Abstract
Purpose:
The microbiome-gut-brain (MGB) axis provides a dynamic model to understand associations between the gut microbiota and psychoneurological comorbidities. The role of the MGB axis in cancer treatment-related psychoneurological symptoms (PNS) remains unknown. The purpose of this study was to conduct a systematic review of the existing literature to identify the influence of the gut microbiota on cancer and cancer treatment-related PNS and toxicities mediated by the MGB axis.
Methods:
We searched the databases of PubMed, Embase, and Web of Science from their earliest records to October 2019. All studies identified in the database searches were screened by title and abstract, followed by a review of the full texts. The Johns Hopkins Nursing Evidence-Based Practice Model was adopted to assess the evidence levels and qualities; the Joanna Briggs Institute critical appraisal tools were used to assess the methodological quality and the possibility of bias for each included study. All the study findings were combined, synthesized, and presented through narrative format.
Results:
Six studies were included in this systematic review. These studies primarily focused on cancer survivorship while receiving chemotherapy, and they were conducted between 2016 and 2019. The gut microbiome was assessed via fecal samples, which were analyzed using 16S rRNA sequencing approaches. With small-scale studies, the gut microbiota was associated with cancer treatment-related PNS, including fatigue, anxiety, depression, sleep disturbance, cognitive impairment, and chemotherapy-induced peripheral neuropathy. A higher relative abundance of Bacteroides was associated with a higher level of fear of cancer recurrence but a higher relative abundance of Lachnospiraceae.g and Ruminococcus was associated with a lower level in fear of cancer recurrence. Changes in fatigue interference were associated with the frequency of genera Faecalibacterium and Prevotella, and changes in anxiety were associated with the frequency of genera Coprococcus and Bacteroides.
Conclusions:
The gut microbiota showed significant associations with cancer treatment-related PNS. Recent work regarding the MGB axis in cancer psychoneurological toxicities focused primarily on individual toxicity and symptoms in cancer survivors with chemotherapy exposure. Associations between the gut microbiota and PNS should be further studied in cancer populations across different ages, cancer types, and treatment modalities.
Keywords: Cancer, microbiome-gut-brain axis, gut microbiota, psychoneurological symptoms, treatment modality, treatment toxicity
Introduction
The human gut hosts tens of trillions of microbial cells, representing 500 bacterial species on average.1,2 The gut microbiome, defined as the collection of all genomes of microbes in the human gastrointestinal (GI) tract,3 plays a critical role in human health and disease.2,4 A dysbiotic gut microbiota (i.e., loss of keystone taxa, loss of diversity, shifts in metabolic capacity, or blooms of pathogens5,6) is not only associated with carcinogenesis and interference with cancer chemotherapeutic metabolism,7–9 but it is also a potential biomarker of toxicities associated with cancer treatment including chemotherapy, radiation therapy, and immunotherapy.10,11 Based on clinically identified symptom clusters,12 disruption to the host microbiota has been suggested to contribute to psychoneurological symptoms (PNS), which is defined as a cluster of symptoms including pain, fatigue, anxiety, depression, sleep disturbance, and cognitive dysfunction.11,13 Experiencing continuous and severe symptoms can lead to delays in cancer treatments, a decrease in tumor response, and a reduction in a patient’s quality of life.14
Recent discovery of the bidirectional signaling network in the microbiome-gut-brain axis (MGB)15 provides a dynamic model to study associations between the gut microbiota and cancer treatment-related PNS and toxicities. Current studies on the MGB axis suggest that the gut microbiota could affect the development of toxicities and PNS through neural (e.g., vagus and spinal), immune (e.g., proinflammatory cytokines), endocrine and metabolic (e.g., short-chain fatty acids), and neurotransmitter pathways (e.g., gamma-aminobutyric acid and serotonin).16,17 Specifically, common cancer chemotherapy protocols containing drugs such as 5-Fluorouracil, irinotecan, methotrexate, and etoposide can cause decreased commensal microbes and increased opportunistic pathogens,14,18 in turn exacerbating treatment toxicities and PNS.19,20 Using preclinical animal models, studies suggest that the gut microbiota is associated with PNS including cancer-related pain,21,22 fatigue and exercise capacity,23 depression,24,25 and cognitive dysfunction.23,26 Until recently, the MGB axis in cancer has been primarily studied in animal models but is rarely studied in human populations.
Cancer treatment such as chemotherapy, radiation therapy, and immunotherapy can interrupt the gut microbiota and the MGB axis. Studies in adult populations have documented alterations of the gut microbiota during chemotherapy and shown that patients’ gut microbiota was significantly dysbiotic during and after chemotherapy. They specifically noted decreases in the abundance of healthy gut microbes including Bacteroides, Bifidobacteria, and Clostridium cluster IV and XIVa, however increases in pathological microbes such as Enterobacteriaceae were noted.18,27,28 Similar findings were reported in childhood cancer.14,29 Multiple studies have found a marked reduction in the number of anaerobic bacteria (i.e., Bacteroides, Clostridium cluster XIVa, Faecalibacterium, and Bifidobacterium) present in childrens’ gut microbiota following chemotherapy, whereas the number of Enterococcus was drastically increased during chemotherapy.14,29 These perturbations of the gut microbiota may potentially heighten cancer treatment toxicity, increase PNS, and decrease treatment efficacy.30 These early-stage findings require further investigation in larger samples controlling for a variety of confounding factors such as demographics, treatment regimens, use of antibiotics and probiotics, and diet and lifestyle behaviors. Current evidence does not definitively show whether cancer and its related treatments cause changes to the gut microbiota composition and function, thereby affecting the MGB axis in cancer patients.9,30
Studies on the MGB axis suggest a link between the gut microbiota and PNS primarily in preclinical models. Compared with specific pathogen free mice, lack of the gut microbiota could lead to exaggerated responses in anxiety (e.g., elevated adrenocorticotropic hormone and corticosterone in plasma and reduced brain-derived neurotrophic factor expression in the cortex and hippocampus)26,31,32 and cognitive dysfunction (e.g., working and reference memory assessed by using novel object test and T-maze) in germ-free (GF) mice.26 Interestingly, infected mice receiving daily combined probiotics (L. rhamnosus + L. helveticus) could reverse their memory dysfunction.26 The gut microbiota may link to these behavioral changes via adjusting the hypothalamic-pituitary-adrenal axis31 and neurochemical changes in amygdala and hippocampus.26,32 Additinally, the absence of commensal gut microbes can lead to lower inflammatory hypernociception associated with reduced tissue inflammation in GF mice.21 Regarding the impact of cancer treatment on the MGB axis, current studies have focused on preclinical models. Specifically, irinotecan-induced gut microbiota alterations may influence chemotherapy-induced pain via toll-like receptor (TLR) 4 signaling;33 paclitaxel chemotherapy concurrently leads to altered gut microbiota, increased fatigue and decreased cognitive performance,34,35 as well as chemotherapy induced peripheral neuropathy36 in mice. Specific pathways associated with the GMB axis such as neuroinflammation34,35 and intestinal barrier integrity36 may lead to chemotherapy-induced behavioral comorbidities. For example, elevated cytokines (e.g., IL-2, IFN-a, and TNFa) and cytokine-based treatments in humans are linked to the development of depression, anxiety, cognitive dysfunction, and fatigue.37,38 Additionally, the intestinal barrier impairment can increase the intestinal permeability and the translocation of the gut microbes, which may lead to an immune response contributing to PNS.39,40 Although these preclinical studies provide evidence of the presence of the MGB axis and specific pathways within the MGB, further data are required to confirm causative relationships between the microbiota and PNS.
