Abstract
Objectives:
To evaluate the relationship between phosphatase of regenerating liver 3 (PRL3) expression and clinical outcome in colorectal cancer (CRC).
Background:
PRL3, a protein tyrosine phosphatase functions as one of the key regulatory enzymes of various signal transduction pathways. PRL3 is highly expressed in a majority of cancers and is a novel potential therapeutic target.
Methods:
PRL3 expression was evaluated by immunohistochemistry in 167 patients with CRC, 37 patients with no disease, and 26 patients with metastatic CRC (mCRC). Phosphorylated Akt at serine 473 (p-Akt S473) expressions was also evaluated by immunohistochemistry in mCRC patients.
Results:
High expression of PRL3 was correlated with CRC progression, and every one unit increase in PRL3 level contributed to an increase in the rate of death by 1 to 1.7%. PRL3 expression was significantly higher in liver metastases compared with primary tumors and showed a significant positive correlation with the expression level of p-Akt S473.
Conclusion:
PRL3 expression levels correlated with CRC progression and metastasis, and positively correlated with activated Akt level in mCRC. Together, these findings indicated that PRL3 might be a potential marker for increased risk of CRC-specific tumor burden and identify PRL3 as an attractive therapeutic target for mCRC treatment.
Keywords: CRC, PRL3, Akt, cell survival, metastasis
INTRODUCTION
Metastasis is the main cause of cancer morbidity and mortality and is estimated to be responsible for about 90% of cancer related deaths [1]. Colorectal cancer (CRC) is the second leading cause of cancer related death in United States and 50–60% of patients will develop metastasis during the course of their disease [2]. Saha et al (2001) performed global gene expression profiles using serial analysis of gene expression (SAGE) technology to identify specific genes involved with CRC metastasis. They reported that phosphatase of regenerating liver 3 (PRL3) was consistently overexpressed in CRC metastases but noted low and intermediate expression in normal colon/benign tumors and early stage CRC respectively [3]. We have also previously reported the relationship between activation of PRL3 mediated cell survival pathway and CRC progression and selective promotion of metastasis [4].
PRL3 belongs to the PRL family of protein tyrosine phosphatases which encodes a small, 22-kD tyrosine phosphatase that is located at the cytoplasmic membrane when prenylated at its COOH-terminus and in the nucleus when it is not conjugated to this lipid [5]. This subfamily of small proteins includes three members; PRL1, PRL2, and PRL3 [6]. PRL1 and PRL2 are ubiquitously expressed, and PRL3 is expressed mainly in heart and skeletal muscle [7,8]. All three sub-groups have been reported to promote processes involved in the hallmarks of cancer such as cell survival and proliferation, tumor growth, invasion, migration and metastasis [7]. Various studies have reported upregulation of PRL3 in CRC [9–11]. PRL3 has also been found to be overexpressed in many other human cancer types such as prostate cancer [12,13], gastric cancer [14,15], ovarian cancer [16], and chronic myeloid leukemia [17]. PRL3 promotes cell survival and epithelial to mesenchymal transition (EMT) by acting upstream of phosphatidylinositol-3 kinase (PI3K)/serine threonine protein kinase Akt signaling [7,18]. PI3K is an oncogene and the PI3K/Akt signaling mediated promotion of cell survival, cell proliferation or EMT is well documented [19,20]. We have also extensively studied the role of cell survival in CRC [21–28], and reported that PRL3 promotes cell survival under growth factor deprivation stress by activating and maintaining the activity of the PI3K ⁄Akt pathway [4].
Aberrant expression of PRL3 has been associated with increased metastatic potential in CRC [29]. However, the correlation of PRL3 expression levels with increased CRC burden, tumor stages, distant recurrence and patients’ survival outcomes have not been fully evaluated. In the present study, we explore the prognostic value of PRL3 expression levels in CRC progression and metastasis.
MATERIALS AND METHODS
Patients and Colon Tissue Microarray (TMA)
Colon tissue microarrays (TMAs’) were purchased from the National Cancer Institute [30]. Details of the TMA were outlined in our previous publication [28]. Briefly, colon TMAs consisted of colon tissue specimens representing different colon cancer stages (obtained from colon cancer patients), normal colon epithelium (obtained from non-cancer diverticulitis surgeries), and tissues from adenomatous colon polyps diagnosed between 1989 to 1996. A total of 167 CRC patients and 37 non-cancer controls were included in the study. Another set of 26 patients with metastatic CRC (mCRC), who underwent surgical resection at the UNMC between 2000 and 2010 for both primary and liver metastatic tumors, were also included. All experiments involving human subjects at UNMC were approved by the UNMC Institutional Review Board (IRB#401–07-EP).
Antibodies
PRL3 antibody (catalog#sc-2045) was purchased from Santa Cruz Biotechnology (Dallas, TX). Phospho-Akt (S473) rabbit polyclonal antibody (catalog#3027) and phospho-Akt (S473) blocking peptide (catalog#1140) were purchased from Cell Signaling Technology (Danvers, MA).
