous efforts to combat the COVID-19 pandemic.
Glossary
Abbreviations: COVID-19, coronavirus disease 2019; ERK, extracellular signal-regulated kinase; FiO2, fraction of inspired oxygen; gp130, glycol protein 130; ICU, intensive care unit; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-6R, IL-6 receptor; JAK, Janus kinases; JNK, Jun n-terminal kinase; MAPK, mitogen-activated protein kinases; mIL-6R, membrane-bound IL-6 receptor; NOS, Newcastle-Ottawa scale; SaO2, oxygen saturation; SARS, severe acute respiratory syndrome; sIL-6R, soluble IL-6 receptor; SOC, standard of care; STAT, signal transducer and activator of transcription; TNF-α, tumor necrosis factor-alpha; WHO, World Health Organization.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.615972/full#supplementary-material.
References
- Alattar R., Ibrahim T. B. H., Shaar S. H., Abdalla S., Shukri K., Daghfal J. N., et al. (2020). Tocilizumab for the treatment of severe COVID-19. J. Med. Virol. 92, 2042–2049. 10.1002/jmv.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony S. J., Davis M. A., Davis M. G., Almaghlouth N. K., Guevara R., Omar F., et al. (2020). Early use of tocilizumab in the prevention of adult respiratory failure in SARS-CoV-2 infections and the utilization of interleukin-6 levels in the management. J. Med. Virol. [Epub ahead of print]. 10.1002/jmv.26288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J. S., Ley S. C. (2013). Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13 (9), 679–692. 10.1038/nri3495 [DOI] [PubMed] [Google Scholar]
- Azkur A. K., Akdis M., Azkur D., Sokolowska M., Van De Veen W., Bruggen M. C., et al. (2020). Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 75 (7), 1564–1581. 10.1111/all.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borku Uysal B., Ikitimur H., Yavuzer S., Ikitimur B., Uysal H., Islamoglu M. S., et al. (2020). Tocilizumab challenge: a series of cytokine storm therapy experiences in hospitalized COVID-19 pneumonia patients. J. Med. Virol. [Epub ahead of print]. 10.1002/jmv.26111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L., Chen C., Bhagat S. S., Parker R. A., Ostor A. J. (2011). Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology 50 (3), 552–562. 10.1093/rheumatology/keq343 [DOI] [PubMed] [Google Scholar]
- Campins L., Boixeda R., Perez-Cordon L., Aranega R., Lopera C. (2020). Early tocilizumab treatment could improve survival among COVID-19 patients. Clin. Exp. Rheumatol. 38 (3), 578 [Epub ahead of print]. [PubMed] [Google Scholar]
- Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., et al. (2020). Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 76, 43–49. 10.1016/j.ejim.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. (2020a). Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J. Infect. 81 (2), 318–356. 10.1016/j.jinf.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F., Niccoli L., Nannini C., Matarrese D., Natale M. E. D., Lotti P., et al. (2020b). Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 81, 647–679. 10.1016/j.jinf.2020.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canziani L. M., Trovati S., Brunetta E., Testa A., De Santis M., Bombardieri E., et al. (2020). Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients. J. Autoimmun. 114, 102511 10.1016/j.jaut.2020.102511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., et al. (2020). Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 146 (1), 137–146. 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra R., De Rossi N., Mattioli F., Romanelli G., Scarpazza C., Sormani M. P., et al. (2020). Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. 76, 31–35. 10.1016/j.ejim.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130 (5), 2620–2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaneri M., Bogliolo L., Valsecchi P., Sacchi P., Zuccaro V., Brandolino F., et al. (2020). Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms 8 (5), 695 10.3390/microorganisms8050695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings M. J., Baldwin M. R., Abrams D., Jacobson S. D., Meyer B. J., Balough E. M., et al. (2020). Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 395 (10239), 1763–1770. 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Torre E., Campochiaro C., Cavalli G., De Luca G., Napolitano A., La Marca S., et al. (2020). Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann. Rheum. Dis. 79, 218122 10.1136/annrheumdis-2020-218122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer J., Vesterbacka J., Svensson A. K., Stojanovic B., Wagrell C., Sönnerborg A., et al. (2020). Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: a retrospective cohort study. J. Intern. Med. [Epub ahead of print]. 10.1111/joim.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz M., López-Medrano F., Pérez-Jacoiste Asín M. A., Maestro De La Calle G., Bueno H., Caro-Teller J. M., et al. (2020). Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: a single-center cohort study. J. Med. Virol. [Epub ahead of print]. 10.1002/jmv.26308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration, and KEVZARA (KEV-za-ra) (2018). (Sarilumab) injection, for intravenous or subcutaneous use Initial U.S. Approval:2010. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761037s001lbl.pdf#page=27 (Accessed April 2018).