Recent studies have been more focused on investigating the gut microbiota in cancer patients; however, the specifics of the MGB axis in cancer and cancer treatment-related symptoms are still unknown. A more complete understanding of the MGB axis in cancer, cancer treatment-related toxicities, and PNS could potentially help identify the biological mechanisms of treatment-related symptoms and toxicities, and this may provide opportunities to design personalized interventions for patients with cancer. The purpose of the present study was to conduct a systematic review of the existing literature to identify the influence of the gut microbiota on PNS outcomes mediated by the MGB axis. We hypothesized that alterations of the gut microbiota would be associated with cancer treatment-related PNS and toxicities, which would be mediated by pathways of the MGB axis.
Methods
Search Strategy
Based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,41 a systematic review was conducted by searching three databases: PubMed, Embase, and Web of Science. A string of key terms used to search these databases included: microbiota; gut-brain-axis; gut microbiome; cancer; radiation therapy; chemotherapy; gastrointestinal microbiome; cognitive function; central nervous system; depression; and anxiety (Supplement Table 1). All databases were searched from their earliest records to October 2019. All search results were filtered by language (English only). The literature search process was overseen by a health science librarian from a large research university in Atlanta, Georgia.
Study Selection
All articles identified from the database searches were initially screened by title and abstract by the primary author (BS) and subsequently confirmed by another author (JB). The full texts were then retrieved and independently reviewed by two reviewers (BS and JB) to determine study eligibility. Ambiguous studies were discussed and reviewed by both authors (BS and JB). Eligibility critera for articles to be included in this study are as follows: 1) study of the gut microbiome in patients receiving cancer treatments (e.g., chemotherapy, radiation therapy, or immunotherapy); 2) primary focus on the MGB axis associated with cancer and cancer treatment; and 3) were published in English. Articles were excluded from this study if they were 1) not associated with cancer treatment; 2) not associated with MGB axis; and 3) conference abstracts without available full texts or unpublished reports. When multiple studies with similar results were found to be authored by the same research team, only the most recent publication was included in this review. A secondary search of reference lists was conducted to decrease the likelihood of omitting eligible papers from this review.
Data Extraction
A standardized extraction form was used to extract data from eligible studies. The extracted information from each study included: 1) information about the studies using the Johns Hopkins evidence level and quality guide (e.g., study design, sample size); 2) participant information (e.g., demographics and clinical information); 3) inclusion and exclusion criteria; 4) variables and measures; 5) data analysis strategies; and 6) research findings. All data were extracted by the first author (BS), and a second author (JB) confirmed data accuracy.
Assessment of Methodological Quality
The Johns Hopkins Nursing Evidence-Based Practice Model42 was adopted to assess the evidence levels and qualities. The evidence levels include five levels (I, II, III, IV, and V). Each level is subdivided into three quality levels: high quality, good quality, or low quality/major flaws. Two students, trained to use the Johns Hopkins Nursing Evidence-Based Practice Model, assessed the evidence level and quality for each study.
To evaluate the research evidence, the Joanna Briggs Institute (JBI) critical appraisal tools43 were used to assess the methodological quality of each study. Additionally, JBI tools were used to determine the possibility of bias in the areas of study design, data collection, and data analysis for each study. All studies included in this systematic review were subjected to rigorous appraisal by one team member (BS). These results were used to synthesize and interpret our findings. The JBI critical appraisal tools were developed by the JBI and its researchers, and these tools are widely used to evaluate a variety of study designs. In this study, the JBI appraisal tools associated with systematic review, cross-sectional, and longitudinal study designs were utilized. For each critical appraisal checklist, every item was evaluated as yes, no, unclear, or not applicable.
Data Synthesis and Analysis
All study results were merged and presented in tables and figures using narrative format.
Results
Literature Search
We initially identified 512 reports for possible inclusion in this systematic review by searching three databases: PubMed (n = 492), Embase (n = 12), and Web of Science (n = 8). After reviewing the articles’ titles and abstracts, two duplicate articles and other irrelevant reports (n = 472) were removed, resulting in 38 reports requiring further review based on the full texts. Only four studies met the inclusion criteria after excluding reports not associated with cancer (n = 11) or the MGB axis (n = 17); further, we removed protocol or conference abstracts (n = 4) and studies of animal models (n = 2). The secondary literature search (by reference lists) and a registered PubMed update identified two additional studies. Finally, six studies were included in this systematic review (Figure 1).
Figure 1.