Immunohistochemical analysis
PRL3 IHC analysis was performed on formalin-fixed paraffin-embedded tissue sections of human CRC (in TMA format or on separate slides) obtained retrospectively from the NCI and UNMC using standard IHC protocols [24,28]. p-Akt (S473) IHC was also performed on sections of human CRC tissues obtained from UNMC. Briefly, tissue slides were deparaffinized in histoclear and rehydrated in descending grades of ethanol. Following deparaffinization, tissue sections were subjected to antigen retrieval with citrate buffer and treatment with 3% H2O2 to block endogenous peroxidase activity. Sections were blocked with 5% normal goat serum at room temperature for 1 hour and immunostaining for PRL3 and p-Akt (S473) at 1:20 and 1:50 dilutions respectively, were done using an indirect detection method. Slides were incubated in either primary antibody or 1X tris-buffered saline with tween 20 (TBST; as a negative control) or blocking peptide (primary antibody and blocking peptide at 1:2 ratio) at 4°C overnight. After washing in 1X TBST, sections were incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories) at room temperature for 30 minutes, followed by incubation with HRP (horseradish peroxidase)-conjugated streptavidin (Invitrogen). Detection used the DAB (3,3’-diaminobenzidine) substrate chromogen kit (DAKO) following the manufacturer’s protocol, and sections were counterstained with hematoxylin (Protocol) and dehydrated with ethanol and histoclear. Specimens were processed on the same day to eliminate any variability in conditions. Slides were scanned using Ventana’s Coreo Au Slide Scanner in the UNMC Tissue Sciences Facility and staining intensity was measured and quantified with NIH Image J [31] and GraphPad Prism version 8 software (GraphPad Software Inc., San Diego, California USA), respectively.
Statistical analysis
To account for the complex sampling design of the NCI colon TMAs, SAS software (SAS Institute Inc., Cary, NC, USA) was used for all statistical analysis. Due to the unavailability of the full TMA for analysis, weights were recalculated based on the available samples to preserve the original stratified sampling design. Stratum weights were calculated as wk=Nk/nk, where Nk is the potential number of samples in the stratum provided in the table of the distribution of cases from the NCI, nk is the number of cases available from each stratum, and wk is the recalculated weight. Weighted sample estimates, standard errors, and 95% confidence limits (CL) were calculated using the Taylor expansion method. T-tests and analysis of variance (ANOVA) models were used to compare PRL3 expression by patient and clinicopathologic characteristics. Cox proportional hazards regression was used for univariate comparison of patient characteristics for overall survival (OS), recurrence free survival (RFS), and disease specific survival (DSS). Variables that were statistically significant in the univariate models were included in a backward selected multivariable Cox regression analysis, and those with p < 0.1 were retained in the multivariable models. All analyses were weighted and stratified except the cumulative incidence analyses. Statistical tests were 2-sided and p < 0.05 were considered statistically significant.
For human metastatic CRC data, paired t-tests was used to compare PRL3 and p-Akt expression status in primary tumors and liver metastases. Correlation of PRL3 and p-Akt (S473) expression levels in metastatic CRC tumors were compared using Pearson correlation coefficient analysis. P < 0.05 were considered statistically significant.
RESULTS
Characteristic of patients with CRC
A cohort of 167 colon cancer patients and 37 individuals without cancer or bearing colon adenomas “designated as normal” were used to generate the TMAs from the NCI. Complete demographic and clinicopathologic features of these 167 patients are summarized in Table 1. One hundred and sixteen patients (67%) were older than 65 years of age, and female patients accounted for 55% of the entire cohort. Of the 167 CRC patients, 112 (66.2%) were deceased at last contact. The median follow-up for patients was 89 months and ranged from 18 to 148 months.
Table 1.