- Food and Drug Administration (2010). ACTEMRA (tocilizumab) injection, for intravenous or subcutaneous use Initial U.S. Approval. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125276s129125472s042lbl.pdf#page=46 (Accessed May 2020).
- Formina D. S., Lysenko M. Y. A., Beloglazova I. P., Mutinova Z. Y., Poteshkina N. G., Samsonova I. V., et al. (2020). Temporal clinical and laboratory response to interleukin-6 receptor blockade with Tocilizumab in 89 hospitalized patients with COVID-19 pneumonia. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.06.12.20122374 (Accessed June 12, 2020). [DOI] [PMC free article] [PubMed]
- Garbers C., Heink S., Korn T., Rose-John S. (2018). Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 17 (6), 395–412. 10.1038/nrd.2018.45 [DOI] [PubMed] [Google Scholar]
- Gokhale Y., Mehta R., Karnik N., Kulkarni U., Gokhale S. (2020). Tocilizumab improves survival in patients with persistent hypoxia in severe COVID-19 pneumonia. EClinicalMedicine 24, 100467 10.1016/j.eclinm.2020.100467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolas M., Cabello A., Prieto Perez L., Villar Alvarez F., Alvarez Alvarez B., Rodriguez Nieto M. J., et al. (2020). Compassionate Use of Tocilizumab in Severe SARS-CoV2 Pneumonia. When late administration is too late. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.06.13.20130088 (Accessed June 16, 2020). [DOI] [PMC free article] [PubMed]
- Gritti G., Raimondi F., Ripamonti D., Riva I., Landi F., Alborghetti L., et al. (2020). IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.04.01.20048561 (Accessed June 20, 2020). [DOI]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., et al. (2020). Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2 (8), e474–e484. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., et al. (2020). Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 9 (1), 1123–1130. 10.1080/22221751.2020.1770129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun A., Thottacherry E. D., Muklewicz J., Aziz Q. U., Edwards J. (2020). Utilizing tocilizumab for the treatment of cytokine release syndrome in COVID-19. J. Clin. Virol. 128, 104443 10.1016/j.jcv.2020.104443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Behrmann I., Haan S., Hermanns H. M., M¨Uller-Newen G., Schaper F. (2003). Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–200. 10.1042/BJ20030407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B. M., De Oliveira M. H. S., Benoit S., Plebani M., Lippi G. (2020). Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 58 (7), 1021–1028. 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indalao I. L., Sawabuchi T., Takahashi E., Kido H. (2017). IL-1β is a key cytokine that induces trypsin upregulation in the influenza virus-cytokine-trypsin cycle. Arch. Virol. 162 (1), 201–211. 10.1007/s00705-016-3093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip A., Berry D. A., Hansen E., Goy A. H., Pecora A. L., Sinclaire B. A., et al. (2020). Hydroxychloroquine and tocilizumab therapy in COVID-19 patients - an observational study. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.05.21.20109207 (Accessed May 25, 2020). [DOI] [PMC free article] [PubMed]
- Issa N., Dumery M., Guisset O., Mourissoux G., Bonnet F., Camou F. (2020). Feasibility of tocilizumab in ICU patients with COVID-19. J. Med. Virol. [Epub ahead of print]. 10.1002/jmv.26110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Brítez G., Ruiz P., Soler X. (2020). Tocilizumab plus glucocorticoids in severe and critically COVID-19 patients. A single center experience. Med. Clin. 155, 410–411. 10.1016/j.medcli.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S. C., Zakowski P., Tran H. P., Smith E. A., Gaultier C., Marks G., et al. (2020). Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clin. Infect. Dis. [Epub ahead of print]. 10.1093/cid/ciaa812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Tanaka T., Narazaki M., Kishimoto T. (2019). Targeting interleukin-6 signaling in clinic. Immunity 50 (4), 1007–1023. 10.1016/j.immuni.2019.03.026 [DOI] [PubMed] [Google Scholar]