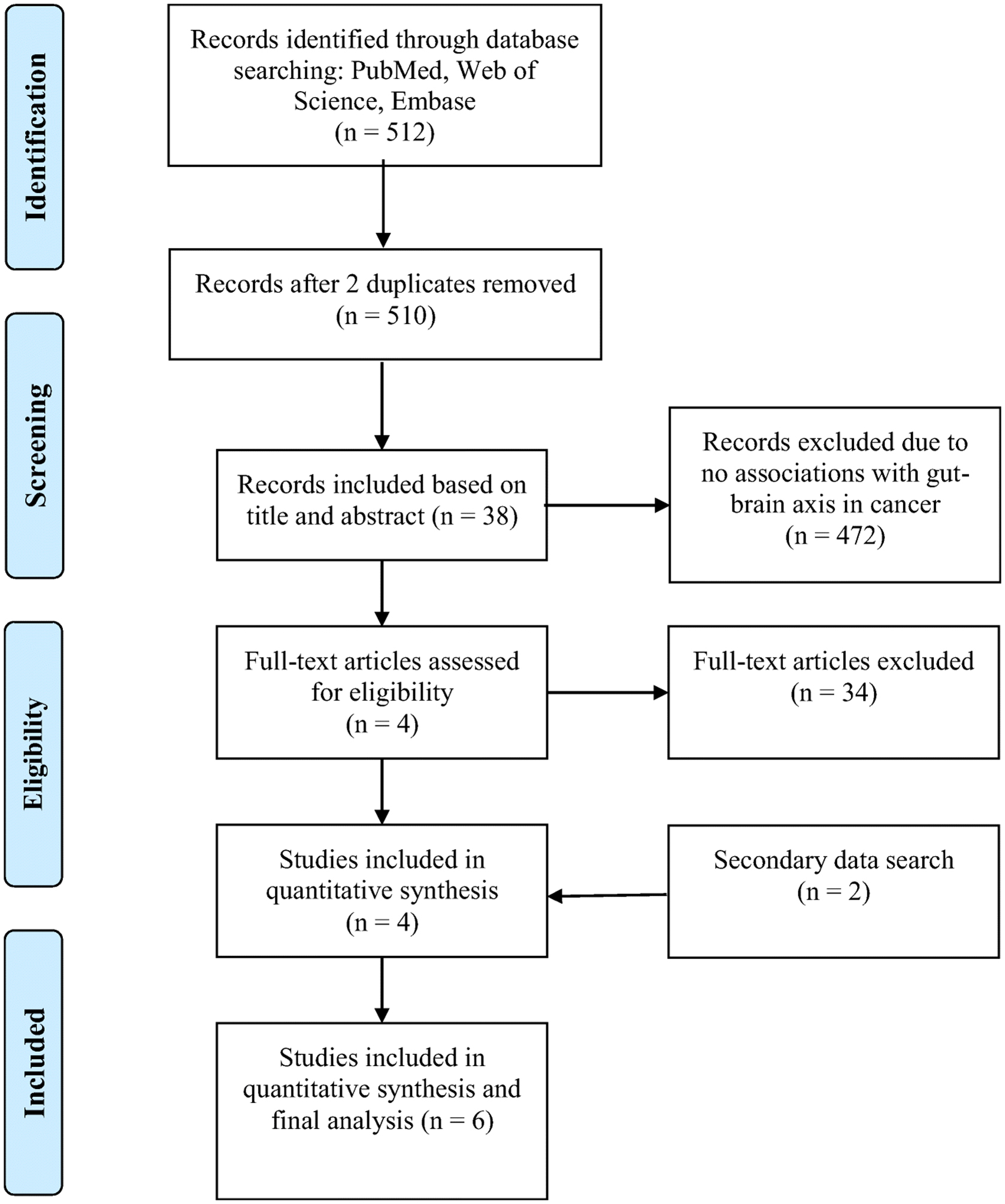
PRISMA diagram of literature search and inclusion process
Study Characteristics and Sampling
Among these six included studies, two were cross-sectional studies,44,45 one longitudinal study,46 and three literature review and synthesis studies (Table 1).47–49 Of the three data-based articles, two studies focused on cancer survivors44,46 and one study focused on women with ovarian cancer.45 The sample size of included studies varied from 1246 to 158.45 These studies were conducted by researchers from the USA (n = 3),45,46,48 Australia (n = 1),47 Japan (n = 1),44 and China (n = 1).49 All studies were conducted between 2016 and 2019.
Table 1.
Characteristics of included studies
| First Author (Yr of Pub.) | Author Origin | Study Design | Participants | Clinical Information | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|---|---|
| Bajic (2018)* | Australia | Review | NA | Preclinical animal models and clinical populations; | NA | NA |
| Donovan (2016) | USA | Cross-sectional | N=158 White (93.7%); age in yrs (Mean=55.49); some college education or above (86.1%); married/partners (77.8%); | Mos since diagnosis (Mean=45.73); number of new chemotherapy regimens (Mean=4.40); days from last chemotherapy (Mean=16.54); minimal to low emetogenicity of current treatments (70.4%); | 1) Women with recurrent ovarian cancer; and 2) being in active chemotherapy treatment; | Subjects with missing data; |
| Jordan (2018)* | USA | Review | NA | Preclinical animal models and clinical populations; | NA | NA |
| Okubo (2019) | Japan | Cross-sectional | N=126 125 female, 1 male; age in yrs (Mean=58); body mass index (Mean=22.0); | History of receiving chemotherapy (45.2%); time since cancer diagnosis (Mean=64.8 mos); stage I disease (47%); | Women who 1) were ≥20 years old; 2) had been diagnosed with invasive breast cancer >1 yr prior; and 3) attended regular and scheduled consultations; | 1) Cognitive impairment or disturbance of consciousness; and 2) Inability to participate as judged by the physician; |
| Paulsen (2017) | USA | Longitudinal | N=12 White (66.7%) and African American (25.0%); age in yr (Mean=55); body mass index (Mean=30.4); waist circumference (Mean=89.1 cm); | Mos since diagnosis (Mean=54); Cancer stage: DCIS (16.7%); 1 (33.3%); 2 (33.3%); and 3 (16.7%); History of chemotherapy (66.7%) History of radiation (58.3%) | 1) 18–70 years old; 2) history of DCIS Stage 1, 2, or 3A breast cancer; 3) English speaking; 4) in the last 6 mos, less than 30 minutes of rigorous activity or 60 minutes of moderate activity per week on average; and 5) physician-reported medical clearance; | 1) Dementia or exhibited other medical, psychological, or social characteristics that interfered with study participation; 2) physical activity contraindication; 3) unable to ambulate; 4) recurrent or metastatic cancer; 5) anticipate elective surgery or travel for >1 week during the first 3 months of the study; 6) used antibiotics during the week prior to sample collection; |
| Zhong (2019)* | China | Review | NA | Preclinical animal models and clinical | NA | NA |
DCIS, ductal carcinoma in situ; Mos, months; NA, not applicable; Yr, year;
NA indicates not applicable for review article with respect to participants, inclusion and exclusion criteria.