Patient and pathologic variables, with comparison of mean PRL3 by groups in CRC primary cases
| Parameters | N | Weighted Percent | Weighted Mean PRL3 | SE of Mean PRL3 | p-value | |
|---|---|---|---|---|---|---|
| Age at diagnosis (years) | < 65 | 51 | 32.6% | 46.29 | 2.25 | 0.65 |
| >=65 | 116 | 67.4% | 45.00 | 1.63 | ||
| Race | White | 160 | 95.5% | 44.91 | 1.32 | - |
| Black | 2 | 1.6% | 64.35 | 6.31 | ||
| Unknown | 5 | 2.9% | 51.82 | 5.05 | ||
| Gender | Male | 77 | 44.7% | 44.29 | 1.98 | 0.47 |
| Female | 90 | 55.3% | 46.33 | 1.81 | ||
| Number of Positive Nodes | 0 | 83 | 55.5% | 40.96 | 1.76 | 0.0002 |
| >=1 | 84 | 44.5% | 50.97 | 1.93 | ||
| Proximal Margin Involvement | Uninvolved by tumor | 167 | 100% | 45.42 | 1.30 | - |
| Distal Margin Involvement | Uninvolved by tumor | 166 | 99.3% | 45.41 | 1.30 | - |
| Involved by tumor | 1 | 0.7% | 46.87 | 0.00 | ||
| Stage | I | 25 | 18.2% | 35.07 | 2.85 | 0.0001 |
| II | 55 | 34.4% | 43.40 | 2.25 | ||
| III | 67 | 28.3% | 48.68 | 2.06 | ||
| IV | 20 | 19.1% | 54.08 | 3.59 | ||
| Histology | Adenocarcinoma | 148 | 89.9% | 46.46 | 1.36 | 0.025^ |
| Mucinous adenocarcinoma | 15 | 8.4% | 36.31 | 4.21 | ||
| Signet ring cell carcinoma | 3 | 1.2% | 29.94 | 3.57 | ||
| Undifferentiated carcinoma | 1 | 0.5% | 47.39 | 0.00 | ||
| Histology Grade | G1 Well differentiated | 85 | 51.2% | 43.25 | 1.69 | 0.26 |
| G2 Moderately differentiated | 59 | 36.8% | 48.25 | 2.46 | ||
| G3 Poorly differentiated | 11 | 5.5% | 40.93 | 4.70 | ||
| G4 Undifferentiated | 12 | 6.5% | 50.21 | 5.99 | ||
| Location | Right (ascending) colon | 22 | 13.4% | 47.80 | 4.09 | 0.23 |
| Hepatic flexure | 10 | 5.1% | 50.07 | 3.23 | ||
| Transverse colon | 18 | 10.9% | 44.36 | 2.80 | ||
| Splenic flexure | 8 | 4.3% | 55.74 | 5.75 | ||
| Left (descending) colon | 7 | 3.3% | 50.16 | 4.59 | ||
| Rectosigmoid junction | 3 | 1.8% | 34.90 | 1.86 | ||
| Cecum | 34 | 21.8% | 47.13 | 3.31 | ||
| Sigmoid colon | 65 | 39.4% | 42.28 | 2.16 | ||
| Blood/lymphatic vessel invasion | Intramural vessel | 13 | 7.5% | 44.25 | 5.94 | 0.22 |
| Extramural vessel | 8 | 4.5% | 57.00 | 6.72 | ||
| Absent | 145 | 87.9% | 44.99 | 1.35 | ||
| Recurrence | No | 116 | 79.6% | 46.01 | 1.54 | 0.28 |
| Yes | 51 | 20.4% | 43.12 | 2.14 | ||
| Type of Recurrence | Local | 3 | 2.7% | 44.38 | 1.45 | <0.0001 |
| Regional | 3 | 2.9% | 39.29 | 2.31 | ||
| Distant | 45 | 46.0% | 43.29 | 2.25 | ||
| Never Disease Free | 20 | 48.4% | 54.08 | 3.59 | ||
| Chemotherapy | No | 156 | 93.2% | 44.83 | 1.34 | 0.14 |
| Yes | 11 | 6.8% | 53.45 | 5.57 | ||
| Radiation Therapy | No | 167 | 100% | 45.42 | 1.30 | |
| Surgery | No | 1 | 1.0% | 81.20 | 0.00 | - |
| Yes | 166 | 99.0% | 45.07 | 1.28 | ||
- No comparison made due to small sample sizes
Comparison Adenocarcinoma vs. Mucinous Adenocarcinoma
Another set of 26 mCRC patients with matched primary tumor and liver metastasis was obtained through the UNMC Tissue Science Facility.
PRL3 expression level serves as a predictive and prognostic biomarker in CRC patients.
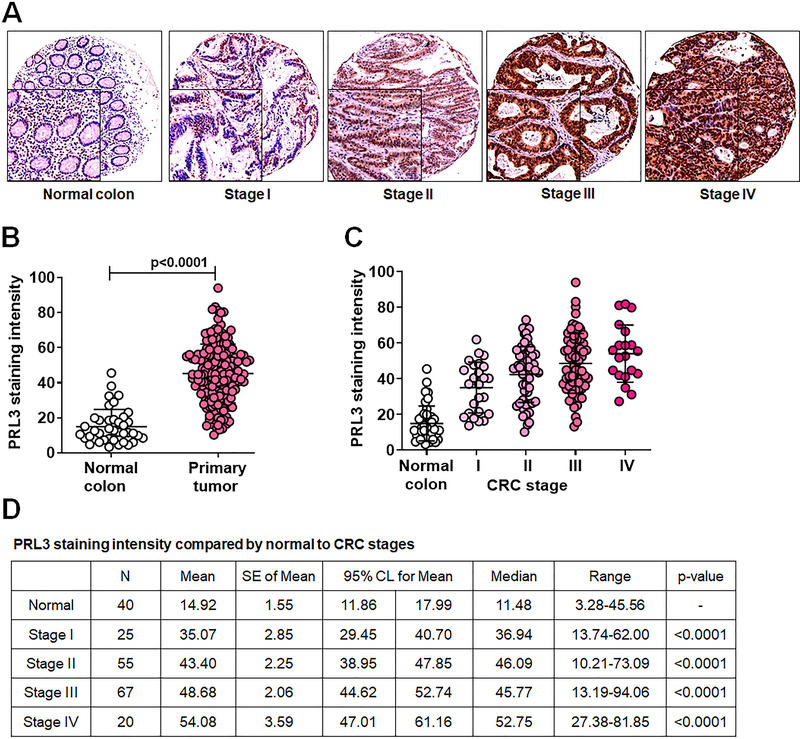
Consistent with previous findings [3], PRL3 expression levels were low/intermediate in normal colon epithelium/early stages of colon cancer tissue samples and significantly elevated in late stage colon cancer (Figure 1A). IHC statistical analysis demonstrated a significant increase in PRL3 expression in primary colon tumors compared with normal colon (p < 0.0001, Figure 1B); furthermore, there was a significant increase in PRL3 levels with CRC progression, with stage IV having the highest expression of the protein (p < 0.0001, Figures 1C and 1D). Patient characteristics and distribution of clinicopathological variables in accordance to PRL3 expression levels are summarized in Table 1. In general, we noted a statistically significant increase in PRL3 expression in cases with one or more positive nodes invasion (p = 0.0002), TNM stage (p = 0.0001), tumor histology (p = 0.025), and type of recurrence (p < 0.0001). However, the levels of PRL3 expression showed no correlation with other clinicopathological features such as age at diagnosis, gender, tumor histology grade, location, blood/lymphatic vessel invasion, and chemotherapeutic status of patients (Table 1).