- Keske Ş., Tekin S., Sait B., İrkören P., Kapmaz M., Çimen C., et al. (2020). Appropriate use of tocilizumab in COVID-19 infection. Int. J. Infect. Dis. 99, 338–343. 10.1016/j.ijid.2020.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kewan T., Covut F., Al-Jaghbeer M. J., Rose L., Gopalakrishna K. V., Akbik B. (2020). Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine 24, 100418 10.1016/j.eclinm.2020.100418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig L. M., Wu D., Gold M., Pettit N. N., Pitrak D., Mueller J., et al. (2020). IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.05.15.20103531 (Accessed September 12, 2020). [DOI] [PMC free article] [PubMed]
- Klopfenstein T., Zayet S., Lohse A., Balblanc J. C., Badie J., Royer P. Y., et al. (2020). Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med. Maladies Infect. 50 (5), 397–400. 10.1016/j.medmal.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr J. P., Colomy V., Mauriello C. M., Ha S. (2020). Tocilizumab in patients with severe COVID-19: a single-center observational analysis. J. Med. Virol. 92, 2813–2820. 10.1002/jmv.26191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosée F., Bremer H. C., Gehrke I., Kehr A., Hochhaus A., Birndt S., et al. (2020). The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia 34 (7), 1805–1815. 10.1038/s41375-020-0891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb Y. N., Deeks E. D. (2018). Sarilumab: a review in moderate to severe rheumatoid arthritis. Drugs 78 (9), 929–940. 10.1007/s40265-018-0929-z [DOI] [PubMed] [Google Scholar]
- Liu B. W., Li M., Zhou Z. G., Guan X., Xiang Y. F. (2020a). Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?. J. Autoimmun. 111, 102452 10.1016/j.jaut.2020.102452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., Liang B., Wang X., Wang H., et al. (2020b). Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55, 102763 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse A., Klopfenstein T., Balblanc J. C., Royer P. Y., Bossert M., Gendrin V., et al. (2020). Predictive factors of mortality in patients treated with tocilizumab for acute respiratory distress syndrome related to coronavirus disease 2019 (COVID-19). Microbes Infect. 22, 500–506. 10.1016/j.micinf.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qiu L., Liu X. L., Liu D., Li J. (2020b). Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 92 (7), 814–818. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Li Y. X., Jiang L. J., Chen Q., Wang T., Ye D. W. (2020a). Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol. Sci. 41 (8), 531–543. 10.1016/j.tips.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyseng-Williamson K. A. (2015). Siltuximab: a review in idiopathic (human herpesvirus-8-negative) multicentric castleman disease. BioDrugs 29 (6), 399–406. 10.1007/s40259-015-0142-5 [DOI] [PubMed] [Google Scholar]
- Marfella R., Paolisso P., Sardu C., Bergamaschi L., D'angelo E. C., Barbieri M., et al. (2020). Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients. Diabetes Metab. 46 (5), 403–405. 10.1016/j.diabet.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Sanz J., Muriel A., Ron R., Herrera S., Ron R., Perez-Molina J. A., et al. (2020). Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicenter cohort study. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.06.08.20125245 (Accessed June 9, 2020). [DOI] [PMC free article] [PubMed]
- Mastroianni A., Greco S., Apuzzo G., De Santis S., Oriolo C., Zanolini A., et al. (2020). Subcutaneous tocilizumab treatment in patients with severe COVID-19–related cytokine release syndrome: an observational cohort study. EClinicalMedicine 24, 100410 10.1016/j.eclinm.2020.100410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulska M., Nicolini L. A., Signori A., Di Biagio A., Sepulcri C., Russo C., et al. (2020). Tocilizumab and steroid treatment in patients with severe. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.06.22.20133413 (Accessed June 26, 2020). [DOI] [PMC free article] [PubMed]
- Moore J. B., June C. H. (2020). Cytokine release syndrome in severe COVID-19. Science 368 (6490), 473–474. 10.1126/science.abb8925 [DOI] [PubMed] [Google Scholar]
- Morena V., Milazzo L., Oreni L., Bestetti G., Fossali T., Bassoli C., et al. (2020). Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur. J. Intern. Med. 76, 36–42. 10.1016/j.ejim.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Garcia E., Rico Caballero V., Albiach L., Aguero D., Ambrosioni J., Bodro M., et al. (2020). Tocilizumab is associated with reduction of the risk of ICU admission and mortality in patients with SARS-CoV-2 infection. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.06.05.20113738 (Accessed June 5, 2020). [DOI] [PMC free article] [PubMed]