Studies included in this analysis primarily focused on chemotherapy exposure or cancer survivorship.44–49 All three data-based papers focused on women’s cancers including breast44,46 and ovarian cancers.45 These studies described the role of the MGB axis in various cancer treatment-related outcomes including neuroimmunological changes,47 GI symptoms (e.g., nausea),45 chemotherapy-induced peripheral neuropathy,49 and psychosocial and behavioral outcomes (e.g., fear, anxiety and depression).44,46,48 To evaluate the MGB axis, the gut microbiome was examined via fecal samples.44,46 All fecal samples were processed using Illumina MiSeq DNA sequencing of the 16S rRNA gene. All gut microbiota data were analyzed using the Quantitative Insights Into Microbial Ecology pipeline (QIIME).44,46
Study Quality Assessment
Based on the Johns Hopkins evidence level and quality guide, the overall level of the studies included: level III (n = 3)44–46 and level V (n = 3).47–49 All three data-based studies were level III and three literature review and synthesis studies were level V. Of the included studies, 3 were high quality studies and 3 were good quality studies (Table 2). The JBI critical appraisal showed acceptable methodological quality for the included studies (Supplement Table 2).
Table 2.
Quality of included studies
| First Author (Yr of Pub.) | Level of Evidence* | Evidence Quality* |
|---|---|---|
| Bajic (2018) | V | A |
| Donovan (2016) | III | A |
| Jordan (2018) | V | B |
| Okubo (2019) | III | B |
| Paulsen (2017) | III | B |
| Zhong (2019) | V | A |
Based on the Johns Hopkins Evidence Level and Quality Guide: Levels include five categories (I, II, III, IV, and V). Evidence quality is categorized into three levels (A, B, and C).
Pathways Associated with the MGB Axis
Three studies reviewed the pathways associated with the MGB axis in chemotherapy (Table 3).47–49 Chemotherapy influences the MGB axis by altering gut microbiota composition and function; disrupting the balance between beneficial and detrimental gut microbes; negatively affecting the gut lining; impairing the enteric nervous system; and activating neuroimmune and pain signaling responses.47–49 Specifically, chemotherapy exposure activates enteroendocrine Cells (EECs) such as secretion of peptide YY (PYY), neuropeptide Y (NPY), glucagon-like peptide 1 (GLP-1), and 5-hydroxytryptamine (5-HT) receptors and related neurotransmitters.49 These neurotransmitters can enter the brain via peripheral circulation,47,49 and the brain can also regulate the permeability of the intestinal barrier (also called “leaky gut”) and the distribution of gut microbiota.47–49
Table 3.
Findings regarding the microbiome-gut-brain axis from three review articles
| First Author (Yr of Pub.) | Study Purpose | Results |
|---|---|---|
| Bajic (2018) | Presented the gut-brain axis dysregulation in the chemotherapy setting and highlighted peripheral-to-central immune signaling mechanisms and their contribution to neuroimmunological changes associated with chemotherapy |
|
| Jordan (2018) | Summarized the clinical and preclinical evidence for the role of intestinal microbiome in mediating behavioral comorbidities through peripheral immune activation in patients with cancer receiving chemotherapy |
|
| Zhong (2019) | Investigated the role of gut microbiome in regulating the incidence and progression of the chemotherapy-induced peripheral neuropathy (CIPN) |
|
5-FU, 5-Fluorouracil; CICI, chemotherapy-induced cognitive impairment; CIGT, chemotherapy-induced gut toxicity; CIPN, chemotherapy-induced peripheral neuropathy; ENS, enteric nervous system; GI, gastrointestinal; TLRs, toll-like receptors;
Associations between the MGB Axis and Psychoneurological Outcomes
Table 4 displays associations between the MGB axis and psychoneurological outcomes. Among breast cancer survivors who have undergone chemotherapy, fear of cancer recurrence was measured by the Concerns About Recurrence Scale.44 A higher relative abundance of the bacterial phylum Bacteroidetes and genera Bacteroides was associated with more fear of cancer recurrence. Additionally, a higher alpha-diversity (Shannon index) and a higher relative abundance of bacterial phylum Firmicutes and genera Lachnospiraceae.g and Ruminococcus were associated with less fear of cancer recurrence.44
Table 4.