Figure 1: PRL3 expression is elevated in human CRC patient specimens.
A, IHC analysis of PRL3 expression in TMAs consisting of normal colon tissues and stage I-IV CRC specimens. B - D, Statistical analysis of PRL3 staining intensity in colon samples. A p < 0.05 is considered statistically significant. PRL3, phosphatase of regenerating liver 3; CRC, colorectal cancer; IHC, immunohistochemistry; TMA, tissue microarray.
To evaluate the association of PRL3 with CRC prognosis, univariate and multivariate Cox proportional hazard regression models were employed to determine whether PRL3 expression was an independent risk factor for CRC prognosis, as shown in tables 2 and 3. In univariate analysis, high levels of PRL3 expression was marginally predictive for RFS (p = 0.082). Interestingly, we observed a significant increase in the risk of death by 1–1.7% for every one unit increase in PRL3 level (p = 0.0396 to 0.077) (Table 2). The analysis also showed that age ≥ 65 was predictive for OS and RFS (p = 0.0274 to 0.028); and number of positive lymph node status (p < 0.0001), TNM stage (p < 0.0001), and blood/lymphatic vessel invasion (p < 0.001 to = 0.001) were predictive of OS, RFS and DSS (Table 2). Multivariate analysis showed a significant correlation of PRL3 level with advanced stage (p < 0.0001) and blood/lymphatic vessel invasion (p = 0.0024 to 0.051) (Table 3).
Table 2.
Univariate Cox regression model analyses of Overall survival, Recurrence free survival and Disease specific survival with PRL3 staining as continuous variable
| Parameters | Overall Survival (OS) | Recurrence free survival (RFS) | Disease Specific Survival (DSS) | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CL) | p-value | HR (95% CL) | p-value | HR (95% CL) | p-value | ||
| PRL3 staining | 1 unit increase | 1.01 (1 – 1.02) | 0.0589 | 1.01 (0.999 – 1.02) | 0.077 | 1.02 (1.00 – 1.03) | 0.0396 |
| PRL3 category | ≥ Median | 0.97 (0.66 – 1.43) | 0.8741 | 0.96 (0.65 – 1.41) | 0.082 | 1.04 (0.60 – 1.79) | 0.89 |
| < Median | 1 | 1 | 1 | ||||
| Age | ≥ 65 | 1.68 (1.06 – 2.66) | 0.0274 | 1.66 (1.06 – 2.62) | 0.028 | 1.08 (0.61 – 1.92) | 0.79 |
| < 65 | 1 | 1 | 1 | ||||
| Gender | Female | 1.07 (0.68 – 1.52) | 0.9336 | 1.01 (0.68 – 1.50) | 0.98 | 1.13 (0.65 – 1.95) | 0.67 |
| Male | 1 | 1 | 1 | ||||
| Number of nodes positive | ≥ 1 | 2.78 (2.07 – 3.74) | <.0001 | 2.85 (2.15 – 3.79) | <.0001 | 7.16 (4.21 – 12.17) | <.0001 |
| 0 | 1 | 1 | 1 | ||||
| Stage | IV | 22.26 (11.48 – 43.17) | <.0001 | 17.45 (9.33 – 32.65) | <.0001 | 11.74 (7.48 – 18.44) | <.0001 |
| III | 2.85 (1.65 – 4.91) | 3.01 (1.74 – 5.20) | |||||
| II | 1.69 (0.94 – 3.04) | 1.70 (0.95 – 3.02) | 1 | ||||
| I | 1 | 1 | |||||
| Histology | Adenocarcinoma | 1.12 (0.65 – 1.93) | 0.68 | 1.11 (0.63 – 1.95) | 0.72 | 1.30 (0.56 – 3.00) | 0.55 |
| Mucinous adenocarcinoma | 1 | 1 | 1 | ||||
| Histology grade | G4 | 1.51 (0.58 – 3.96) | 0.82 | 1.42 (0.54 – 3.77) | 0.87 | 2.06 (0.65 – 6.58) | 0.20 |
| G3 | 1.23 (0.41 – 3.67) | 1.18 (0.39 – 3.57) | 2.35 (0.76 – 7.24) | ||||
| G2 | 1.1 (0.73 – 1.71) | 1.10 (0.72 – 1.69) | 1.61 (0.90 – 2.87) | ||||
| G1 | 1 | 1 | 1 | ||||
| Blood/lymphatic vessel invasion | Intramural vessel | 2.56 (1.16 – 5.66) | 0.0008 | 2.55 (1.14 – 5.67) | 0.001 | 3.98 (1.64 – 9.66) | <0.0001 |
| Extramural vessel | 4.22 (1.72 – 10.36) | 5.14 (1.80 – 14.68) | 6.35 (2.55 – 15.77) | ||||
| Absent | 1 | 1 | 1 | ||||
G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; G4, undifferentiated
Table 3.