- Moreno-Pérez O., Andres M., Leon-Ramirez J. M., Sánchez-Payá J., Rodríguez J. C., Sánchez R., et al. (2020). Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: a retrospective cohort study. J. Autoimmun. 114, 102523 10.1016/j.jaut.2020.102523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. R., Johnson J. M., Griebe K. M., Jones M. C., Stine J. J., Hencken L. N., et al. (2020). Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. J. Autoimmun. 114, 102512 10.1016/j.jaut.2020.102512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narain S., Stefanov D., Chau A. S., Weber A. G., Marder G. S., Kaplan B., et al. (2020). Comparative survival analysis of immunomodulatory therapy for COVID-19 'cytokine storm': a retrospective observational cohort study. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.06.16.20126714 (Accessed June 19, 2020). [DOI] [PMC free article] [PubMed]
- Patel A., Shah K., Dharsandiya M., Patel K., Patel T., Patel M., et al. (2020b). Safety and efficacy of tocilizumab in the treatment of severe acute respiratory syndrome coronavirus-2 pneumonia: a retrospective cohort study. Indian J. Med. Microbiol. 38 (1), 117–123. 10.4103/ijmm.IJMM_20_298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K., Gooley T. A., Bailey N., Bailey M., Hegerova L., Batchelder A., et al. (2020a). Use of the IL-6R antagonist tocilizumab in hospitalized COVID-19 patients. J. Intern. Med. https//.org/10.1111/joim.13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone F., Piccirillo M. C., Ascierto P. A., Salvarani C., Parrella R., Marata A. M., et al. (2020). Tocilizumab for patients with COVID-19 pneumonia. The TOCIVID-19 prospective phase 2 trial. medRxiv: the preprint server for health sciences [Preprint] Available at: 10.1101/2020.06.01.20119149 (Accessed July 2, 2020). [DOI]
- Potere N., Di Nisio M., Cibelli D., Scurti R., Frattari A., Porreca E., et al. (2020a). Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case–control study. Ann. Rheum. Dis. [Epub ahead of print]. 10.1136/annrheumdis-2020-218243 [DOI] [PubMed] [Google Scholar]
- Potere N., Di Nisio M., Rizzo G., La Vella M., Polilli E., Agostinone A., et al. (2020b). Low-Dose subcutaneous tocilizumab to prevent disease progression in patients with moderate COVID-19 pneumonia and hyperinflammation. Int. J. Infect. Dis. 100, 421–424. 10.1016/j.ijid.2020.07.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. C., Altice F. L., Shyr Y., Koff A., Pischel L., Goshua G., et al. (2020). Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. Chest 158, 1397–1408. 10.1016/j.chest.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartuccio L., Sonaglia A., Mcgonagle D., Fabris M., Peghin M., Pecori D., et al. (2020). Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J. Clin. Virol. 129, 104444 10.1016/j.jcv.2020.104444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy M., Mannam P., Comer R., Sinclair E. (2020). Off-label real World experience using tocilizumab for patients hospitalized with COVID-19 disease in a regional community health system: a case-control study. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.05.14.20099234 [DOI]
- Rojas-Marte G. R., Khalid M., Mukhtar O., Hashmi A. T., Waheed M. A., Ehrlich S., et al. (2020). Outcomes in patients with severe COVID-19 disease treated with tocilizumab - a case- controlled study. QJM 113, 546–550. 10.1093/qjmed/hcaa206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S., Winthrop K., Calabrese L. (2017). The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat. Rev. Rheumatol. 13 (7), 399–409. 10.1038/nrrheum.2017.83 [DOI] [PubMed] [Google Scholar]
- Rossi B., Nguyen L. S., Zimmermann P., Boucenna F., Baucher L., Dubret L., et al. (2020). Effect of tocilizumab in hospitalized patients with severe pneumonia COVID-19: a cohort study. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.06.06.20122341 (Accessed June 9, 2020). [DOI] [PMC free article] [PubMed]
- Roumier M., Paule R., Groh M., Vallee A., Ackermann F. (2020). Interleukin-6 blockade for severe COVID-19. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.04.20.20061861 (Accessed April 22, 2020). [DOI]