Findings regarding the microbiome-gut-brain axis from three data-based articles
| First Author (Yr of Pub.) | Study Purpose | Primary Measure (Variable) | Bioinformatics and Statistical Analysis | Results |
|---|---|---|---|---|
| Donovan (2016) | Compared women reporting nausea to women not reporting nausea with regard to the severity of other commonly reported symptoms in ovarian cancers | Symptom Representation Questionnaire (symptom severity for cancer- and treatment-related symptoms and side effects); | Independent sample t-test; simple linear regression analyses; SPSS version 22 | Seventeen symptoms were significantly related to nausea, including those with strong evidence linking to the gut-brain axis (e.g., abdominal bloating, bowel disturbances, vomiting) and others with emerging links to the gut-brain axis (e.g., sleep problems, fatigue, memory problems, and mood swings); Nausea severity significantly predicted 13 symptoms after controlling for age, number of previous chemotherapy regimens, and time since most recent treatment. Bowel disturbances, mood swings, and sleep disturbances showed a trend associations with nausea; |
| Okubo (2019) | Examined the association between fear of cancer recurrence and the gut microbiota in breast cancer survivors | Concerns About Recurrence Scale (fear of cancer recurrence used); Hospital Anxiety and Depression Scale (depressive symptoms); fecal samples using 16S rRNA V3-V4 gene region by an Illumina MiSeq instrument (gut microbiome); | Multiple regression analysis; QIIME version 2017.10; R 3.2.1; | Among all participants, no association of bacterial compositions with the overall fear index in phylum-level. At the genus level, a higher relative abundance of Bacteroides was significantly associated with a higher overall fear index; Among all participants, no significant associations of other genera or the alpha diversity (Shannon index) with the overall fear index; For participants without chemotherapy, no significant association of bacterial compositions with the fear index were found in phylum- and genus-level; For participants with chemotherapy, a higher alpha diversity (Shannon index) was significantly associated with a lower fear index; For participants with chemotherapy, a higher relative abundance of Bacteroidetes was associated with a higher fear index and a higher relative abundance of Firmicutes was significantly associated with a lower fear index at the phylum level. At the genus level, a higher relative abundance of Bacteroides was associated with a higher fear index and higher relative abundances of Lachnospiraceae.g and Ruminococcus were associated with a lower fear index; |
| Paulsen (2017) | Determined correlations between the gut microbiota composition and alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors | A submaximal treadmill test (quantified as predicted VO2, cardiorespiratory fitness); Hospital Anxiety and Depression Scale (Anxiety and depression); Fatigue Symptom Inventory (fatigue intensity and interference). Pittsburgh Sleep Quality Index (Sleep dysfunction); fecal samples at baseline and at the end of 3 months using Illumina MiSeq DNA Sequencing of the 16S rRNA gene (gut microbiota); 3-day diet record; |
Permutational multivariate analysis; t test and Wilcoxon rank sum test; Kruskal-Wallis testing with false discovery rate (FDR); principal coordinate analysis; QIIME; | Increased fatigue interference was associated with increased Shannon diversity index. Increased/no changes in depression scores showed a trend association with changes in Shannon diversity index. Changes in anxiety, fatigue intensity, and sleep dysfunction were not associated with changes in Shannon diversity index; Using weighted UniFrac distance matrix, significant differences were found for anxiety and fatigue interference at the end of 3 months, while sleep dysfunction and fatigue intensity trended toward significance; There were no significant differences for depression and fatigue intensity for weighted and unweighted UniFrac clustering; Kruskal-Wallis testing without FDR correction found that the quartile of magnitude change in fatigue interference was associated with the frequency of genera Faecalibacterium and Prevotella; quartile of magnitude change in anxiety was associated with the frequency of genera Coprococcus and Bacteroides; quartile of magnitude change in cardiorespiratory fitness was associated with the frequency of genera Roseburia and SMB53, a subset of the family Clostridiaceae; Kruskal-Wallis testing with FDR correction showed no associations for bacterial taxa analyses; |
FDR, false discovery rate; QIIME, Quantitative Insights Into Microbial Ecology
Paulsen et al.46 examined associations between the gut microbiota composition and alterations in psychosocial outcomes among breast cancer survivors. Survivors’ anxiety and depression were assessed using Hospital Anxiety and Depression Scale; fatigue intensity and interference using Fatigue Symptom Inventory; and sleep quality using Pittsburgh Sleep Quality Index. An increase in fatigue interference was associated with an increased alpha-diversity, and an increase/no change in depression showed a trend association with alpha-diversity changes. Conversely, changes in anxiety, fatigue intensity, and sleep dysfunction were not associated with alpha-diversity changes.46 Bacterial beta-diversity was reported using weighted and unweighted UniFrac distance matrices. Among breast cancer survivors, differences were found in anxiety and fatigue interference, while sleep dysfunction and fatigue intensity showed trends toward significance using weighted UniFrac distance matrices. No differences in depression were found for weighted and unweighted UniFrac distance matrices. The fatigue interference change from baseline to 3-month follow up was associated with the frequency of genera Faecalibacterium and Prevotella; the anxiety change from baseline to 3-month follow up was associated with the frequency of genera Coprococcus and Bacteroides.46 The gut microbiota may contribute to chemotherapy-induced peripheral neuropathy through the immune-nervous-endocrine axis.49
Discussion
The MGB axis,50 including the central nervous system (CNS), the enteric and autonomic nervous system, the neuro-endocrine system (e.g., serotonin), the neuro-immune pathways (e.g., proinflammatory cytokines), and the gut microbiota,50,51 provides a signaling network to study associations between the gut microbiota and psychoneurological outcomes in cancer patients. A dysbiotic gut microbiota is associated with the development of PNS in non-cancer3 and adult cancer populations.10,27,21,25,26 In this study, six studies were systematically reviewed to describe the role of the MGB axis in cancer and treatment-related psychoneurological outcomes. Few studies have focused on the MGB axis in cancer and cancer treatment-related outcomes, and these studies have primarily focused on female cancer patients receiving chemotherapy.
Multiple studies suggest that a dysbiotic gut microbiota is associated with PNS in cancer patients receiving chemotherapy.44,48,52 In this review, chemotherapy-induced nausea showed correlations, not only with GI symptoms (e.g., abdominal bloating, bowel disturbances, vomiting), but also with other symptoms that may be linked to the MGB axis (e.g., fatigue, sleep disturbance, memory problems, and mood swings).45 Furthermore, gut microbiota alpha-diversity (based on the Shannon index) was significantly associated with patient-reported PNS such as fear of cancer relapse,44 fatigue interference, and depression.46 The beta-diversity of the gut microbiota further suggested the gut microbiota associates with improvement of fatigue and sleep disturbance over time (from baseline to 3-month follow up) for breast cancer survivors.46 Different taxa associated with PNS were reported by Okubo et al44 and Paulsen et al.46 Specifically, a higher relative abundance of Bacteroides was associated with a higher fear of cancer recurrence (measured by the Concerns About Recurrence Scale), while a higher relative abundance of Lachnospiraceae.g and Ruminococcus was associated with a lower fear of cancer recurrence.44 The change in fatigue interference from baseline to 3-month follow up was associated with the frequency of genera Faecalibacterium and Prevotella; the change in anxiety was associated with the frequency of genera Coprococcus and Bacteroides.46 However, the direction of these associations were not reported. These small-scale studies corroborated the role of the gut microbiota in PNS via the MGB axis.50,51 As a unknown but promsing field, several large-scale studies are currently undergoing for young adult cancer surviors (18–39 year old)53 and children (7–17 year old) with solid tumors54. These ongoing studies would further confirm the role of the gut microbiota in the PNS and treatment toxicities (e.g., chemotherapy) mediated by the MGB axis.