Multivariate Cox regression model analyses of Overall survival, Recurrence free survival and Disease specific survival with PRL3 staining as continuous variable
| Parameters | Overall survival (OS) | Recurrence free survival (RFS) | Disease specific survival (DSS) | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CL) | p-value | HR (95% CL) | p-value | HR (95%CL) | p-value | ||
| PRL3 Staining | 1 unit increase | 1.00 (0.99 – 1.02) | 0.56 | 1.00 (0.99 – 1.01) | 0.69 | 1.00 (0.99 – 1.02) | 0.76 |
| Age | ≥ 65 | 1.44 (0.94 – 2.21) | 0.094 | ||||
| < 65 | 1 | ||||||
| Stage | III/IV | 2.75 (1.99 – 3.80) | <0.0001 | 2.92 (2.16 – 3.95) | <0.0001 | 10.50 (6.30 – 17.49) | <0.0001 |
| I/II | 1 | 1 | 1 | ||||
| Blood/lymphatic vessel invasion | Intramural vessel | 1.85 (0.93 – 3.69) | 0.051 | 2.02 (1.01 – 4.05) | 0.026 | 2.41 (1.06 – 5.45) | 0.0024 |
| Extramural vessel | 2.42 (0.91 – 6.41) | 2.93 (0.99 – 8.68) | 2.60 (1.01 – 6.72) | ||||
| Absent | 1 | 1 | 1 | ||||
Correlation of PRL3 and phosphorylated Akt expression in human mCRC specimens
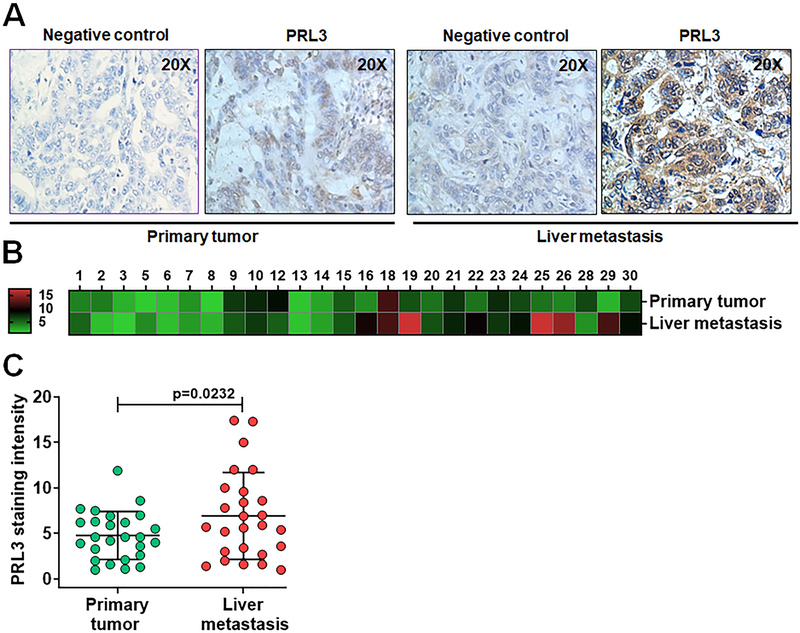
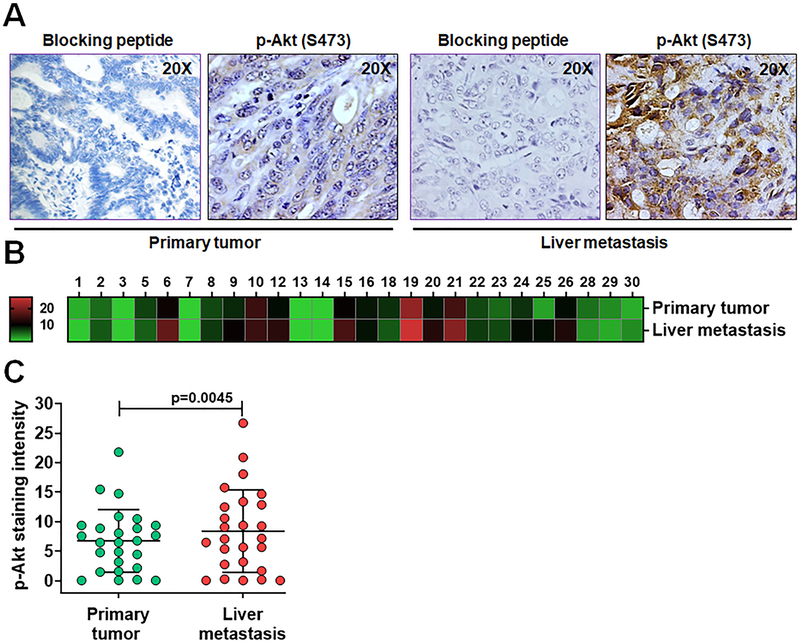
Studies have shown that PRL3 is associated with increased metastatic potential in several cancers including CRC [9,32,33]. PRL3 has been reported to activate PI3K/Akt signaling in different cancer cell models [18,34] and we have shown that PRL3 activates Akt through phosphorylation at serine 473 in colon cancer cells [4]. In the present study, we investigated the levels of PRL3 and phosphorylated Akt (S473) expressions in 26 paired human CRC primary tumor and liver metastatic tissue specimens using IHC methods. As expected, weak and homogenous PRL3 expression was detected in uninvolved normal colorectal epithelium similar to the observation in TMA analysis in Figure 1 (data not shown). However, both primary and metastatic CRC display significant variation in PRL3 expression among cases and PRL3 staining distributed heterogeneously (Figure 2A). Heat map dendogram was generated to provide visual representation of score for relative PRL3 expression levels analyzed on primary tumors and liver metastatic tissues (Figure 2B). There was a significantly higher PRL3 expression in liver metastatic tissues compared with primary tumor tissues (p = 0.0232, Figure 2C). Interestingly, p-Akt (S473) expression levels were also significantly higher in liver metastasis compared with the matched primary tumors as represented by IHC pictures and the corresponding heat map generated (Figures 3A and 3B). The difference of p-Akt (S473) expression is statistically significant between two groups (p = 0.0045, Figure 3C).