- Sardu C., D'onofrio N., Balestrieri M. L., Barbieri M., Rizzo M. R., Messina V., et al. (2020c). Hyperglycaemia on admission to hospital and COVID-19. Diabetologia 63 (11), 2486–2487. 10.1007/s00125-020-05216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C., D'onofrio N., Balestrieri M. L., Barbieri M., Rizzo M. R., Messina V., et al. (2020a). Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control?. Diabetes Care 43 (7), 1408–1415. 10.2337/dc20-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C., Gargiulo G., Esposito G., Paolisso G., Marfella R. (2020b). Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19. Cardiovasc. Diabetol. 19 (1), 76 10.1186/s12933-020-01047-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C., Maggi P., Messina V., Iuliano P., Sardu A., Iovinella V., et al. (2020d). Could anti-hypertensive drug therapy affect the clinical prognosis of hypertensive patients with COVID-19 infection? Data from centers of southern Italy. J. Am. Heart Assoc. 9 (17), e016948 10.1161/JAHA.120.016948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C., Marfella R., Maggi P., Messina V., Cirillo P., Codella V., et al. (2020e). Implications of AB0 blood group in hypertensive patients with covid-19. BMC Cardiovasc. Disord. 20 (1), 373 10.1186/s12872-020-01658-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R., et al. (2020). Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin. Exp. Rheumatol. 38 (3), 529–532. [Epub ahead of print]. [PubMed] [Google Scholar]
- Sinha P., Mostaghim A., Bielick C. G., Mclaughlin A., Hamer D. H., Wetzler L., et al. (2020). Early administration of Interleukin-6 inhibitors for patients with severe Covid-19 disease is associated with decreased intubation, reduced mortality, and increased discharge. Int. J. Infect. Dis. 99, 28–33. 10.1016/j.ijid.2020.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. S., Aletaha D., Koeller M., Weisman M. H., Emery P. (2007). New therapies for treatment of rheumatoid arthritis. Lancet 370 (9602), 1861–1874. 10.1016/S0140-6736(07)60784-3 [DOI] [PubMed] [Google Scholar]
- Somers E. C., Eschenauer G. A., Troost J. P., Golob J. L., Gandhi T. N., Wang L., et al. (2020). Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. ciaa954 10.1093/cid/ciaa954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6 (10), a016295 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. (2016). Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 8 (8), 959–970. 10.2217/imt-2016-0020 [DOI] [PubMed] [Google Scholar]
- Tomasiewicz K., Piekarska A., Stempkowska-Rejek J., Serafińska S., Gawkowska A., Parczewski M., et al. (2020). Tocilizumab for patients with severe COVID-19: a retrospective, multi-center study. Expert Rev. Anti Infect. Ther. 2020, 1–7 10.1080/14787210.2020.1800453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., et al. (2020). Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 19, 102568 10.1016/j.autrev.2020.102568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai A., Diawara O., Nahass R. G., Brunetti L. (2020). Impact of tocilizumab administration on mortality in severe COVID-19. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.07.30.20114959 (Accessed August 2, 2020). [DOI] [PMC free article] [PubMed]
- Wadud N., Ahmed N., Mannu Shergil M., Khan M., Krishna M. G., Gilani A., et al. (2020). Improved survival outcome in SARs-CoV-2 (COVID-19) Acute Respiratory Distress Syndrome patients with Tocilizumab administration. medRxiv: the preprint server for health sciences [Preprint]. Available at: 10.1101/2020.05.13.20100081 (Accessed May 16, 2020). [DOI]
- World Health Organization (2019) Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed December 31, 2019).
- World Health Organization (2020) Corticosteroids for COVID-19. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (Accessed September 2, 2020).
- Xu X. L., Han M. F., Li T. T., Sun W., Wang D. S., Fu B. Q., et al. (2020). Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 117 (20), 10970–10975. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhao Y., Zhang F., WangLi Q. T., Liu Z., et al. (2020). The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin. Immunol. 214, 108393 10.1016/j.clim.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.