The MGB axis plays a critical role in determining cancer’s psychoneurological outcomes and treatment toxicities. Multiple pathways have been examined in the MGB axis, however these pathways have primarily been studied in preclinical models. The peripheral immune system is one of the most relevant pathways between the GI tract and the brain during chemotherapy.48 Cancer treatments (e.g., chemotherapy and immunotherapy) can lead to a reduction in microbial diversity as represented by a change in the Shannon index;44,46 additionally, treatments can cause a reduction in species (e.g., L. rhamnosus and L. acidophilus)55 associated with anti-inflammatory activities, which can induce intestinal inflammation and permeability. Cancer treatment also leads to increased peripheral inflammation, neuroinflammation, and impaired CNS function.48 Specifically, in chemotherapy-induced peripheral neuropathy, a dysbiotic gut microbiota activates inflammatory cells (e.g., T lymphocytes and macrophages), glial cells (e.g., astrocytes, microglia, and Schwann cells), and cell surface receptors (e.g., TLRs and PD-1), thereby inducing the production of pro- and anti-inflammatory cytokines or chemokines, all of which may contribute to the development of psychoneurological outcomes. Therefore, the immune-nervous-endocrine axis48 was conceptualized to strengthen the understanding of the MGB axis in cancer treatment-related PNS and toxicities.
The gut microbiota is associated with psychoneurological outcomes in cancer, including anxiety, depression, cognitive impairment, sleep disturbance, and peripheral neuropathy.44,46–49 These outcomes may be caused by the gut microbiota increasing neuroinflammation and psychoneurological behaviors via activating a peripheral immune response during chemotherapy.48 Further, a dysbiotic gut microbiota could activate inflammatory cells, glial cells, and cell surface receptors.48,49 Activations of these cells and cell receptors could induce the secretion of pro- and anti-inflammatory cytokines, which could bind to related receptors on nociceptors to change the excitability threshold, membrane potential, and other properties of ion channels.49 The gut microbiota could communicate with the brain via endocrine and neuroinflammation pathways, however, the fastest way for the microbiota to influence the brain is through hijacking vagus nerve.56,57 To date, vagus nerve pathway has not been well examined during cancer treatment and therefore is preceived as a potential mechanism in the MGB axis dysregulation.47 Current mechanistic studies have primarily focused on rodent models. More work is needed to understand the role of specific pathways (e.g., neuroinflammation, vagus nerve, and blood brain barriers) involved in the MGB axis in cancer treatment-related toxicitites and symptoms among human populations. In addition, cancer treatments can disrupt the gut barrier and allow lipopolysaccharide (LPS) to leak into the blood stream (“leaking gut”53,58), which can cause systemic inflammation with subsequent activation of sickness behaviors (e.g., lethargy, depression, reduced social exploration, appetite loss, sleepiness, hyperalgesia, and confusion59,60). Further confirmation of the “leaking gut” and its relevant mechanisms leading to PNS and sickness behaviors are particularly in need.
Understanding the pathways of the MGB axis can help identify innovative therapies for PNS and can improve treatment-related outcomes in cancer. Recent work points to promising interventions using prebiotics (i.e., nondigestible ingredients that support beneficial bacterial growth), probiotics (i.e., live microorganisms that provide health benefits by improving or restoring the gut flora), synbiotics (i.e., the combinations of probiotics and prebiotics), or fecal microbiota transplantation (FMT).48 Among patients with cancer, most of current work supported the preventive effect of probiotics on chemotherapy61,62 or radiation-related63,64 diarrhea65,66 and other side effects.67,68 Use of probiotics could help maintain the integrity of intestinal barrier, activate cyto-protective pathways, reduce reactive oxygen species, and replace pathogenic bacteria.69 Following these preliminary studies, clinical practice guidelines have been published to advocate the use of Lactobacillus-related probiotics to prevent chemotherapy- or radiation-related diarrhea in patients with pelvic malignancies.70 However, interventions targeting at PNS or the MGB axis are still in its infancy.48,71 Only one study demonstrated that L. rhamnosus and L. acidophilus treatments reduced symptoms of depression, anxiety, and fatigue in colorectal cancer survivors who had received chemotherapy.48 Furthermore, current clinical trials involving probiotics are limited by small sample sizes and differing combinations of probiotic strains and dosages, making meaningful comparisons between studies difficult. Prebiotic supplementation and FMT are even less studied than probiotic treatments.48 All of these innovative treatments require further investigation in cancer populations.
This study has several limitations. Due to only a few studies meeting our inclusion criteria, three review articles were included in this study. These review articles provided critical information to understand the pathways involved in the MGB axis. Additionally, this study only included studies published in English and may miss publications in other languagues. Lastly, this study only focuses on clinical studies. Future work should synthesize findings from both preclinical and clinical work to promote the knowledge translations between the bench and the bedside. With respect to the small sample size and restrictive inclusion criteria for these included studies (i.e., breast cancer and gynecological cancers), further investigation of the relationships between the MGB axis and treatment-related psychoneurological toxicities is warranted in diverse and large cancer populations. Future work should evaluate the effects of diverse cancer treatments such as chemotherapy, radiation therapy, and immunotherapy on the MGB axis and treatment-related PNS and toxicities.