Figure 2: IHC analysis of PRL3 expression in human metastatic CRC patient specimens.
A, IHC analyses of PRL3 expression in human stage IV primary CRCs and matched liver metastatic tumor tissues. B, Heat map of PRL3 staining in human stage IV primary CRCs and matched liver metastatic specimens. C, Densitometric analysis of human stage IV CRCs and matched liver metastases stained for PRL3 by Image J, followed by statistical analysis. A p < 0.05 is considered statistically significant. IHC, immunohistochemistry; PRL3, phosphatase of regenerating liver 3, CRC, colorectal cancer.
Figure 3: IHC analysis of p-Akt (S473) expression in human metastatic CRC patient specimens.
A, IHC analyses of p-Akt (S473) expression in human stage IV CRCs and matched liver metastatic tumor tissues. B, Heat map of p-Akt (S473) staining in human stage IV primary CRCs and matched liver metastatic specimens. C, Densitometric analysis of human stage IV CRCs and matched liver metastases stained for p-Akt (S473) by Image J, followed by statistical analysis. A p < 0.05 is considered statistically significant. IHC, immunohistochemistry; p-Akt (S473), phosphorylated Akt at serine 473; CRC, colorectal cancer.
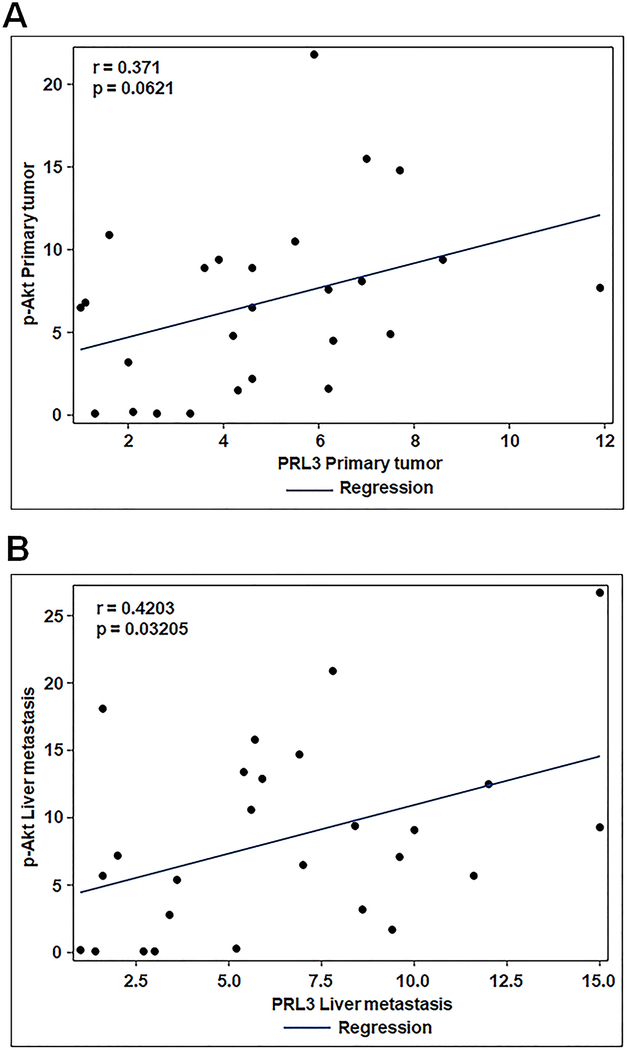
To further understand the relation between PRL3 and phosphorylated Akt (S473) in mCRC, Pearson correlation was performed. We observed a significant positive correlation between PRL3 and p-Akt (S473) expression levels in liver metastasis (r = 0.4203, p = 0.03205; Figure 4A) and a marginal positive correlation in primary CRC (r = 0.371, p = 0.0621; Figure 4B).
Figure 4: Correlation of PRL3 and p-Akt (S473) expression levels in human metastatic CRC patient specimens.
A, B, Correlation of co-efficient analysis of PRL3 and p-Akt (S473) levels in human stage IV CRCs and matched liver metastatic tumor tissues. A p < 0.05 is considered statistically significant. PRL3, phosphatase of regenerating liver 3, p-Akt (S473), phosphorylated Akt at serine 473.
DISCUSSION
The study presented here investigates the potential role of PRL3 to serve as prognostic and metastatic predictive biomarker in CRC patients. We showed the association of high PRL3 expression with CRC progression and metastasis, and the positive correlation of PRL3 with PI3K/Akt cell survival signaling in metastatic CRC.
PRL3, together with other protein tyrosine phosphatases functions as a key regulatory enzyme in various signal transduction pathways [35]. High PRL3 level is associated with the development of many cancers including CRC [9–11]. Increased PRL3 expression in CRC induces epithelial to mesenchymal translocation (EMT) and undergoes progressive alterations of expression during the CRC adenoma-carcinoma development [18]. Experiments we performed in a cohort of 167 patients with CRC and 37 control subjects provided strong evidence that PRL3 levels in patients with CRC were significantly higher than in healthy non-cancer controls. Moreover, PRL3 expression levels were correlated with CRC progression and significantly higher in patients with stage III/IV CRC than in stage I/II CRC. Interestingly, for every one unit increase in PRL3 level we observed 1 to 1.7% increase risk of death in CRC patients (p = 0.0396 to 0.077). In another subset of 26 matched human primary and mCRC tissue samples, we observed a significant increase in PRL3 expression in 50% of liver metastatic tissue samples, indicating PRL3 may also contribute to CRC metastasis.
PRL3 has been reported to play a role in the regulation of several pathways, such as the PI3K/AKT [18], Src [36], and ERK [37] pathways. Aberrant cell survival is one of the hallmarks of cancer [38], and PI3K/Akt and its downstream signaling components are critical for cancer cell survival [20]. PRL3 activates PI3K/Akt signaling via inhibition of PTEN and activation of Akt [18]. We have also reported that PRL3 activates Akt as reflected by increased phosphorylation at serine 473 [4]. In the present study, we demonstrated that high PRL3 expression level correlated with activated Akt expression in mCRC. Therefore, we speculate that high PRL3 level in CRC cells increase their survival capabilities in tumor microenvironment, which is partly mediated through the Akt signaling pathway, thereby, leading to tumor progression and metastatic colonization.
High expression of PRL3 in biopsied and surgical colorectal cancer specimens may provide clinicians with useful information for identifying patients with CRC occult metastases. The findings in the present study is limited due to the small sample size of metastatic CRC, and more samples are required to validate the findings. Additional investigations are also warranted to clarify the role(s) of PRL3/Akt mediated aberrant cell survival in the process of CRC metastasis and to develop novel anti-metastatic therapeutic strategies against PRL3.
CONCLUSIONS
This study demonstrates PRL3 as a probable metastatic-predictive biomarker in CRC patients. PRL3 level correlated with CRC progression and there was a significant association of PRL3 expression levels with CRC metastatic phenotype. Our data also suggest a positive correlation of PRL3 with cell survival marker, p-Akt (S473) indicating that PRL3 positive tumor cells might partly contribute to CRC progression and metastasis. Therefore, PRL3 may be a novel potential prognostic biomarker for CRC progression and might aid in the identification of patients with potential liver metastasis.
SYNOPSIS.
Phosphatase of regenerating liver 3 (PRL3) is reported to be upregulated in many cancers including colorectal cancer (CRC). We analyzed PRL3 expression in two sets of CRC patient specimens and reported a significant association between PRL3 levels and CRC tumor burden. We also demonstrated a strong positive correlation between PRL3 and activated Akt expression levels in metastatic CRC specimens.
ACKNOWLEDGEMENT
This study was financially supported by the National Institute of Health (NIH) grants R01CA038173 and R01CA054807 to MGB, R01CA208063, R01CA212241 and R01CA215389 to JW and by the Fred and Pamela Buffett Cancer Center grant P30 CA036727.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVIALABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCE
- 1.Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Science. 2011;331(6024):1559–1564. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Saha S, Bardelli A, Buckhaults P, et al. A Phosphatase Associated with Metastasis of Colorectal Cancer. Science. 2001;294(5545):1343–1346. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Liu X-Q, Rajput A, et al. Phosphatase PRL-3 Is a Direct Regulatory Target of TGFβ in Colon Cancer Metastasis. Cancer Research. 2011;71(1):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Q, Si X, Horstmann H, et al. Prenylation-dependent Association of Protein-tyrosine Phosphatases PRL-1, −2, and −3 with the Plasma Membrane and the Early Endosome. Journal of Biological Chemistry. 2000;275(28):21444–21452. [DOI] [PubMed] [Google Scholar]
- 6.Al-aidaroos AQO, Yuen HF, Guo K, et al. Metastasis-associated PRL-3 induces EGFR activation and addiction in cancer cells. The Journal of Clinical Investigation. 2013;123(8):3459–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rios P, Li X, Köhn M. Molecular mechanisms of the PRL phosphatases. The FEBS Journal. 2013;280(2):505–524. [DOI] [PubMed] [Google Scholar]
- 8.Hardy S, Kostantin E, Hatzihristidis T, et al. Physiological and oncogenic roles of the PRL phosphatases. The FEBS Journal. 2018;285(21):3886–3908. [DOI] [PubMed] [Google Scholar]
- 9.Bardelli A, Saha S, Sager JA, et al. PRL-3 Expression in Metastatic Cancers. Clinical Cancer Research. 2003;9(15):5607–5615. [PubMed] [Google Scholar]
- 10.Sundar J, Gnanasekar M. Can dehydroepiandrostenedione (DHEA) target PRL-3 to prevent colon cancer metastasis? Medical Hypotheses. 2013;80(5):595–597. [DOI] [PubMed] [Google Scholar]
- 11.Lan Q, Lai W, Zeng Y, et al. CCL26 Participates in the PRL-3–Induced Promotion of Colorectal Cancer Invasion by Stimulating Tumor-Associated Macrophage Infiltration. Molecular Cancer Therapeutics. 2018;17(1):276–289. [DOI] [PubMed] [Google Scholar]
- 12.Andersen S, Richardsen E, Rakaee M, et al. Expression of phosphatase of regenerating liver (PRL)-3, is independently associated with biochemical failure, clinical failure and death in prostate cancer. PLOS ONE. 2017;12(11):e0189000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandsemb EN, Bertilsson H, Abdollahi P, et al. Phosphatase of regenerating liver 3 (PRL-3) is overexpressed in human prostate cancer tissue and promotes growth and migration. Journal of Translational Medicine. 2016;14(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miskad UA, Semba S, Kato H, et al. High PRL-3 expression in human gastric cancer is a marker of metastasis and grades of malignancies: an in situ hybridization study. Virchows Archiv. 2007;450(3):303–310. [DOI] [PubMed] [Google Scholar]