Conclusions
The MGB axis provides a promising biological network for understanding associations between the gut microbiota and psychoneurological outcomes. Few studies have focused on the MGB axis and its affect on PNS, including fatigue, anxiety, depression, sleep disturbance, and cognition, in cancer populations. Current work on the MGB axis in cancer treatment-related outcomes primarily focuses on individual symptoms in cancer survivors who have undergone chemotherapy. A systematic evaluation of the links between the gut microbiota and PNS should include investigating cancer populations across different ages, different cancer types, and different treatment modalities. Further investigation of therapies such as prebiotics, probiotics, synbiotics, and FMT is needed, particularly in cancer populations.
Supplementary Material
Funding
Jinbing Bai was supported by grants from the National Institute of Health/National Institute of Nursing Research (1K99NR017897-01), NCI R25CA203650 (PI: Melinda Irwin), and the Oncology Nursing Foundation Grant from the Oncology Nursing Society.
Abbreviations
- 5-HT
5-hydroxytryptamine
- CNS
Central nervous system
- EEC
Enteroendocrine cell
- FMT
Fecal microbiota transplantation
- GI
Gastrointestinal
- GLP-1
Glucagon-like peptide 1
- JBI
Joanna Briggs Institute
- MGB
Microbiome-Gut-Brain Axis
- NPY
Neuropeptide Y
- PNS
Psychoneurological symptoms
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PYY
Peptide YY
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Declaration of Conflict of Interests The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. [DOI] [PubMed] [Google Scholar]
- 2.Knight R, Buhler B. Follow your gut : the enormous impact of tiny microbes. First TED Books hardcover edition. ed 2015. [Google Scholar]
- 3.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. [DOI] [PubMed] [Google Scholar]
- 4.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375(24):2369–2379. [DOI] [PubMed] [Google Scholar]
- 5.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. [DOI] [PubMed] [Google Scholar]
- 6.Vangay P, Ward T, Gerber Jeffrey S, Knights D. Antibiotics, Pediatric Dysbiosis, and Disease. Cell host & microbe. 2015;17(5):553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrett WS. Cancer and the microbiota. Science. 2015;348(6230):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zitvogel L, Daillere R, Roberti MP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol. 2017. [DOI] [PubMed] [Google Scholar]
- 9.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–285. [DOI] [PubMed] [Google Scholar]
- 10.Stringer AM, Al-Dasooqi N, Bowen JM, et al. Biomarkers of chemotherapy-induced diarrhoea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(7):1843–1852. [DOI] [PubMed] [Google Scholar]
- 11.Kelly DL, Lyon DE, Yoon SL, Horgas AL. The Microbiome and Cancer: Implications for Oncology Nursing Science. Cancer nursing. 2016;39(3):E56–62. [DOI] [PubMed] [Google Scholar]
- 12.Xiao C The state of science in the study of cancer symptom clusters. European Journal of Oncology Nursing. 2010;14(5):417–434. [DOI] [PubMed] [Google Scholar]
- 13.Touchefeu Y, Montassier E, Nieman K, et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40(5):409–421. [DOI] [PubMed] [Google Scholar]
- 14.van Vliet MJ, Tissing WJ, Dun CA, et al. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(2):262–270. [DOI] [PubMed] [Google Scholar]
- 15.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature reviews Gastroenterology & hepatology. 2009;6(5):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montiel-Castro AJ, Gonzalez-Cervantes RM, Bravo-Ruiseco G, Pacheco-Lopez G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Frontiers in integrative neuroscience. 2013;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strasser B, Becker K, Fuchs D, Gostner JM. Kynurenine pathway metabolism and immune activation: Peripheral measurements in psychiatric and co-morbid conditions. Neuropharmacology. 2016. [DOI] [PubMed] [Google Scholar]
- 18.Zwielehner J, Lassl C, Hippe B, et al. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PloS one. 2011;6(12):e28654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson G, Dobish R. Chemotherapy induced diarrhea. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2007;13(4):181–198. [DOI] [PubMed] [Google Scholar]
- 20.Morturano R Managementof chemotherapy-induced diarrhea. In. OncoLink, Penn Medicine,. Vol 2016 2012. [Google Scholar]
- 21.Amaral FA, Sachs D, Costa VV, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen S, Lim G, You Z, et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci. 2017;advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu YJ, Chiu CC, Li YP, et al. Effect of intestinal microbiota on exercise performance in mice. Journal of strength and conditioning research / National Strength & Conditioning Association. 2015;29(2):552–558. [DOI] [PubMed] [Google Scholar]
- 24.Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26(8):1155–1162. [DOI] [PubMed] [Google Scholar]
- 25.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. [DOI] [PubMed] [Google Scholar]
- 26.Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. [DOI] [PubMed] [Google Scholar]
- 27.Galloway-Pena JR, Smith DP, Sahasrabhojane P, et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montassier E, Batard E, Massart S, et al. 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microbial ecology. 2014;67(3):690–699. [DOI] [PubMed] [Google Scholar]
- 29.Rajagopala SV, Yooseph S, Harkins DM, et al. Gastrointestinal microbial populations can distinguish pediatric and adolescent Acute Lymphoblastic Leukemia (ALL) at the time of disease diagnosis. BMC genomics. 2016;17(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nature reviews Gastroenterology & hepatology. 2017;advance online publication. [DOI] [PubMed] [Google Scholar]
- 31.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology. 2004;558(Pt 1):263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23(3):255–264, e119. [DOI] [PubMed] [Google Scholar]
- 33.Wardill HR, Gibson RJ, Van Sebille YZ, et al. Irinotecan-Induced Gastrointestinal Dysfunction and Pain Are Mediated by Common TLR4-Dependent Mechanisms. Mol Cancer Ther. 2016;15(6):1376–1386. [DOI] [PubMed] [Google Scholar]
- 34.Loman BR, Jordan KR, Haynes B, Bailey MT, Pyter LM. Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice. Scientific reports. 2019;9(1):16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan KR, Loman BR, Bever SR, et al. Abstract # 3083 The role of gut-brain axis in chemotherapy-induced behavioral comorbidities. Brain, behavior, and immunity. 2019;76:e13. [Google Scholar]
- 36.Ramakrishna C, Corleto J, Ruegger PM, et al. Dominant Role of the Gut Microbiota in Chemotherapy Induced Neuropathic Pain. Scientific reports. 2019;9(1):20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(10):2143–2151. [DOI] [PubMed] [Google Scholar]
- 38.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26(5):643–652. [DOI] [PubMed] [Google Scholar]
- 39.Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Experimental & Molecular Medicine. 2018;50(8):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Frontiers in cellular neuroscience. 2015;9:392–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–130. [PMC free article] [PubMed] [Google Scholar]
- 42.Otten RA. Johns Hopkins Nursing Evidence-Based Practice Model and Guidelines. Nursing Education Perspectives. 2008;29(4):234. [Google Scholar]
- 43.Porritt K, Gomersall J, Lockwood C. JBI’s Systematic Reviews: Study selection and critical appraisal. The American journal of nursing. 2014;114(6):47–52. [DOI] [PubMed] [Google Scholar]
- 44.Okubo R, Kinoshita T, Katsumata N, Uezono Y, Xiao J, Matsuoka YJ. Impact of chemotherapy on the association between fear of cancer recurrence and the gut microbiota in breast cancer survivors. Brain, behavior, and immunity. 2019. [DOI] [PubMed] [Google Scholar]
- 45.Donovan H, Hagan T, Campbell G, et al. Nausea as a sentinel symptom for cytotoxic chemotherapy effects on the gut-brain axis among women receiving treatment for recurrent ovarian cancer: an exploratory analysis. Supportive Care in Cancer. 2016;24(6):2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulsen JA, Ptacek TS, Carter SJ, et al. Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bajic JE, Johnston IN, Howarth GS, Hutchinson MR. From the bottom-up: Chemotherapy and gut-brain axis dysregulation. Frontiers in Behavioral Neuroscience. 2018;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordan KR, Loman BR, Bailey MT, Pyter LM. Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong S, Zhou Z, Liang Y, et al. Targeting strategies for chemotherapy-induced peripheral neuropathy: does gut microbiota play a role? Crit Rev Microbiol. 2019;45(4):369–393. [DOI] [PubMed] [Google Scholar]
- 50.Dinan TG, Cryan JF. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterology clinics of North America. 2017;46(1):77–89. [DOI] [PubMed] [Google Scholar]
- 51.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2016. [DOI] [PubMed] [Google Scholar]
- 52.Bai J, Jhaney I, Daniel G, Watkins Bruner D. Pilot Study of Vaginal Microbiome Using QIIME 2 in Women With Gynecologic Cancer Before and After Radiation Therapy. Oncology nursing forum. 2019;46(2):E48–E59. [DOI] [PubMed] [Google Scholar]
- 53.Deleemans JM, Chleilat F, Reimer RA, et al. The chemo-gut study: investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC cancer. 2019;19(1):1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai J Exploring the Microbiome-Gut-Brain Axis in Psychoneurological Symptoms for Children with Solid Tumors In. Emory University, Atlanta, GA: National Institute of Health; 2020. [Google Scholar]
- 55.Lee JY, Chu SH, Jeon JY, et al. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014;46(12):1126–1132. [DOI] [PubMed] [Google Scholar]
- 56.Fülling C, Dinan TG, Cryan JF. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron. 2019;101(6):998–1002. [DOI] [PubMed] [Google Scholar]
- 57.Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Frontiers in neuroscience. 2018;12:49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollander D, Kaunitz JD. The “Leaky Gut”: Tight Junctions but Loose Associations? Digestive diseases and sciences. 2020;65(5):1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prather AA. Sickness Behavior In: Gellman MD, Turner JR, eds. Encyclopedia of Behavioral Medicine. New York, NY: Springer New York; 2013:1786–1788. [Google Scholar]
- 60.D’Mello C, Ronaghan N, Zaheer R, et al. Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain. J Neurosci. 2015;35(30):10821–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osterlund P, Ruotsalainen T, Korpela R, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007;97(8):1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pouncey AL, Scott AJ, Alexander JL, Marchesi J, Kinross J. Gut microbiota, chemotherapy and the host: the influence of the gut microbiota on cancer treatment. Ecancermedicalscience. 2018;12:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delia P, Sansotta G, Donato V, et al. Use of probiotics for prevention of radiation-induced diarrhea. World journal of gastroenterology : WJG. 2007;13(6):912–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urbancsek H, Kazar T, Mezes I, Neumann K. Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus in patients with radiation-induced diarrhoea. European journal of gastroenterology & hepatology. 2001;13(4):391–396. [DOI] [PubMed] [Google Scholar]
- 65.Hassan H, Rompola M, Glaser A, Kinsey SE, Phillips R. Systematic review and meta-analysis investigating the efficacy and safety of probiotics in people with cancer. Supportive Care in Cancer. 2018;26(8):2503–2509. [DOI] [PubMed] [Google Scholar]
- 66.Ciorba MA, Hallemeier CL, Stenson WF, Parikh PJ. Probiotics to prevent gastrointestinal toxicity from cancer therapy: an interpretive review and call to action. Current opinion in supportive and palliative care. 2015;9(2):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wada M, Nagata S, Saito M, et al. Effects of the enteral administration of Bifidobacterium breve on patients undergoing chemotherapy for pediatric malignancies. Supportive Care in Cancer. 2010;18(6):751–759. [DOI] [PubMed] [Google Scholar]
- 68.Zheng S, Steenhout P, Kuiran D, et al. Nutritional support of pediatric patients with cancer consuming an enteral formula with fructooligosaccharides. Nutrition Research. 2006;26(4):154–162. [Google Scholar]
- 69.Scott AJ, Merrifield CA, Younes JA, Pekelharing EP. Pre-, pro- and synbiotics in cancer prevention and treatment-a review of basic and clinical research. Ecancermedicalscience. 2018;12:869–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bai J, Behera M, Bruner D. A Systematic Review of the Gut Microbiome, Treatment-Related Symptoms and Targeted Interventions in Children with Cancer. Pediatric blood & cancer. 2017;64:S151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