- 15.Xing X, Lian S, Hu Y, et al. Phosphatase of regenerating liver-3 (PRL-3) is associated with metastasis and poor prognosis in gastric carcinoma. Journal of Translational Medicine. 2013;11(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polato F, Codegoni A, Fruscio R, et al. PRL-3 Phosphatase Is Implicated in Ovarian Cancer Growth. Clinical Cancer Research. 2005;11(19):6835–6839. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Cheong L-L, Liu S-C, et al. The pro-metastasis tyrosine phosphatase, PRL-3 (PTP4A3), is a novel mediator of oncogenic function of BCR-ABL in human chronic myeloid leukemia. Molecular Cancer. 2012;11(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Quah SY, Dong JM, et al. PRL-3 Down-regulates PTEN Expression and Signals through PI3K to Promote Epithelial-Mesenchymal Transition. Cancer Research. 2007;67(7):2922–2926. [DOI] [PubMed] [Google Scholar]
- 19.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Current Opinion in Oncology. 2006;18(1):77–82. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Kuropatwinski K, Hauser J, et al. Colon carcinoma cells harboring PIK3CA mutations display resistance to growth factor deprivation induced apoptosis. Mol Cancer Ther. 2007;6(3):1143–1150. [DOI] [PubMed] [Google Scholar]
- 22.Guo XN, Rajput A, Rose R, et al. Mutant PIK3CA-bearing colon cancer cells display increased metastasis in an orthotopic model. Cancer Res. 2007;67(12):5851–5858. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Yang L, Yang J, et al. Transforming growth factor beta induces apoptosis through repressing the phosphoinositide 3-kinase/AKT/survivin pathway in colon cancer cells. Cancer Res. 2008;68(9):3152–3160. [DOI] [PubMed] [Google Scholar]
- 24.Leiphrakpam PD, Rajput A, Mathiesen M, et al. Ezrin expression and cell survival regulation in colorectal cancer. Cell Signal. 2014;26(5):868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leiphrakpam PD, Agarwal E, Mathiesen M, et al. In vivo analysis of insulin-like growth factor type 1 receptor humanized monoclonal antibody MK-0646 and small molecule kinase inhibitor OSI-906 in colorectal cancer. Oncol Rep. 2014;31(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leiphrakpam P Role of Ezrin in Colorectal Cancer Cell Survival Regulation. Theses & Dissertations. 2017;241 https://digitalcommons.unmc.edu/etd/241. Accessed July 2019. [Google Scholar]
- 27.Leiphrakpam PD, Brattain MG, Black JD, Wang JJ. TGFβ and IGF1R signaling activate protein kinase A through differential regulation of ezrin phosphorylation in colon cancer cells. Journal of Biological Chemistry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leiphrakpam PD, Lazenby AJ, Chowdhury S, et al. Prognostic and therapeutic implications of NHERF1 expression and regulation in colorectal cancer. Journal of Surgical Oncology. 2019;n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weidle UH, Birzele F, Krüger A. Molecular targets and pathways involved in liver metastasis of colorectal cancer. Clinical & Experimental Metastasis. 2015;32(6):623–635. [DOI] [PubMed] [Google Scholar]
- 30.National Cancer Institute. Cancer Diagnostic Program, Division of Cancer Treatment & Diagnosis. 2012; https://cdp.cancer.gov/. Accessed July 2019.
- 31.Rasband WS, Image J, U. S. National Institutes of Health, Bethesda, Maryland, USA; 1997–2008. https://imagej.nih.gov/ij/. Accessed December 2019. [Google Scholar]
- 32.Al-Aidaroos AQO, Zeng Q. PRL-3 phosphatase and cancer metastasis. Journal of Cellular Biochemistry. 2010;111(5):1087–1098. [DOI] [PubMed] [Google Scholar]
- 33.Khapare N, Kundu ST, Sehgal L, et al. Plakophilin3 Loss Leads to an Increase in PRL3 Levels Promoting K8 Dephosphorylation, Which Is Required for Transformation and Metastasis. PLOS ONE. 2012;7(6):e38561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R, Bhattacharya R, Ye X, et al. Endothelial Cells Promote Colorectal Cancer Cell Survival by Activating the HER3-AKT Pathway in a Paracrine Fashion. Molecular Cancer Research. 2019;17(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krndija D, Münzberg C, Maass U, et al. The phosphatase of regenerating liver 3 (PRL-3) promotes cell migration through Arf-activity-dependent stimulation of integrin α5 recycling. Journal of Cell Science. 2012;125(16):3883–3892. [DOI] [PubMed] [Google Scholar]
- 36.Liang F, Liang J, Wang W-Q, et al. PRL3 Promotes Cell Invasion and Proliferation by Down-regulation of Csk Leading to Src Activation. Journal of Biological Chemistry. 2007;282(8):5413–5419. [DOI] [PubMed] [Google Scholar]
- 37.Ming J, Liu N, Gu Y, et al. PRL-3 facilitates angiogenesis and metastasis by increasing ERK phosphorylation and up-regulating the levels and activities of Rho-A/C in lung cancer. Pathology. 2009;41(2):118–126. [DOI] [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]